Abstract
Introduction
Penile carcinoma has an incidence of 4,000 cases in Europe. The therapy and prognosis depend decisively on the lymph node status. Lymph node metastases are detected in 23–65% cases depending on the histopathological pattern. Due to improved diagnostic methods an early detection of tumor stage is possible. Multimodal therapeutic concepts can offer curability for a subset of patients, even those suffering from advanced disease.
Material and methods
Current data on penile cancer based on a selective review of the literature by PubMed and the EAU guidelines 2009.
Results
Invasive diagnostic tools, such as fine–needle biopsy (FNB) and dynamic sentinel node biopsy (DSNB), improved the diagnosis of lymph node status considerably and reduced the morbidity in specialized centers. The application of 18F–FDG–PET/CT for metastases detection needs further evaluation due to inconsistent results. Inguinal lymphadenectomy is the therapeutic standard in case of metastases proof. It was possible to reduce the complications due to the new modified operation techniques. Patients with extended lymph node and distant metastases have a poor prognosis. Different systemic polychemotherapy regimes are applied currently and are associated with poor outcome (response rates <50%) and high morbidity. Neoadjuvant chemotherapy is recommended in patients with unresectable and relapsing lymph node metastases.
Conclusions
Currently, inconsistent therapy regimens are applied for metastatic penile cancer. Standardization is urgently needed through the development of high–quality studies and long–term registers in order to lower the morbidity and increase the efficiency of diagnosis and therapy.
Keywords: penile cancer, diagnostic imaging, sentinel lymph node biopsy, lymphadenectomy, adjuvant chemotherapy
INTRODUCTION
Penile squamous cell carcinoma (SCC) is a rare but challenging urological tumour with a potentially devastating course of disease. Only a few studies with larger scale patient cohorts have been published due to the low prevalence. The heterogeneous clinical appearance and different diagnostic and therapeutic strategies make it difficult to perform randomized trials. Furthermore, the analysis is complicated by the fact that in earlier decades, different tumour classifications were used and that the now widely used TNM–classification has undergone changes through regular “updates”.
MATERIAL AND METHODS
The literature search for this article was based on the PubMed database. No prospective randomized clinical trials were found (Jul 2013), therefore there is an overall low level of evidence. The current EAU Guidelines on penile cancer (2009) were used as a standard reference [1].
RESULTS
Frequency and etiology
Penile carcinoma occurs in Western countries, depending on the region, with an incidence of 0.5 to 1.6 cases per 100,000 men and represents approximately 0.5% of all malignant tumours in men. Higher prevalence of the disease was detected in several Asian, African and South American countries. Penile carcinoma is a tumour of aging men, with a strong increase of incidence in the 6th decade of life and a peak around the 80s [2].
SCC is the most common malignant tumour of the penis, in addition to the very rare malignant lesions such as basal cell carcinoma, malignant melanoma, sarcoma, M. Paget, lymphoreticular tumours and metastases. The possible risk factors are low standards of hygiene, phimosis, recurrent balanitis, high number of sexual partners (early age at first sexual intercourse), presence of HPV infection, circumcision practice, strong tobacco consumption, genital ultraviolet radiation and penile trauma. The known data suggests that the critical exposure period for certain etiologic factors is before puberty. Phimosis was found to correlate with invasive penile cancer. However, international scientific societies (including the American Academy of Pediatrics) have not yet declared a general recommendation for neonatal circumcision because of insufficient data for its preventive effect. Human papillomavirus (HPV) infection was identified to play a role in the development of penile cancer by interfering with the cell cycle regulation. DNA sequences of HPV (most frequently types 16 and 18) were detected in about 40–80% of tumour tissue samples in primary penile and metastatic lesions. The current data regarding the prognostic importance of HPV DNA in tumour tissue is still contradictory. Tobacco products seem to support the malignant cell transformation in existing HPV infection or local bacterial chronic inflammation [1–5].
Clinical course
The diagnosis is delayed in up to 50% of patients with SCC because of shame, guilt, fear, ignorance and carelessness. The reasons for delay on the medical side are often prolonged systemic and local treatment before the biopsy of the lesion (Figure 1). Two–thirds of the patients have an organ–confined disease at the time of initial diagnosis. Primary metastatic sites are the regional inguinal lymph nodes (up to 45%, depending on the histological pattern) and later, the pelvic nodes. Therefore, a proper examination of the inguinal lymph node status is crucial for the evaluation of the prognosis and overall survival. An overall stage–independent 5–year survival rate is 50%, but only 27% of patients with lymph node metastases survive the first five years after diagnosis. Detectable distant metastases (lung, liver, bone, or brain) are rare and occur late in the course of the disease. Penile carcinoma is characterized by a permanent progressive course that leads to death within two years in the majority of untreated patients. Spontaneous remissions have not been reported in the literature [6, 7].
