Abstract
Psoriasis is one of the most common immune-mediated chronic, inflammatory skin diseases characterized by hyperproliferative keratinocytes and infiltration of T cells, dendritic cells, macrophages and neutrophils. Although the pathogenesis of psoriasis is not fully understood, there is ample evidence suggesting that the dysregulation of immune cells in the skin, particularly T cells, plays a critical role in psoriasis development. In this review, we mainly focus on the pathogenic T cells and discuss how these T cells are activated and involved in the disease pathogenesis. Newly identified ‘professional' IL-17-producing dermal γδ T cells and their potential role in psoriasis will also be included. Finally, we will briefly summarize the recent progress on the T cell and its related cytokine-targeted therapy for psoriasis treatment.
Keywords: Psoriasis,TH1/TH17 cells; Gammadelta T cells; T cell-targeted theraphy
Introduction
Psoriasis is a common, chronic inflammatory skin disease, affecting about 2% of the worldwide population.1 Psoriasis vulgaris is the most common type of psoriasis, manifested as dry, red raised plaques with adherent silvery scales. Histologically, psoriasis is characterized by hyperproliferation and aberrant differentiation of keratinocytes, dilated, hyperplastic blood vessels as well as an inflammatory infiltration of leukocytes, predominantly into the dermis.2 Until the late 1970s, psoriasis was originally thought to be a disease primarily of dysfunctional epidermal keratinocytes.3 However, substantial clinical and basic research observations indicate that the cellular innate and adaptive immune responses, especially the activation of T cells, play a critical role in the pathogenesis of psoriasis. The successful treatment of psoriasis patients with cyclosporine A, an immunosuppressive agent that inhibits T-cell proliferation and cytokine production, was the first clinical evidence to suggest a potential role of T cells in psoriasis pathogenesis.4 Other T cell-targeted drugs such as anti-CD4 monoclonal antibody and cytotoxic T lymphocyte-associated antigen 4-immunoglobulin were also observed to have a significant therapeutic efficacy in psoriasis treatment.5,6,7 In addition, an earlier in vitro study showing that activated CD4+ T cells from psoriatic lesions could enhance keratinocyte proliferation via secretion of interferon-γ (IFN-γ)8 and the establishment of psoriasis xenograft animal model in severe combined immunodeficient mouse9,10 further confirm the importance of T cells in psoriasis development. Thus, psoriasis is considered to be an organ-specific T cell-driven inflammatory disease and T cells play a dominant pathogenic role in the initiation and maintenance of psoriasis. In the past few years, many new findings on T cells and their contributions to the disease development have challenged our conventional views regarding psoriasis as a T helper (Th) 1-mediated skin disease and prompted us to reassess T-cell functions in psoriasis. In this review, we will summarize recent progress on T cells as well as some important innate immune cells and their roles in the pathogenesis of psoriasis. Finally, we will briefly discuss some newly developed biological agents targeting T cells and the related cytokines, and their therapeutic efficacy in the treatment of psoriasis patients.
What causes the activation of pathogenic T cells in psoriasis?
As a T cell-mediated autoimmune skin disease, an important question in psoriasis that attracts researchers' interest is to understand how the pathogenic T cells become activated during disease development. The close relationship between streptococcal infection and psoriasis have made antigen(s) from streptococcus the main candidate responsible for activating T cells.11,12 The concept of superantigen was initially proposed based on several research findings where psoriasis patients had a restricted T-cell receptor (TCR) Vβ usage in the peripheral blood and lesions.13,14 Additionally, streptococcal exotoxins could induce expression of a skin-homing receptor, cutaneous lymphocyte-associated antigen, on T cells.15 However, an oligoclonal T-cell expansion found in psoriasis lesions has been reported by several independent groups when analyzing TCR usage on the infiltrated T cells, which provides clear evidence in favor of an antigen-specific T-cell response in the local lesion.16,17 Furthermore, the identification of conserved clonal TCR rearrangement within skin lesions from different patients and continuous presence of the same T-cell clone over a prolonged period of time, even in relapsing disease, suggest that the T cells, which mediate chronic psoriasis, are driven by common antigen(s).18,19 These findings along with the fact that streptococcal M protein shares a high similar structure with type I keratins propose a new theory in psoriasis, named molecular mimicry, or disease mimicry. The concept of molecular mimicry explains how anti-streptococcal T cells can be activated in the skin to target-specific tissue antigen(s) and subsequently causing disease.20,21 Indeed, peripheral blood T cells from psoriatic patients but not healthy controls have been shown to respond to several synthetic peptides corresponding to these shared homologous sequence motifs for the production of IFN-γ.22,23 Further study demonstrated that CD8+ T cells in the peripheral blood from psoriasis patients carrying the HLA–Cw6 allele, which has been reported to have a strong association with psoriasis, had a significant IFN-γ response to the peptide selected from the shared sequence by keratin 17 and M6 protein and the predicted HLA–Cw6 binding.24 Additionally, the majority (>90%) of these responding cells express the skin homing cutaneous lymphocyte-associated antigen determinant.24 Autoantigen candidates Ezrin, Maspin, Peroxiredoxin 2 and Heat Shock Protein 27, all of which have homologies to streptococcal proteins, have been shown to react with the sera from psoriasis patients.25
