Abstract
Post-traumatic epilepsy (PTE) is a consequence of traumatic brain injury (TBI), occurring in 10–25% of patients with moderate to severe injuries. The development of animal models for testing antiepileptogenic therapies and validation of biomarkers to follow epileptogenesis in humans necessitates sophisticated understanding of the subtypes of PTE, which is the objective of this study. In this study, retrospective review was performed of patients with moderate to severe TBI with subsequent development of medically refractory epilepsy referred for video-electroencephalography (EEG) monitoring at a single center over a 10-year period. Information regarding details of injury, neuroimaging studies, seizures, video-EEG, and surgery outcomes were collected and analyzed. There were 123 patients with PTE identified, representing 4.3% of all patients evaluated in the epilepsy monitoring unit. Most of them had localization-related epilepsy, of which 57% had temporal lobe epilepsy (TLE), 35% had frontal lobe epilepsy (FLE), and 3% each had parietal and occipital lobe epilepsy. Of patients with TLE, 44% had mesial temporal sclerosis (MTS), 26% had temporal neocortical lesions, and 30% were nonlesional. There was no difference in age at injury between the different PTE subtypes. Twenty-two patients, 13 of whom had MTS, proceeded to surgical resection. At a mean follow-up of 2.5 years, Engel Class I outcomes were seen in 69% of those with TLE and 33% of those with FLE. Our findings suggest PTE is a heterogeneous condition, and careful evaluation with video-EEG monitoring and high resolution MRI can identify distinct syndromes. These results have implications for the design of clinical trials of antiepileptogenic therapies for PTE.
Key words: : EEG, post-traumatic epilepsy, seizure, surgical resection, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a heterogeneous condition, resulting in both focal and diffuse neuronal injury, and is a leading cause of death and disability worldwide, resulting in major social, economic, and health problems.1,2 Data from the Centers for Disease Control in 2006 indicated that in the United States, there was an annual incidence of about 1.4 million emergency department visits and close to 300,000 hospitalizations for TBI. Annually, TBI contributes to a substantial burden of lifelong disability.3 Physical, cognitive, emotional, and behavioral sequelae can result from TBI, resulting in life-altering consequences.4
Post-traumatic epilepsy (PTE), defined as recurring seizures occurring more than 7 days after injury, complicate 3–5% of moderate TBIs and as many as 25–50% of severe TBIs.5 TBI accounts for approximately 4% of focal epilepsy in the general population and is the leading cause of epilepsy with onset in young adulthood (age 15–24 years).6,7
The onset of PTE can be up to several years after the brain injury. Most patients are empirically started on antiepileptic medications after the first late post-traumatic seizure. In most cases, treatment significantly reduces the frequency of seizures.8 In other cases, however, seizures are refractory to medical management, which often prompts evaluations in epilepsy monitoring units (EMU). Confirmed PTE accounts for 5% of all referrals to specialized epilepsy centers.9
In some cases, medically intractable PTE arises from the mesial temporal structures (the hippocampus, amygdala, and parahippocampal gyrus), presumably resulting from diffuse injury mechanisms that result in damage to vulnerable neuronal or axonal populations and may result in mesial temporal sclerosis (MTS).10 In such cases, resection of the anterior temporal structures can be curative,11–13 although previous studies have reported less favorable surgical outcomes than in cryptogenic MTS.14
Many patients with PTE do not have MTS, however.10 Other refractory epilepsies can occur from focal neocortical scars resulting from localized contusions and intracranial hemorrhages from such mechanisms as penetrating head trauma. The pre-surgical evaluation for such cases is more time intensive and invasive, because seizure evaluations using subdural grids and strips as well as functional mapping of the sensory, motor, and language areas are often needed. Despite the time investment and higher risk, this approach is often less successful in localizing and curing the epilepsy.15
An interesting point is that occasional patients with previous TBI have idiopathic or symptomatic generalized epilepsy, which may be inadvertently treated with narrow spectrum antiepileptic drugs used for focal seizures because their seizures started after their TBI.16 Successful treatment of these patients usually necessitates tailored broad spectrum antiepileptic therapy. Another issue is that frontal lobe epilepsy (FLE) is often misdiagnosed, historically, as primary sleep disorders, psychogenic nonepileptic seizures, or other primary psychiatric illnesses.17–19 As a result, these patients with a misdiagnosis are not successfully treated.
