Abstract
Liver fibrosis and its end-stage consequence, cirrhosis, represent the final common pathway of virtually all chronic liver diseases. Research into hepatic stellate cell activation, imbalance of the extracellular matrix synthesis and degradation and the contribution of cytokines and chemokines has further elucidated the mechanisms underlying fibrosis. Furthermore, clarification of changes in host adaptive and innate immune systems has accelerated our understanding of the association between liver inflammation and fibrosis. Continued elucidation of the mechanisms of hepatic fibrosis has provided a comprehensive model of fibrosis progression and regression. This review summarizes the current concepts of improvements that have been made in the field of fibrosis.
Keywords: cirrhosis, hepatic stellate cell, liver fibrosis
Introduction
Hepatic fibrosis represents a ubiquitous response of the liver to acute or chronic injury. Current evidence suggests that the process of hepatic fibrosis is driven primarily by the development of inflammation in response to parenchymal injury, with variable clinical manifestations that are determined by the type and extent of liver damage, the underlying liver disease and the capacity of the whole body to respond.1 Fibrosis accompanies progressive liver injury and varies from mild to extensive, while cirrhosis, which is the end stage of fibrosis of hepatic parenchyma, is characterized by architectural disruption, aberrant hepatocyte regeneration, nodule formation and vascular changes.2 Cirrhosis is also associated with an increased risk of liver failure, portal hypertension and liver cancer. Recent studies suggest that cirrhosis is associated with a high risk of complications that accrue over time. In patients infected with the chronic hepatitis C virus, the annual mortality rate is greater than 4%.3 Among patients with cirrhosis, approximately 70% of deaths are directly attributable to liver disease.4 Fibrosis and cirrhosis of the liver remain major causes of morbidity and mortality worldwide and are associated with increasing economic and social impact.
Liver regeneration is an extremely complex process. Recent research in human and animal models has shown that liver fibrosis is potentially reversible in specific circumstances and resolution with a restoration of near normal architecture has been demonstrated.5, 6 It is hoped that a clearer understanding of the etiology of liver fibrosis will provide improved diagnostic tools and potential therapeutic approaches for the treatment of liver fibrosis and cirrhosis. Currently, liver transplantation is the only effective treatment available for cirrhosis.7 However, shortages of organs, the presence of concurrent disease affecting other tissues and recurrence of the original disease in transplant recipients limit the impact of this approach. Continued elucidation of the mechanisms of hepatic fibrosis has rendered the possibility of arresting fibrosis progression a more realistic prospect.
Cell targets contributing to liver fibrosis
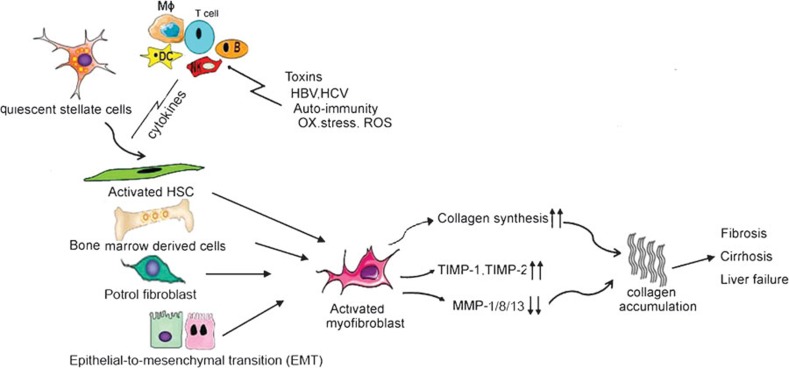
Hepatic fibrosis results from a dynamic process regarded as the result of an imbalance in extracellular matrix (ECM) synthesis and degradation. With ongoing liver damage, fibrosis may progress to cirrhosis, which is characterized by a distortion of the liver vasculature and architecture, and which is the major determinant of morbidity and mortality in patients with liver disease, predisposing to liver failure and primary liver cancer. Several reviews have emphasized the essential role of hepatic stellate cell (HSC) and myofibroblasts (MF) activation in the pathogenesis of hepatic fibrosis (Figure 1).
