Abstract
Treatment of traumatic brain injury (TBI) is still an unmet need. Cell therapy by human umbilical cord blood (HUCB) has shown promising results in animal models of TBI and is under evaluation in clinical trials. HUCB contains different cell populations but to date, only mesenchymal stem cells have been evaluated for therapy of TBI. Here we present the neurotherapeutic effect, as evaluated by neurological score, using a single dose of HUCB-derived mononuclear cells (MNCs) upon intravenous (IV) administration one day post-trauma in a mouse model of closed head injury (CHI). Delayed (eight days post-trauma) intracerebroventricular administration of MNCs showed improved neurobehavioral deficits thereby extending the therapeutic window for treating TBI. Further, we demonstrated for the first time that HUCB-derived pan-hematopoietic CD45 positive (CD45+) cells, isolated by magnetic sorting and characterized by expression of CD45 and CD11b markers (96–99%), improved the neurobehavioral deficits upon IV administration, which persisted for 35 days. The therapeutic effect was in a direct correlation to a reduction in the lesion volume and decreased by pre-treatment of the cells with anti-human-CD45 antibody. At the site of brain injury, 1.5-2 h after transplantation, HUCB-derived cells were identified by near infrared scanning and immunohistochemistry using anti-human-CD45 and anti-human-nuclei antibodies. Nerve growth factor and vascular endothelial growth factor levels were differentially expressed in both ipsilateral and contralateral brain hemispheres, thirty-five days after CHI, measured by enzyme-linked immunosorbent assay. These findings indicate the neurotherapeutic potential of HUCB-derived CD45+ cell population in a mouse model of TBI and propose their use in the clinical setting of human TBI.
Key words: : brain trauma, CD45+ hematopoietic cells, cell transplantation, cord blood, neurotherapy
Introduction
Traumatic brain injury (TBI) represents a major health care problem and a significant socio-economic challenge worldwide. According to the Centers for Disease Control and Prevention, approximately 1.5 million patients are affected each year in the United States alone and the mortality of severe TBI remains as high as 35–40%.1 There is an unmet need for efficient treatment modalities for TBI patients. Post-traumatic brain damage is determined by a combination of primary and secondary insults.2 The primary damage is instantaneous and results from the mechanical forces applied at the time of impact, while the secondary brain damage evolves over time and shares mechanisms similar to those occurring after cerebral ischemia.3 A useful pharmacological model of TBI is represented by the closed head injury (CHI) model, in which a standardized weight-drop device is causing a focal blunt injury to the brain through an intact skull.4,5 The resulting mechanical impact triggers a profound neuronal inflammatory response within the brain, leading to neurological and cognitive impairment.6 This model was developed and validated in our laboratories by characterizing neuroprotective drugs, as well as by unraveling of pharmacological mechanisms of neurotoxicity and neuroprotection.1,7
Stem cell–based clinical trials hold promise for the treatment of various human diseases, including TBI.8 Clinical-grade stem cells from human umbilical cord blood (HUCB) might provide an abundant and convenient source of pluripotent progenitor cells devoid of ethical controversies9 for cell therapy.10 The HUCB contains multiple populations of stem cells, capable of giving rise to hematopoietic, epithelial, endothelial, myotubes, and neural cells.10,11 These cell populations can be selected based on their expression of various lineage-specific markers and may be divided into hematopoietic and non-hematopoietic stem cells, according to the expression of hematopoietic-related markers such as CD34 (glycoprotein involved in cell-cell adhesion),12 CD133 (glycoprotein, also named Prominin 1, PROM1)13 and CD45.14 The cell membrane protein tyrosine phosphatase, receptor type C (PTPRC) CD45 is abundantly expressed on all nucleated hematopoietic cells and is critical for classical antigen receptor signaling indicated by the arrested development of B and T cells in CD45 deficient mice.15,16 CD45 isoforms are expressed on human hematopoietic cells at different stages of development14 and also are involved in regulation of adhesion and motility.17 Currently, HUCB is a clinically acceptable source of hematopoietic stem cells for transplantations in patients with malignant and genetic or metabolic diseases.18 In addition, neurological disorders such as trauma, stroke and neurodegenerative diseases may represent another target for cell-based regenerative medicine using HUCB-derived cells. The therapeutic potential of whole HUCB and derived mononuclear cells (MNCs) in neurological disorders was investigated in animal models of central nervous system damage induced by hemorrhagic brain injury,19 heatstroke,20 middle cerebral artery occlusion stroke model,21 spinal cord injury,22 as well as TBI.23 Given the fact that mesenchymal stem cells (MSCs) are frequently used for cell therapy of neurodegenerative disorders in clinical trials,8,24 a recent study demonstrated the efficacy of HUCB-derived MSCs in protecting mice brains after trauma.25 However, the percentage of MSCs in HUCB is very low and their purification requires multiple cell-sorting steps, presenting a serious disadvantage for translation of this concept into a clinical relevance.26 By contrast, the significantly more abundant population of pan-hematopoietic CD45-positive (CD45+) cells, which can be isolated in a single step by magnetic sorting, has not yet been evaluated for its therapeutic potential in TBI.
In the present study, we sought to evaluate the neurotherapeutic potential of HUCB-derived MNCs and CD45+ cells for the treatment of TBI using the CHI mouse model. We found that the CD45+population homed to the site of the brain injury, decreased the lesion volume, and induced a significant beneficial effect on neurobehavioral recovery up to 35 days, after intravenous (IV) administration one day post-trauma. Further, treatment of the cells with anti-human-CD45 antibody was shown to reduce the beneficial effect. The present pre-clinical evaluations of HUCB-derived CD45+ cells neurotherapeutic effect in TBI model propose their consideration for translational therapy in the clinic.
Materials and Methods
HUCB collection and separation of CD45+ cells
HUCB was collected according to a protocol approved by Sheba Medical Center and Israel's Ministry of Health after obtaining informed consent of the mothers. Placental blood was stored in ethylenediaminetetraacetic acid–containing bags immediately after delivery and was used within 24 h. The blood sample volume was in the range of 70–120 mL, with a median volume of 75 mL. HUCB-derived MNCs were separated twice on a sterile Ficoll-Hypaque gradient (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) to increase their purity. Isolated cells were washed with phosphate buffered saline (PBS; Invitrogen, Carlsbad, CA) and the cells were counted by hemocytometer. CD45+ cell separation was achieved via immunomagnetic sorting using mouse monoclonal anti-human-CD45 antibody and MACS CD45 microbeads (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions. The purity of the separated cells was verified by flow cytometry analysis using anti-human-CD45 antibody (murine immunoglobulin G [IgG]1 anti-human-CD45-fluorescein isothiocyanate [FITC], IQproducts, Groningen, the Netherlands) as described below. After their separation, HUCB-derived MNCs, CD45+ cells, or non-hematopoietic CD45-negative (CD45-) cells were evaluated for cell viability using Trypan Blue exclusion and used immediately for administration. Cell concentration was adjusted in PBS to 1000, 10,000 or 100,000 cells/μL for intracerebroventricular (ICV) transplantation or 10,000 cells/μL for IV administration. Unless otherwise stated, the dose of cells for ICV transplantation was 1×105 cells and for IV administration was 1×106 cells.
Fluorescence-activated cell sorting (FACS)
After magnetic separation, HUCB-derived MNCs, CD45+ or CD45- cells (1×106) were washed in FACS buffer (3% fetal bovine serum and 0.01% sodium azide in PBS) and incubated with one of the following fluorophore-conjugated mouse anti-human antibodies: CD4-APC, CD11b-FITC, CD20-PE-Cy5, CD90-PerCP-eFlour 710, CD105-APC, CD184-PE (all from eBioscience, San Diego, CA), CD34-PE, CD45-FITC (all from IQproducts), CD73-PE (R&D systems, Minneapolis, MN) and CD133-PE (Miltenyi Biotec). Isotype-matched immunoglobulins were used as negative controls. All the steps involving fluorescence were carried-out in a dark room. Ten thousand events were acquired with a BD LSRII flow cytometer (BD Biosciences Immunocytometry Systems, BD Biosciences, San Jose, CA) and plots were generated using the FCS express 4 plus analysis software (De Novo Software, Los Angeles, CA).27 Each FACS experiment was repeated independently five times.