However, several studies from Fry's group suggested that streptococcal M protein might not be the target for the lesional skin T cells.26,27 In these studies, T-cell lines grown from lesional dermis did not respond by proliferation to recombinant M protein.28 Alternatively, they found that at least half of the streptococcal cell wall-specific Th1 cells in psoriasis lesions were specific for streptococcal peptidoglycan (PG), a major component of the Gram-positive bacterial cell wall.29 In dermal lesions, there were observations of increased numbers of macrophages, containing streptococcal PG, within clusters of dermal T cells or in the dermal papillae.29 It is well known that PG is a strong pro-inflammatory stimulus in chronic inflammation through its interaction with dendritic cells (DCs) and monocytes via pattern recognition receptors including Toll-like receptor 2, nucleotide-binding oligomerization domains 1 and 2 and PG recognition proteins 1–4.30,31 Interestingly, the genes encoding the above PG recognition receptors are all located within linkage sites associated with psoriasis.32 On the basis of these findings, the authors proposed that PG is a major etiological factor in psoriasis and an altered innate response to PG might contribute to the enhanced pathogenic T-cell activation and expansion in the psoriasis lesion.33 However, this schema does not well address the role of CD8+ T-cell function in psoriasis, which has been found to be essential for the development of psoriatic lesions.34 Thus, Valdimarsson et al.35 recently proposed a modified schematic model to explain the potential role of CD4+ and CD8+ T cells in the development of psoriasis lesions and how streptococcal infection triggers and maintains psoriasis. In addition to the streptococcal PG, we also found that streptococcal CpG DNA could enhance the proliferation and activation of peripheral T cells from psoriasis patients upon stimulated with streptococcal antigen, indicating that integral function of streptococcal antigen, particularly streptococcal DNA, in the pathogenesis of psoriasis.36 Thus far, the (auto)antigen(s) responsible for activating psoriatic T cells is still controversial and remains to be determined. Future studies should focus on identifying the autoantigen triggering psoriatic T cells, as it could lead to the use of a vaccine therapy for the treatment of psoriasis.
Is psoriasis a Th1 and/or Th17 cell-mediated inflammatory skin disease?
For over 30 years, T cells were classified as Th1 or Th2 cells by production of defining cytokines, IFN-γ and interleukin (IL)-4, respectively. In psoriatic plaques and peripheral blood of psoriatic patients, there were large numbers of CD4+ Th1 and CD8+ cytotoxic T cells type 1 (Tc1) cells as well as elevated cytokine levels of IFN-γ, tumor-necrosis factor (TNF)-α and IL-12, which well defined psoriasis as a Th1 cell-mediated disease.37,38,39 Since then, it has been widely recognized that the interaction of T cells and DCs creates a ‘type 1' inflammatory environment by secreting large amounts of Th1 type cytokines, leading to the development of psoriasis.40
Recently, a new population of IL-17-producing CD4+ Th cells, named Th17, has been identified and shown to be involved in the models of inflammatory and autoimmune diseases.41,42,43 It has been well documented that IL-23 functions as a key cytokine for the maintenance and development of both murine and human Th17 cells.44,45,46,47 IL-23 is a heterodimeric cytokine that has unique subunit IL-23p19 combined with IL-12p40, shared with IL-12.48 There is growing evidence to suggest that Th17 cells and their related cytokines such as IL-17A, IL-17F, IL-22, IL-21 and IL-26 play essential roles in a variety of chronic inflammatory diseases, including psoriasis. Th17 cells and their downstream effector molecules, which include IL-17A, IL-17F, IL-22, IL-21 and TNF-α, are found at increased levels in psoriatic skin and circulation.49,50,51,52 Moreover, psoriatic skin lesions also contain high mRNA and protein levels of IL-23 compared with non-lesional and normal skin and IL-23 has been found to be produced mainly by the activated macrophages and DCs.53,54,55 Intradermal injection of IL-23 or IL-21 in mice can stimulate keratinocytes proliferation and cause epidermal hyperplasia (acanthosis), which is one of the most significant features in human psoriasis.51,56 Further studies demonstrated that IL-22, IL-17A as well as CC chemokine receptor (CCR) 6 are all required for IL-23-induced psoriasis-like skin inflammation.57,58,59 In addition, IL-22 was also shown to be critical in another murine psoriasiform model triggered by topical application of Toll-like receptor 7/8 agonist Imiquimod.60 More importantly, these released Th17-related cytokines can further act on keratinocytes and other inflammatory cells infiltrated in the skin, thus amplifying the local inflammation as well as causing keratinocyte hyperproliferation. It has been reported that IL-17A can upregulate keratin 17 expression on keratinocytes, which was regarded as a hallmark of psoriasis.61 Meanwhile, IL-17A plus IL-22 can synergistically enhance keratinocyte expression of antimicrobial peptides, although each cytokine modulates distinct inflammatory and keratinocyte-response pathways that compensatively contribute to the psoriatic phenotype.62,63,64
Collectively, these studies indicate the important role of IL-23/Th17 axis in psoriasis development, which challenges the definition of psoriasis to be a Th1 cell-mediated disease. Th1 and Th17 cells are known as distinct polarized Th cell types, but they are often colocalized in pathological environments, as both Th1 and Th17 cells are increased in the psoriatic lesions and peripheral blood.50,52 To address these questions, Kryczek et al.65 found that IFN-γ is a potent promoter of human IL-17+ T-cell trafficking, induction and function. IFN-γ synergized with IL-17 could enhance human normal keratinocyte to produce human β-defensin 2, suggesting that Th1 and Th17 cells may collaboratively interact with each other and contribute to the autoimmune disease pathogenesis.65 This concept is well supported by another study, in which the authors found that there was accumulation of Th1/Th17 cell-polarizing myeloid DCs in the psoriatic lesions.66 Additionally, intradermal injection of IFN-γ into the non-lesional skin of psoriasis patients could induce significant infiltration of T cells and inflammatory DCs as well as the production of chemokines and cytokines, including IL-23.67
Recently, another distinct population of Th cells, which preferentially express CCR10, CCR6 and CCR4 and produce only IL-22, but not IL-17 or IFN-γ, has been characterized and called IL-22-producing Th cells (Th22).68,69 This unique subset of human skin-homing memory T cells may be involved in epidermal immunity and remodeling and dedicated to the skin homeostasis and pathology.70 It has been reported that there were more Th22 cells along with Th1 and Th17 cells in the circulation of psoriatic patients.52 Res et al.71 also demonstrated that there were more IL-22-producing CD8+ T cells (Tc22) along with Tc17 (IL-17-producing CD8+ T cells) in the psoriatic lesions. They found these Th22 and Tc22 cells might arise from IL-17-producing cells (Th17 and Tc17 cells), suggesting these cells acquire plastic ability in their development. Therefore, psoriasis cannot be simply defined as one subset of Th cells-mediated disease. Instead, all these pathogenic Th cells are implicated in the disease development, which interact with other types of T cells, DCs and neutrophils to create a chronic inflammatory environment for the maintenance of psoriatic plaque.