The objective of this study is to characterize the subtypes of epilepsy that arise as a consequence of TBI. A sophisticated understanding of these subtypes of PTE will potentially aid in the development of clinically relevant animal models and human biomarkers of epileptogenesis.20 Some of the patients included in the study were reported in two previous publications from our group.16,21 The current study extends the previous reports from 5 to 10 years of experience from a single epilepsy center and includes results of resective surgical treatments that were not published previously.
Methods
This is a retrospective, Institutional Review Board approved, chart review of patients referred to the EMU at Parkland Memorial Hospital/University of Texas Southwestern Medical Center at Dallas, Texas, from January 1998 through June 2008 for definitive diagnosis of presumed epilepsy. During the admission, patients and their families were questioned about possible epilepsy risk factors, including perinatal injuries, febrile seizures, meningitis and/or encephalitis, family history of epilepsy, and TBI.
On admission, antiepileptic medications were suspended during the assessment period, and all patients had prolonged continuous video and scalp electroencephalography (EEG) recording. Scalp EEG data were collected using a BMSI 5000 system sampling at 400 Hz (1998–2001) and a Stellate Harmonie system (2002–2008) sampling at 200 Hz, both systems using the international 10–20 and modified combinatorial nomenclature system of electrode placement. Ictal events were identified by the patient, family, medical staff, or by computerized spike and seizure detection algorithms.
Patients were included in this study if they had a documented history of moderate to severe TBI (defined as TBI associated with prolonged loss of consciousness or amnesia lasting ≥30 min or prolonged hospitalization, skull fracture, intracerebral or intracranial hematoma, or traumatic encephalomalacia on neuroimaging studies obtained nonacutely during their epilepsy evaluation) preceding the onset of epilepsy that was proven during the EMU evaluation. Patients who had nondiagnostic evaluations or who had exclusive psychogenic nonepileptic seizures were excluded.
An MRI of the brain using a technique sensitive for detecting MTS was obtained. Sequences obtained included sagittal T1-weighted, axial gradient echo, and coronal T1-, T2-, and fluid attenuated inversion recovery (FLAIR) images obtained at 3-mm slice thickness through the region of the hippocampus. MTS was defined by hippocampal atrophy, T2 hyperintensity, and/or FLAIR hyperintensity, all assessed by visual inspection by board-certified neuroradiologists who were blinded to patient risk factors. When the MRI of the brain was unavailable or contraindicated, a CT scan of the brain was used. A study was classified as nonlesional if the imaging study obtained during the epilepsy workup did not show any abnormalities.
A subset of these patients had a more intensive pre-surgical evaluation that included neuropsychometric testing, Wada testing (intracarotid amobarbital), and/or intracranial/subdural EEG monitoring with or without functional mapping of the sensory, motor, and language areas. The intracranial electrode implantations were individualized to each patient depending on their suspected seizure focus. Subsequent surgical resection of the epileptogenic focus was performed if all of the pre-surgical workup was of adequate concordance in regard to seizure localization.
Anteriomesial temporal lobectomy with hippocampectomy was performed in patients with mesial temporal lobe seizures or with an anterior temporal neocortical focus. Lesionectomies and tailored resections guided by results of intracranial electrode monitoring were performed in the remaining patients with lesional and nonlesional neocortical epilepsy. The surgical margins were defined in part by the intracranial EEG findings. Pathologic analysis of the resected tissue was performed using standard methods. Clinical assessment of surgical outcome was graded using the Engel classification (I, seizure-free; II, rare disabling seizures; III, worthwhile seizure reduction; and IV, no improvement) at least 1 year after operation.
Information regarding details of the TBI, the latency from injury to onset of epilepsy, the neuroimaging studies, the EEG and video recordings of seizures, and the post-surgical follow-up were collected and analyzed via retrospective chart review.
Results
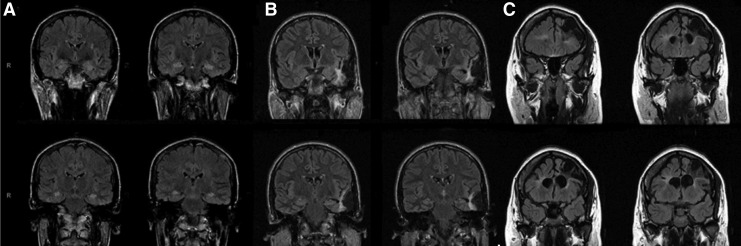
Of the 2886 consecutive unique patients referred to the EMU over a 10-year period, 123 (4.3%) patients fulfilled the inclusion criteria, of whom 60% were male. Table 1 shows the demographic information, mechanism of injury, age at injury, and latency to onset of epilepsy. The majority of the TBIs involved motor vehicle accidents or falls. Figure 1 shows imaging examples of lesions seen in this study.