Figure 1.
Initiation and maintenance of fibrogenesis. Activated hepatic stellate cells, bone marrow derived cells, portal fibroblasts, epithelial-to-mesenchymal transition (EMT) are source of myofibroblasts. The pathways of HSC activation include those that provoke initiation and those that maintain perpetuation. Initiation is stimulated by soluble stimuli such as toxins, HBV or HCV infection and oxidant stress signals. Following activation, these myofibroblasts are characterized by increased proliferation, migration and a relative resistance to apoptosis. Imbalances in collagen synthesis and degradation result in cirrhosis and liver failure. HBV, hepatitis B virus; HCV, hepatitis C virus; HSC, hepatic stellate cell; ROS, reactive oxygen species; TIMP, tissue inhibitor of metalloproteinase.
HSCs are resident perisinusoidal cells located in the subendothelial space between hepatocytes and sinusoidal endothelial cells.8 These cells are characterized by vitamin A autofluorescence, perisinusoidal orientation and variable expression of a number of cytoskeletal proteins including desmin, glial fibrillary acid protein, vitamin and nestin.9 Under pathological conditions, including injury, inflammation, hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, HSCs are activated to undergo a phenotypic switch from a quiescent, vitamin A storing cell into proliferative, α-smooth muscle actin-positive, myofibroblast-like cells, exhibiting upregulated collagen synthesis.10 Furthermore, morphological observations imply that the so-called activation of HSCs is facilitated even in the absence of contact with the sinusoids or hepatic plates.11 HSCs exhibit a wide repertoire of activities that emphasize the dynamic nature of the liver wound-healing response. These activities include the synthesis of fibrillar collagens, contractile activity, secretion of chemotactic and vasoactive factors, migratory activity and the secretion of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs).12, 13 There is evidence that a substantial number of proinflammatory cytokines regulate the function of HSCs. Foremost among these is transforming growth factor (TGF)-β, which has been identified as the most profibrotic cytokine, promoting HSC expression of collagen I, transition to a myofibroblast-like phenotype and inhibition of ECM degradation through the expression of TIMPs.14 In parallel, platelet-derived growth factor has emerged as the most potent proproliferative cytokine for HSCs, whereas tumor-necrosis factor (TNF)-related apoptosis ligand expressed by Kupffer cells (KCs) is thought to mediate stellate cell apoptosis.15 Activated HSCs play a significant role, not only in the remodeling of sinusoidal walls necessary for the regeneration of hepatocytes, but also in the formation of central–central bridging fibrosis at the site of the incomplete regeneration.
Initiation of HSC activation is stimulated by soluble factors such as oxidant stress signals (reactive oxygen intermediates), apoptotic bodies, lipopolysaccharide (LPS) and paracrine stimuli from neighboring cell types including hepatic macrophages (KCs), sinusoidal endothelium and hepatocytes.16, 17 Sustained activation of HSCs is characterized by specific phenotypic changes including proliferation, contractility, fibrogenesis, altered matrix degradation, chemotaxis and inflammatory signaling. Loss of activated HSCs is associated with resolution of hepatic fibrosis, which occurs following clearance of the primary liver disease. Spontaneous resolution of liver fibrosis has been reported in a number of models of chronic liver injury, although whether this results from selective apoptosis of activated HSC or reversion to a quiescent state remains to be determined.18, 19
Myofibroblasts, the prototypical mesenchymal cell type that regulates repair of injury in a range of tissues, are defined primarily by their ability to produce ECM and exhibit contractile activity. Although HSCs are the primary source of MF in the liver, the contribution of other cells is increasingly being appreciated.20 The primary cell source is derived from bone marrow and following liver injury, these mobilized MF-like cells participate in the progression of liver fibrosis.21 However, the collagen production capacity of these bone marrow-derived cells is limited. Portal fibroblasts, which are resident within the connective tissue of portal areas, are also recruited and activated into MF and are especially relevant in diseases associated with ischemia and cholestasis. The contribution of these cells to fibrosis is particularly important in biliary diseases. The third source of MF is derived from cells that undergo epithelial-to-mesenchymal transition (EMT).22 This process allows a closely attached epithelial cell with apical–basal polarity to migrate and accumulate in the interstitium of the tissue and acquire a mesenchymal cell phenotype, including migratory capacity, invasiveness, resistance to apoptosis and production of ECM. EMT has been associated not only with tissue regeneration and fibrosis, but also with embryonic development and cancer progression. However, whether these cells simply express mesenchymal markers or contribute substantively to ECM production remains to be clarified. In contrast to EMT, which is a dynamic and bidirectional process, fibrogenic cells can revert to an epithelial phenotype. The actual contribution of these cells to hepatic regeneration and fibrosis requires further investigation.