Innate immune T cells or innate effector cells in psoriasis pathogenesis
In addition to these conventional T cells, more attention has been drawn recently on the innate immune cells, such as gamma delta (γδ) T cells, natural killer (NK) cells and natural killer T (NKT) cells and their potential roles in psoriasis.
Murine skin contains abundant γδ T cells in the epidermis, called dendritic epidermal γδ T cells.72,73 The development and function of dendritic epidermal γδ T cells have been well defined for decades.74,75,76 Recently, three laboratories including ours have identified a novel γδ T-cell subset in the dermis.55,77,78 Unlike dendritic epidermal γδ T cells and conventional αβ T cells, dermal γδ T cells constitutively express IL-23 receptor, CCR6 and transcriptional factor RORγt. More importantly, these cells are demonstrated to be the major IL-17 producer in the skin upon IL-23 stimulation. It has been demonstrated that γδ T cells from other anatomical sites have important pathogenic roles in some infectious and autoimmune diseases through their ability to rapidly produce IL-17 upon IL-23 and IL-1β or danger signal stimulation even in the absence of TCR ligation.79,80,81 Thus, γδ T cells are considered as innate-like cells and can amplify the conventional acquired immune response. Not surprisingly, as ‘professional' IL-17-producing cells, dermal γδ T cells are also found very critical in the psoriasis pathogenesis.55 In TCR δ-deficient (TCRd−/−) mice, the epidermal hyperplasia and inflammation induced by IL-23 and Imiquimod were significantly decreased. Consistent with our finding, Mabuchi et al.82 also showed IL-23 induced less severe skin pathology in TCRd−/− mice, but they claimed there were specific epidermal CCR6 positive γδ T cells which were responsible in this murine skin inflammation model. The discrepancy between their findings and ours could be explained by the possibility that dermal γδ T cells might migrate into the epidermis during the inflammation. This notion is supported by the finding that this subset of γδ T cells is scarce in epidermis under a steady condition.55 We also found that dermal γδ T cells expanded upon IL-23 stimulation. Importantly, high frequency of γδ T cells was found in the lesions of psoriatic patients, which closely link the murine model system to the clinics. These γδ T cells in the human skin were also capable of producing large amounts of IL-17 upon IL-23 stimulation as the murine counterpart did. Recently, Nestle's group also reported a novel skin homing Vγ9Vδ2 T-cell subset in humans, which expressed cutaneous lymphocyte-associated antigen and other skin homing chemokine receptors. In psoriasis patients, this population was found to be significantly decreased in the peripheral blood, but increased in the lesion.83 Taken together, we assume these γδ T cells present in the skin may become the first line of defense against the invasion of foreign pathogens or the danger signals released upon infection. The released cytokines after γδ T-cell activation can further trigger the downstream immune response, thus causing the chronic inflammation in the local skin. Thus far, the function of dermal γδ T cells, especially in humans, is not fully understood and need to be further investigated in the future.
Additional innate immune cells involved in psoriasis are NK and NKT cells. NKT cells are a heterogenous subset of T cells that share features of both T cells and NK cells. Activated NKT cells can induce psoriasis in xenograft mouse model by using non-lesional psoriatic skin.84,85 Both NK and NKT cells infiltrations have been found significantly increased in psoriatic lesions.86,87 Infiltrating NK cells express CXCR3, CCR5 and CCR6 as well, which are in response to the corresponding chemokines CXCL10, CCL5 or CCL20 secreted by keratinocytes. Additionally, psoriatic NK cells might have increased cytotoxicity through the release of cytotoxic granules such as perforin, which were found upregulated in the psoriatic lesions.87 Recently, NKT cells have been demonstrated to be a potent IL-17 producer, suggesting that these cells may act similarly as dermal γδ T cells in psoriasis pathogenesis.88
In addition to these unconventional T cells, Lin et al.89 reported that mast cells and neutrophils were prominent cells that produced IL-17 in the skin of healthy controls as well as psoriasis patients. In addition, it has been shown that there is a specific IL-23-responsive innate lymphoid population in the intestine, which mediates intestinal immunopathology in inflammatory bowel disease.90 It is possible there might be a similar cell population in psoriatic lesions.