Figure 1.
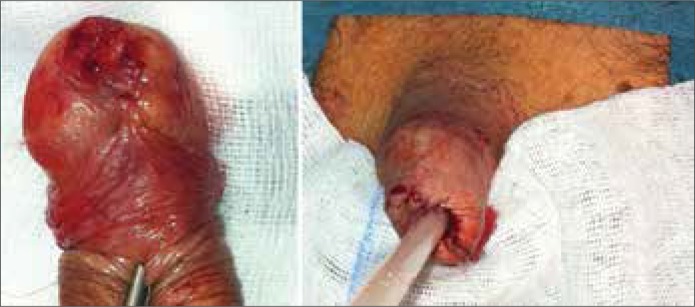
56yr patient with pT3G3 penile SCC (ulcer with infiltration of urethra) and clinical enlarged inguinal LNs on the left side. The right figure shows the result after partial penectomy.
Histology and staging
Penile SCC is classified histopathologically according to the growth pattern and degree of differentiation. These criteria reflect different risks for development of metastases. In addition, it has been found that the presence of vascular invasion is of significant prognostic importance. The initial classification scheme from 1921, by Broders, was revised in 1991 by Maiche, adding that the assessment of poorly differentiated tumour components is particularly important [8]. The mostly used classifications (e.g. Jackson system) have not been standardized. Recently there was an alignment of the TNM system of UICC (Union Internationale Contre le Cancer) and that of the AJCC (American Joint Committee on Cancer).
Clinical examination
In addition to the inspection and palpation of the external genitalia, the careful examination of the bilateral inguinal region for enlarged lymph nodes is crucial. It may be difficult to detect lymph nodes in obese patients and in patients previously operated in the groin. The enlarged inguinal lymph nodes can cause skin necrosis, chronic inflammation and lead to death due to sepsis or erosion of the femoral vessels in advanced metastatic disease [9].
Laboratory parameters
There are no tumour–specific serum markers. The SCC antigen, a glycoprotein belonging to the family of serine protease inhibitors, is a serological marker for SCC (lung, cervix, esophagus, etc.), but also increases in benign diseases (e.g. psoriasis). SCC antigen serum level correlates with tumour burden. Significant increases in serum values were measured only in patients with widespread lymph node metastases or organ metastases. Further prospective studies should analyse the value of SCC examination for therapy control or follow–up [10]. Several studies detected hypercalcemia without the presence of bone metastases, but associated with a lymph node involvement. The reason for this could be paraneoplastic production of parathyroid hormone.
Imaging
Although the inguinal lymph nodes are well accessible for palpation, the clinical examination makes a false prediction of the true pathological status in about 40% of patients. 30–60% of patients have enlarged inguinal lymph nodes at the primary presentation. Half of these cases are enlarged due to metastasis, the other half by a nonspecific inflammation. This means that in about 50% of cases an unnecessary lymphadenectomy, which is associated with high morbidity, is performed. On the other hand, 10–25% of patients without clinical signs have occult lymph node metastases. The correct detection of lymph node status is essential for the further treatment decisions. Ultrasound examination, although easy to use, has been shown to be imprecise due to high examiner variability. CT and MRI imaging techniques are able to detect lymph nodes >0.5–1 cm (sensitivity 36%). Only the size of the node can be reliably assessed but inflammation and metastasis cannot be differentiated. However, CT and MRI are useful for lymph node evaluation in obese patients or those after inguinal operations and for detection of pelvic lymph node metastases or distant metastases. Positron emission tomography (PET) is already used successfully for the detection of malignant tumours and metastases of other tumour entities. Some studies reported an increased 18F–FDG (18F–fluorodeoxyglucose) uptake in penile carcinoma and its metastases. In combination with CT, sensitivities of 75–88% were detected but large randomized series are still missing. The known general problems of FDG PET are the lack of possibility to detect micro metastases and the false positive results in inflamed lymph nodes [11, 11, 13].