Scanning electron microscopy (SEM)
HUCB-derived cells were cultured on plastic 8-well chamber slides (Nunc, Rochester, NY) and fixed with 2% glutaraldehyde in PBS, pH 7.4, for 2 h. Samples were post-fixed in 1% osmium tetroxide, dehydrated, sputter-coated with gold, and examined in a Philips 505 SEM (30 kV).28
Closed head injury model
The study was conducted according to a protocol approved by the Hebrew University Faculty of Medicine Animal Care and Ethic Committee, in compliance with National Institutes of Health guidelines. Adult Sabra male mice weighing 40 g were used for these experiments and transplanted with HUCB-derived cells or treated with vehicle (PBS). Five sham-operated mice served as controls. Food and water were provided ad libitum. Experimental CHI was induced by using a weight-drop device method developed4 and modified5 in our laboratory. This is a convenient model, which provides fronto-parietal cortical injury. Briefly, surgical anesthesia was induced and maintained with 4% isoflurane gas (Terrell™, Piramal Critical Care, Bethlehem, PA) O2 flow rate 1.5 L/min for 8 min, administered through a nose mask. Respiration rate and depth, as well as palpebral and pedal withdrawal reflexes, monitored depth of anesthesia. A midline longitudinal incision was performed, the skin was retracted, and the skull was exposed. The left anterior frontal area was identified and a tipped Teflon cone was placed 1 mm lateral to the midline. The head was fixed and a 95 g weight was dropped on the cone from 18 cm height. After trauma, the animals received supporting oxygenation with 95% O2 for no longer than 2 min. After recovery from anesthesia, the mice were returned to their home cages with post-operative care and free access to food and water. Sham controls received anesthesia and skin incision only, but no head injury was induced.5
HUCB-derived cells administration to CHI mice
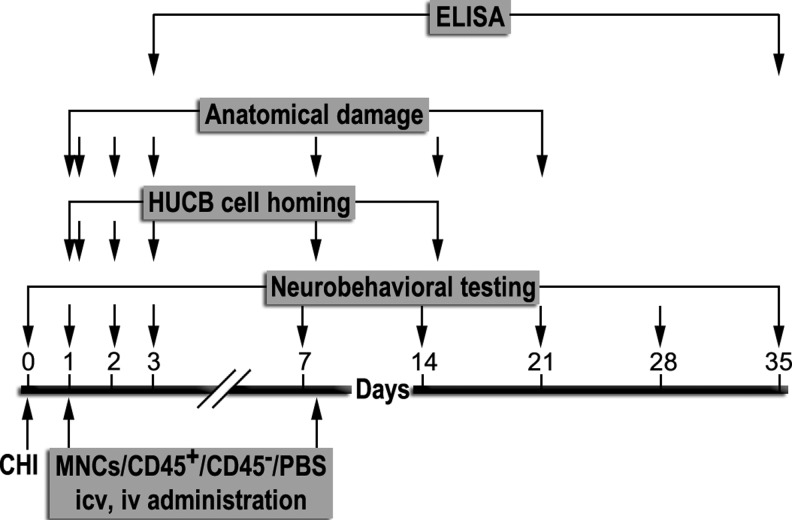
One day and/or eight days post-trauma, the mice were anesthetized and administered HUCB-derived MNCs, CD45+ cells, or CD45- cells, or treated with vehicle. Cells were either transplanted ICV (10 μL) or administered intravenously (100 μL) into the tail vein (n=7-14 mice per group). Each experiment was repeated three times. All surgeries and injuries were performed by the same investigator who was blinded to the treatment allocation following injury. The experimental paradigm illustrated in Figure 1 shows the different post-trauma time points in which various tests were performed.
FIG. 1.
Experimental design. Mice were exposed to closed head injured or sham surgery on Day 0. Human umbilical cord blood-derived mononuclear cells (MNCs), CD45 positive (CD45+) or negative (CD45-) cells, or vehicle (phosphate buffered saline [PBS]) was transplanted intracerebroventricularly (ICV) or administered intravenously (IV) in the tail vein one and/or eight days post-trauma. Neurobehavioral tests, cell homing, anatomical damage, and enzyme-linked immunosorbent assay (ELISA) experiments were performed at the time points indicated.
Neutralization of CD45+ antigen from HUCB-derived MNCs was performed as previously described using anti-human-CD45 antibody (Dako, Glostrup, Denmark), which blocks human CD45 specifically and does not cross-react with mouse CD45.29 Briefly, cells were neutralized using 0.5 μg anti-human-CD45 antibody/106 cells or anti-human-IgG-control antibody/106 cells (Dako) by incubation for 1 h at 37°C before their administration into CHI mice.
Neurobehavioral evaluation and determination of the neurological score
Mice were weighed and examined with a standardized neurologic severity score (NSS) up to 35 days post-trauma. During the first week, testing was performed on Days 1, 2, 3 and 7, and then once a week until the end of the experiment. Ten different tasks were used to evaluate motor ability, balance, and alertness of the tested mice and score points were given for failure to perform a task, as previously described.30 Loco-motor/balance activity of the CHI mice was estimated by measuring their walking ability on a 1-, 2-, and 3-cm wide beam.30 The improvement in this measure is represented by the percentage of mice in the experimental group that successfully completed the task of walking the full length of the beam. The pathologic scores correlate well with clinical disability scores and with the degree of brain edema.5 The first NSS was obtained at 1 h post-trauma and reflects the initial severity of injury. NSS at 1 h is predictive of both morbidity and mortality and also correlates well with the extent of damage seen by MRI.31 While most of the mice (86%) were moderately injured, with a NSS of 6–7, we included in the study all mice in the NSS range of 6–9 at 1 h post-trauma, reflecting the heterogeneity in the initial severity of injury. After determination of the initial NSS, mice were randomly separated into the different treatment groups, while keeping similar mean values of the NSS between the groups. The extent of recovery is calculated as the difference between the NSS at 1 h and at any subsequent time point (ΔNSS).30
Measurements of HUCB-derived cells homing to the brain using near infrared (NIR) scanning and CD45 immunohistochemistry
NIR scanning
To trace the administered cells homing to the brain lesioned area, we employed a technology using epidermal growth factor (EGF) labeled with NIR dye,32 since HUCB-derived MNCs were previously shown to respond to EGF.33 Briefly, 0.5-2×106 HUCB-derived MNCs were treated for 30 min at 37°C with 7 nM of the tagged EGF-IRDye800CW (NIR-TAG; LI-COR Biosciences, Lincoln, NE). The cells were then washed three times with PBS and 150,000 cells/well were plated in 12-well tissue culture plates (Nunc). Cell-associated NIR intensity was estimated using Odyssey® Infrared Imager (LI-COR Biosciences) under the following conditions: resolution, 170-340 microns; pixel area, 0.03 mm2; quality, medium-low; focus offset, 1-3; channels, 800 nm; intensity, 1-3. NIR intensity in control groups was measured by treatment of HUCB-derived MNCs with 7 nM of the untagged IRDye800CW (NIR-control) under the same conditions (providing information about nonspecific absorption of the NIR-dye to the cells) and by measuring the NIR-TAG binding to tissue culture plates (noise, background) in the absence of HUCB-derived MNCs. One day post-trauma mice were intravenously administered with HUCB-derived MNCs either NIR-TAG-labeled or left unlabeled (1×106 cells/mouse), or treated with vehicle. Additional control groups included sham, non-injured mice either administered HUCB-derived MNCs NIR-TAG-labeled (1×106 cells/mouse), or left untreated. All mice were anesthetized throughout the in vivo imaging procedures.33 To minimize the NIR background during the in vivo NIR imaging, the snout and head of the mice were shaved. Mice were placed in a supine position on the LI-COR Biosciences small-animal imager of the Odyssey® Imager equipped with the MousePOD instrument, while constant temperature of 37°C was maintained in the chamber.32In vivo NIR images were taken 1.5 and 5 h as well as one, two, and seven days post-administration, using the conditions previously described. At each time point, five mice from each group were randomly sacrificed and whole brains were dissected. The brains were then placed in PBS in a 12-well plate (BD Falcon™, BD Biosciences) and immediately scanned on the Odyssey Imager. The NIR intensity was estimated semi-quantitatively using Odyssey software.
Immunohistochemistry
Immunohistochemistry was performed using a floating section staining procedure. For the experiments in which cells homing to the brain were evaluated, brains were removed 2 h after administration. Mice were transcardially perfused, under anesthesia, with instilled PBS followed by fixation with 4% paraformaldehyde in PBS. After perfusion, the brains were quickly removed and immersed in the same fixative overnight, and then cryoprotected by immersion in 30% sucrose for 48 h at 4°C. The brains were then frozen on dry ice and cut serially into 30 μ-thick coronal sections using a cryostat (Leica CM 1850, Leica Biosystems, Nussloch, Germany) and stored at −20°C in cryoprotectant (28% glycerol, 29% ethylene glycol in 0.1 M phosphate buffer) until assayed. Briefly, the brain sections were washed three times for 10 min each with PBST (0.1% Triton in PBS, pH 7.4), treated with blocking buffer (10% normal donkey serum in PBST) for 2 h at room temperature and incubated with primary antibodies at 4°C overnight. The following antibodies were used: FITC-conjugated mouse anti-human-CD45 (1:500; IQproducts), FITC-conjugated mouse anti-human-IgG control (1:500; IQproducts) and mouse anti-human-nuclei monoclonal antibody (1:100; Millipore), which stains nuclei of all human cell types and does not react with nuclei from mouse or rat.34 Donkey anti-mouse Alexa Fluor®555 (1:400) was purchased from Invitrogen, Eugene, OR. Antibodies were diluted in 2% normal donkey serum dissolved in PBST. The next day, brain sections were washed with PBST three times for 10 min each, and thereafter incubated with the corresponding secondary antibody. Finally, free-floating brain sections were carefully mounted on slides, air dried and covered with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). All the steps involving fluorescence were carried out in a dark room. Immunostained slices were examined in a fluorescent microscope (Nikon 50i) at magnifications of 100×or 200×. Photographs of different random fields around the trauma were taken and analyzed for CD45 intensity levels (mean gray level) using the image J software (National Institutes of Health, Bethesda, MD). Representative fields are displayed.