Imbalance in extracellular matrix synthesis and degradation
In normal liver, ECM is a highly dynamic substratum with a precisely regulated balance between synthesis and degradation. However, this balance is disrupted in liver fibrosis. The ECM becomes progressively insoluble and resistant to protease digestion due to thickening of fibrotic septae and increased crosslinking. Normally, the hepatic ECM comprises less than 3% of the relative area of a liver tissue section and approximately 0.5% of the wet weight. During chronic liver injury, both the quality and quantity of hepatic ECM are altered by disruption of this matrix and replacement by fibrillar collagen,23 with collagen types I and III24 being the predominant forms that are accumulated in advanced fibrosis.
The turnover of collagen and other ECM proteins is controlled by regulation of MMPs and TIMPs. Fibrosis occurs when this balance tips in favour of TIMPs, whereas resolution is associated with reduced TIMP expression. MMPs are a family of zinc-dependent endoproteinases that degrade various ECM components and are highly expressed in HSCs and KCs. It is therefore unsurprising that research is focused on discovery of the identity and source of key MMPs that mediate the resolution of fibrosis. The first of these molecules to be identified, and the best characterized interstitial collagenase in humans, MMP-1, is widely expressed in human tissues including liver. However other human interstitial collagenases with more limited cellular expression including neutrophil collagenase (MMP-8) and collagenase 13 (MMP-13) are also being investigated.25 The recently described enzymes, MMP-2 and MMP-14, also have interstitial collagenolytic activity.26 Studies in animal models and human liver fibrosis indicate that interstitial collagenolytic activity decreases in liver extracts in advanced fibrosis, thus promoting net collagen deposition.
TIMPs play an important role in preventing degradation of the accumulating matrix during liver injury by antagonizing the activity of metalloproteinases and it is speculated that activated HSCs are an important source of TIMPs in injured liver. Expression of both TIMP-1 and TIMP-2 is increased in human fibrotic liver and in a rat model of liver fibrosis. Moreover, the degree of TIMP-1 expression correlates with the extent of fibrosis in human liver.27 The resulting increase in the TIMP/MMP ratio in liver may promote fibrosis by protecting deposited ECM from degradation by MMPs. The balance between MMP-2/TIMP-2 has been shown to be critical for the turnover of ECM.28 The contribution of other MMP inhibitory mechanisms in fibrosis cannot be discounted.
Continued elucidation of the mechanisms of hepatic fibrosis which has made stopping the cirrhosis getting worse is becoming more and more realistic. Preclinical studies have highlighted a number of therapies that specifically could abrogate fibrogenesis. Such therapies have been targeted at inhibition of collagen synthesis, matrix deposition, modulation of stellate cell activation, stimulation of matrix degradation or stimulation of stellate cell death. Those treatments including anti TGF-β approach, cytokines including interleukin-10 (IL-10) and hepatocyte growth factor (HGF), and some herbal medicines, have also been shown to have antifibrotic properties in animal models, but the long-term efficiency and safety need to be confirmed in a larger cohort of clinical trial and tracer studies.
