Abstract
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed neoplasms in elderly men. The precancerous lesion of PCa is considered a high-grade prostate intraepithelial neoplasm (HG-PIN), while atypical small acinar proliferation (ASAP) is commonly considered as an under-diagnosed cancer. The aim of the study was to establish the impact of ASAP and extensive HG-PIN on pre-biopsy prostate-specific antigen (PSA) levels and the risk of cancer development in subsequent biopseis.
Material and methods
The 1,010 men suspected for PCa were included in the study based on elevated PSA, and/or positive rectal examination. Transrectal ultrasound (TRUS) guided 10 core biopsy was performed. In those with extensive HG-PIN or ASAP on the first biopsy, and/or elevated PSA value, a second biopsy was performed.
Results
In the second biopsy, PCa was diagnosed in 6 of 19 patients (31.57%) with extensive HG-PIN, in four of 40 (10%) with BPH, and in 4 of 18 (22.22%) with ASAP.
There was a statistically significant difference between the values of PSA in the group of patients with ASAP in comparison to those with benign prostate hyperplasia (BPH) (p = 0.005) as well as in patients with HG-PIN in comparison to BPH (p = 0.02).
Conclusions
A precancerous lesion diagnosed upon biopsy causes a statistically significant increase in the values of PSA in relation to BPH, as well as in the case of ASAP and extensive HG-PIN.
The estimate of risk of PCa diagnosis in patients with ASAP and those with extensive HG-PIN in the first biopsy is comparable, which is why there are no reasons for different treatment of patients with the above-mentioned diagnoses. Both should be subjected to urgent second biopsy in around the 4-6 weeks following the initial biopsy.
Keywords: prostate cancer, biopsy, prostatic intraepithelial neoplasia
INTRODUCTION
Prostate cancer (PCa) is one of the most commonly diagnosed neoplasms in elderly men. In Western Europe and the USA, it is the most frequent malignant neoplasm in men and accounts for 30% of all newly diagnoses cases [1, 2].
The precancerous lesion of PCa is considered a high–grade of intraepithelial neoplastic growth (also known as intraepithelial anaplasia of high degree – High–Grade Prostatic Intraepithelial Neoplasia – HG–PIN) [3, 4, 5]. The incidence of HG–PIN among patients subjected to biopsy due to cancer suspicion ranges from 1.5% to around 16% [6–10]. In meta–analysis of a number of studies, Epstein and Bostwick calculated the mean risk of diagnosing cancer during second biopsy in patients in whom HG–PIN was diagnosed on first biopsy to be 18.1% and 30%, respectively [11, 12, 13].
Atypical small acinar proliferation (ASAP) can be considered to be an element in the progression of changes in cell morphology between healthy tissue of the prostate and PCa. In most clinical studies, ASAP is diagnosed in 0.5–2% of patients subjected to biopsy of the prostate due to the suspicion of cancer [14, 15]. Diagnosis of ASAP is proposed, when it is not possible to find certain significant changes in the morphology of cells to diagnose PCa [16, 17]. Therefore, ASAP is presently considered to be an under diagnosed cancer. The frequency of diagnosing PCa in the second biopsy in patients with ASAP in the first biopsy ranges from 17–70% (mean 40.2%) [18, 19].
As a result of the lower estimate of risk of PCa diagnosis in subsequent biopsies in patients with HG–PIN in the first biopsy and normal levels of PSA, in comparison to men with benign changes and the associated increased levels of PSA, the European Association of Urology Guidelines of 2010 excluded HG–PIN, without elevated levels of PSA, from the indications for subsequent prostate biopsy. Only extensive HG–PIN can be a reason to re–biopsy, because the risk of subsequent PCa is increased [20]. Concurrently, due to the opinion that at least some cases of ASAP diagnosis in biopsy specimens are actually undiagnosed cancers, the indication to second biopsy in such patients has been preserved [21].
Some studies stand at least partially in contradiction to the opinion that patients with ASAP have a worse prognosis in comparison to HG–PIN. A study by Schoenfield (2007) found that the risk of cancer development in patients with HG–PIN was 33% while it was only 25% in ASAP [22]. The practice of undeserved exclusion of HG–PIN in the prognosis of cancer development occurs more and more frequently but it should be evaluated.