Measurements of brain lesion area and volume
NIR approach
To evaluate the size of the brain lesion area, mice heads were scanned at 800 nm with Odyssey Imager, as previously described, 1.5 and 5 h as well as two and seven days post-administration. The images were transferred to SigmaScan software, the boundary of the lesioned area was marked and the lesion areas in identical regions of interest were calculated in mm2. These conditions enabled evaluation of hemorrhage on the injury area by measuring the endogenous NIR fluorescence of intracranial hematoma.35
Histological approach
Twenty one days post-trauma, mice were deeply anesthetized and perfusion fixed with instilled 4% paraformaldehyde in PBS. The mice were decapitated and brains were quickly removed and frozen on powdered dry ice. Frozen brains were serially sectioned to slices (n=23-31 per group) of 10-μm thickness using a cryostat (Leica CM 1850, Leica Biosystems). Thereafter, the slices were stained with Giemsa stain-modified solution (1:1 in double distilled water; Fluka, Sigma-Aldrich Corporate, St. Louis, MO) and digitally photographed. The volume of injured tissue was measured with Image J software (National Institutes of Health). Damaged tissue volume was calculated by dividing the volume of the injured/ipsilateral hemisphere (Left) by that of the non-lesioned/contralateral hemisphere (Right). The results are expressed as a percentage of hemispheric tissue. Calculations were performed according to the following formula:
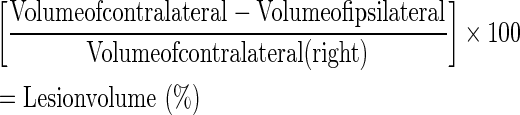 |
Enzyme-linked immunosorbent assay (ELISA) measurements of neurotrophins
On Days 3 and 35 post-trauma, ipsilateral and contralateral cortical and hippocampal tissues (including the tissue above the rhinal fissure)36 were dissected separately, rapidly frozen in liquid nitrogen and stored at -80°C until analysis (n=3-4 each group per time point), each experiment was repeated three times. Frozen samples were thawed and homogenized at a speed of 30,000 rpm (Polytron PT-MR2100 homogenizer, Kinematica AG, Lucerne, Switzerland) at room temperature for 5 min in cell lysis buffer (Cell Signaling Technology, Danvers, MA) together with a cocktail of proteases and phosphatases inhibitors (Sigma-Aldrich). Homogenates were kept on ice for 30 min and centrifuged at 14,000 g for 10 min at 4°C. Lysates were transferred to a new tubes and protein concentration was determined using the Lowry method (Protein Assay; Bio-Rad, Hercules, CA) and stored at −80°C until assayed. Samples were analyzed for brain-derived neurotrophic factor (BDNF), nerve growth factor (β-NGF) and vascular endothelial growth factor (VEGF-A, both the 165 and 121 isoforms) using a human BDNF ELISA Kit (Boster Biological Technology, Fremont, CA), human β-NGF ELISA Development Kit and VEGF ELISA Development Kit (both from PeproTech, Rocky Hill, NJ). The amount of neurotrophins in pg/mg wet tissue was calculated from a standard curve of recombinant BDNF, β-NGF and VEGF (with sensitivity of 31.2 pg/mL, 16 pg/mL and 63 pg/mL, respectively) and normalized to protein concentrations. Preparation of plates, solutions and ELISA protocols was performed according to the manufacturers' instructions.
Statistical analysis
Statistical analysis was performed using a commercial statistics package (IBM SPSS Statistics Software, SPSS Inc. Chicago, IL). Data are presented as mean±standard error of the mean. NSS and ΔNSS values in the neurobehavioral evaluations were compared using the non-parametric Kruskal-Wallis one-way analysis of variance followed by the Mann-Whitney test between the selected groups. Data for brain hemorrhage measurements, lesion volume analysis, NIR intensity, CD45 intensity, and neurotrophin profiles were evaluated by the Student's t-test. A p value of 0.05 or less was considered significant for all comparisons.
Results
Isolation and characterization of HUCB-derived CD45+ hematopoietic cells
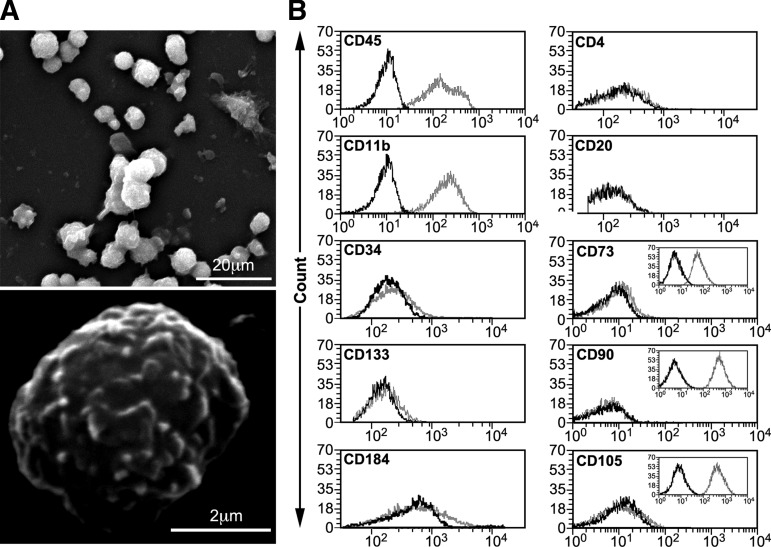
HUCB-derived MNCs isolated from fresh HUCB units are small, round, and express a rough cell surface morphology (Fig. 2A). Freshly isolated cells were separated by immunomagnetic sorting using anti-CD45 MicroBeads with a purity of 96% (Fig. 2B) for the positive cell population and 95.4% for the negative one (data not shown). Their identification was made by FACS analysis using a large set of cluster of differentiation (CD) markers. Approximately 99% of the CD45+ cells expressed CD11b (integrin alpha M, ITGAM), a typical cell-surface antigen of monocyte cells.27 A minority expressed the stem/progenitor cell markers CD3412 and CD13313 (Fig. 2B). The expression of the C-X-C chemokine receptor type 4 (fusin, CXCR4) CD184,37 and of CD4 (glycoprotein, leu-3, T4) and CD20 (B-lymphocyte antigen), which are T and B lymphocyte markers,38 was very low, as were the expression levels of MSCs-specific cell-surface antigens39 such as CD73 (5′-nucleotidase, 5′-NT, 5.9%), CD90 (Thy1, 0.3%), and CD105 (Endoglin, END, 4.4%). These results suggest a pan-hematopoietic, non-mesenchymal origin of the sorted CD45+ cells. Of the CD45− cells, only 4.6%, 3.4% and 4.2% expressed the hematopoietic markers CD45, CD11b, and CD34, respectively (data not shown), indicating the non-hematopoietic origin of the cells. A very low percentage of these CD45- cells were found positive for CD133 (5%), CD184 (1.3%), CD4 (2.6%) and CD20 (0.9%) and for the mesenchymal markers CD73 (1.2%), CD90 (0.6%), and CD105 (0.5%) (data not shown). An enriched population of MSCs isolated from human bone marrow, which expressed CD73, CD90 and CD105 at levels of 98.5%, 99.3% and 99%, respectively, served as positive control (Fig. 2B, inserts).
FIG. 2.
Human umbilical cord blood (HUCB)-derived CD45 positive (CD45+) cells isolation and characterization. (A) Representative scanning electron microscopy image of HUCB-derived mononuclear cells immediately upon isolation. (B) Representative fluorescence-activated cell sorting analysis (n=5) of HUCB-derived CD45+ cells immunophenotype profile after magnetic sorting separation using CD45 marker. CD73, CD90, and CD105 mesenchymal markers were validated with bone marrow-derived mesenchymal cells (inserts).
Validation of the neurotherapeutic effect of cord blood derived mononuclear cells
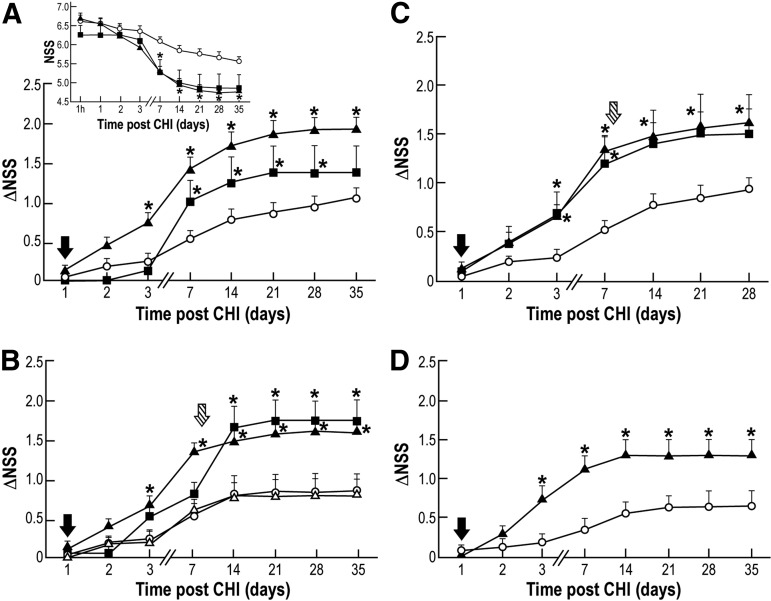
To characterize the neurotherapeutic effect of HUCB-derived MNCs, we transplanted these cells into the left ventricle of CHI mice brains one day following CHI and evaluated neurobehavioral deficits (NSS) at pre-determined time points post-trauma. The more rapid, pronounced, and statistically significant decline in NSS, as seen in the MNCs-transplanted mice compared with those that received only the vehicle from Day 7 until Day 35 post-trauma (Fig. 3A insert), is indicative of a beneficial effect. ΔNSS, which is a measure of recovery (each mouse serving as its own control) was calculated from Day 1 to Day 35 in groups of mice transplanted with three doses (1×104, 1×105 or 1×106) of MNCs or treated with vehicle. The most profound functional improvement was observed in mice transplanted with 1×105 cells and was statistically significant, compared with vehicle from Day 3 until Day 35 post-trauma using Kruskal-Wallis and Mann-Whitney tests (Fig. 3A). Remarkably, the increase in ΔNSS persisted until the end of the follow-up, at 35 days, although the cells were transplanted in a single dose one day post-trauma. No further increase in the neurotherapeutic effect was achieved by transplanting 1×106 cells (data not shown).
FIG. 3.