Cytokines and chemokines in fibrosis
Cytokines, which mediate many immune and inflammatory reactions, are soluble peptides secreted by several types of cells including immunocytes, parenchymal and non-parenchymal cells. Hepatocyte damage resulting from factors such as viral agents, alcohol consumption, hepatotoxins and autoimmunity, leads to recruitment of macrophages and neutrophils and local production of cytokines and chemokines that mediate the inflammatory response that leads to the regeneration of liver tissue and ultimately to the deposition of extracellular matrix by activation of HSCs. The most important cytokines produced in the liver include TGF-β, TNF-α, IL-1, IL-6, IL-10 and interferon (IFN)-γ29 and these are predominantly produced by resident macrophages, approximately 80%–90% of which are KCs. Alteration in cytokines is observed in different liver diseases. In chronic alcoholic liver disease, the production of TNF-α, IL-1, IL-6, as well as the chemokine IL-8/CXCL8, is increased, while TNF-α is identified as an important mediator of non-alcoholic fatty liver disease.30
TGF-β1, which has been identified as the most profibrotic cytokine, stimulates its own production by myofibroblasts, thereby establishing an autocrine cycle of myofibroblast differentiation and activation. Furthermore, anti-TGF-β approaches have been established and successfully utilized for the treatment of experimental fibrogenesis. However, broad pharmacological targeting of the TGF-β pathway to combat disease is likely to be problematic due to the pleiotropic nature of TGF-β activities.31 Therefore, elucidation of the fundamental mechanism underlying TGF-β stimulation of fibrogenesis is essential for the development of approaches that specifically target profibrogenic TGF-beta signaling. Other compounds with mitogenic activity toward stellate cells include vascular endothelial cell growth factor, thrombin, epidermal growth factor, keratinocyte growth factor and basic fibroblast growth factor. Furthermore, IL-8 acts as a mediator of HSC differentiation by providing a stop signal to migrating HSCs that have reached a fibrosis site and inducing a contractile phenotype associated with focal adhesion and upregulation of α-smooth muscle actin production.32
Due to the critical involvement of the chemokine system in lymphocyte recruitment, several studies have analyzed the role of chemokines in the pathogenesis of hepatic fibrosis. Although a large number of chemokine signaling pathways are involved, the CC- and CXC-chemokine receptor families have consistently exhibited important regulatory roles. CCL2 (MCP-1) and its receptor, CCR2 have been widely investigated. Interference with the action of CCL2 through adenovirus-mediated expression of a mutated ligand (7ND-CCL2) revealed a marked suppression of macrophage and lymphocyte infiltration into injured livers, reduction of HSC activation and prevention of hepatic fibrogenesis.33 CCR5, a chemokine receptor which binds CCL5, and CCL3 or CCL4, mediates both recruitment of monocyte/macrophages and liver fibrosis by induction of proliferation and migration of human HSCs.34 In patients with hepatitis C infection, CXCR3-associated chemokines may be an important determinant of liver injury and fibrogenesis. Moreover, expression of chemokines interacting with CXCR3 and particularly CXCL10, is upregulated in patients with advanced inflammation and fibrosis.35
In patients with primary biliary cirrhosis, expression of CCR7 is upregulated and immunostaining for its ligand, CCL21, is detected around inflammatory lymphoid follicles.36 Involvement in the fibrogenic process was indicated by the observation that activation of CCR7 on cultured HSCs stimulates cell migration and accelerates wound healing. HSCs also express functional CXCR4, the activation of which induces expression of α-smooth muscle actin and collagen I and increased proliferation.37 The liver is the first tissue to come in contact with bacterial products, including endotoxins, derived from the gastrointestinal tract. Toll-like receptors (TLRs) comprise a highly conserved family of receptors that recognize pathogen-associated molecular patterns and allow the host to detect microbial infection. Recently, the role of the TLR system in liver fibrogenesis has been extensively investigated. Activation of TLR4 by lipopolysaccharide (LPS) upregulates chemokine secretion and sensitizes HSCs to the action of TGF-β.38 TLR4 signaling induces expression of several profibrogenic cytokines, including TNF-α, IL-1 and IL-6. A single-nucleotide polymorphism of the TLR4 gene has recently emerged as a factor conferring protection from fibrosis in patients with chronic hepatitis C.39 These single-nucleotide polymorphisms were shown to reduce TLR4-mediated inflammatory and fibrogenic signaling and lower the apoptotic threshold of activated HSCs in vitro.40 TLR9, another widely investigated fibrogenic TLR, functions as a sensor of degraded nuclear DNA. Following apoptotic hepatocyte DNA ligation, activated TLR9 has been shown to modulate the biology of HSC, resulting in inhibited cell migration and upregulated collagen production.41 Furthermore, the development of biliary fibrosis is delayed in mice lacking TLR9.42
Innate and adaptive immunity in liver fibrosis
Chronic inflammation and fibrosis are inextricably linked and the interactions between immune effector cells, local fibroblasts and tissue macrophages at sites of scar formation determine the outcome of liver injury. With improved understanding of the processes that govern inflammation and fibrosis, it has become clear that both the adaptive and innate immune systems are involved in the regulation of fibrosis (Table 1).