Therefore, the aim of this prospective study was to determine the incidence of PCa, intraepithelial anaplasia of high–grade (HG–PIN), atypical small acinar proliferation (ASAP), and other pathological states – in men subjected to subsequent and repeat prostate biopsy due to suspicion of PCa, and to assess whether there is any difference in risk of cancer development depending on type of changes identified in the first biopsy (ASAP versus HG–PIN). Another aim was to asses if ASAP or HG–PIN change the value of PSA.
MATERIAL AND METHODS
This prospective study included 1010 men who were evaluated from April 2006 to September 2007, after approval of the local ethical committee. The study's inclusion criteria included the suspicion of PCa on the basis of elevated values of prostate specific antigen (PSA, threshold set at 4 ng/ml), changes in digital rectal examination (DRE), and/or presence of changes on transrectal ultrasound (TRUS).
Patients who were qualified to join the study were referred to two urology departments where, with quinolone chemoprophylaxis and local anesthesia, 10–12 core biopsy using a Tru Cut Cook 18G needle under TRUS control was performed. In general, ten specimens were collected, but the protocol was sometimes expanded to include additional biopsies from suspicious areas of the prostate as seen in TRUS imaging.
If the results of the biopsy specimen revealed PCa then the patient was qualified for further treatment or observation depending on the severity of the disease. If the results revealed ASAP or extensive HG–PIN then the patient entered the outpatient observation program and a second biopsy was proposed three months later. The same protocol was applied for those with benign changes or prostatitis in the first biopsy with accompanying rising or persistent elevation of PSA levels. They were subjected to 10–12 core biopsy scheme, depending on the prostate volume.
The Shapiro–Wilk test was used to evaluate the compliance of the parameters with the normal distribution. The analyzed parameters did not have a normal distribution, which is why the non–parametric Mann–Whitney U test was used to compare the sum of the rank in two samples, which were presented using the median (Me), upper (Q1) and lower quartiles (Q3), the range of minimums and maximums, as well as mean and standard deviation. For comparison of more than three groups, the Kruskal–Wallis ANOVA by ranks test was used. The value of significant difference was set as p <0.05.
RESULTS
Results of the first prostate biopsy
Among 1,010 men who were subjected to first time biopsy, PCa was diagnosed in 336 patients (33.27%).