Neurotherapeutic effect of human umbilical cord blood (HUCB)-derived mononuclear cells (MNCs) upon intracerebroventricular transplantation or intravenous administration. Neurologic disability was evaluated at different time points post-trauma for the extent of recovery (ΔNSS). *p≤0.05, compared with the phosphate buffered saline (PBS)-treated group. (A) Mice were intracerebroventricularly transplanted with 1×104 (black square), 1×105 (black triangle) HUCB-derived MNCs or with PBS (white circle) one day post-trauma (black arrow); insert- temporal changes in neurological severity score (NSS) (n=7-11; each group repeated three times). (B) Mice were intracerebroventricularly transplanted with MNCs (1×105) one day (black arrow; black triangle) or eight days (striped arrow; black square) post-trauma or with PBS at either one day (white circle) or eight days (white triangle) post-trauma (n=7-11; each group repeated three times). (C) Mice were intracerebroventricularly transplanted with MNCs (1×105), at a single dose one day (black arrow; black triangle) or a double dose at one day (black arrow) and at eight days (striped arrow; black square) post-trauma or with PBS at one day (black arrow; white circle) post-trauma (n=7-11; each group repeated three times). (D) Mice were intravenously administered at day one (black arrow) post-trauma with MNCs (1×106; black triangle) or with PBS (white circle); (n=7-14; each group repeated three times).
To date, the narrow window for therapeutic intervention, which is in the range of up to 6 h after TBI or stroke poses several limitations on the efficacy of drug-based therapies.40 Identification of novel treatments that might extend the window for intervention to days and even weeks post-trauma is of tremendous need and value. To address this issue, we compared the effect of HUCB-derived MNCs treatment given one or eight days post-trauma on functional recovery for 35 days. Even when transplanted eight days post-trauma, the cells were able to facilitate recovery, as depicted in Figure 3B, suggesting a wider window of opportunity to intervene with HUCB-derived MNCs transplantation than other modalities currently under consideration. As seen in Figure 3B, the recovery rate of mice administered vehicle and MNCs-transplantation eight days post-trauma was similar until Day 7. When MNCs were transplanted at Day 8 post-trauma, the recovery reached at Day 14 approximately the same level as that in the mice transplanted at day one. This level was maintained until Day 35, when the follow-up was terminated. Further, ΔNSS of one day-transplanted mice was found significant from Day 3 post-trauma.
In the current clinical use of HUCB transplantation, transplantation of two (double) HUCB units is the most promising strategy to augment the total transplanted cell dose.41 To further identify HUCB-derived MNCs dosage requirements for therapy against CHI-induced damage, we transplanted two doses of 1×105 cells at one and eight days post-trauma (Fig. 3C). The recovery was statistically significant at Days 3–28 for the single dose transplantation, while the double dose transplanted mice yielded significant ΔNSS only at Days 3–7 post-trauma. The lack of significance in the double dose transplanted mice, at Days 14–28, is probably due to the small sized group and high variability of standard errors, therefore requiring additional experimentation. No additional benefit was observed in ΔNSS after the second transplantation (Fig. 3C), compared with a single dose transplanted one day post-trauma. Therefore, these results suggest that in this mouse model of CHI, a maximal therapeutic effect of HUCB-derived MNCs may be achieved upon transplantation at a single dose, one to eight days after the onset of injury.
In spite of the improved neurological function after HUCB-derived MNCs ICV transplantation (Fig. 3A-C), this cell route of administration was less investigated than IV. Therefore, to validate our model, we examined the effect of HUCB-derived MNCs (1×106) upon IV administration to the CHI mice tail vein. As evident in Figure 3D, the peripheral administration of HUCB-derived MNCs demonstrated a significant improvement on ΔNSS at Days 3–35 post-trauma, compared with vehicle, analyzed by Mann-Whitney test, as previously shown.23
Neurotherapeutic effect of HUCB-derived CD45+ cells upon IV administration
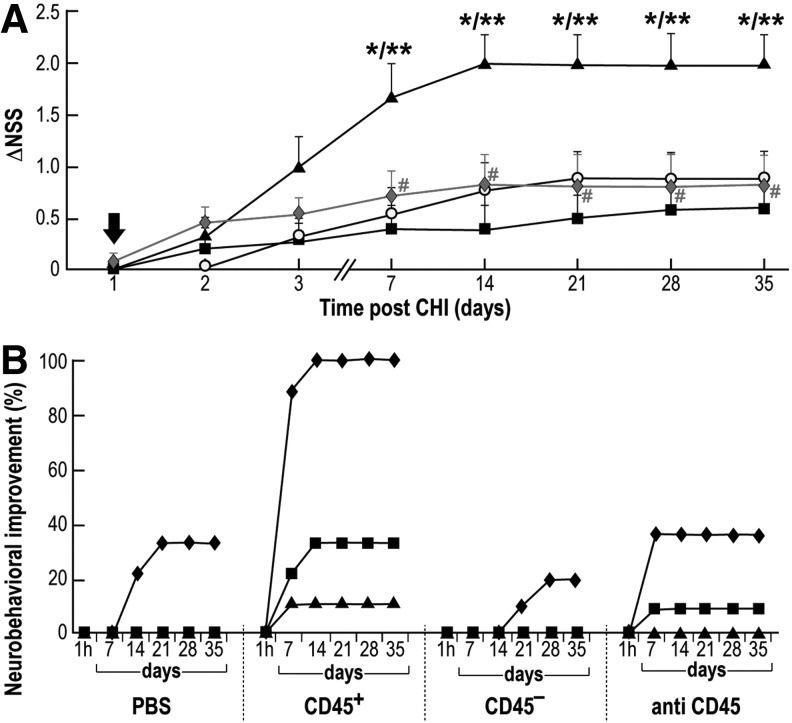
Transplantation of HUCB for translational therapy may include the use of whole HUCB sample, the isolated MNCs, or subclasses of MNCs-derived cell populations. Although the therapeutic potential of HUCB-derived MNCs for the treatment of brain trauma has been experimentally demonstrated in rats23 and mice (this study), the identity of the cells responsible for the neurotherapeutic effect out of the heterogeneous MNCs population warrants further exploration. HUCB-derived MNCs were separated according to their CD45 expression and the isolated populations were evaluated for their ability to reduce neurobehavioral deficits upon their IV administration into CHI mice. Figure 4A depicts the significant effect (expressed as ΔNSS) at Days 7–35 in mice administered CD45+ cells, compared with mice that received CD45- cells or vehicle, only. We then used human-CD45-blocking antibody to directly inhibit CD45-mediated migration and homing capabilities of the HUCB-derived MNCs.29 These cells were incubated with anti-human-CD45 antibody before IV administration into CHI mice. Under these conditions, HUCB-derived MNCs lost their ability to reduce neurological deficits (Fig. 4A). Upon pre-treatment with anti-human-CD45 antibody, the ΔNSS values were similar to those obtained of mice that received CD45- cells or vehicle and were statistically significant at Days 7–35, compared with CD45+-treated mice. Upon administration of a control group, in which HUCB-derived MNCs were pre-treated with an IgG-control, a significant improvement in ΔNSS was observed, similar to that improvement seen with non-treated HUCB-derived MNCs (data not shown). These findings suggest that the CD45+ pan-hematopoietic cell population is the major contributor to the neurotherapeutic effect described in Figure 3D.
FIG. 4.
Neurotherapeutic effect of human umbilical cord blood (HUCB)-derived CD45 positive (CD45+) cells upon intravenous administration. (A) One day post-trauma (black arrow) mice were intravenously administered (1×106) with HUCB-derived CD45+ cells (black triangle), CD45- cells (black square), mononuclear cells treated with anti-human-CD45 antibody (solid diamond) or treated with phosphate buffered saline (PBS; white circle). Neurologic disability was evaluated at different time points post-trauma for the extent of recovery (changes in neurologic severity score [ΔNSS]; n=11; each group repeated three times). *p≤0.05, compared with the PBS-treated group. **p≤0.05, compared with the CD45--treated group. #p≤0.05, compared with the CD45+-treated group. (B) Loco-motor/balance tests of the four experimental groups described in A. Mice were examined for their ability to walk on a beam of 3- (diamond), 2- (square), and 1- (triangle) cm wide.
To identify the individual neurobehavioral tests contributing to the significant effect shown in Figure 4A, we separately analyzed at different time points the success rate of the 10 tests composing the NSS in CHI mice administered either CD45 positive or negative cells, MNCs-treated with anti-human-CD45 antibody, or vehicle. Of all these tests we saw a clear effect only in the loco-motor/balance activity of CHI mice administered CD45+ cells, as measured by walking on 1-, 2-, and 3-cm wide beams. This improvement was evident with an onset of seven days post-trauma in the group of CD45+-transplanted mice and partially inhibited in the group of mice transplanted with cells treated with anti-human-CD45 antibody (Fig. 4B). The effect of treatment on other tests of the NSS was less pronounced, and not significant (data not shown). These cumulative observations further support the neurotherapeutic contribution of CD45+ cells to the improvement of loco-motor/balance activity of CHI injured mice as reflected by the increase in the ΔNSS.
Reduction of brain lesion volume upon HUCB-derived CD45+ cells IV administration
The severity of brain damage was assessed by different morphometric methods. As an indirect approach, we evaluated the size of hemorrhage on the area of injury by measuring the endogenous NIR fluorescence of intracranial hematoma.35 In vivo non-invasive laser scanning of CHI mice heads revealed a significant decrease in head lesion area 48 h post-administration of HUCB-derived MNCs, compared with vehicle (Fig. 5A). These data suggest that in HUCB-derived MNCs-treated brains, less hemorrhage was measured, implying healing of brain tissue around the site of the lesion.
FIG. 5.