Invasive diagnostics
In patients without evidence of lymph nodes involvement, but with histopathological risk factors, a clarification of the situation should be achieved with minimal morbidity [14]. Patients with detectable lymphadenopathy benefit from an exact diagnosis when inflammatory changes can be reliably found and operations avoided. There are several current approaches, such as the fine–needle biopsy, the lymph node biopsy, dynamic sentinel lymph node biopsy with lymph node mapping, the sentinel lymph node resection, and the superficial or modified lymph node resection [15].
The fine–needle biopsy (FNB) with aspiration cytology is easily done and especially useful in patients with palpable lymph nodes. The inguinal lymph nodes are evaluated with high–resolution ultrasound (7.5 MHz at least). Subsequently, an ultrasound or CT–guided biopsy and the cytopathological examination of aspirate are done. If the result is negative, FNB can be repeated after a course of antibiotic treatment. Due to the large number of false–negative results and an unsatisfactory sensitivity (39–71%), this method is considered to be unreliable for tumour staging in patients with negative clinical findings [16].
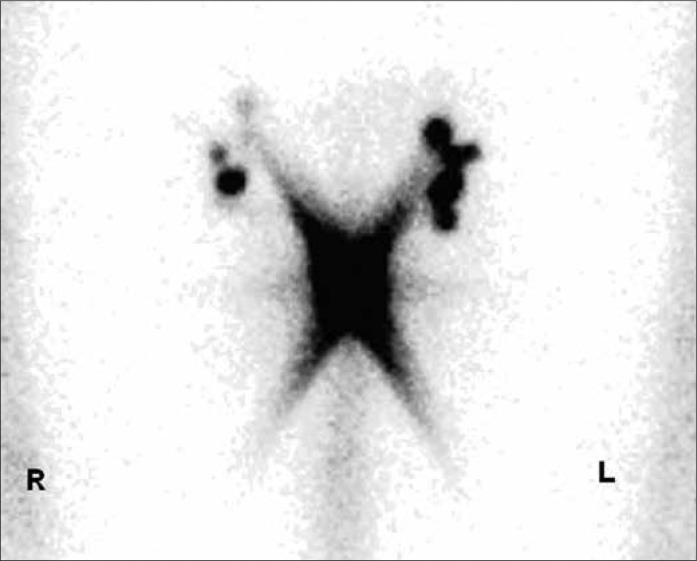
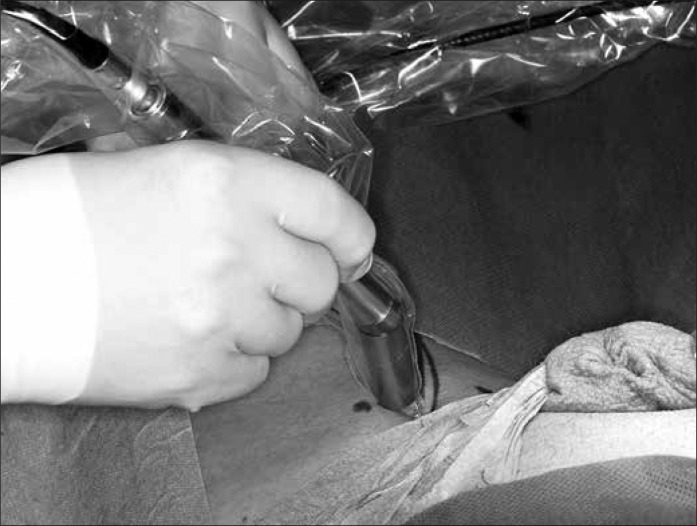
The principle of the SLN (sentinel lymph node) is based on the theory that there are one or more lymph nodes in a primary metastatic region of tumour. The exclusion of metastases in these lymph nodes assumes an organ–confined disease. The DSNB (dynamic sentinel node biopsy) can be easily done and has significantly fewer complications than the radical inguinal lymphadenectomy. It is represented in the current EAU Guidelines as a staging procedure for non–palpable lymph nodes [1]. Lymphoscintigraphy with Tc–99m labelled colloidal human albumin is performed a day before the surgery (Figure 2A). Intraoperative measurement of the radioactive activity is performed with a gamma camera, and the SLNs are localised and excised (mapping) (Figure 2B). In addition, patent blue can be injected at the primary lesion in order to simplify the detection of the lymph nodes. The complication rate is 5–6% (radical lymphadenectomy up to 40%) and most complications can be treated conservatively. The inguinal lymph node dissection is performed only if DSNB is positive, in order to prevent overtreatment. This method is less useful in patients with palpable lymph nodes due to the high false–negative rates caused by the blockage of lymph vessels by tumour cells [17, 18]. There are only a few studies that have analysed the reliability of the intraoperative lymph node mapping as a staging procedure in penile carcinoma (M.D. Anderson Cancer Center, University of Texas and The Netherlands Cancer Institute). False–negative rates of 0% to 29% with a significant fluctuation of the sensitivity (71– 93%) were detected. The good results (overall 7% false–negative, 93% sensitivity) in the largest two–center study (n = 323) by Leijte et al. are explained by the modification and technical improvement of the method over years [19]. In addition, FNB and the histopathological work–up with standardized application of immunohistochemistry were introduced. In this method, the initial false–negative rate of 22% could be reduced to less than 5%. The value of this technique for not–specialised clinics is limited by the small number of patients and complexity of the procedure [14–21].