Table 1. Role of immunocyte cell types in liver fibrosis.
| Cell type | Cytokine production | Role in liver fibrosis | Reference |
|---|---|---|---|
| Kuffer cells | IL-1, IL-6, TNF-α | Secrete large number of pro-inflammatory and fibrogenic mediators | 43 |
| Depletion of KC reduces histological fibrosis | 44 | ||
| NK cells | IFN-γ, | Induce HSC apoptosis in a TRAIL-dependent manner | 46 |
| TNF-α, GM-CSF | Depletion of NK cells enhances liver fibrosis | 48 | |
| NKT cells | IFN-γ, IL-4, IL-13 | Drive progression of fibrosis through secretion of type 2 profibrotic cytokines | 51 |
| Depletion of NKT cells decreases liver damage and fibrosis | 52 | ||
| DC cells | IL-12, IL-10 | Change in DC quality and quantity during liver fibrosis. Gain ability to stimulate NKT and HSCs | 54 |
| IL-6, IFN-α | DC depletion completely abrogates inflammatory mediators in fibrotic liver | ||
| T cells | IL-2, IFN-γ | Th1 cells produce antifibrotic cytokines | 60, 61 |
| IL-4, IL-5, IL-6, IL-10 | Th2 cells produce profibrotic cytokines | ||
| B cells | IL-12, IL-8, IL-1, TNF-α | B-cell depletion reduces collagen deposition | 62 |
Abbreviations: DC, dendritic cell; GM-CSF, granulocyte/macrophage colony-stimulating factor; HSC, hepatic stellate cell; IFN, interferon; KC, Kupffer cell; NK, natural killer; NKT, natural killer T; Th, T helper; TNF, tumor-necrosis factor; TRAIL, tumor-necrosis factor-related apoptosis ligand.