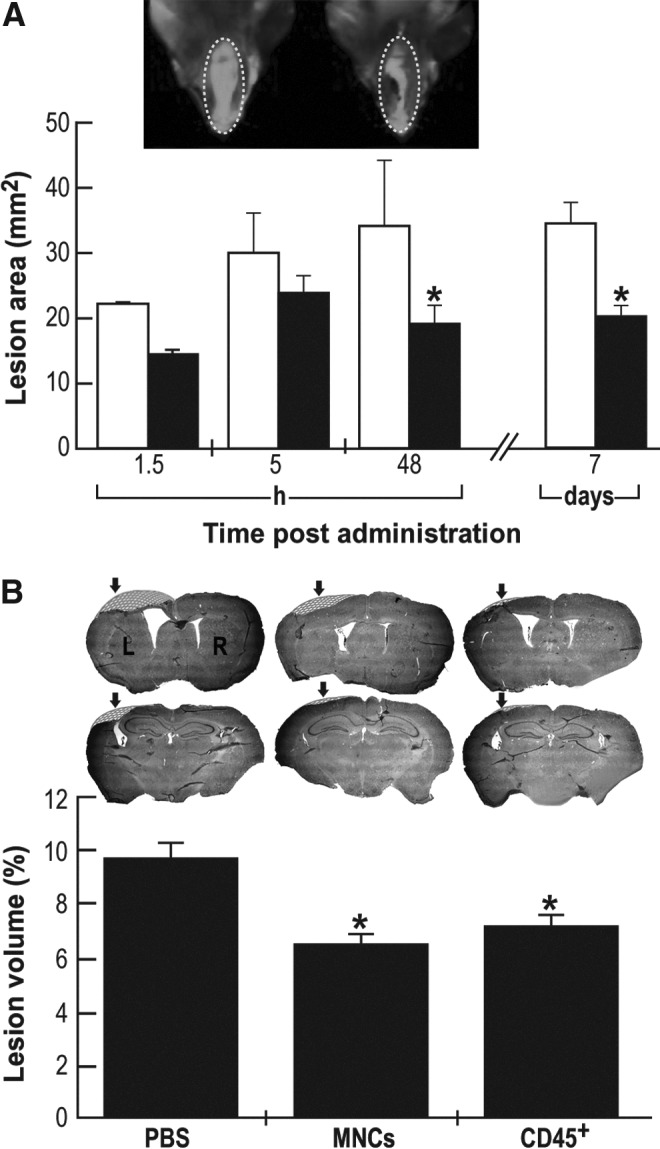
Morphometric analysis of brain lesions upon intravenous administration of human umbilical cord blood (HUCB)-derived mononuclear cells (MNCs) and CD45 positive (CD45+) cells (1×106). (A) Hemorrhage lesion area (mm2) was calculated upon mice head non-invasive near infrared (NIR) scanning, at different time points: white bars, phosphate buffered saline (PBS); black bars, HUCB-derived MNCs. Insert: Typical NIR-fluorescent micrographs of mice head taken seven days after HUCB-derived MNCs (right) or PBS (left) administration. (n=5 each group per time point). (B) Twenty one days after closed head injury, mice (n=6 each group) were sacrificed and brain slices were stained with Giemsa. Representative frontal (upper panel) and parietal (lower panel) brain sections of HUCB-derived MNCs, CD45+ cells and PBS treated mice. Marked area is the extrapolated missing brain piece in the injured area (arrow). Evaluation of lesion volume was calculated as percentage of contralateral hemispheric tissue. *p≤0.05, compared with the PBS-treated mice for both panels.
As an alternative, direct approach, brains from mice administered either HUCB-derived MNCs or CD45+ cells, or vehicle-treated controls were removed 21 days post-trauma. Isolated brains were fixed, sliced, stained with Giemsa, and evaluated for lesion volume as percentage of the uninjured contralateral hemispheric tissue, as described in Materials and Methods. As seen in Figure 5B, treatment with HUCB-derived MNCs or CD45+ cells yielded a significant reduction in brain lesion volume by 30–40%, compared with vehicle-treatment.
Brain homing of HUCB-derived CD45+ cells upon IV administration
We further exploited a NIR imaging platform and used immunohistochemistry of brain slices stained with anti-human-CD45 antibody to assess the presence of intravenously-administered cells in the brain. In the first approach, staining of 0.5×106 HUCB-derived MNCs with NIR-TAG generated a distinct fluorescent signal, which was increased at a higher cell density of 2×106 (Fig. 6A). The NIR intensity of the cells was very low in control slices incubated with the NIR dye alone (NIR-control), indicating low, non-specific absorption by the cells. Also, in the absence of cells the non-specific adhesion of NIR-TAG to the plate was negligible (Fig. 6A). NIR-TAG labeling of the cells in vitro was found to be stable for a week. One day post-trauma, NIR-TAG labeled 1×106 HUCB-derived MNCs were administered intravenously, and mice heads in vivo (Fig. 6B) and brains ex vivo (Fig. 6C) were laser scanned at different time points post-administration. The NIR images of CHI-injured heads upon administering either labeled cells (Fig. 6B; right) or vehicle (Fig. 6B; left) suggest an increased NIR signal in the vicinity of the injury site in the brain of mice administered cells. A more pronounced difference in the NIR-TAG fluorescence intensity is seen in ex vivo images of isolated brains (Fig. 6C), in which there is no background fluorescence originating from the bleeding around the site of injury. Analysis of NIR intensity of the isolated brains (Fig. 6D) indicates a significant increase in brain fluorescence only after 1.5 h post-administration, compared with vehicle. No further significant fluorescence change was seen at the different time points post-administration (from 5 h up to seven days; Fig. 6D). A similar fast homing effect was observed upon IV administration of NIR labeled CD45+ cells (data not shown).
FIG. 6.
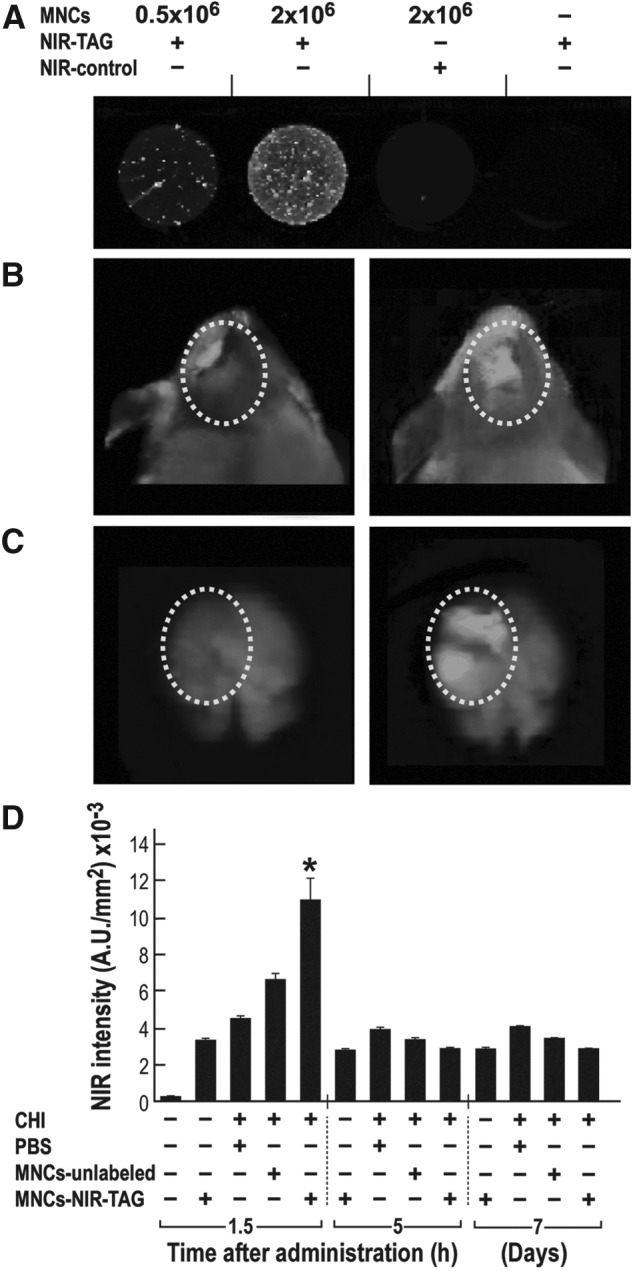
Homing of human umbilical cord blood (HUCB)-derived mononuclear cells (MNCs) (1×106) to the brain upon intravenous administration. (A) HUCB-derived MNCs were labeled with near infrared (NIR)-TAG and maintained in culture. Representative photos from three days after labeling indicate the specificity of cell labeling, compared with the very low background in control. (B-D) Mice were intravenously administered one day post-trauma with labeled (closed head injury [CHI+] MNCs-NIR-TAG+) or unlabeled (CHI+ MNCs-unlabeled+) HUCB-derived MNCs or with phosphate buffered saline (PBS; CHI+ PBS+). Sham animals, without CHI also were administered labeled HUCB-derived MNCs (CHI- MNCs-NIR-TAG+) or untreated (CHI- MNCs-) and served as control groups (n=5 each group per time point). Representative micrographs of mice heads (B) and isolated brains (C) scanned at 800 nm 1.5 h after administration with labeled HUCB-derived MNCs (right) or with PBS (left). The marked circle indicates the lesion area in the presence or absence of administered cells visualized in green. (D) At 1.5 and 5 h and seven days post-administration, whole brains were removed and scanned immediately. NIR intensity was calculated as a ratio to background fluorescence and is presented in arbitrary units (A.U.). *p≤0.05, compared with the PBS-treated group.