Figure 2A.
53yr patient with pT1G3 penile SCC and clinical inapparent inguinal LNs (histology: no malignancy). Lymphoscintigraphy with 120 MBq Tc–99m nanocoll. Sentinel lymph nodes are marked on both sides (the illustration was kindly provided by MD Hautzel and MD Antke, Nuclear Medicine Clinic, University of Duesseldorf).
Figure 2B.
Intraoperative measurement of activity with a gamma probe (own operation photo).
THERAPY
Lymph node surgery
The extent and the complete removal of the inguinal lymph node metastases is the most important prognostic factor in patients with penile carcinoma. Primary lesions and regional lymph nodes are usually treated separately [22].
Bilateral inguinal lymphadenectomy was the standard therapy for intermediate and high–risk tumours for a long time; however, the indication has changed in recent years due to the further development of diagnostic options such as DSNB.
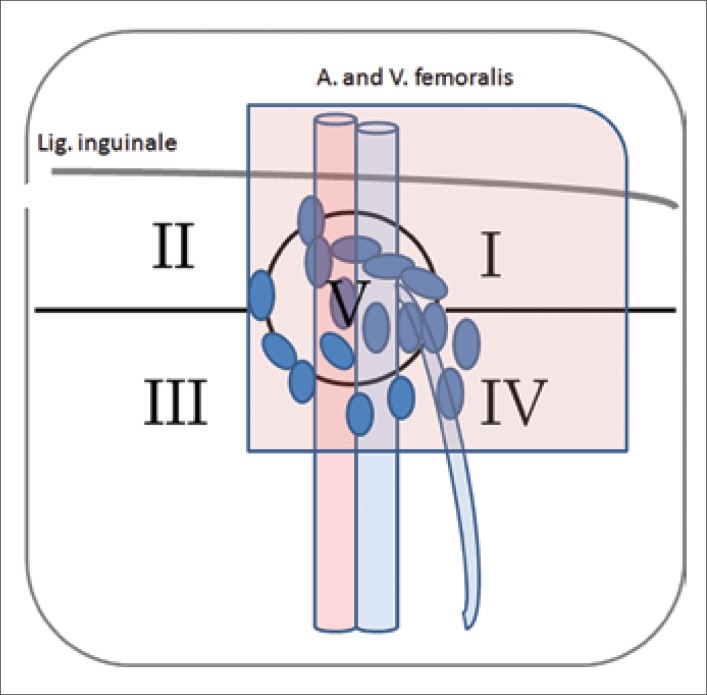
The regional inguinal lymph nodes are divided into superficial and deep lymph nodes (divided by fascia lata). The inguinal region is sectored in five clinically relevant zones (Daseler–zones, Figure 2C). Bilateral LN dissection is indicated in the presence of inguinal lymph node metastases. However, extended LN dissection is associated with high perioperative complications (30–70%), including prolonged lymphatic fistula, skin oedema and necrosis, wound infections, thrombosis and embolization. In recent series, the morbidity is significantly lower due to improved perioperative care, as well as advances in modified resection technique with careful preservation of the dermis, the Scarpa's fascia, and the great saphenous vein. Additionally, prophylactic antibiotics, compression stockings, and early mobilisation are important [23].
Figure 2C.
53yr patient with pT1G3 penile SCC and clinical inapparent inguinal LNs (histology: no malignancy). Inguino–femoral lymphatic drainage regions (Daseler zones). The region is divided into five zones: central zone (V), superior (I) and inferior (IV) medial zones, serior (II) and inferior (III) lateral zones. In penile carcinoma, the most common lymph node metastases were detected in the superior and medial zones. The red marked zone shows the region of modificated lymphadenectomy.