Table 1.
Demographic Information
| Demographics | Mean | SD | Median |
|---|---|---|---|
| Sex (% male) | 74 (60%) | ||
| Age at injury (years) | 20.0 | 12.7 | 19.0 |
| Age at seizure onset (years) | 23.1 | 12.7 | 21.5 |
| Latency to seizure (years) | 3.5 | 6.6 | 0.0 |
| Age at EMU evaluation (years) | 38.7 | 11.7 | 39.0 |
| Mechanisms of brain injury (%) | |||
| Motor vehicle accident | 61 (49.6) | ||
| Motor pedestrian collision | 1 (0.8) | ||
| Fall | 32 (26.0) | ||
| Assault | 17 (13.8) | ||
| Gunshot wound | 6 (4.9) | ||
| Unknown | 6 (4.9) | ||
| Total | 123 | ||
SD, standard deviation; EMU, epilepsy monitoring unit.
FIG. 1.
Three imaging examples of lesions seen in this study. (A) Patient with bilateral mesial temporal sclerosis. Magnetic resonance imaging also shows global atrophy but no focal scars; (B) patient with left temporal neocortical lesion; (C) patient with left >right frontal encephalomalacia.
Localization related epilepsy was the most common subtype of PTE, diagnosed in 115 (93%) patients and arising most commonly from the temporal lobes and frontal lobes. Of those patients with temporal lobe epilepsy (TLE), just under half had MTS and about one-third were nonlesional. Of patients with MTS after TBI, 83% (24/29) had their injury after the age of 5 years. Four patients were found to have bilateral MTS.
Multifocal epilepsy was found in one patient. A small subset had primary generalized epilepsy, most of whom had clinical, including semiology, and EEG features indicative of idiopathic generalized epilepsy. Of those with idiopathic generalized epilepsy, 33% (2/6) had a positive family history of epilepsy. The others had features indicative of a symptomatic generalized epilepsy syndrome (Table 2).
Table 2.
Epilepsy Syndromes for the 115 Patients with Focal, Localization-Related Epilepsy
| Mesial temporal | Lesional neocortical | Nonlesional neocortical | Unknown | ||
|---|---|---|---|---|---|
| Focal | n (%) | n (%) | n (%) | n (%) | Total |
| Temporal | 29 (44.0) | 17 (25.8) | 20 (30.3) | - | 66 |
| Frontal | - | 32 (80.0) | 6 (15.0) | 2 (5) | 40 |
| Parietal | - | 3 | 1 | - | 4 |
| Occipital | - | 3 | 1 | - | 4 |
| Multifocal | - | 1 | - | - | 1 |
| Total | 29 (25.2) | 56 (48.7) | 28 (24.35) | 2 (1.74) | 115 |
| Idiopathic | Symptomatic | Total | |
|---|---|---|---|
| Generalized | 6 | 2 | 8 |
Syndromic diagnosis was based on seizure semiology, scalp or intracranial EEG pattern, and imaging.
Among the subtypes of epilepsy, the average latency to epilepsy for MTS (n=29) was 2.1 years (standard deviation [SD] 4.1 years, median <1 year) compared with 5.1 years (SD 8.4 years, median 1 year) for all lesional neocortical cases (n=56) (two-tailed p value=0.07; Mann-Whitney rank order test).
Of the 123 patients in the study, 22 proceeded to a surgical resection (Table 3). Most of the surgical patients had TLE of whom the majority had MTS. One of the four patients with bilateral MTS had exclusive unilateral temporal seizures and underwent resection. The other surgical patients had FLE of whom all had a lesional neocortical focus.
Table 3.
Epilepsy Subtypes and Outcomes for 22 Surgically Treated Patients
| Overall surgical outcome at latest clinical follow-up | ||||||
|---|---|---|---|---|---|---|
| Temporal | Frontal | |||||
| Total | MTS | Lesional neocortical | Nonlesional neocortical | Lesional neocortical | Nonlesional neocortical | |
| Engel Class | n (%) | n (%) | n | n | n | n |
| I | 13 (59.1) | 10 (76.9) | 0 | 1 | 2 | - |
| II | 4 (18.2) | 2 (15.4) | 1 | 0 | 1 | - |
| III | 2 (9.1) | 0 | 0 | 0 | 2 | - |
| IV | 3 (13.6) | 1 (7.7) | 0 | 1 | 1 | - |
| Total | 22 | 13 | 1 | 2 | 6 | - |
MTS, mesial temporal sclerosis.