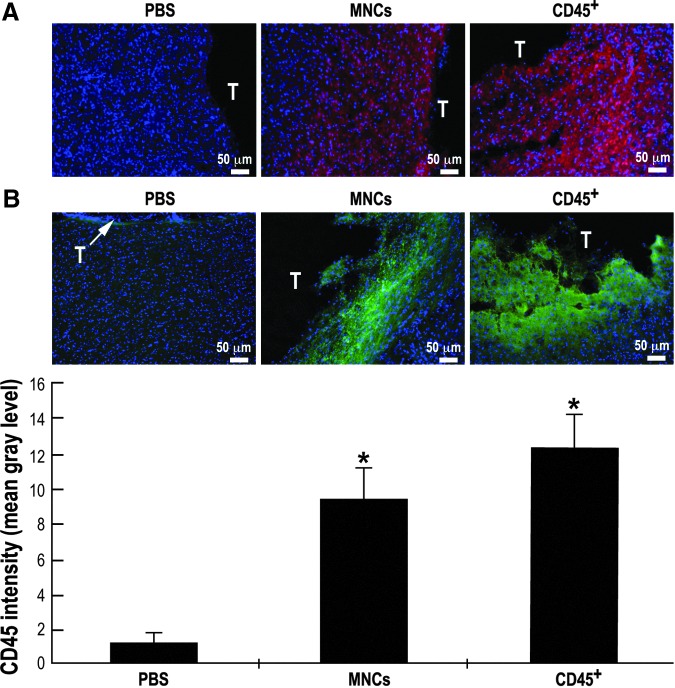
In the second approach, brain slices from the different experimental groups were stained with anti-human-nuclei antibody (red, Fig. 7A) and anti-human-CD45 antibody (green, Fig. 7B) to identify the administered HUCB-derived MNCs or CD45+ cells. Typical photos of brain slices focusing on the border line of the traumatic injury (T, Fig. 7) of mice administered either vehicle or HUCB-derived MNCs or CD45+ cells stained also with DAPI (blue), providing an unambiguous proof of the human origin of the cells. Analysis of CD45 intensity of the brain slices (Fig. 7B) indicates a significant increase in CD45 staining in MNCs- and CD45+-treated mice, compared with vehicle.
FIG. 7.
Homing of human umbilical cord blood (HUCB)-derived mononuclear cells (MNCs) and CD45 positive (CD45+) cells (1×106) to the brain upon intravenous administration. Mice were intravenously administered one day post-trauma with HUCB-derived MNCs or CD45+ cells or with phosphate buffered saline (PBS). Two hours later mice were sacrificed and brains were removed (n=4 each group). Brain slices stained with anti-human-nuclei antibody (red) (A) or anti-human-CD45 antibody (green) (B). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Representative photographs around the trauma. (B) CD45 intensity was calculated as a ratio to background fluorescence and is presented in mean gray level. *p≤0.05, compared with the PBS-treated group.
Altogether, these results indicate that intravenously-administered cells home to the lesion site of the brain.
Brain levels of neurotrophins upon IV administration of HUCB-derived cells
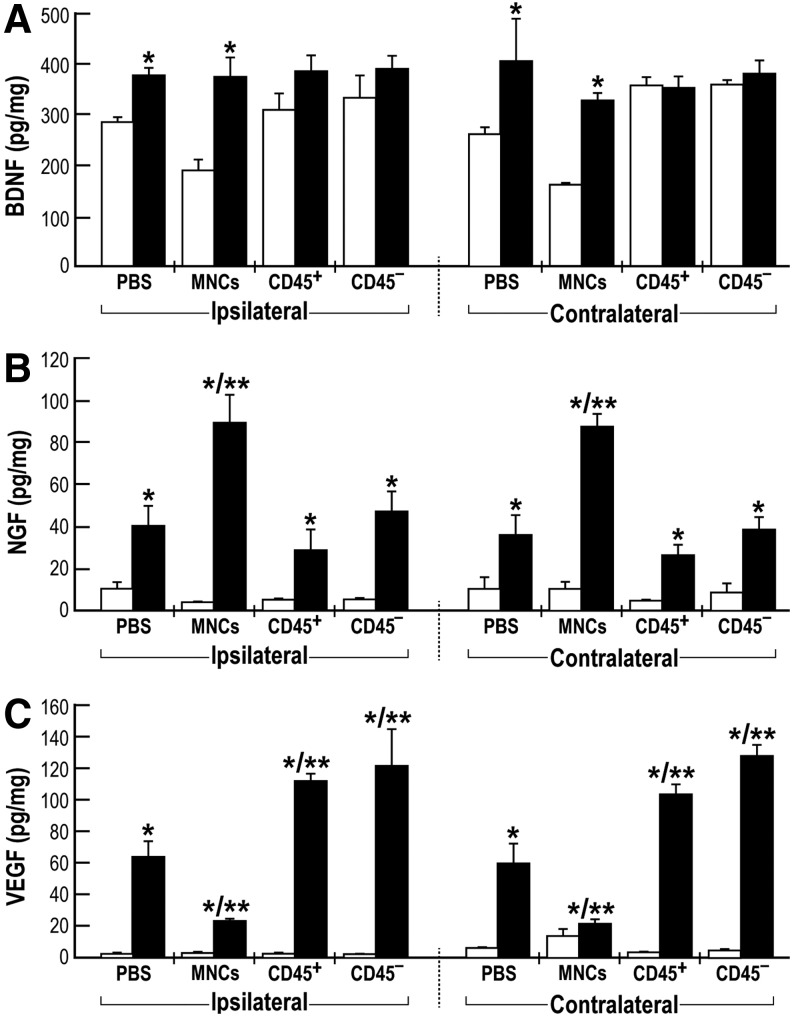
HUCB-derived cells secrete neurotrophic and angiogenic factors which may account, in part, for the observed neurotherapeutic effects.42 We examined whether intravenously-administered cells could indeed induce changes in BDNF, NGF, and VEGF levels in the acute (three days) and chronic (35 days) phases after injury. CHI mice were intravenously administered HUCB-derived MNCs, CD45+ or CD45- cells or with vehicle. The cortical contusion, the bordering hippocampus (both ipsilateral) and the contralateral areas were dissected and protein levels were analyzed by selective ELISA assays. BDNF levels in both the ipsilateral and contralateral brain cortex were significantly increased in the chronic versus acute phases only in MNCs and vehicle-treated mice. No significant changes induced by CD45+ or CD45- cells administration were detected (Fig. 8A). In contrast to BDNF levels, NGF and VEGF levels were very low in the acute phase and significantly increased at the chronic phase in all groups (Fig. 8B, C). Interestingly, NGF levels in MNCs-administered mice were twice as high as the levels seen in mice administered CD45+ cells, CD45- cells, or with vehicle (Fig.8B). An opposite pattern was observed with VEGF levels: In brains of CD45+ cells– and CD45- cells–administered mice, the levels were higher than those in MNCs- or vehicle-treated animals (Fig. 8C). Similar patterns of neurotrophins were measured in the isolated hippocampus (data not shown). These findings may suggest complex interactions in the secretion of neurotrophins by the purified CD45+ and CD45- cells when present together in the original MNCs population.
FIG. 8.
Neurotrophins levels in the closed head injured mice brains. Mice were intravenously administered one day post-trauma with human umbilical cord blood-derived mononuclear cells (MNCs), CD45 positive (CD45+) or CD45 negative (CD45−) cells (1×106) or with phosphate buffered saline (PBS). Three (white bars) and 35 days (black bars) post-trauma mice were sacrificed, whole brains were removed and ipsilateral and contralateral cortex tissues were separated and homogenized for protein extraction. Brain-derived neurotrophic factor (BDNF; A), nerve growth factor (NGF; B), and vascular endothelial growth factor (VEGF; C) levels were measured using enzyme-linked immunosorbent assays. (n=3-4, repeated three times). *p≤0.05, compared with three days respective groups. **p≤0.05 35 days, compared with the PBS-treated group.
Discussion
Our long-term goal is to identify novel therapeutic modalities for and the neurological outcomes of TBI. Using a mouse model of CHI, the two major findings in this study are a) the existence of a longer-term window of opportunity (at least eight days post-trauma) for treating TBI with HUCB-derived MNCs and b) identification of HUCB-MNCs derived CD45+ cells as the active cell population of MNCs that significantly improves the neurological status. This effect was evident as early as seven days and was persistent for five weeks post-trauma, when follow-up was terminated. To the best of our knowledge, this is the first study to show long-lasting neurotherapeutic effects of HUCB-derived CD45+ pan-hematopoietic cells in TBI.