Pelvic lymph nodes should not be excised if inguinal lymph nodes are tumour–free because there are no direct lymph vessels from the primary tumour to the pelvic lymph nodes. If the lymph node of Cloquet or at least two superficial inguinal LNs are affected a rate of metastatic pelvic lymph nodes of 23–56% was detected. In these cases the pelvic LN dissection is indicated [24–29].
Chemotherapy
Limited experience has shown that adjuvant chemotherapy may improve the long–term survival of patients after radical resection of positive lymph nodes and that neoadjuvant chemotherapy permits resection after “downsizing” in about 50% of patients with primary inoperable inguinal metastases. However, chemotherapy alone in metastatic disease is not curative. The response rates are low (≤50%). Commonly occuring severe side effects which can restrict or significantly complicate the use of chemotherapy in elderly patients with impaired organ function, should be taken into account. Currently, there are many competing treatment regimens. Protzel et al. have found that a total of 18 different chemotherapy regimens (mean response rates <30%) were used in surveyed German hospitals in 2009. High–quality multi–centre studies are urgently needed to evaluate the success of therapy modalities and to develop new regimens.
The EAU guidelines recommend adjuvant chemotherapy in patients with at least two lymph node metastases after lymphadenectomy (≥pN2). Neoadjuvant chemotherapy is recommended in patients with non–resectable primary LN metastases for downstaging and decreased LN recurrence after surgery [1].
The previously used substances are vincristine, bleomycin, methotrexate, cisplatin, 5–fluorouracil, leucovorin, and paclitaxel used as single drug therapy or in various combinations (Table 1). The traditionally used Pizzocaro–regimen (VBM) in the adjuvant and neoadjuvant setting has acceptable toxicity and 50% long–time response rate. 5–year survival rate of patients after adjuvant treatment with VBM was about 84% (n = 25, relapse rate 16%) compared to 39% in the control group (n = 38, relapse rate 45%) after lymphadenectomy alone. The new promising therapies should be compared with this standard regimen. Cisplatin–based modalities deliver comparable response rates but with higher side effects (mortality up to 20%) and should be applied restrictively in aging patients. Toxicity leads to poor tolerance and hence poor patient compliance. The results for the same chemotherapy protocols are often significantly divergent (e.g. the adjuvant application of CBM: cisplatin, bleomycin, methotrexate) with response rate of 32.5–50%. The reasons are small and in homogeneous patient groups (n <10). In most series of CBM, dose reductions and interval prolongations were used, at the cost of efficacy.
Table 1.
Adjuvant and neoadjuvant chemotherapy regimens for metastatic penile cancer
| Adjuvant chemotherapy | Cycle | n | Response | Follow–up | Toxicity | Author |
|---|---|---|---|---|---|---|
| Vinblastin 1 mg/m2, d 1 methotrexat 30–50 mg/m2 d 3 bleomycin 15 mg/m2, d 1 and 2 |
Every week (12 cycles) |
12, 25 | 50% | 42 mos (median remission) 84% (5–year SR) | Max. grade 3 | [22, 35] |
| Cisplatin 20 mg/m2, d 2–6 methotrexat 200 mg/m2, d 1, 15, 22bleomycin 10 mg/m2, d 2–6 |
Every 4 weeks | 8, 40 | 32.5–50% | 26 mos (median remission) CR possible | Grade 4 (15%) mortality (12.5%) | [30, 36] |
| Neoadjuvant chemotherapy | ||||||
| Vinblastin 1 mg/m2, d 1 methotrexat 30–50 mg/m2, d 3 bleomycin 15 mg/m2, d 1 and 2 |
Every week (12 cycles) |
16 | 56% | 31% (5–year disease free) | Max. grade 3 | [22, 35] |
| Cisplatin 20 mg/m2, d 2–6 methotrexat 200 mg/m2, d 1, 15, 22bleomycin 10 mg/m2, d 2–6 |
Every 4 weeks (4 cycles) |
10 | 60% | Mortality (20%) | [30] | |
| Paclitaxel 120 mg/m2, d 1 cisplatin 50 mg/m2, d 1, 2 5–FU 1 g/m2, d 2–5 |
Every 3 weeks (2–4 cycles) |
6 | 80% (remission 50%) | 11 mos (median survival) | Max. grade 2 | [34] |
SR – survival rate; CR – complete remission
The best recent results for the neoadjuvant regimen were achieved with the combination taxol/cisplatin/5FU with moderate toxicity under co–medication. Pizzocaro et al. reported response rates >80% and lasting remissions in 50% of six patients in an small uncontrolled pilot series.