Clinical assessment of surgical outcome at least 1 year after the surgical procedure was performed using the Engel classification: I, seizure-free; II, rare disabling seizures; III, worthwhile seizure reduction; and IV, no improvement.
At the latest post-operative clinical assessment (mean 2.52 years, SD 2.26 years, median 2.07 years), most patients had a good outcome (Engel Class I or II) (Table 3). Eleven (69%) of the temporal lobe epilepsy syndromes were Engel Class I compared with 2 (33%) of the FLE syndromes. Of note, the patient who underwent unilateral temporal resection in the setting of bilateral MTS was an Engel Class I outcome at the latest post-operative clinical assessment (21 months).
Discussion
PTE is a common, heterogenous, and disabling sequela of TBI. It is likely that successful development of antiepileptogenic therapies will necessitate a detailed understanding of the PTE subtypes. Comprehensive clinical evaluations, including video-EEG monitoring and neuroimaging, can identify distinct PTE syndromes. The reviewed data are concordant with previous smaller studies,12,13,16,21 and indicate that PTE commonly arises from the temporal lobes, predominately from the mesial structures. It also commonly arises from the frontal lobes and relatively rarely from occipital or parietal lobes. This increasing awareness of the high prevalence of temporal and frontal lobe PTE should guide practitioners to consider this diagnosis early in the course of the illness.
Of note, only one patient had dual areas of epileptogenicity, which may be lower than expected for PTE and could be the result of sampling error in part because of uncertainty that all seizure types (both clinical and subclinical) were captured during their EMU stay. It is plausible that some patients in our data series in fact had more than one epileptogenic zone, with the other zones producing microseizures, which are too focal to either show up on scalp EEG or to cause clinical manifestation. Microseizures have been identified using depth electrodes in the acute setting of TBI.22 Another possibility is selection bias because patients from our center with already known multifocal epileptogenicity (characterized elsewhere) would not have met the study inclusion criteria. Also of note, the correlation, if any, between the presumed idiopathic and symptomatic generalized epilepsies seen is unclear and is likely an unrelated occurrence, but it is plausible that more severe TBI could have led to earlier expression of underlying susceptibility.
In this study, the latency to referral to our epilepsy center averaged 15 years. Decreasing the latency to surgical referral may lead to earlier diagnosis and optimal treatment, potentially leading to better outcomes. Optimal treatment considerations, contrary to previous data, should include both pharmacological as well as surgical options when appropriate because these data suggest that the success rate of surgical resection appears to be comparable to that in the nontrauma population. In addition, early referral may permit surgical interventions that may not be feasible after late referral because of progressive secondary injury from recurrent seizures. For example, early referral may allow surgical therapy of patients in whom surgically intractable bilateral hippocampal injury and memory dysfunction would otherwise develop.
The overall poorer epilepsy surgical outcomes for FLE when compared with TLE are similar to those seen in the nontraumatic population. The exact reason for this is unclear. Mesial temporal structures, including the amygdala and hippocampus, are highly epileptogenic and are readily resectable. In addition, a standard temporal lobe resection that removes mesial and lateral temporal structures encompasses more than epileptogenic margins. In contrast, in many frontal lobe cases, the epileptogenic zone is more widespread and the margins are difficult to define, especially in nonlesional cases. The tailored resections may, therefore, be inadequate.
Although not statistically significant in this study, the trend for a shorter latency to onset of PTE with MTS is a potential novel finding that must be replicated in larger prospective cohorts. If proven with statistical significance, this could potentially help identify early on those patients who might be good surgical candidates given the high seizure-free success rate of anteriomesial temporal lobectomy with hippocampectomy.
The higher number of MTS cases in post-traumatic epilepsy, seen in this retrospective study and others, is interesting and may be the result of injury from repeated temporal seizures or may be implicated in actual epileptogenesis. Proinflammatory cytokines, such as interleukins (IL-1α IL-1β), play a role in the molecular cascade leading to neuronal injury after brain trauma, and expression of these genes are induced by trauma.23–25 There is also experimental evidence that implicates these proinflammatory cytokines in epilepsy.26,27 Therefore, it is possible that in diffuse brain injury that may lead to MTS, there might be activation of cytokines that might tip the balance in a patient predisposed to epilepsy.