The presence of ASAP and HG–PIN lesion were diagnosed in 159 men (15.47%) (Table 1). The mean value of PSA in the studied men was 19.17 ng/ml (median 9.16 ng/ml). The highest PSA levels were found in the group of patients with PCa (mean 34.93 ng/ml, median 13.5 ng/ml) and the lowest in the group of patients with low–grade prostatic intraepithelial neoplasia (LG–PIN) (mean 9.3 ng/ml, median 6.90 ng/ml).
Table 1.
Results of second prostate biopsy
| Results of first biopsy | n | % |
|---|---|---|
| PCa | 336 | 33.27 |
| BPH | 387 | 38.32 |
| ASAP | 46 | 4.55 |
| ASAP + LG PIN | 8 | 0.79 |
| ASAP + prostatitis | 4 | 0.40 |
| ASAP + HG-PIN | 19 | 1.88 |
| HG-PIN | 76 | 7.52 |
| HG-PIN + prostatitis | 6 | 0.59 |
| LG-PIN | 23 | 2.28 |
| Prostatitis | 101 | 10 |
| Prostate atrophy | 4 | 0.40 |
| Total | 1,010 |
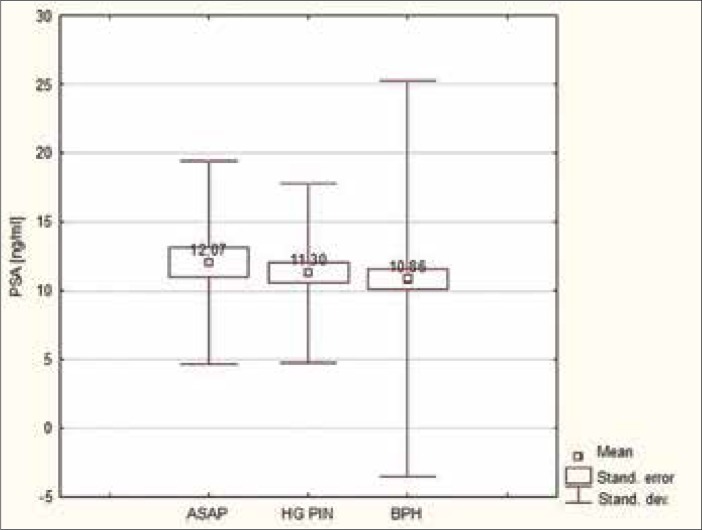
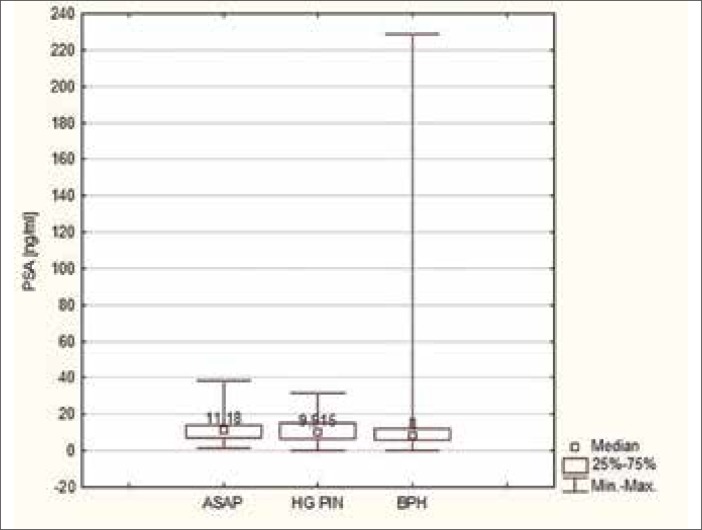
Comparing the mean values of PSA in patients with ASAP and HG–PIN in relation to patients with benign prostatic hyperplasia (BPH), a higher mean value was observed. A similar correlation is observed when comparing medians with only the case of co–occurrence of ASAP and HG–PIN; it was found that the value was equal to the median of patients with BPH. The differences in PSA levels between these lesions (ASAP and HG–PIN) were not statistically significant from each other (p = 0.6139), similar to the levels of PSA in the group of patients with ASAP in comparison to those with ASAP occurring together with HG–PIN (p = 0.1684) and HG–PIN in comparison to ASAP occurring together with HG–PIN (p = 0.4737). There was, however, a statistically significant difference between the values of PSA in the group of patients with ASAP in comparison to those with BPH (p = 0.005) as well as in patients with HG–PIN in comparison to BPH (p = 0.02) (Figures 1 and 2).
Figure 1.
Mean values of PSA in the group of patients with ASAP and HG-PIN in relation to patients with BPH.
Figure 2.
Median PSA in the group of patients with ASAP and HG-PIN in relation to patients with BPH.
Second prostate biopsy
The most common diagnosis made in the course of second biopsy was BPH (32 patients – 32.65%) and PCa was diagnosed in 16 (16.33%) patients in whom it was not previously found. In the second biopsy, ASAP (17 men – 17.34%) was diagnosed more frequently than HG–PIN (14 patients – 14.28%) (Table 2).
Table 2.
Results of second prostate biopsy
| Diagnosis | n | % |
|---|---|---|
| PCa | 16 | 16.33 |
| ASAP | 17 | 17.34 |
| ASAP + HG-PIN | 3 | 3.06 |
| HG-PIN | 14 | 14.28 |
| BPH | 32 | 32.65 |
| Prostatitis | 11 | 11.22 |
| LG-PIN | 4 | 4.08 |
| Prostate atrophy | 1 | 1.02 |
| Total | 98 |
PCa was diagnosed in six of 19 patients subjected to the second biopsy (31.57%) in whom first biopsy revealed the presence of HG–PIN, in four of 40 (10%) in whom BPH was diagnosed, and in four of 18 (22.22%) with ASAP or changes of LG–PIN type together with ASAP, and two of five (40%) in whom first biopsy revealed coexistence of ASAP and HG–PIN (Table 3).
Table 3.