The prognosis of patients with distant metastases is still very poor (median survival of 6 mo). Haas et al. have treated the largest group (n = 40) in a phase II multicenter study with the CBM regimen. Only one patient with retroperitoneal lymph node metastases achieved a complete remission and two patients with lung metastases achieved a partial remission. The median survival time was 28 months [1, 30–36].
Radiotherapy
SCC is characterized by radio resistance. The doses required for effective tumour therapy (60 Gy) lead to high rates of major complications (e.g. fistula of urethra, stricture, necrosis, fibrosis of corpora cavernosa, pain, and oedema). Sometimes a secondary penectomy is necessary. External radiation therapy or brachytherapy are performed for the treatment of the primary tumour lesions in selected cases.
According to the EAU guidelines, prophylactic irradiation of the palpable lymph nodes is not recommended because of radiogenic complications. In addition, it complicates the evaluation of a possible recurrence in the post radiation fibrotic tissue. Palliative radiation therapy in inoperable lymph node metastases seems to be useful [1].
Follow up
For not enlarged inguinal lymph nodes at low–risk stage and in patients with negative SLN–biopsy results, a close follow up should be done, if an appropriate compliance of the patient is provided. Most important are the clinical examination of the groins, ultrasonography, and in selected cases additional imaging techniques such as PET–CT. The cancer follow up depends on the primary treatment. About 90% of recurrences occur within the first five years and mostly at the site of the primary lesion [1, 14, 15, 37].
CONCLUSIONS
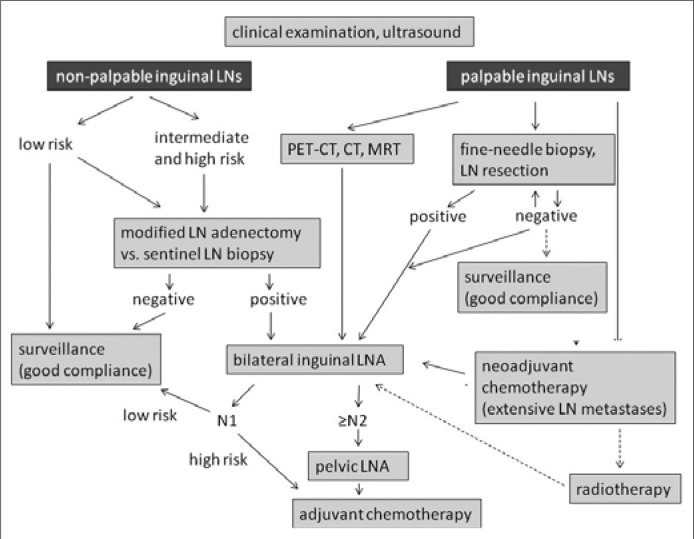
SCC of the penis is a rare, biologically aggressive tumour. In advanced stages, multimodal treatment options are available in specialized centres. The prevention and early detection are crucial, as an advanced disease with metastatic lymph node involvement worsens the prognosis considerably. A correct detection of the tumour stage by imaging and invasive diagnosis is important. The previously used radical bilateral inguinal lymphadenectomy can be avoided in most cases of early detection. In patients with unsuspicious clinical palpation of the inguinal region, intensive diagnostics (FNB, DSNB) should be done to exclude the metastases before these patients can be qualified for follow–up regimen. In the case of lymph node metastases modified inguinal lymph node dissection is indicated and if necessary, followed by the consecutive pelvic lymph node dissection. A cisplatin–based chemotherapy is indicated in adjuvant, neoadjuvant or advanced palliative setting but the individual benefit and risk of toxicity should be evaluated prior to therapy (Figure 3). A practical approach to provide larger scale studies with good evidence would be necessary to establish national and international penile SCC tumour registries.
Figure 3.
Algorithm for the treatment of LN metastases [according to EAU Guidelines 2009 (1)]. LN, lymph node; LNA, lymph node adenectomy
ACKNOWLEDGEMENTS
Special thanks go to Volker Mueller–Mattheis from the University Hospital of Duesseldorf for scientific advice.