To date, translation from basic research to clinical application in TBI patients has invariably failed. Results from prospective clinical trials are disappointing, in part due to the short temporal window for intervention and the failure of many candidate drugs to cross the blood-brain barrier.43 Therefore, an ongoing clinical trial using allogeneic HUCB therapy for chronic TBI (NCT01451528, ClinicalTrials.gov) raises many hopes. To date, single and double HUCB units are used clinically for the treatment of hematopoietic-related disorders; however, there is no U.S. Food and Drug Administration (FDA) approval yet for the clinical utilization of HUCB-derived isolated populations such as MSCs, since minimally manipulated, unrelated allogeneic placental/HUCB are intended for hematopoietic reconstitution for specified indications (Guidance for Industry and FDA Staff, June 2013). HUCB-derived MSCs also were found to stimulate the injured brain of TBI mice, evoking trophic events and the phenotypic switch of microglia/macrophages, inhibiting glial scarring and leading to significant improvement of neurological outcomes.25 Currently, a variety of MSCs,8 including HUCB-derived MSCs,24,25 represent the most active stem cell population used for pre-clinical and clinical trials of brain injury. While the majority of these regenerative clinical trials using MSCs have proved safety, phase II clinical trials are still ongoing and are facing issues of efficacy. The HUCB cells are used for therapy of hematopoietic and non-hematopoietic disorders that require reconstitution of the hematopoietic system.18 This HUCB can be easily isolated from the cord of the donor. So far, HUCB has been shown to reduce neurological deficits in rats after TBI: Following IV administration, the cells preferentially entered the brain and homed into the boundary zone of the injured area.23
In this study, we confirmed some prior results and provided novel evidence that HUCB-derived MNCs improve the neurobehavioral deficits upon both ICV transplantation and IV administration in the CHI mouse model. Previously, Lu and colleagues reported a reduction of neurological deficit after IV administration of MNCs in a rat TBI model.23 In the present study, the effect was dependent on the number of ICV transplanted cells and was maximal with 1×105 cells/mice (Fig. 3A). One of the major challenges in the clinical management of TBI is the narrow therapeutic window, which is in the range of up to 4–6 h.40 Part of the failure to bring promising drug candidates from pre-clinical studies to the clinic is thought to be the failure to meet this window of opportunity, and the administration of the tested drug at a time when it is not effective anymore.7 A novel and important message emanating from the findings in this study is that, ICV transplantation of HUCB-derived MNCs eight days after TBI still can improve functional recovery as effectively as when given 24 h post-trauma. Both treatment regimens demonstrated a similar increase in ΔNSS values, which were sustained up to three weeks (Fig. 3B). Following the primary mechanical insult, TBI results in delayed events, such as neurochemical, metabolic and cellular changes, which lead to the secondary injury that accounts for many of the observed neurological deficits. The phase that leads up to the secondary injury represents a new window of opportunity for therapeutic intervention to prevent progressive tissue damage and loss of function after injury.44 Our approach may target this new window: The ability to improve function even when treatment is given eight days post-trauma holds promise for future clinical developments.
An important question addressed in this study relates to the possibility of donor-dependent variability of HUCB-derived MNCs and CD45+ cells in terms of their beneficial effect in the CHI mouse model. We performed these experiments over a three-year period using more than 30 cord blood units from different donors, and all were efficient in reducing the neurological deficits in the mice after CHI. These findings indicate that the effect is independent of a specific cord blood donor, a conclusion that is relevant for future translational HUCB-derived MNCs-based therapies of TBI.
Another major novel finding of this study is the demonstration of a neurotherapeutic effect of CD45+ cells upon IV administration in CHI mice. The pan-leukocyte CD45 phosphatase plays an essential role in trafficking and repopulation of the bone marrow by CD34+ hematopoietic stem cells. Inhibition of CD45 negatively affects development of hematopoietic progenitors ex vivo and their recovery in transplanted recipients in vivo, supporting the central role of CD45 in the regulation of hematopoiesis.17,29 The ability of HUCB to improve neurological deficits in the CHI mice may be explained by a direct effect of the CD45+ cells homing to the brain (Fig. 7). A direct role of the CD45+ cells is further emphasized by the neutralization experiments with an anti-human-CD45 antibody, which significantly decreased their neurotherapeutic efficacy (Fig. 4A). It appears that CD45+ cells provide higher benefit than MNCs transplantation by comparing the neurotherapeutic effect of CD45+ cells (Fig. 4) with that of MNCs (Fig. 3D) at a similar IV administration dose of 1×106 cells. Considering that the CD45+ population represents about 50% of the MNCs fraction, we can propose that transplantation with enriched pan-hematopoietic CD45+ cells might provide an enhanced neurotherapeutic effect by comparison to whole HUCB transplantation in TBI patients.
As previously reported for HUCB-derived MSCs25 and MNCs,23 in this study, HUCB-derived MNCs and CD45+ cells intravenously administered one day post-trauma rapidly home (within 1.5–2 h; Fig.6D and Fig. 7) to the brain lesion site. In control, non-injured mice, the intravenously-administered cells did not home to the brain. These findings are consistent with many studies indicating homing ability of IV-infused stem cells to the site of injury,45 although a majority of these cells will end up in the lung, spleen, stomach, and small and large intestines.46 Disruption of the blood brain–barrier, which occurs rapidly (within 1–4 h after injury),4,5 and other mechanisms promoting homing to the injury site,47 are probably involved in the enhanced homing to and engraftment in the injured brain, in particular at the site of lesion and its close vicinity. Our finding of rapid homing of intravenously-administered HUCB-derived MNCs and CD45+ cells to the injured site is in line with previous reports tracking the integration of bone marrow-derived MSCs into traumatic injured brain.48,49 The decrease in brain NIR fluorescence of CHI mice treated with labeled MNCs cells at later time points post-administration, from 5 h to seven days (Fig. 6D) may indicate a very short-term retention of labeled cells at the injured site. However, this may also be a consequence of a loss of the fluorescent signal, an issue that warrants further research. Homing of the HUCB-derived cells to an injured region also may be targeted by neurotransmitters. Gamma-aminobutyric acid, for instance, has been shown to attract both hematopoietic and non-hematopoietic cord blood cells.50 Indeed, there is a strong cross-talk between the nervous system and hematopoiesis, for example, hematopoietic niches are controlled by sympathetic innervations.51 Alternatively, the human administered HUCB-derived CD45+cells may also stimulate/recruit endogenous murine bone marrow hematopoietic stem cells, resulting in the indirect activation of endogenous therapeutic effects.29 The basic mechanism by which the neurotherapeutic effect is achieved is as yet unknown. The temporal and causal relationships between immunomodulation, neurotherapeutic effect and homing of administered cells deserve future investigation.
Based on the ability of HUCB-derived MNCs and CD45+ cells to induce a rapid early reduction in the observed neurological deficits in mice after CHI, we hypothesize that the neurotherapeutic effect is due to stimulation of endogenous neuroprotective mechanisms by an autocrine/paracrine effects mediated by these cells. Potential trophic factors repeatedly reported to be involved in neuroprotection include BDNF, NGF, and VEGF.45,52,53 Present data show that upon CD45+ cells administration, BDNF levels in both the ipsilateral and contralateral brain cortex were not significantly changed, as was shown by comparing acute with chronic cell implantation effects. In contrast to BDNF levels, NGF and more VEGF levels were significantly increased at the chronic phase (35 days). Interestingly, the level of NGF was reduced in the CD45+- or CD45--treated mice, compared with MNCs-treated mice. This effect may suggest that the both CD45+ and CD45- subpopulations contribute to the enhanced effect of MNCs. In contrast, the VEGF level in CD45+ and CD45- was higher than in MNCs-treated mice, indicating that in the MNCs an inhibitory effect is occurring between the two subpopulations. However, since in our hands we have not been able to determine a direct correlation of the above growth factors between the ipsilateral and contralateral brain sites we might propose that other growth factors and cytokines are involved. Alternatively, we cannot exclude the possibility that differential levels of these growth factors between the ipsilateral and the contralateral hemispheres might be due to a short time window between three and 35 days after CHI. Also, future availability of ELISA assays selective for either human or mice derived neurotrophic factors may further enlighten and differentiate between the contributions of exogenous and/or endogenous factors to the neurotherapeutic effect.
In conclusion, administration of HUCB-derived MNCs or CD45+ pan-hematopoietic cells reduced brain damage in CHI mouse model, as reflected by reduced neurological deficits, starting seven days post-trauma and lasting for several weeks. This neurotherapeutic effect was induced by HUCB-derived cells upon rapid homing to the site of brain injury. Based on the minimal manipulation required for the isolation of CD45+ cells from HUCB and their ability to efficiently reduce neurological deficits, we propose that (HUCB-derived) CD45+ hematopoietic cells should be considered for translational therapy in treating TBI patients.