Results of first biopsy in patients in whom PCa was diagnosed in second biopsy
| Diagnosis in first biopsy | Number of patients subjected to second biopsy | Number of cancers identified in second biopsy | |
|---|---|---|---|
| ASAP | 13 | ASAP→ PCa | 2 |
| ASAP + HG-PIN | 5 | ASAP + HG-PIN→ PCa | 2 |
| ASAP + LG-PIN | 5 | ASAP + LG-PIN→ PCa | 2 |
| ASAP + inflammation | 1 | ASAP + inflammation→ PCa | 0 |
| HG-PIN | 19 | HG-PIN→ PCa | 6 |
| HG-PIN + inflammation | 3 | HG-PIN + inflammation→ PCa | 0 |
| LG-PIN | 2 | LG-PIN→ PCa | 0 |
| BPH | 40 | BPH→ PCa | 4 |
| BPH + inflammation | 10 | BPH + inflammation→ PCa | 0 |
| Total | 98 | 16 |
There were no statistically significant differences in the incidence of PCa diagnosis in repeat biopsy between patients with the diagnosis of ASAP and HG–PIN in first biopsy (p = 0.2929). Also, there was no statistically significant difference in the incidence of diagnosing cancer in repeat biopsies after diagnosing ASAP coexisting together with HG–PIN in first biopsy (p = 0.3512) in comparison to ASAP and HG–PIN occurring independently.
DISCUSSION
Currently, the only recognized precancerous lesion for PCa is considered to be HG–PIN. The incidence of HG–PIN among patients subjected to biopsy due to suspicion of cancer ranges from 1.5% to about 16.5% with an average around 6% [23, 24].
ASAP is a condition associated with the occurrence of lesions of atypical proliferation in the prostatic glandular epithelium, which is likely to be associated with the development of under–appreciation of PCa. ASAP has been recognized in the results of prostate biopsy since the end of the 90s. In many studies its frequency varies and is about 0.5–2% (up to 6% is some studies) [25, 26]. The correct classification of the changes observed in histopathological preparation is extremely difficult, but more and more authors classify changes found in the ASAP as an under diagnosed cancer. This can lead to the idea of radical treatment of patients with ASAP. Braussi, among such patients, post–operatively confirmed the presence of PCa in case of patients, and almost all of them with clinical significance [27]. Therefore it is to be discussed, whether ASAP is still a valid term, or if it should be classified as a cancer, and radical treatment should be proposed.
In most studies, ASAP or HG–PIN alone does not cause a rise in PSA levels, and it is believed that only cancer and acute inflammation can account for such a rise. However, in some works, especially the older ones, opposing opinions can be found [28, 29]. In our study, among patients in whom ASAP and extensive HG–PIN were diagnosed, median values of PSA were statistically higher in comparison to healthy men (those with BPH) (p = 0.005 for the group with ASAP and p = 0.02 for the group with HG–PIN).
Such a difference can, however, suggest that at least a portion of the patients with ASAP and HG–PIN, already in the moments of the first biopsy, have already developed an undiagnosed PCa, which caused the elevated values of PSA. Unfortunately, it is impossible to fully assess the impact of all the histopathological changes of the prostate on the level of PSA, as well as to exclude the lack of possible impact of accidentally missing the cancer. In fact, such a correlation could only be established after examining the whole prostate on autopsy and correlating its results with levels of PSA. That is why it is impossible to definitively exclude that a coexisting undiagnosed PCa could cause the observed elevation of PSA level in men with precancerous lesions.
In recently published studies it is observed that somewhat less frequent diagnoses of PCa in second biopsy occur in patients with HG–PIN in comparison to studies from previous years. It seems that one of the causes may be the increased number of specimens collected during the first biopsy, and, therefore, increased probability of cancer diagnosis already in the first biopsy. Currently, only a few centers still perform sextant biopsies and the suggested biopsy standard has become a 10 or 16–core collection. Herawi proposed an explanation of this fact, and outlined that if the first and second biopsies were sextant, then the risk of PCa diagnosis was 14.1% for each biopsy. If the first sextant biopsy diagnosed HG–PIN and the second was performed with eight cores then the risk of PCa rose to 31.9%. If, however, both the first and subsequent biopsies were performed using eight cores then the chance of diagnosing cancer in both groups fell to 14.6%. He claims that the decrease in diagnostic ability in the last group is probably caused by the greater frequency of diagnosing cancer in the first biopsy and he proposes not subjecting patients to subsequent biopsies in such cases [30].