Regulatory T cells (Tregs)
Tregs are a subset of T lymphocytes that suppress not only autoimmune responses but also other aberrant or excessive immune responses to non-self-antigens.91 Although similar proportions of Tregs have been found in normal and psoriatic peripheral blood, both in vitro and in vivo experiments have suggested that Tregs did not function properly in psoriatic plaques. Psoriatic Tregs, in which cells were isolated from lesional psoriatic skin or sorted from peripheral blood of psoriatic patients, are functionally deficient in suppressing effector T-cell responses in either alloantigen-specific or polyclonal TCR stimulation assays.92 Furthermore, in a psoriasis mouse model which was established by knocking out CD18, the primary dysfunction of Tregs has been found to allow subsequent hyperproliferation of pathogenic T cells.93 The possible mechanism by which Tregs exhibit decreased suppression function is partially due to the pro-inflammatory cytokine milieu in the psoriasis lesion, especially high levels of IL-6 secreted from endothelial cells, DCs and Th17 cells, which inhibit Treg activity and enable infiltrated T effector cells escape from the suppression.94,95 Thus, the dysfunctional Treg activity in the blood and psoriatic plaques may eventually result in the reduced restraint and consequent hyperproliferation of psoriatic pathogenic T cells in vivo.92 It has been noticed that CD4+CD25highFoxp3+ Tregs can be converted into inflammation-associate Th17 cells under certain condition. Based on these findings, Bovenschen et al.96 demonstrated that this subset of Tregs from patients with severe psoriasis were more prone to differentiate into IL-17-producing cells compared to healthy controls upon stimulation. Most importantly, they found that there was a specific population of CD4+IL-17A+Foxp3+ cells in the skin lesions, which they assume would probably contribute to the disease development.
T cell-targeted therapy in psoriasis
To date, the Food and Drug Administration has approved various biologic agents for psoriasis therapy include an anti-IL-12/IL-23 common chain p40 antibody,97,98 TNF-α inhibitors99 and T cell-targeted agents. A human antibody against IL-17A or IL-17 receptor is now under phase II clinical trial and has showed a promising therapeutic efficacy.100,101,102 Here, we briefly summarize the recent progress on these biological agents in the treatment of psoriasis.
The pathogenic role of TNF-α in the psoriasis development has made it one of the most important targets for psoriasis therapy. Thus far, there are three Food and Drug Administration-approved anti-TNF-α agents in the market, including etanercept, infliximab and adalimumab and another one, called golimumab, used for the psoriatic arthritis treatment.99 Although all these inhibitors showed the significant therapeutic efficacy, the adverse effects cannot be neglected, especially the increased risk for tuberculosis infection during treatment.103 Another unexpected side effect of TNF-α antagonist treatment from clinical reports is the induction or exacerbation of psoriatic skin lesions.104 This could be caused by the dysregulation of cytokine milieu with the increased production of IFN-α and other pro-inflammatory cytokines, such as IL-1β, IL-6, IL-17, IL-21 and IL-22. Additionally, TNF-α antagonists are also found to enhance Th17 function, but suppress FoxP3+ Tregs in the skin in the murine psoriasis-like model.105 Therefore, the effects of anti-TNF-α agents can be systemic and non-tissue specific, thus caution must be taken when utilizing these agents for therapy.
Recently, the recognition of the importance of IL-23/Th17 axis in psoriasis pathogenesis has prompted the development of new biological agents for psoriasis therapy. Ustekinumab and briakinumab both target IL-12/IL-23 common chain p40 and showed superior efficacy to etanercept in a large clinical study, focusing on the treatment of moderate-to-severe psoriasis.97,98,106 However, serious adverse events have been reported during therapy, which might be due to the broad biological effects of IL-23. In this case, targeting the downstream effector cytokine like IL-17 or IL-22 would be a more logical choice. With great promise, anti-IL-17 antibodies AIN457 (secukinumab) and LY2439821 (ixekizumab) and anti-IL-17 receptor antibody AMG 827 (brodalumab) have shown remarkable therapeutic efficacy in the phase II clinical trials directed towards the treatment of chronic plaque psoriasis.100,101,102 However, their efficacy and safety need to be further assessed in the future.107 Unfortunately, anti-IL-22 antibody (ILV 095) treatment failed in the phase I clinical trial due to lack of efficacy. Although IL-22 plays a critical role in the murine psoriasis development as well as in psoriasis patients, the significance of targeting IL-22 for the disease treatment remains questionable.
Another important therapeutic approach in the treatment of psoriasis is targeting T cells. In addition to anti-CD4 antibodies and T lymphocyte-associated antigen 4-immunoglobulin, here we focus on Efalizumab and Alefacept, which are two recently developed drugs to inhibit T-cell function.
Efalizumab is a humanized, chimeric monoclonal anti-CD11a antibody that binds to the α subunit of leukocyte function-associated antigen (LFA)-1, thereby blocking the interaction of LFA-1 with intercellular adhesion molecule, leading to a disruption of the interaction between DCs and T cells at tissue sites and in lymph nodes.108 It also blocks adhesion molecules between T cells and endothelial cells, thus preventing circulating T cells from entering the skin. However, Efalizumab is no longer marketed due to the required boxed warning by the Food and Drug Administration, which highlights the risk of bacterial sepsis, viral meningitis, invasive fungal disease, progressive multifocal leukoencephalopathy109 and other infection risks after Efalizumab treatment.
