Abstract
Introduction
Near infrared (NIR) technology has recently garnered much interest as a tool for intraoperative image–guided surgery in various surgical sub–disciplines. In urology, although nascent, NIR technology is also fostering much enthusiasm.
This review discusses the two major fluorophores, indocyanine green (ICG) and methlyene blue (MB), with NIR guidance in experimental and clinical urology. The authors aim to illustrate and analyze the currently available initial studies to better understand the potential and practicability of NIR–guided imaging in the diagnosis and surgical outcome improvement.
In the first part of the study we analyzed problems associated with sentinel lymph node biopsy, NIR–guided detection and imaging of tumors.
Material and methods
PubMed and Medline databases were searched for ICG and MB use in urological settings, along with data published in abstracts of urological conferences.
Results
Although NIR–guided ICG and MB are still in their initial phases, there have been significant developments in major domains of urology, including uro–oncological surgery: 1) sentinel lymph node biopsy, 2) detection and imaging of tumors
Conclusions
Much like in other fields of surgical medicine, the application of NIR technology in urology is at its early stages. Therefore, more studies are needed to assess the true potential and limitations of the technology. However, initial developments hint towards a pioneering tool that may influence various aspects of urology.
Keywords: indocyanine green, methylene blue, near infrared fluorescence, sentinel node
INTRODUCTION
There are currently five main diagnostic imaging modalities routinely used in urology; they include radiography, magnetic resonance imaging (MRI), ultrasound (US), single–photon emission computed tomography (SPECT), and positron emission tomography (PET). However, out of the aforementioned modalities, only X–ray fluoroscopy and US have shown the capability of facilitating image–guided surgery. Intraoperative imaging of anatomical structures can possibly facilitate and improve the final outcome of the surgery. Regrettably, the current ability to perform image–guided surgery is limited. Nevertheless, novel imaging methods are constantly being devised; perhaps at the forefront of this paradigm are those technologies utilizing near–infrared light (NIR).
Near–infrared light is electromagnetic radiation with a wavelength that lies between ∼700–850 nm and is invisible to the human eye. In the 18th century, William Herschel first coined the term infrared radiation as a type of invisible radiation in the light spectrum beyond red light. It is absorbed or emitted by molecules when they change their rotational and vibrational movement. It is often used, especially in medicine.
Special hardware must be employed in order to display a visual image when using NIR. There are a number of commercial and experimental systems currently available but the principle of obtaining the image is very similar: first, a fluorophore, i.e. a dye capable of fluorescence by excitation, is administered; then a light source (usually a light emitting diode or LED) excites the fluorophore with one wavelength, and the fluorophore in turn emits a different wavelength that is detected by a charged–couple device (CCD) camera with special filters. This information is interpreted and an image is generated on a screen. To date, the fluorophore that has incited the most interest has been indocyanine green (ICG), a molecule with a diameter of 1.2 nm, 776Da, a tricarbocyanine amphiphilic probe, with an excitation peak of 807 nm and an emission peak of 822 nm. Another fluorophore of interest has been methylene blue (MB), which is a heterocyclic aromatic compound with a diameter of 1.43 nm, 320Da, with an excitation peak of 670 nm and an emission peak of 690 nm. Currently, the only fluorophores approved for use by the US Food and Drug Administration (FDA) and the European Medicals Agency are ICG and MB. For many years, ICG has been used in ophthalmic angiography and to determine cardiac output and hepatic function. Recently there has been a trend towards increased use of ICG in surgery. Although the first reports of ICG usage in medicine date back to the mid–1950s, the first use of ICG in surgery was in the 1970s when Krajka et al. [1, 2] used ICG in determining liver function, and the efficiency of elimination of ICG from the blood of patients during the course of obstructive jaundice. However, with the advent of NIR technology in the last 5– 6 years, the usability of ICG has widened dramatically: its usage has been reported in procedures such as sentinel node biopsy (SLNB) for breast cancer, melanoma, stomach, and other cancers, in angiography of intraoperative microvascular free flaps in reconstructive surgery, and in intraoperative tumor imaging [3–6]. In urology, the use of NIR fluorescence has also begun garnering much interest in various domains such as 1) SLNB, 2) tumor imaging, 3) kidney allograft perfusion, 4) real–time visualization of reconstructed vessels, 5) angiography perfusion of tissue flaps, 6) visualization of ureters, 7) visualization of urinary calcifications, and 8) cancer targeting and imaging. In this review, the authors attempt to present and summarize this ever–growing field, as well as to assess and analyze the current developments and possible future of NIR technology in the context of urology.