Data regarding the risk of PCa diagnosis in subsequent biopsies if HG–PIN is diagnosed on first biopsy is ambiguous. In earlier studies published before the “era” of ASAP (studies published up to the end of the nineties), it is believed that this risk is high and even reaches 50%. However, this assessment was conducted on small groups of men (150–200 patients). Among them, analyzing only the studies that included a large number of patients, the mean risk of PCa development among patients with HG–PIN was 24.1% (range: 21.7% to 28.9%) [31–33]. In more recent studies, the estimated risk is currently estimated at 18.1% (range: 16.7% to 19.6%) [34, 35, 36]. In the analyzed study, a repeat biopsy was performed in 19 patients with ASAP, and in four of them (21.05%) PCa was diagnosed. The proportion of patients with HG–PIN diagnosed in the first biopsy and PCa in the second was slightly higher. A biopsy was performed in 22 patients, and PCa was found in six of them (27.27%). In all of the cases, HG–PIN leading to a second biopsy was considered as extensive. The difference between the risk of PCa diagnosis after the diagnosis of ASAP or HG–PIN in the first biopsy is statistically insignificant and also stands in contradiction to the majority of presently published studies, in which the assessed risk of PCa development in the second biopsy after ASAP being discovered in the first is significantly (sometimes even two–fold) greater than after HG–PIN in the first. In our work, risk is comparable for both diseases and the slightly more frequent finding of PCa after HG–PIN in the first biopsy does not clearly establish a worse prognosis in these patients. It should be worth mentioning that, if the results of this analyzed study were compared to older data from before the era of ASAP, the risk of PCa development after HG–PIN in the first biopsy would be similar to that presented in the literature. Very few studies confirm our results and from recent works, only Schoenfield presents a risk of PCa development that is similar to our study, indicating it as 33% for HG–PIN and only 25% for ASAP [22].
In light of these results it seems that HG–PIN cannot be clearly rejected as a precancerous lesion and, as other authors propose, performing subsequent prostate biopsies should not be abandoned, especially when HG–PIN is extensive, like in our study. Such patients have a similar risk of PCa diagnosis in the second biopsy as those with ASAP in the first and at a much higher risk than those with benign changes found in the first biopsy (the presence of PCa was confirmed in our study in four of 50 patients with BPH – 8%).
It seems that patients with both histopathologic entities – ASAP and extensive HG–PIN – should be subjected to an urgent second prostate biopsy. It is very likely that these are patients in whom, at the moment of the first biopsy, the PCa had already developed, but was not explicitly confirmed. This is also why there seems to be no reason to subject these patients to different treatment protocols as they both should be subjected to an urgent second biopsy in around 4–6 weeks following the initial procedure.
CONCLUSIONS
A precancerous lesion diagnosed upon biopsy causes a statistically significant increase in the values of PSA in relation to BPH, likewise in the case of ASAP as well as extensive HG–PIN.
The estimate of risk of PCa diagnosis in patients with ASAP and those with extensive HG–PIN in the first biopsy is comparable, which is why there is no reason for different treatment of patients with the above–mentioned diagnoses. Both should be subjected to an urgent second biopsy in around 4–6 weeks following the initial procedure.