Alefacept is a human LFA-3/IgG1 Fc fusion (recombinant) protein that binds to CD2 on memory/effector T cells, selectively blocking the interaction between CD2 on T cells and LFA-3 on antigen-presenting cells, interfering with the function of antigen-presenting cells and T-cell activation.108 It also induces antibody-dependent cellular cytotoxicity in T cells bound to Alefacept and leads to apoptosis of memory-effector CD45RO-positive T cells in the skin. A recent AWARE (Amevive Wisdom Acquired from Real-World Evidence) study supports the safety of alefacept used alone or in combination with other antipsoriatic therapies, in a broad population of chronic plaque psoriasis patients in Canada.110
Another novel T cell-targeted biological drug, called Siplizumab, which is a humanized anti-CD2 monoclonal antibody that interferes with costimulation necessary for T-cell activation and proliferation, has been tested in the clinical trial.111 However, two independent randomized, double-blind, placebo-controlled phase II studies showed that Siplizumab only had modest effects and some clinical activity against inflammatory processes in psoriasis patients, suggesting that targeting CD2+ cells in psoriasis may not yield a therapeutic benefit.
Table 1 is the summary of different subsets of T cells involved in the pathogenesis of psoriasis, including their released pathogenic cytokines and related biological agents for psoriasis therapy.
Table 1. Summary of different subsets of T cells and related cytokines involved in psoriasis pathogenesis.
| T cell | Cytokine (s) produced | Major role in the pathogenesis of psoriasis | Cytokine-target therapy | |
|---|---|---|---|---|
| Type/subtypes | ||||
| CD4 | Th1 | IFN-γ40,50 | KHEH, SI, DCM | — |
| Th17 | IL-1750,112 | KHEH, SI, AIRA | Aia, Lya, Ama | |
| IL-22113 | KHEH, SI | ILb | ||
| IL-2151 | KHEH | — | ||
| IL-694 | KHEH, TrI | — | ||
| Th22 | IL-22114,115 | KHEH, SI | ILb | |
| FoxP3+ Treg | IL-1796 | KHEH, SI, AIRA, IRD | Aia, Lya, Ama | |
| CD8 | IL-1771,116 | KHEH, SI, AIRA | Aia, Lya, Ama | |
| IL-2271 | KHEH, SI | ILb | ||
| IFN-γ116 | KHEH, SI, IM, DCM | — | ||
| TNF-α116 | KHEM, DCM | Ad, Et, In, Goc | ||
| γδ | Dermal | IL-1755,112,117 | KHEH, SI, AIRA | Aia, Lya, Ama |
| IL-2255 | KHEH | ILb | ||
| TNF-α55 | KHEM, DCM | Ad, Et, In, Goc | ||
| NKT | IL-1788 | KHEH, SI, AIRA | Aia, Lya, Ama | |
Abbreviations: Roles in psoriasis pathogenesis: AIRA, acquired immune response amplification; DCM, dendritic cell maturation; IFN, interferon; IRD, immune regulation dysfunction; KHEH, keratinocyte hyperproliferation and epidermal hyperplasia; NKT, natural killer T; SI, skin inflammation; Th, T helper; TNF, tumor-necrosis factor; TrI, Treg inhibition. Therapy: Ad, Adalimumab; Ai, AIN 457; Am, AMG 827—IL-17 receptor targeted; Et, Etanercept; Go, Golimumab; IL, ILV 095; IM, inflammatory migration; In, Infliximab; Ly, LY2439821.
In clinical trials.
Phase 1 clinical trial discontinued March 2011.
Approved for psoriatic arthritis.
Future directions
The new T-cell subsets, like dermal γδ T cells and Tregs, which are involved in psoriasis pathogenesis, as we mention above, can become the candidates for the future immunotherapy of psoriasis. Current research is focusing on specific subsets of γδ T cells, such as CCR6-positive Vγ9Vδ2, in hopes of expanding our knowledge of the T cells involved in disease development but also in search for more specific targets for future drug therapies. As therapeutic approaches become more specific towards the major players of psoriasis, keen observations of the unexpected, therapeutic side effects will provide greater knowledge pertaining to these newcomers of psoriasis. As more human genomic data from psoriasis patients become available, investigators can begin looking into the genetic underpinnings to the above T-cell interactions and modulations leading to disease development. Perhaps new areas of study such as immunogenomics will give us the tools necessary to clarify elusive problems such as the identity of the autoantigen(s) triggering psoriatic T-cell activation and expansion since these answers could lead to the development of breakthrough immunotherapies.