Acknowledgments
This study was supported by the Israel Ministry of Science, Technology and Space. PL holds the Jacob Gitlin Chair in Physiology and is affiliated with and supported by the David R. Bloom Center for Pharmacy and the Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at the Hebrew University of Jerusalem, Israel. HAZ was supported by “VATAT” fellowship from the Israeli Council for Higher Education. GC is supported by “Eshkol fellowship” from the Israeli Ministry of Science, Technology and Space. PIL is the Laura H. Carnell Professor in the Department of Bioengineering, Temple University.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beauchamp K., Mutlak H., Smith W.R., Shohami E, and Stahel P.F. (2008). Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 14, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafa A.G. and Alshboul O.A. (2013). Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 18, 222–234 [PubMed] [Google Scholar]
- 3.Leker R.R. and Shohami E. (2002). Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res. Brain Res. Rev. 39, 55–73 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 5.Yatsiv I., Grigoriadis N., Simeonidou C., Stahel P.F., Schmidt O.I., Alexandrovitch A.G., Tsenter J., and Shohami E. (2005). Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 19, 1701–1703 [DOI] [PubMed] [Google Scholar]
- 6.Liraz-Zaltsman S., Alexandrovich A.G., Trembovler V., Fishbein I., Yaka R., Shohami E., and Biegon A. (2011). Regional sensitivity to neuroinflammation: in vivo and in vitro studies. Synapse 65, 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shohami E. and Biegon A. (2011). Controversial findings on the role of NMDA receptors in traumatic brain injury. In: Traumatic brain and spinal cord injury. Morganti-Kossman , Raghupathi , Maas (eds). Cambridge University Press: UK [Google Scholar]
- 8.Trounson A., Thakar R.G., Lomax G., and Gibbons D. (2011). Clinical trials for stem cell therapies. BMC Med. 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuckin C.P. and Forraz N. (2008). Potential for access to embryonic-like cells from human umbilical cord blood. Cell Prolif. 41Suppl 1, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arien-Zakay H., Lazarovici P. and Nagler A. (2010). Tissue regeneration potential in human umbilical cord blood. Best Pract. Res. Clin. Haematol. 23, 291–303 [DOI] [PubMed] [Google Scholar]
- 11.Harris D.T. and Rogers I. (2007). Umbilical cord blood: a unique source of pluripotent stem cells for regenerative medicine. Curr. Stem Cell Res. Ther. 2, 301–309 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen J.S. and McNagny K.M. (2008). Novel functions of the CD34 family. J. Cell Sci. 121, 3683–3692 [DOI] [PubMed] [Google Scholar]
- 13.Meregalli M., Farini A., Belicchi M., and Torrente Y. (2010). CD133(+) cells isolated from various sources and their role in future clinical perspectives. Expert Opin. Biol. Ther. 10, 1521–1528 [DOI] [PubMed] [Google Scholar]
- 14.Craig W., Poppema S., Little M.T., Dragowska W., and Lansdorp P.M. (1994). CD45 isoform expression on human haemopoietic cells at different stages of development. Br. J. Haematol. 88, 24–30 [DOI] [PubMed] [Google Scholar]
- 15.Dahlke M.H., Larsen S.R., Rasko J.E., and Schlitt H.J. (2004). The biology of CD45 and its use as a therapeutic target. Leuk. Lymphoma 45, 229–236 [DOI] [PubMed] [Google Scholar]
- 16.Huntington N.D. and Tarlinton D.M. (2004). CD45: direct and indirect government of immune regulation. Immunol. Lett. 94, 167–174 [DOI] [PubMed] [Google Scholar]
- 17.Shivtiel S., Kollet O., Lapid K., Schajnovitz A., Goichberg P., Kalinkovich A., Shezen E., Tesio M., Netzer N., Petit I., Sharir A., and Lapidot T. (2008). CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J. Exp. Med. 205, 2381–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanevsky A., Shimoni A., Yerushalmi R., and Nagler A. (2011). Cord blood stem cells for hematopoietic transplantation. Stem Cell Rev. 7, 425–433 [DOI] [PubMed] [Google Scholar]
- 19.Nan Z., Grande A., Sanberg C.D., Sanberg P.R., and Low W.C. (2005). Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann. N. Y. Acad. Sci. 1049, 84–96 [DOI] [PubMed] [Google Scholar]
- 20.Chen S.H., Chang F.M., Tsai Y.C., Huang K.F., Lin C.L., and Lin M.T. (2006). Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Exp. Neurol. 99, 67–76 [DOI] [PubMed] [Google Scholar]
- 21.Chen J., Sanberg P.R., Li Y., Wang L., Lu M., Willing A.E., Sanchez-Ramos J., and Chopp M. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 22.Chen C.T., Foo N.H., Liu W.S., and Chen S.H. (2008). Infusion of human umbilical cord blood cells ameliorates hind limb dysfunction in experimental spinal cord injury through anti-inflammatory, vasculogenic and neurotrophic mechanisms. Pediatr. Neonatol. 49, 77–83 [DOI] [PubMed] [Google Scholar]
- 23.Lu D., Sanberg P.R., Mahmood A., Li Y., Wang L., Sanchez-Ramos J., and Chopp M. (2002). Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant 11, 275–281 [PubMed] [Google Scholar]
- 24.Wang S., Cheng H., Dai G., Wang X., Hua R., Liu X., Wang P., Chen G., Yue W., and An Y. (2013). Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 1532, 76–84 [DOI] [PubMed] [Google Scholar]
- 25.Zanier E.R., Montinaro M., Vigano M., Villa P., Fumagalli S., Pischiutta F., Longhi L., Leoni M.L., Rebulla P., Stocchetti N., Lazzari L., and De Simoni M.G. (2011). Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit. Care Med. 39, 2501–2510 [DOI] [PubMed] [Google Scholar]
- 26.Wexler S.A., Donaldson C., Denning-Kendall P., Rice C., Bradley B. and Hows J.M. (2003). Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 121, 368–374 [DOI] [PubMed] [Google Scholar]
- 27.Gorczyca W., Sun Z.Y., Cronin W., Li X., Mau S., and Tugulea S. (2011). Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell Biol. 103, 221–266 [DOI] [PubMed] [Google Scholar]
- 28.Arien-Zakay H., Nagler A., Galski H., and Lazarovici P. (2007). Neuronal conditioning medium and nerve growth factor induce neuronal differentiation of collagen-adherent progenitors derived from human umbilical cord blood. J. Mol. Neurosci. 32, 179–191 [DOI] [PubMed] [Google Scholar]
- 29.Shivtiel S., Lapid K., Kalchenko V., Avigdor A., Goichberg P., Kalinkovich A., Nagler A., Kollet O., and Lapidot T. (2011). CD45 regulates homing and engraftment of immature normal and leukemic human cells in transplanted immunodeficient mice. Exp. Hematol. 39, 1161–1170 [DOI] [PubMed] [Google Scholar]
- 30.Beni-Adani L., Gozes I., Cohen Y., Assaf Y., Steingart R.A., Brenneman D.E., Eizenberg O., Trembolver V. and Shohami E. (2001). A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 296, 57–63 [PubMed] [Google Scholar]
- 31.Tsenter J., Beni-Adani L., Assaf Y., Alexandrovich A.G., Trembovler V. and Shohami E. (2008). Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J. Neurotrauma 25, 324–333 [DOI] [PubMed] [Google Scholar]
- 32.Cohen G., Lecht S., Arien-Zakay H., Ettinger K., Amsalem O., Oron-Herman M., Yavin E., Prus D., Benita S., Nissan A., and Lazarovici P. (2012). Bio-imaging of colorectal cancer models using near infrared labeled epidermal growth factor. PLoS One 7, e48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhoff S., Moers J., Rieks M., Grunwald T., Jensen A., Dermietzel R., and Meier C. (2007). Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp. Hematol. 35, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Pernaute R., Studer L., Ferrari D., Perrier A., Lee H., Vinuela A., and Isacson O. (2005). Long-term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells 23, 914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiyama T., Kuroda S., Osanai T., Shichinohe H., Kuge Y., Ito M., Kawabori M., and Iwasaki Y. (2011). Near-infrared fluorescence labeling allows noninvasive tracking of bone marrow stromal cells transplanted into rat infarct brain. Neurosurgery 68, 1036–1047 [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G. and Franklin K.B.J. (2004). The Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press: Waltham, Massachusetts [Google Scholar]
- 37.Teicher B.A. and Fricker S.P. (2010). CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927–2931 [DOI] [PubMed] [Google Scholar]
- 38.Zola H. (2006). Medical applications of leukocyte surface molecules—the CD molecules. Mol. Med. 12, 312–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augello A., Kurth T.B. and De Bari C. (2010). Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur. Cell Mater. 20, 121–133 [DOI] [PubMed] [Google Scholar]
- 40.Davis S.M. and Donnan G.A. (2009). 4.5 hours: the new time window for tissue plasminogen activator in stroke. Stroke 40, 2266–2267 [DOI] [PubMed] [Google Scholar]
- 41.Stanevsky A., Shimoni A., Yerushalmi R., and Nagler A. (2010). Double umbilical cord blood transplant: more than a cell dose? Leuk. Lymphoma 51, 975–982 [DOI] [PubMed] [Google Scholar]
- 42.Arien-Zakay H., Lecht S., Bercu M.M., Tabakman R., Kohen R., Galski H., Nagler A., and Lazarovici P. (2009). Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp. Neurol. 216, 83–94 [DOI] [PubMed] [Google Scholar]
- 43.Menon D.K. (2009). Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 37, S129–S135 [DOI] [PubMed] [Google Scholar]
- 44.Kumar A. and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 45.Gincberg G., Arien-Zakay H., Lazarovici P., and Lelkes P.I. (2012). Neural stem cells: therapeutic potential for neurodegenerative diseases. Br. Med. Bull. 104, 7–19 [DOI] [PubMed] [Google Scholar]
- 46.Maurya D.K., Doi C., Pyle M., Rachakatla R.S., Davis D., Tamura M., and Troyer D. (2011). Non-random tissue distribution of human naive umbilical cord matrix stem cells. World J. Stem Cells 3, 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andres R.H., Choi R., Pendharkar A.V., Gaeta X., Wang N., Nathan J.K., Chua J.Y., Lee S.W., Palmer T.D., Steinberg G.K., and Guzman R. (2011). The CCR2/CCL2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke 42, 2923–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng J., Zou Z.M., Zhou T.L., Su Y.P., Ai G.P., Wang J.P., Xu H., and Dong S.W. (2011). Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol. Sci. 32, 641–651 [DOI] [PubMed] [Google Scholar]
- 49.Yoon J.K., Park B.N., Shim W.Y., Shin J.Y., Lee G., and Ahn Y.H. (2010). In vivo tracking of 111In-labeled bone marrow mesenchymal stem cells in acute brain trauma model. Nucl. Med. Biol. 37, 381–388 [DOI] [PubMed] [Google Scholar]
- 50.Zangiacomi V., Balon N., Maddens S., Tiberghien P., Versaux-Botteri C., and Deschaseaux F. (2009). Human cord blood-derived hematopoietic and neural-like stem/progenitor cells are attracted by the neurotransmitter GABA. Stem Cells Dev. 18, 1369–1378 [DOI] [PubMed] [Google Scholar]
- 51.Spiegel A., Shivtiel S., Kalinkovich A., Ludin A., Netzer N., Goichberg P., Azaria Y., Resnick I., Hardan I., Ben-Hur H., Nagler A., Rubinstein M., and Lapidot T. (2007). Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 8, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 52.Longhi L., Zanier E.R., Royo N., Stocchetti N., and McIntosh T.K. (2005). Stem cell transplantation as a therapeutic strategy for traumatic brain injury. Transpl. Immunol. 15, 143–148 [DOI] [PubMed] [Google Scholar]
- 53.Mahmood A., Lu D., and Chopp M. (2004). Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 21, 33–39 [DOI] [PubMed] [Google Scholar]