References
- 1.Pizzocaro G, Algaba F, Horenblas S, Solsona E, Tana S, Van Der Poel H, Watkin NA. EAU Penile Cancer Guidelines 2009. Eur Urol. 2010;57:1002–1012. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 2.Wein A, Kavoussi L, Novick A, Partin A, Peters C. 9th ed. Philadelphia: Saunders; 2007. Campbell–Walsh Urology. [Google Scholar]
- 3.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Penile cancer: importance of circumcision, human papillomavirus, and smoking for in situ and invasive disease. Int J Cancer. 2005;116:606–616. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 4.Santos–Cortes J, Shapiro E, Ghavamian R. Penile carcinoma: a single institution experience. Cent European J Urol. 2010;63:117–120. [Google Scholar]
- 5.Nasierowska–Guttmejer A. Histological images of precancerous lesions and penile cancer in situ. Cent European J Urol. 2009;62:15–17. [Google Scholar]
- 6.Cubilla AL. The role of pathologic prognostic factors in squamous cell carcinoma of the penis. World J Urol. 2008;27:169–177. doi: 10.1007/s00345-008-0315-7. [DOI] [PubMed] [Google Scholar]
- 7.Pizzocaro G, Piva L, Bandieramonte G, Tana S. Up–to–date management of carcinoma of the penis. Eur Urol. 1997;32:5–15. [PubMed] [Google Scholar]
- 8.Maiche AG, Pyrhönen S, Karkinen M. Histological grading of squamous cell carcinoma of the penis: a new scoring system. Br J Urol. 1991;67:522–526. doi: 10.1111/j.1464-410x.1991.tb15199.x. [DOI] [PubMed] [Google Scholar]
- 9.Lont AP, Besnard AP, Gallee MP, van Tinteren H, Horenblas S. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int. 2003;91:493–495. doi: 10.1046/j.1464-410x.2003.04119.x. [DOI] [PubMed] [Google Scholar]
- 10.Hungerhuber E, Schlenker B, Schneede P, Stief CG, Karl A. Squamous cell carcinoma antigen correlates with tumor burden but lacks prognostic potential for occult lymph node metastases in penile cancer. Urology. 2007;70:975–979. doi: 10.1016/j.urology.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Horenblas S, Van Tinteren H, Delemarre JF, Moonen LM, Lustig V, Kröger R. Squamous cell carcinoma of the penis: Accuracy of tumor, nodes and metastases classification system and role of lymphangiography, computerized tomography scan, and fine needle aspiration cytology. J Urol. 1991;146:1279–1283. doi: 10.1016/s0022-5347(17)38068-0. [DOI] [PubMed] [Google Scholar]
- 12.Kochhar R, Taylor B, Sangar V. Imaging in primary penile cancer: current status and future directions. Eur Radiol. 2010;20:36–47. doi: 10.1007/s00330-009-1521-4. [DOI] [PubMed] [Google Scholar]
- 13.Mueller–Lisse UG, Scher B, Scherr MK, Seitz M. Functional imaging in penile cancer: PET/computed tomography, MRI and sentinel lymph node biopsy. Curr Opin Urol. 2008;18:105–110. doi: 10.1097/MOU.0b013e3282f151fd. [DOI] [PubMed] [Google Scholar]
- 14.Ficarra V, Zattoni F, Artibani W, Fandella A, Martignoni G, Novara G, et al. Nomogram predictive of pathological inguinal lymph node involvement in patients with squamous cell carcinoma of the penis. J Urol. 2006;175:1700–1704. doi: 10.1016/S0022-5347(05)01003-7. [DOI] [PubMed] [Google Scholar]
- 15.Hungerhuber E, Schlenker B, Karl A, Frimberger D, Rothenberger KH, Stief CG, Schneede P. Risk stratification in penile carcinoma: 25–year experience with surgical inguinal lymph node staging. Urology. 2006;68:621–625. doi: 10.1016/j.urology.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Kroon BK, Horenblas S, Deurloo EE, Nieweg OE, Teertstra HJ. Ultrasonography–guided fine–needle aspiration cytology before sentinel node biopsy in patients with penile carcinoma. BJU Int. 2005;95:517–521. doi: 10.1111/j.1464-410X.2005.05330.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroon BK, Valdés Olmos RA, van Tinteren H, Nieweg OE, Horenblas S. Reproducibility of lymphoscintigraphy for lymphatic mapping in patients with penile carcinoma. J Urol. 2005;174:2214–2217. doi: 10.1097/01.ju.0000181813.43631.e5. [DOI] [PubMed] [Google Scholar]