References
- 1.Jensen OM, Esteve J, Moller H, Renard H. Cancer in European community and its member states. Eur J Cancer. 1990;26:1167–1256. doi: 10.1016/0277-5379(90)90278-2. [DOI] [PubMed] [Google Scholar]
- 2.Salagierski M, Sosnowski M, Schalken JA. How accurate is our prediction of biopsy outcome? PCA3-based nomograms in personalized diagnosis of prostate cancer. Cent European J Urol. 2012;65:110–112. doi: 10.5173/ceju.2012.03.art1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987;15:788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Montironi R, Mazzucchelli R, Lopez-Beltran A, Scarpelli M, Cheng L. Prostatic intraepithelial neoplasia: its morphological and molecular diagnosis and clinical significance. BJU Int. 2011;108:1394–1401. doi: 10.1111/j.1464-410X.2011.010413.x. [DOI] [PubMed] [Google Scholar]
- 5.Di Francesco S, Tenaglia RL. Obesity, diabetes and aggressive prostate cancer hormone-naïve at initial diagnosis. Cent European J Urol. 2014;66:423–427. doi: 10.5173/ceju.2013.04.art7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamczyk P, Wolski Z, Butkiewicz R, Nussbeutel J, Drewa T. Inflammatory changes in biopsy specimens from patients with suspected prostate cancer. Cent European J Urol. 2013;66:256–262. doi: 10.5173/ceju.2013.03.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novis DA, Zarbo RJ, Valenstein PA. Diagnostic uncertainty expressed in prostate needle biopsies. A College of American Pathologists Q-probes Study of 15,753 prostate needle biopsies in 332 institutions. Arch Pathol Lab Med. 1999;123:687–692. doi: 10.5858/1999-123-0687-DUEIPN. [DOI] [PubMed] [Google Scholar]
- 8.Nowicki A, Sporny S, Duda-Szymanska J. β-catenin as a prognostic factor for prostate cancer (PCa) Cent European J Urol. 2012;65:119–223. doi: 10.5173/ceju.2012.03.art4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dovey Z, Corbishley CM, Kirby RS. Prostatic intraepithelial neoplasia: a risk factor for prostate cancer. Can J Urol. 2005;12:49–52. [PubMed] [Google Scholar]
- 10.Lefkowitz GK, Taneja SS, Brown J, Melamed J, Lepor H. Follow-up interval prostate biopsy 3 years after diagnosis of high grade prostatic intraepithelial neoplasia is associated with high likelihood of prostate cancer, independent of change in prostate specific antigen levels. J Urol. 2002;168:1415–1418. doi: 10.1016/S0022-5347(05)64463-1. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820–834. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 12.Bostwick DG. High grade intraepithelial neoplasia. The most likely precursor of prostate cancer. Cancer. 1995;75:1823–1836. [Google Scholar]
- 13.Bostwick DG, Cheng L. Precursors of prostate cancer. Histopathology. 2012;60:4–27. doi: 10.1111/j.1365-2559.2011.04007.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheville JC, Reznicek MJ, Bostwick DG. The focus of „atypical glands, suspicious for malignancy” in prostatic needle biopsy specimens: incidence, histologic features, and clinical follow-up of cases diagnosed in a community practice. Am J Clin Pathol. 1997;108:633–640. doi: 10.1093/ajcp/108.6.633. [DOI] [PubMed] [Google Scholar]
- 15.Borboroglu PG, Sur RL, Roberts JL, Amling CL. Repeat biopsy strategy in patients with atypical small acinar proliferation or high grade prostatic intraepithelial neoplasia on initial prostate needle biopsy. J Urol. 2001;166:866–869. [PubMed] [Google Scholar]
- 16.Venigalla S, Zhao C, Miyamoto H. Histopathologic features of atypical glands on prostate biopsy: nucleolar size is a predictor of subsequent detection of prostatic adenocarcinoma. Prostate. 2013;73:376–381. doi: 10.1002/pros.22577. [DOI] [PubMed] [Google Scholar]
- 17.Iczkowski KA, Casella G, Seppala RJ. Needle core length in sextant biopsy influences prostate cancer detection rate. Urology. 2002;59:698–703. doi: 10.1016/s0090-4295(02)01515-7. [DOI] [PubMed] [Google Scholar]