MATERIAL AND METHODS
A literature review was performed from PubMed and Medline as well as a search of abstracts from urology conferences.
Sentinel lymph node biopsy
SLNB in prostate and bladder cancer continues to remain controversial. Nonetheless, lymph node dissection (LND) is a reliable staging method for localized prostate and bladder cancer. Instead of being the sole method for staging, SLNB has been proposed by Wawroshek et al. [7] as an additional tool to decrease the morbidity of extended pelvic lymph node dissection (PLND). This procedure may improve staging and finding more micrometastases because of better pathological examination of the sentinel lymph nodes (SLNs), as well as standard examination of the other nodes. Originally, ICG was used as a simple dye to perform SLNB in breast cancer. In the study conducted by Motomura et al. [8], the sensitivity of this method was 73.8%, with a moderately high false negative of 11.1%. The revolution in the use of ICG in SLNB came with the ability to utilize its fluorescent properties. Kitai et al. [9] used the NIR system with a CCD camera and LEDs in order to visualize the SLNs. In this initial study, a group of 18 patients underwent SLNB using ICG as a simple dye, and resulted in the visualization of 83.3% of the lymphatic tracts and 50% of SLNs, while the use of ICG fluorescence properties increased the detection rate of SLN to 94.4%. Since then, a number of studies have been conducted to explore, better understand, and to optimize NIR guided SLNB using ICG in different cancers [3, 4, 5, 9–12].
Knapp et al. [13] performed NIR–guided SLNB in animal models (dogs and pigs) with naturally occurring invasive bladder cancer. This experiment featured HSA800 and NIR quantum dots, which at this time are not available for human studies. Within 10 seconds of fluorophore injection, lymphatic routes were visualized, with subsequent SLN visualization at 3 minutes. This initial animal study was an important step forward for a number of reasons. First, it assessed the optimum volume per injection, which was deemed to be 0.3 ml. Furthermore, the manner of administration was carefully analyzed and determined the superiority of superficial subserosal application of the dye over the superficial mucosal application or deep into the serosa. Finally, the importance of bladder pressure difference from 20 to 40 cm H2O in determining the flow of the marker in the lymphatic system was discovered. In this study, the NIR–guided method illustrated a number of different paths to different lymph node stations, and not just within the pelvis. Other reports of varying fluorescent patterns utilizing NIR–guided surgery describe variable patterns of ICG fluorescence in tumors of the liver correlating to the tumor's level of histological differentiation [14]. In this study, intra–tumoral injection of HSA800 resulted in no visualization of SLN and lymph routes, whereas peri–tumoral injection gave the visualization, coinciding with other cancer NIR–guided SLNBs studies. In the study by Knapp et al. [13], lymphatic metastases were found in 4 of 5 dogs, with SLN visualization in all of these. No SLN were visualized for 1 dog, but this animal was diagnosed with metastases to the lungs, in the absence of metastasis to the lymphatic system.