Hepatic KC, which constitute 15% of the total liver cell population, are tissue macrophages derived from circulating monocytes. Macrophage phenotype and function are critical determinants of fibrotic scarring or resolution of injury. Once activated by bacterial products, KCs secret a large number of pro-inflammatory and fibrogenic mediators. Activation of fibroblasts by macrophage-derived TGF-β1 or insulin-like growth factor is an early feature of fibrogenesis which promotes a switch in fibroblast gene expression to initiate matrix remodeling.43 Gadolinium chloride-mediated depletion of KC has been shown to result in attenuation of CCl4-induced hepatic fibrosis in rats in a process associated with reduced histological fibrosis and prevention of the increased TGF-β expression.44
Macrophage ablation has been shown to attenuate fibrosis in various conditions, suggesting that these cells are among the main producers of this growth factor. In response to inflammatory stimuli, monocytes undergo distinctive pathways of differentiation into classically activated M1 macrophages or the alternative M2 phenotype. Activation of M1 inflammatory macrophages by classical immune pathways leads to the expression of MHC class II antigens and release of pro-inflammatory cytokines. In response to ongoing injury, M1 macrophages propagate inflammation and ultimately the development of fibrosis. Depending on micro-environmental cues, M2 macrophages are recruited from the circulation or activated in situ as a result of an M1-to-M2 phenotype switch. M2 anti-inflammatory macrophages secrete regenerative trophic factors that promote cell proliferation and reduce apoptosis. KCs elicit divergent effects on liver fibrosis by promoting stellate cell activation in the face of continued injury or conversely, induce stellate cell apoptosis during regression. These divergent roles suggest that either different populations of macrophages are involved at each stage or, more likely, that macrophage function switches during fibrogenesis in response to cytokines in the microenvironment.45
The liver, with its strategic position between the intestine and the systemic circulation is continuously exposed to bacterial products, toxins and food-derived antigens. Natural killer (NK) cells and natural killer T (NKT) cells provide a first line of defense again invading infectious microbes and neoplastic cells. The functions of NK cells are controlled by a balance of signals from stimulatory and inhibitory receptors. In contrast to the relatively small percentage of NK cells in the peripheral lymphatics, these cells constitute a large proportion of liver-resident lymphocytes via induction of HSC apoptosis in a TNF-related apoptosis ligand-dependent manner and production of antifibrotic mediators that protect against fibrosis.46 As previously described, NK cells express a variety of TLRs, which participate in the pathogenesis of hepatic fibrosis. Activation of TLR3 by poly(I:C) has been shown to inhibit liver fibrosis through NK cell-mediated killing of activated HSCs and IFN-γ production.47 Depletion of NK cells enhances liver fibrosis.48 However, it is speculated that imbalances in the hepatic cytokine profile in patients with chronic hepatitis B virus infection drive NK cells towards a hypercytolytic phenotype responsible for liver damage.49 In vitro studies have demonstrated that HCV RNA expression is suppressed by NK cells. Moreover, CD56+ cells isolated from the liver, but not peripheral blood mononuclear cell (PBMC), of HCV cirrhotic patients produced significantly less IFN-γ and exhibited decreased anti-tumor cytotoxicity following stimulation with IL-2. Cytolytic activity had an inverse association with the stage of liver fibrosis suggesting that the presence of cytolytically active peripheral NK cells protects against liver disease progression.50
NKT cells are a heterogeneous group of cells that express both T-cell markers (αβ-TCR) and NK cell markers (NK1.1 and CD161). More recently, NKT cells have been defined as cells that have an invariant Vα14-Jα18 (Vα24-Jα18 in humans) rearrangement and reactivity to the glycosphingolipid, α-galactosylceramide presented by the MHC class I-like molecule CD1d that presents hydrophobic/lipid antigens. NKT cells are found in normal liver tissues, although numbers increase with necro-inflammatory activity and in the context of chronic viral hepatitis these cells have been shown to secrete type 2 profibrotic cytokines including IL-4 and IL-13, capable of driving the progression of fibrosis.51 Furthermore, depletion of NKT cells protected mice against CCl4-induced liver damage and fibrosis.52 In humans, an increase in the ratio of intrahepatic to peripheral NKT cells in viral cirrhosis has been reported, although the percentage of intrahepatic NKT cells reported in this study was low. Peripheral and intrahepatic NKT cells from cirrhotic patients stimulated with PMA/ionomycin also displayed increased production of IFN-γ, IL-4 and IL-13 and increased expression of CD1d in liver.53 The authors concluded that NKT cells respond to progressive liver damage caused by chronic hepatitis viral infection via a CD1d-mediated pathway and contribute to the pathogenesis of cirrhosis by expressing a set of cytokines involved in the progression of fibrosis.