- 18.Leijte JA, Hughes B, Graafland NM, Kroon BK, Olmos RA, Nieweg OE, et al. Two–center evaluation of dynamic sentinel node biopsy for squamous cell carcinoma of the penis. J Clin Oncol. 2009;27:3325–3329. doi: 10.1200/JCO.2008.20.6870. [DOI] [PubMed] [Google Scholar]
- 19.Kroon BK, Horenblas S, Estourgie SH, Lont AP, Valdés Olmos RA, Nieweg OE. How to avoid false–negative dynamic sentinel node procedures in penile carcinoma. J Urol. 2004;171:2191–2194. doi: 10.1097/01.ju.0000124485.34430.15. [DOI] [PubMed] [Google Scholar]
- 20.Crawshaw JW, Hadway P, Hoffland D, Bassingham S, Corbishley CM, Smith Y, et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound–guided fine needle aspiration in penile carcinoma. Br J Radiol. 2009;82:41–48. doi: 10.1259/bjr/99732265. [DOI] [PubMed] [Google Scholar]
- 21.Hughes B, Leijte J, Shabbir M, Watkin N, Horenblas S. Non–in¬vasive and minimally invasive staging of regional lymph nodes in penile cancer. World J Urol. 2009;27:197–203. doi: 10.1007/s00345-008-0288-6. [DOI] [PubMed] [Google Scholar]
- 22.Pizzocaro G, Piva L, Nicolai N. Treatment of lymphatic metastasis of squamous cell carcinoma of the penis: experience at the National Tumor Institute of Milan. Arch Ital Urol Androl. 1996;68:169–172. [PubMed] [Google Scholar]
- 23.Protzel C, Alcaraz A, Horenblas S, Pizzocaro G, Zlotta A, Hakenberg OW. Lymphadenectomy in the surgical management of penile cancer. Eur Urol. 2009;55:1075–1088. doi: 10.1016/j.eururo.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Bevan–Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: The M.D. Anderson Cancer Center experience. J Urol. 2002;167:1638–1642. [PubMed] [Google Scholar]
- 25.Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: Technique and preliminary results. J Urol. 1988;140:306–310. doi: 10.1016/s0022-5347(17)41589-8. [DOI] [PubMed] [Google Scholar]
- 26.Horenblas S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 1: diagnosis of lymph node metastasis. BJU Int. 2001;88:467–472. doi: 10.1046/j.1464-410x.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 27.Horenblas S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int. 2001;88:473–483. doi: 10.1046/j.1464-410x.2001.00379.x. [DOI] [PubMed] [Google Scholar]
- 28.Hughes BE, Leijte JA, Kroon BK, Shabbir MA, Swallow TW, Heenan SD, et al. Lymph node metastasis in intermediate–risk penile squamous cell cancer: A two–centre experience. Eur Urol. 2010;57:688–692. doi: 10.1016/j.eururo.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005;173:816–819. doi: 10.1097/01.ju.0000154565.37397.4d. [DOI] [PubMed] [Google Scholar]
- 30.Haas GP, Blumenstein BA, Gagliano RG, Russell CA, Rivkin SE, Culkin DJ, et al. Cisplatin, methotrexate, and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J Urol. 1999;161:1823–1825. [PubMed] [Google Scholar]
- 31.Heidenreich A, Jakse G. Neoadjuvant and adjuvant chemotherapy in patients with advanced penile cancer. Urologe A. 2007;46:1398–1399. doi: 10.1007/s00120-007-1545-9. 1395–1396. [DOI] [PubMed] [Google Scholar]
- 32.Leijte JA, Kerst JM, Bais E, Antonini N, Horenblas S. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur Urol. 2007;52:488–494. doi: 10.1016/j.eururo.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. JCO. 2010;28:3851–3857. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzocaro G, Nicolai N, Milani A. Taxanes in combination with cisplatin and fluorouracil for advanced penile cancer: preliminary results. Eur Urol. 2009;55:546–551. doi: 10.1016/j.eururo.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–824. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 36.Protzel C, Hakenberg OW. Chemotherapy in patients with penile carcinoma. Urol Int. 2009;82:1–7. doi: 10.1159/000176016. [DOI] [PubMed] [Google Scholar]
- 37.Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow–up based on a two–centre analysis of 700 patients. Eur Urol. 2008;54:161–168. doi: 10.1016/j.eururo.2008.04.016. [DOI] [PubMed] [Google Scholar]