- 18.O'Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553–559. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 19.Postma R, Roobol M, Schroder FH, van der Kwast TH. Lesions predictive for prostate cancer in a screened population: first and second screening round findings. Prostate. 2004;61:260–266. doi: 10.1002/pros.20105. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 21.Iczkowski KA, MacLennan GT, Bostwick DG. Atypical small acinar proliferation suspicious for malignancy in prostate needle biopsies: clinical significance in 33 cases. Am J Surg Pathol. 1997;21:1489–1495. doi: 10.1097/00000478-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfield L, Jones JS, Zippe CD, Reuther AM, Klein E, Zhou M, Magi-Galluzzi C. The incidence of high-grade prostatic intraepithelial neoplasia and atypical glands suspicious for carcinoma on first-time saturation needle biopsy, and the subsequent risk of cancer. BJU Int. 2007;99:770–774. doi: 10.1111/j.1464-410X.2006.06728.x. [DOI] [PubMed] [Google Scholar]
- 23.Bostwick DG, Qian J, Frankel K. The incidence of high grade prostatic intraepithelial neoplasia in needle biopsies. J Urol. 1995;54:1791–1794. [PubMed] [Google Scholar]
- 24.Epstein JI. Pathology of prostatic neoplasia. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campell's Urology. 8th Ed. Philadelphia: Saunders; 2002. pp. 3025–3037. [Google Scholar]
- 25.Moore CK, Karikehalli S, Nazeer T. Prognostic significance of high grade prostatic intraepithelial neoplasia and atypical small acinar proliferation in the contemporary era. J Urol. 2005;173:70–72. doi: 10.1097/01.ju.0000148260.69779.c5. [DOI] [PubMed] [Google Scholar]
- 26.Iczkowski KA, MacLennan GT, Bostwick DG. Atypical small acinar proliferation suspicious for malignancy in prostate needle biopsies: clinical significance in 33 cases. Am J Surg Pathol. 1997;21:1489–1495. doi: 10.1097/00000478-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Brausi M, Castagnetti G, Dotti A, De Luca G, Olmi R, Cesinaro AM. Immediate radical prostatectomy in patients with atypical small acinar proliferation. Over treatment? J Urol. 2004;172:906–915. doi: 10.1097/01.ju.0000134622.54235.93. [DOI] [PubMed] [Google Scholar]
- 28.Catalona WC, Smith DS, Wolfert RL, Wang TJ, Rittenhouse HG, Ratliff TL, Nadler RB. Evaluation of percentage of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274:1214–1220. [PubMed] [Google Scholar]
- 29.Wills ML, Hamper UM, Partin AW, Epstein JI. Incidence of high-grade prostatic intraepithelial neoplasia in sextant needle biopsy specimens. Urology. 1997;49:367–373. doi: 10.1016/S0090-4295(96)00622-X. [DOI] [PubMed] [Google Scholar]
- 30.Herawi M, Kahane H, Cavallo C, Epstein JI. Risk of prostate cancer on first re-biopsy within 1 year following a diagnosis of high grade prostatic intraepithelial neoplasia is related to the number of cores sampled. J.Urol. 2006;175:121–124. doi: 10.1016/S0022-5347(05)00064-9. [DOI] [PubMed] [Google Scholar]
- 31.Wroński S. Radical perineal prostatectomy – the contemporary resurgence of a genuinely minimally invasive procedure: Procedure outline. Comparison of the advantages, disadvantages, and outcomes of different surgical techniques of treating organ-confined prostate cancer (PCa). A literature review with special focus on perineal prostatectomy. Cent European J Urol. 2012;65:188–194. doi: 10.5173/ceju.2012.04.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawlikowski M, Minias R, Sosnowski M, Zielinski KW. Immunohistochemical detection of angiotensin AT 1 and AT 2 receptors in prostate cancer. Cent European J Urol. 2011;64:252–255. doi: 10.5173/ceju.2011.04.art14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickinson SI. Premalignant and malignant prostate lesions: pathologic review. Cancer Control. 2010;17:214–222. doi: 10.1177/107327481001700402. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Khalek M, El-Baz M, Ibrahiem el-H. Predictors of prostate cancer on extended biopsy in patients with high grade prostatic intraepithelial neoplasia: a multivariate analysis model. BJU Int. 2004;94:528–533. doi: 10.1111/j.1464-410X.2004.04996.x. [DOI] [PubMed] [Google Scholar]
- 35.Alsikafi NF, Brendler CB, Gerber GS, Yang XJ. High-grade prostatic intraepithelial neoplasia with adjacent atypia is associated with a higher incidence of cancer on subsequent needle biopsy than high-grade prostatic intraepithelial neoplasia alone. Urology. 2001;57:296–301. doi: 10.1016/s0090-4295(00)00912-2. [DOI] [PubMed] [Google Scholar]
- 36.Borboroglu PG, Sur RL, Roberts JL, Amling CL. Repeat biopsy strategy in patients with atypical small acinar proliferation or high grade prostatic intraepithelial neoplasia on initial prostate needle biopsy. J Urol. 2001;166:866–869. [PubMed] [Google Scholar]