Dendritic cells (DCs) are central to the processes that modulate liver immunity. Although DCs mediate powerful immune responses in most contexts, liver DCs exhibit a distinctly tolerogenic phenotype and are believed to form the basis of hepatic tolerance. DCs represent approximately 25% of leukocytes in fibrotic hepatic tissue. A transformation of DC function from tolerogenic to immunogenic underlies the immunological and inflammatory changes in liver fibrosis. Studies in a mouse model of liver fibrosis have demonstrated that the hepatic DC population expanded fivefold, acquired an activated surface phenotype and, most importantly, gained marked ability to stimulate NK cells, T cells and HSCs.54 Furthermore, DC depletion completely abrogated the elevated levels of many inflammatory mediators that are produced in the fibrotic liver.
The complement system represents an effective innate immune mechanism of host defense for eradication of microbial pathogens, but is also extensively involved in many forms of acute and chronic inflammatory diseases. Complement activation exerts its destructive functions through the generation of complement protein cleavage products. The liver (mainly hepatocytes) is responsible for biosynthesis of approximately 80%–90% of plasma complement components and expresses a variety of complement receptors. Recent evidence from several studies suggests that the complement system is also involved in the pathogenesis of a variety of liver disorders including liver injury, repair and fibrosis. The gene encoding complement factor 5 (C5) has been identified as a quantitative trait that modifies liverfibrogenesis in mouse and human HCV infection.55 Furthermore, the expression of C5aRl was detected on HSCs at high levels and increased significantly during transdifferentiation to myofibroblasts in culture. Upregulation of fibronectin but not of entactin, collagen IV and smooth muscle actin by anaphylatoxin C5a was also detected in rat HSCs.[56] C5-deficient mice also exhibit impaired liver regeneration.57 Complement factor 3 (C3) contributes to ethanol-induced liver steatosis in mice.58 Furthermore, serum complement factor 4 (C4) levels significantly correlate with liver biopsy findings and may be a useful indicator of disease activity and/or damage in chronic hepatitis B CHB patients with high transaminase levels.59 Although the functions of complement have been studied extensively in animal models, the role of complement in human liver disease remains unclear.
Growing evidence has revealed the importance of the adaptive immune system in hepatic fibrosis. T helper (Th) 1 lymphocytes express high levels of antifibrotic cytokines such as IFN-γ, whereas Th2 lymphocytes express high levels of profibrotic cytokines such as interleukin IL-4. Therefore, the Th1/Th2 ratio can alter the balance in favor of or against fibrosis.60, 61 The role of B cells in the pathogenesis of fibrosis was identified by the reduction in collagen deposition observed in CCl4-induced fibrosis in B cell-deficient mice.62 It is possible that several mechanisms are responsible for the impact of B cells on liver fibrosis. It is speculated that B-cell production of the profibrotic cytokine IL-6 contributes to liver fibrosis through induction of HSC differentiation into myofibroblasts, fibroblast proliferation and increased collagen and TIMP synthesis. Furthermore, the absence of autoantibody production may affect profibrogenic cytokine activity and alterations in T-cell function in B cell-deficient mice may contribute to the observed impact of B cells on this process. Thus, B cells are no longer considered to be ‘bystanders' in the pathogenesis of liver fibrosis.
Conclusion
Despite extensive research and advances in our understanding of the mechanisms responsible for liver fibrosis, no antifibrotic agents have yet emerged for the effective treatment of cirrhosis. However, identification of the main cellular effectors of liver fibrosis, the key cytokines regulating the fibrotic process, the determinants of ECM turnover and advances in serological and molecular research techniques have highlighted a number of potential therapeutic approaches that are suitable for future development in this area.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 31170865).
The authors declare no financial or commercial conflict of interest.