Conclusions
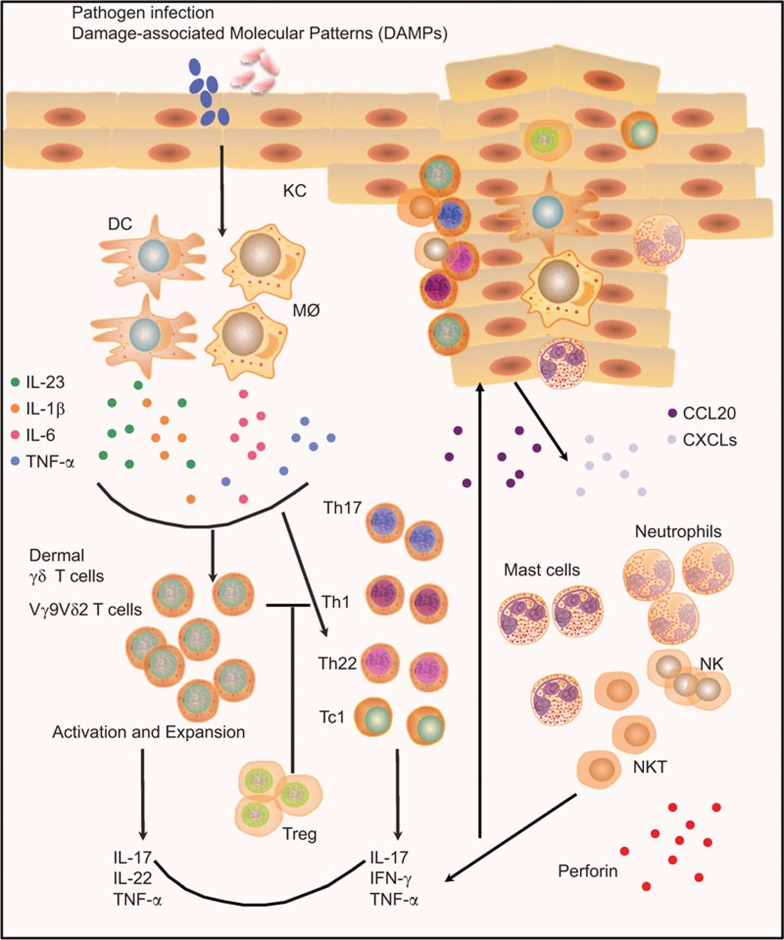
Over the past few years, there have been great advances on T cells and their roles in the inflammatory and autoimmune diseases that help us understand more deeply the pathogenesis of psoriasis. Instead of the traditional view regarding psoriasis as a Th1 type disease, it is clearer that Th1, Th17, Treg and Th22 cells interplay with each other and all contribute to the disease development. Additionally, newly identified ‘professional' IL-17-producing dermal γδ T cells also play critical roles in psoriasis pathogenesis. In the schematic model of psoriasis immunopathogenesis depicted in Figure 1, we propose that the pathogens carrying foreign antigens or danger signals first activate the DCs and macrophages to release IL-23, IL-1β and other pro-inflammatory cytokines. These cytokines can activate dermal γδ T cells and other IL-17-producing cells to secrete abundant IL-17 further promoting the conventional acquired immune responses. IL-17, IL-22 and TNF-α can act on keratinocytes and induce keratinocyte hyperproliferation. The activated keratinocytes also release chemokines, such as CCL20 and CXCL1, 3, 8–11, to attract more immune effector cells into the skin. These immune effector cells including neutrophils, mast cells, NK and NKT cells further contribute to the pro-inflammatory environment producing cytokines and chemokines. Thus, this amplified positive feedback loop leads to the development of psoriatic lesions.
Figure 1.
Dysregulated immune responses in psoriasis. Pathogen components or DAMPs activate the DCs and macrophages to produce IL-23, IL-1β and other pro-inflammatory cytokines including IL-6 and TNF-α. These cytokines induce dermal γδ T cells activation and expansion to secrete IL-17, IL-22 and TNF-α, which in turn further promote the conventional CD4+ T cell-mediated (Th1, Th17 and Th22) and CD8+ T cell-mediated (Tc1) acquired immune responses. In addition, skin infiltrating inflammatory cells, such as mast cells, neutrophils, NK cells and NK T cells, also contribute to the disease development via producing cytokines (IL-17), antimicrobial peptides and cytotoxic granules. Additionally, the dysfunctional Tregs lose their suppressive activity and some of them are able to convert to Th17 effector cells, further enhancing the inflammatory reaction in the local skin. The pro-inflamamtory cytokines and chemokines act on keratinocytes and induce keratinocyte hyperproliferation. The activated keratinocytes also produce some chemokines, such as CCL20 and CXCL1, 3, 8–11, to attract more immune effector cells infiltrating into skin, forming the amplified positive feedback loop, leading to the development of psoriatic lesions. DAMP, damage-associated molecular pattern; DC, dendritic cell; NK, natural killer; Th, T helper; TNF, tumor-necrosis factor; Treg, regulatory T cell.
Acknowledgments
This work was supported by the NIH, the National Psoriasis Foundation and the Arthritis National Research Foundation (JY).