Of note, some cases in the study were initially classified as nonlesional based on current imaging techniques. On microscopic examination of the resected tissue, however, abnormalities were identified consistent with contusions and axonal injury. This demonstrates that currently used imaging standards for patients with TBI may be suboptimal. Studies have shown that developing MRI technologies, including phased array coils and higher strength MRI magnets, improves signal-to-noise ratio, which leads to higher quality images with improved spatial resolution and contrast.28 Incorporating such technologies into current practice might increase the possibility of detecting subtle structural changes with current MRI imaging sequences (i.e, T1-weighted, T2-weighted, and FLAIR sequences) and with novel, sophisticated techniques, such as diffusion tensor imaging and tractography,29 and this may lead to a more accurate clinical impression and clinical outcome.
Limitations of this retrospective analysis were realized in addition to the expected inherent recall bias and inconsistent data recording. This study had a selection bias toward cases that were more difficult in terms of diagnosis and management (i.e., pharmacoresistant). In addition, scalp EEG lacks sensitivity in identifying focal electrographic seizures, especially frontal, and historically FLE is often misdiagnosed17–19 and therefore may not get referred for epilepsy evaluation. Thus, estimating the unbiased prevalence of the subtypes of PTE cannot be done. The exclusion of patients whose spells predated the TBI makes it impossible to exclude the possibility that pre-morbid conditions contributed to PTE. In addition, the lack of nonlesional frontal surgical cases in this study was a result of the rigorous patient selection for surgery that is done at our epilepsy center, and the high percent of Engel Class I outcomes may be biased by conservative patient selection for surgery.
Overall, the available retrospective data are still limited because they represent only those referred to a single epilepsy center, which is a fraction of the total populationwith PTE. Therefore, prospective studies are needed to fully understand the endophenotypes of PTE. It is hopeful that this will lead to better design of clinical trials of antiepileptogenic therapies for PTE and to the development of appropriate animal models and biomarkers to follow the epileptogenic process in humans.
Acknowledgment
This study was sponsored by NIH R01 HD78179, U01 HD42652, NIDRR H133 A0205206.
Author Disclosure Statement
Dr. Ramon Diaz-Arrastia is on the speaker's bureau for UCB Pharma. For the remaining authors, no competing financial interests exist.
References
- 1.Melvin J.W. (2002). Brain-injury biomechanics. in: Accidental Injury: Biomechanics and Prevention. Nahum A.M., Melvin J.W. (eds). Springer: Berlin, pp 277–302 [Google Scholar]
- 2.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 3.Coronado V.G., Xu L., Basavaraju S.V., McGuire L.C., Wald M.M., Faul M.D., Guzman B.R., and Hemphill J.D.; Centers for Disease Control Prevention. (2011). Surveillance for traumatic brain injury-related deaths—United States, 1997–2007. MMWR Surveil. Summ. 60, 1–32 [PubMed] [Google Scholar]
- 4.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A., Timothy J., Pandit L., and Manju M. (2006). Post-traumatic epilepsy: an overview. Clin. Neurol. Neurosurg. 108, 433–439 [DOI] [PubMed] [Google Scholar]
- 6.Annegers J.F. (1996). The epidemiology of epilepsy, in, The Treatment of Epilepsy: Principles and Practice. Wyllie E. (ed). Williams and Wilkins: Baltimore, p. 165 [Google Scholar]
- 7.Annegers J.F., Hauser W.A., Coan S.P., and Rocca W.A. (1998). A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 338, 20–24 [DOI] [PubMed] [Google Scholar]
- 8.Sillanpaa M., Jalava M., Kaleva O., and Shinnar S. (1998). Long-term prognosis of seizures with onset in childhood. N. Engl. J. Med. 338, 1715–1722 [DOI] [PubMed] [Google Scholar]