References
- Balabaud C, Bioulac-Sage P, Desmouliere A. The role of hepatic stellate cells in liver regeneration. J Hepatol. 2004;40:1023–1026. doi: 10.1016/j.jhep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2010;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Sangiovanni A, Prati GM, Fasani P. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40:860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield JA, Iredale JP. Targeted treatments for cirrhosis. Expert Opin Ther Targets. 2004;8:423–435. doi: 10.1517/14728222.8.5.423. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Berg T. Isolation and cultivation of rat liver stellate cells. Methods Enzymol. 1990;190:58–71. doi: 10.1016/0076-6879(90)90009-p. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Alison MR, Vig P, Russo F, Bigger BW, Amofah E, Themis M, et al. Hepatic stem cells: from inside and outside the liver. Cell Prolif. 2004;37:1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K. Three-dimensional structure of the sinusoidal wall in the liver: a Golgi study. Prog Clin Biol Res. 1989;295:257–262. [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Gores GJ, Kaufmann SH. Is TRAIL hepatotoxic. Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- Fischer R. Cariers A, Reinehr R, Häussinger D. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123:845–861. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide (LPS) in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- Higashiyama R, Moro T, Nakao S, Mikami K, Fukumitsu H, Ueda Y, et al. Negligible contribution of bone marrow-derived cells to collagen production during hepatic fibrogenesis in mice. Gastroenterology. 2009;137:1459–1466. doi: 10.1053/j.gastro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2011;21:351–372.2001. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase—inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases-1 and -2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- Arthur MJ, Fibrosigenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:4820–4828. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- Maher JJ. Interactions between hepatic stellate cells and the immune system. Semi Liver Dis. 2001;21:417–426. doi: 10.1055/s-2001-17555. [DOI] [PubMed] [Google Scholar]
- McGaha TL, Bona CA. Role of profibrogenic cytokines secreted by T cells in fibrotic processes in scleroderma. Autoimmun Rev. 2002;1:174–181. doi: 10.1016/s1568-9972(02)00027-7. [DOI] [PubMed] [Google Scholar]
- Pinzani M, Vizzutti F. Fibrosis and cirrhosis reversibility: clinical features and implications. Clin Liver Dis. 2008;12:901–913. doi: 10.1016/j.cld.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Mahmood S, Sho M, Yasuhara Y, Kawanaka M, Niiyama G, Togawa K, et al. Clinical significance of intrahepatic interleukin-8 in chronic hepatitis C patients. Hepatol Res. 2002;24:413–419. doi: 10.1016/s1386-6346(02)00136-5. [DOI] [PubMed] [Google Scholar]
- Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128:138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003;125:1060–1076. doi: 10.1016/s0016-5085(03)01194-6. [DOI] [PubMed] [Google Scholar]
- Hong F, Tuyama A, Lee TF, Loke J, Agarwal R, Cheng X, et al. Hepatic stellate cells express functional CXCR4: role in stromal cell-derived factor-1alpha-mediated stellate cell activation. Hepatology. 2009;49:2055–2067. doi: 10.1002/hep.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, de Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, et al. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- Gäbele E, Mühlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, et al. Attenuation of CCl4-induced hepatic fibrosis by GdCl3 treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2002;281:G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Facchetti F, Dusi S, Luini W, Lissandrini D, Simmelink M, et al. Induction of functional IL-8 receptors by IL-4 and IL-13 in human monocytes. J Immunol. 2000;164:3862–3869. doi: 10.4049/jimmunol.164.7.3862. [DOI] [PubMed] [Google Scholar]
- Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;117:3417–3425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-α. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, et al. Complement factor 5 is a quantitative trait gene that modifies liver. Nat Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- Schlaf G, Schmitz M, Heine I, Demberg T, Schieferdecker HL, Götze O. Upregulation of fibronectin but not of entactin, collagen IV and smooth muscle actin by anaphylatoxin C5a in rat hepatic stellate cells. Histol Histopathol. 2004;19:1165–1174. doi: 10.14670/HH-19.1165. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–2486. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med. 2006;38:280–286. doi: 10.1080/07853890600664608. [DOI] [PubMed] [Google Scholar]
- Bugdaci MS, Alkim C, Karaca C, Kesici B, Bayraktar B, Sokmen M. Could complement C4 be an alternative to biopsy for chronic hepatitis b histopathologic findings. J Clin Gastroenterol. 2011;45:449–455. doi: 10.1097/MCG.0b013e31820f7ee5. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, et al. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]