References
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- Voorhees JJ. Pathophysiology of psoriasis. Annu Rev Med. 1977;28:467–473. doi: 10.1146/annurev.me.28.020177.002343. [DOI] [PubMed] [Google Scholar]
- Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:555. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- Nicolas JF, Chamchick N, Thivolet J, Wijdenes J, Morel P, Revillard JP. CD4 antibody treatment of severe psoriasis. Lancet. 1991;338:321. doi: 10.1016/0140-6736(91)90465-2. [DOI] [PubMed] [Google Scholar]
- Prinz J, Braun-Falco O, Meurer M, Daddona P, Reiter C, Rieber P, et al. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet. 1991;338:320–321. doi: 10.1016/0140-6736(91)90464-z. [DOI] [PubMed] [Google Scholar]
- Abrams JR, Kelley SL, Hayes E, Kikuchi T, Brown MJ, Kang S, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–694. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bata-Csorgo Z, Hammerberg C, Voorhees JJ, Cooper KD. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture. Cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J Clin Invest. 1995;95:317–327. doi: 10.1172/JCI117659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T. Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol. 1999;155:145–158. doi: 10.1016/S0002-9440(10)65109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, Kristinsson KG, Valdimarsson H. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530–534. doi: 10.1046/j.1365-2133.2003.05552.x. [DOI] [PubMed] [Google Scholar]
- Lewis HM, Baker BS, Bokth S, Powles AV, Garioch JJ, Valdimarsson H, et al. Restricted T-cell receptor V beta gene usage in the skin of patients with guttate and chronic plaque psoriasis. Br J Dermatol. 1993;129:514–520. doi: 10.1111/j.1365-2133.1993.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Leung DY, Travers JB, Giorno R, Norris DA, Skinner R, Aelion J, et al. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J Clin Invest. 1995;96:2106–2112. doi: 10.1172/JCI118263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Gately M, Trumble A, Ferguson-Darnell B, Schlievert PM, Picker LJ. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen, via stimulation of interleukin 12 production. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menssen A, Trommler P, Vollmer S, Schendel D, Albert E, Gürtler L, et al. Evidence for an antigen-specific cellular immune response in skin lesions of patients with psoriasis vulgaris. J Immunol. 1995;155:4078–4083. [PubMed] [Google Scholar]
- Chang JC, Smith LR, Froning KJ, Schwabe BJ, Laxer JA, Caralli LL, et al. CD8+ T cells in psoriatic lesions preferentially use T-cell receptor V beta 3 and/or V beta 13.1 genes. Proc Natl Acad Sci USA. 1994;91:9282–9286. doi: 10.1073/pnas.91.20.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz JC, Vollmer S, Boehncke WH, Menssen A, Laisney I, Trommler P. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur J Immunol. 1999;29:3360–3368. doi: 10.1002/(SICI)1521-4141(199910)29:10<3360::AID-IMMU3360>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vollmer S, Menssen A, Prinz JC. Dominant lesional T cell receptor rearrangements persist in relapsing psoriasis but are absent from nonlesional skin: evidence for a stable antigen-specific pathogenic T cell response in psoriasis vulgaris. J Invest Dermatol. 2001;117:1296–1301. doi: 10.1046/j.0022-202x.2001.01494.x. [DOI] [PubMed] [Google Scholar]
- Prinz JC. Disease mimicry—a pathogenetic concept for T cell-mediated autoimmune disorders triggered by molecular mimicry. Autoimmun Rev. 2004;3:10–15. doi: 10.1016/S1568-9972(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Prinz JC. Psoriasis vulgaris—a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol. 2001;26:326–332. doi: 10.1046/j.1365-2230.2001.00831.x. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Sigurgeirsson B, Troye-Blomberg M, Good MF, Valdimarsson H, Jonsdottir I. Circulating T cells of patients with active psoriasis respond to streptococcal M-peptides sharing sequences with human epidermal keratins. Scand J Immunol. 1997;45:688–697. doi: 10.1046/j.1365-3083.1997.d01-438.x. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, Good MF, Valdimarsson H, Jonsdottir I. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis. Clin Exp Immunol. 1999;117:580–586. doi: 10.1046/j.1365-2249.1999.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8+ T cells. Clin Exp Immunol. 2004;138:83–93. doi: 10.1111/j.1365-2249.2004.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besgen P, Trommler P, Vollmer S, Prinz JC. Ezrin, maspin, peroxiredoxin 2, and heat shock protein 27: potential targets of a streptococcal-induced autoimmune response in psoriasis. J Immunol. 2010;184:5392–5402. doi: 10.4049/jimmunol.0903520. [DOI] [PubMed] [Google Scholar]
- Brown DW, Baker BS, Ovigne JM, Fischetti VA, Hardman C, Powles AV, et al. Non-M protein(s) on the cell wall and membrane of group A streptococci induce(s) IFN-gamma production by dermal CD4+ T cells in psoriasis. Arch Dermatol Res. 2001;293:165–170. doi: 10.1007/s004030100218. [DOI] [PubMed] [Google Scholar]
- Baker BS, Ovigne JM, Fischetti VA, Powles A, Fry L. Selective response of dermal Th-1 cells to 20–50 kDa streptococcal cell-wall proteins in chronic plaque psoriasis. Scand J Immunol. 2003;58:335–341. doi: 10.1046/j.1365-3083.2003.01309.x. [DOI] [PubMed] [Google Scholar]