- 9.Semah F., Picot M.C., Adam C., Broglin D., Arzimanoglou A., Bazin B., Cavalcanti D., and Baulac M. (1998). Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51, 1256–1262 [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Arrastia R., Agostini M.A., Madden C.J., and Van Ness P.C. (2009). Posttraumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia 50, Suppl 2, 14–20 [DOI] [PubMed] [Google Scholar]
- 11.Mathern G.W., Babb T.L., Vickrey B.G., Melendez M., and Pretorius J.K. (1994). Traumatic compared to non-traumatic clinical-pathologic associations in temporal lobe epilepsy. Epilepsy Res. 19, 129–139 [DOI] [PubMed] [Google Scholar]
- 12.Swartz B.E., Houser C.R., Tomiyasu U., Walsh G.O., DeSalles A., Rich J.R., and Delgado-Escueta A. (2006). Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia 47, 1373–1382 [DOI] [PubMed] [Google Scholar]
- 13.Hartzfeld P., Elisevich K., Pace M., Smith B., and Gutierrez J.A. (2008). Characteristics and surgical outcomes for medial temporal post-traumatic epilepsy. Br. J. Neurosurg. 22, 224–230 [DOI] [PubMed] [Google Scholar]
- 14.Schuh L.A., Henry T.R., Fromes G., Blaivas M., Ross D.A., and Drury I. (1998). Influence of head trauma on outcome following anterior temporal lobectomy. Arch. Neurol. 55, 1325–1328 [DOI] [PubMed] [Google Scholar]
- 15.Marks D.A., Kim J., Spencer D.D., and Spencer S.S. (1995). Seizure localization and pathology following head injury in patients with uncontrolled epilepsy. Neurology 45, 2051–2057 [DOI] [PubMed] [Google Scholar]
- 16.Hudak A.M., Trivedi K., Harper C.R., Booker K., Caesar R.R., Agostini M., Van Ness P.C., and Diaz-Arrastia R. (2004). Evaluation of seizure-like episodes in survivors of moderate and severe traumatic brain injury. J. Head Trauma Rehabil. 19, 290–295 [DOI] [PubMed] [Google Scholar]
- 17.Williamson P.D., Spencer D.D., Spencer S.S., Novelly R.A., and Mattson R.H. (1985). Complex partial seizures of frontal lobe origin. Ann. Neurol. 18, 497–504 [DOI] [PubMed] [Google Scholar]
- 18.Williamson P.D., and Spencer S.S. (1986). Clinical and EEG features of complex partial seizures of extratemporal origin. Epilepsia 27, S46–S63 [DOI] [PubMed] [Google Scholar]
- 19.Williamson P.D. (1992). Frontal lobe seizures. Problems of diagnosis and classification. Adv. Neurol. 57, 289–309 [PubMed] [Google Scholar]
- 20.Herman S.T. (2002). Epilepsy after brain insult: targeting epileptogenesis. Neurology 59, Suppl 5, S21–S26 [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Arrastia R., Agostini M.A., Frol A.B., Mickey B., Fleckenstein J., Bigio E., and Van Ness P.C. (2000). Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol 57, 1611–1616 [DOI] [PubMed] [Google Scholar]
- 22.Waziri A., Claassen J., Stuart R.M., Arif H., Schmidt J.M., Mayer S.A., Badjatia N., Kull L.L., Connolly E.S., Emerson R.G., and Hirsch L.J. (2009). Intracortical electroencephalography in acute brain injury. Ann. Neurol. 66, 366–377 [DOI] [PubMed] [Google Scholar]
- 23.Rothwell N.J. (1999). Annual review prize lecture cytokines—killers in the brain? J Physiol. 514, 3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Arrastia R., and Baxter V.K. (2006). Genetic factors in outcome after traumatic brain injury: what the human genome project can teach us about brain trauma. J. Head Trauma Rehabil. 21, 361–374 [DOI] [PubMed] [Google Scholar]
- 25.Frugier T., Morganti-Kossmann M.C., O'Reilly D., and McLean C.A. (2010). In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J. Neurotrauma 27, 497–507 [DOI] [PubMed] [Google Scholar]
- 26.D'Ambrosio R., and Perucca E. (2004). Epilepsy after head injury. Curr. Opin. Neurol. 17, 731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao R.S., Prakash A., and Medhi B. (2009). Role of different cytokines and seizure susceptibility: a new dimension towards epilepsy research. Ind. J. Exp. Biol. 47, 625–634 [PubMed] [Google Scholar]
- 28.Grant P.E. (2005). Imaging the developing epileptic brain. Epilepsia 46, Suppl 7, 7–14 [DOI] [PubMed] [Google Scholar]
- 29.Wang J.Y., Bakhadirov K., Devous M.D., Sr., Abdi H., McColl R., Moore C., Marquez de la Plata C.D., Ding K., Whittemore A., Babcock E., Rickbeil T., Dobervich J., Kroll D., Dao B., Mohindra N., Madden C.J., and Diaz-Arratis R. (2008). Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 65, 619–626 [DOI] [PubMed] [Google Scholar]