- Baker BS, Brown DW, Fischetti VA, Ovigne JM, Porter W, Powles A, et al. Skin T cell proliferative response to M protein and other cell wall and membrane proteins of group A streptococci in chronic plaque psoriasis. Clin Exp Immunol. 2001;124:516–521. doi: 10.1046/j.1365-2249.2001.01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BS, Laman JD, Powles A, van der Fits L, Voerman JS, Melief MJ, et al. Peptidoglycan and peptidoglycan-specific Th1 cells in psoriatic skin lesions. J Pathol. 2006;209:174–181. doi: 10.1002/path.1954. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Liu C, Dziarski R. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem. 2000;275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Peptidoglycan recognition proteins (PGRPs) Mol Immunol. 2004;40:877–886. doi: 10.1016/j.molimm.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H. The genetic basis of psoriasis. Clin Dermatol. 2007;25:563–567. doi: 10.1016/j.clindermatol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Baker BS, Powles A, Fry L. Peptidoglycan: a major aetiological factor for psoriasis. Trends Immunol. 2006;27:545–551. doi: 10.1016/j.it.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494–501. doi: 10.1016/j.it.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Cai YH, Lu ZY, Shi RF, Xue F, Chen XY, Pan M, et al. Enhanced proliferation and activation of peripheral blood mononuclear cells in patients with psoriasis vulgaris mediated by streptococcal antigen with bacterial DNA. J Invest Dermatol. 2009;129:2653–2660. doi: 10.1038/jid.2009.153. [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102:145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–759. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K. Flow cytometric characterization of lesional T cells in psoriasis: intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res. 2000;292:519–521. doi: 10.1007/s004030000167. [DOI] [PubMed] [Google Scholar]
- Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25:295–305. doi: 10.1016/j.it.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev. 2008;226:132–146. doi: 10.1111/j.1600-065X.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15:1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Renauld JC, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- Shi X, Jin L, Dang E, Chang T, Feng Z, Liu Y, et al. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol. 2011;131:2401–2408. doi: 10.1038/jid.2011.222. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Huang LM, Suarez-Farinas M, Pierson KC, Fuentes-Duculan J, Cueto I, Lentini T, et al. A single intradermal injection of IFN-gamma induces an inflammatory state in both non-lesional psoriatic and healthy skin. J Invest Dermatol. 2012;132:1177–1187. doi: 10.1038/jid.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstresser PR, Tigelaar RE, Dees JH, Streilein JW. Thy-1 antigen-bearing dendritic cells populate murine epidermis. J Invest Dermatol. 1983;81:286–288. doi: 10.1111/1523-1747.ep12518332. [DOI] [PubMed] [Google Scholar]
- Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Jameson J, Havran WL. Skin gammadelta T-cell functions in homeostasis and wound healing. Immunol Rev. 2007;215:114–122. doi: 10.1111/j.1600-065X.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. Eur J Immunol. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol. 2011;187:5026–5031. doi: 10.4049/jimmunol.1101817. [DOI] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Wrone-Smith T, Bonish B, Porcelli SA. Response of murine and normal human skin to injection of allogeneic blood-derived psoriatic immunocytes: detection of T cells expressing receptors typically present on natural killer cells, including CD94, CD158, and CD161. Arch Dermatol. 1999;135:546–552. doi: 10.1001/archderm.135.5.546. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Bonish B, Huang BB, Porcelli SA. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212–225. doi: 10.1016/s0923-1811(00)00120-1. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16− NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- Kastelan M, Prpic Massari L, Gruber F, Zamolo G, Zauhar G, Coklo M, et al. Perforin expression is upregulated in the epidermis of psoriatic lesions. Br J Dermatol. 2004;151:831–836. doi: 10.1111/j.1365-2133.2004.06168.x. [DOI] [PubMed] [Google Scholar]
- Cosmi L, de Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Peters T, Sindrilaru A, Kess D, Oreshkova T, Yu XZ, et al. TGF-beta-dependent suppressive function of Tregs requires wild-type levels of CD18 in a mouse model of psoriasis. J Clin Invest. 2008;118:2629–2639. doi: 10.1172/JCI34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165:661–668. doi: 10.1111/j.1365-2133.2011.10419.x. [DOI] [PubMed] [Google Scholar]
- Kircik LH, del Rosso JQ. Anti-TNF agents for the treatment of psoriasis. J Drugs Dermatol. 2009;8:546–559. [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- Bachmann F, Nast A, Sterry W, Philipp S. Safety and efficacy of the tumor necrosis factor antagonists. Semin Cutan Med Surg. 2010;29:35–47. doi: 10.1016/j.sder.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233–240. doi: 10.1016/j.semarthrit.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Ma HL, Napierata L, Stedman N, Benoit S, Collins M, Nickerson-Nutter C, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–440. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304–314. doi: 10.1038/jid.2011.304. [DOI] [PubMed] [Google Scholar]
- Waisman A. To be 17 again—anti-interleukin-17 treatment for psoriasis. N Engl J Med. 2012;366:1251–1252. doi: 10.1056/NEJMe1201071. [DOI] [PubMed] [Google Scholar]
- Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370:272–284. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- Korman BD, Tyler KL, Korman NJ. Progressive multifocal leukoencephalopathy, efalizumab, and immunosuppression: a cautionary tale for dermatologists. Arch Dermatol. 2009;145:937–942. doi: 10.1001/archdermatol.2009.175. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Searles G, Landells I, Shear NH, Papp K, Lui H, et al. The AWARE study: methodology and baseline characteristics. J Cutan Med Surg. 2009;13 Suppl 3:S113–S121. doi: 10.2310/7750.2009.00029. [DOI] [PubMed] [Google Scholar]
- Bissonnette R, Langley RG, Papp K, Matheson R, Toth D, Hultquist M, et al. Humanized anti-CD2 monoclonal antibody treatment of plaque psoriasis: efficacy and pharmacodynamic results of two randomized, double-blind, placebo-controlled studies of intravenous and subcutaneous siplizumab. Arch Dermatol Res. 2009;301:429–442. doi: 10.1007/s00403-009-0961-7. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2012. [DOI] [PubMed]
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi: 10.1016/j.sder.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–443. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359:419–429. doi: 10.1007/s11010-011-1036-6. [DOI] [PubMed] [Google Scholar]