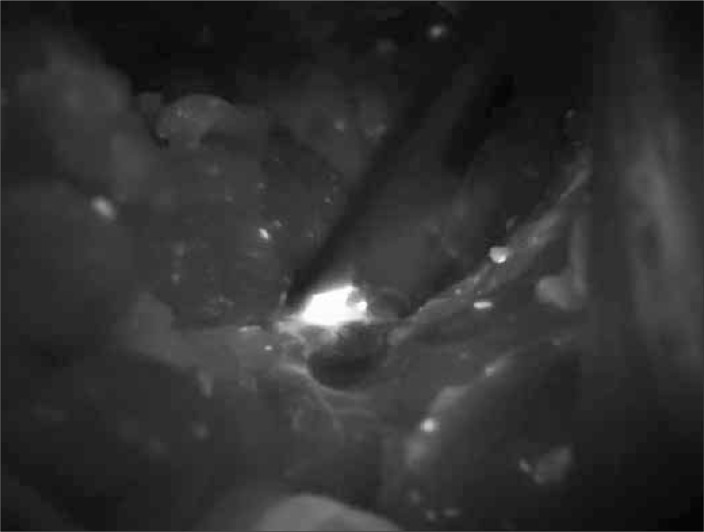
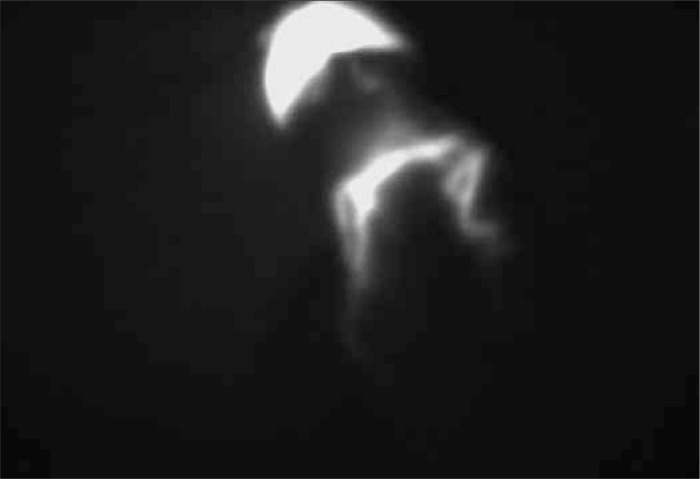
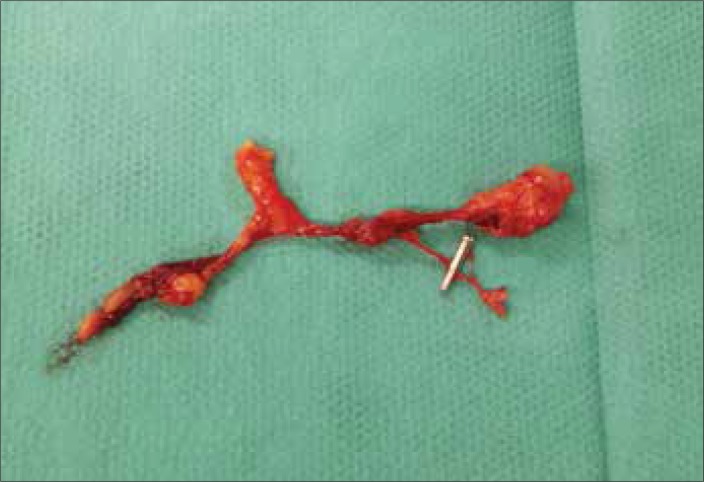
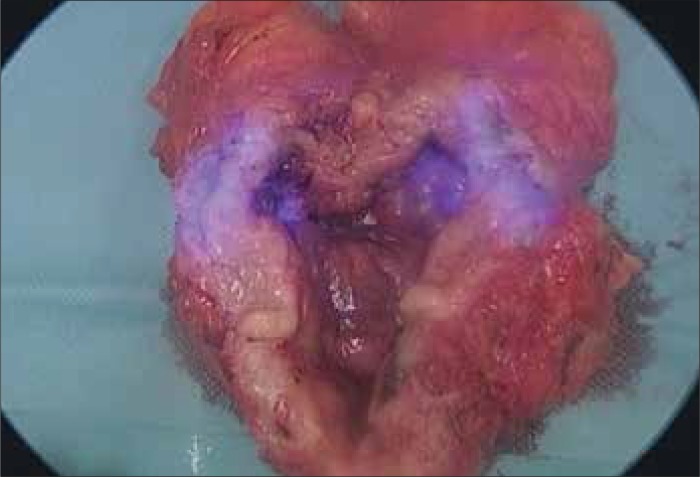
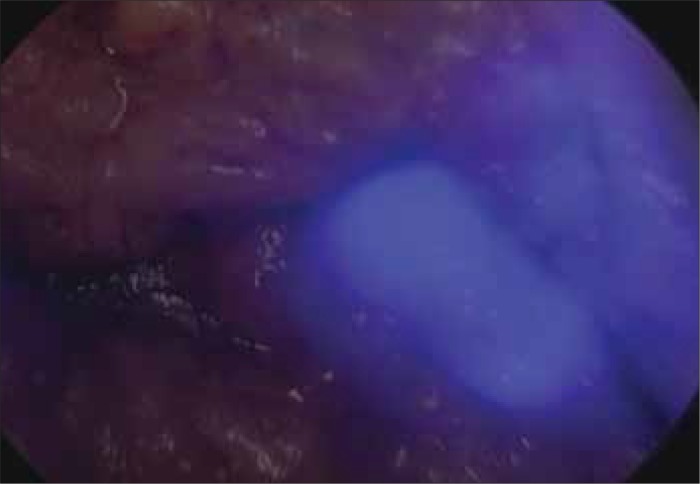
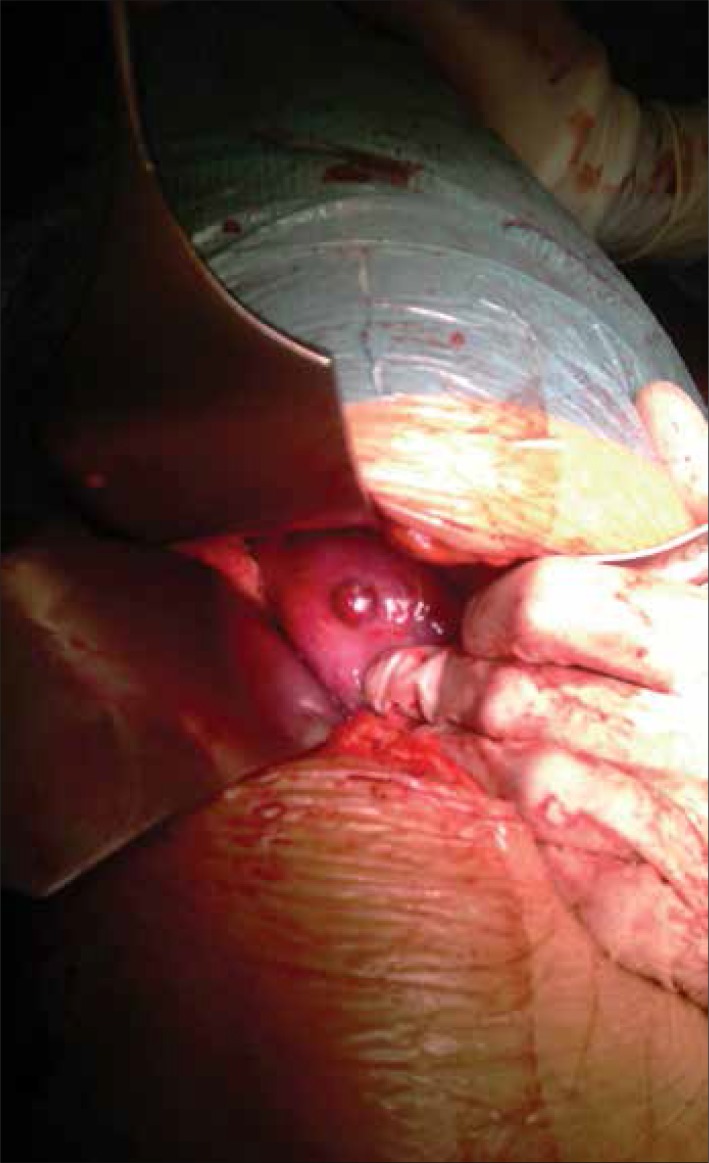
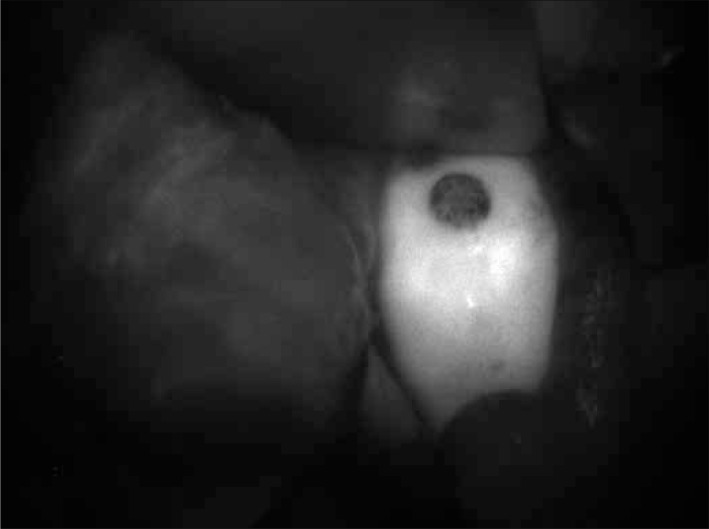
The first study using NIR–guided SLNB in human prostate cancer was by Van der Poel et al. [15]. This study used a combination marker – ICG–99mTc multimodal NanoColl. This study combined the benefits offered by real–time visualization of NIR with the well–established gamma emitting 99mTc guided SLNB. In 11 patients, the combination marker was injected into the peripheral zone of both lobes of the prostate 3 hours prior to surgery, and SLNB was performed by robot–assisted laparoscopic prostatectomy (RALP). SPECT was performed in all patients. SLNs were found in 10 of the 11 patients (0–4 nodes). Of the total number of 112 lymph nodes removed, 27 nodes were SLNs. Node metastases were found in 3 SLNs and were detected by both parts of the marker (ICG and 99mTc). Despite the administration of a mixture of both markers in the same syringe, intraoperative visualization of SLNs using both markers was possible in the case of 16 SLNs (59.2%), using only fluorescence in 6 SLNs (22.2%), and using only 99mTc in 4 nodes (14.8%). One SLN was visible only in SPECT. The problem in the intraoperative identification of SLN was deemed to be the thickness of the adipose tissue and the proximity of blood vessels, which made it difficult to detect the dye with NIR light. This study was also important in its originality, as it was first to use combined 99mTc with ICG. A combination of ICG with human serum albumin (ICG:HSA) is most frequently attempted in other cancer studies, with a number of possible advantages over ICG alone observed, and this phenomenon may be comparable to the ICG–99mTc combination [16–18] (Figures 1–7).
Figure 1.
Lymph node in case of prostate cancer seen in NIR light during surgery.
Figure 7.
Penile cancer – lymph flow in NIR light after ICG injection.
Figure 2.
Lymph node in case of prostate cancer in white light after excision.
Figure 3.
Lymph node in case of prostate cancer in NIR light after excision.
Figure 4.
Bladder cancer – fluorophore ICG injection site seen in white light.
Figure 5.
Bladder cancer – fluorophore ICG injection site seen in NIR light.
Figure 6.
Bladder cancer – lymph node in NIR light during surgery.
Near InfraRed – guided tumor imaging
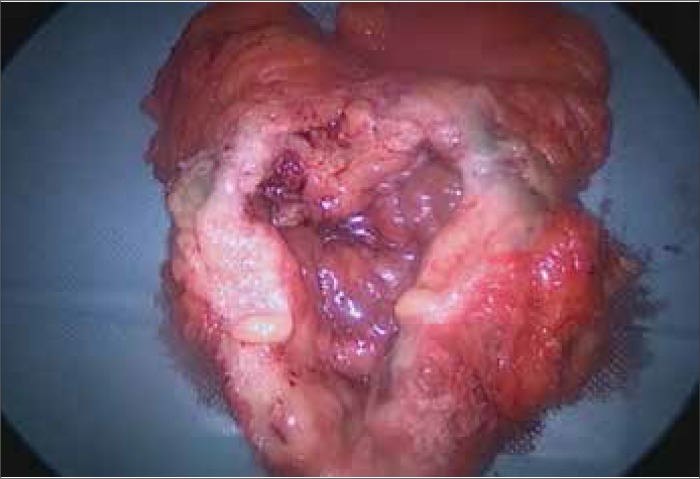
Partial nephrectomy in the case of renal cancer has become standard. However, one of the major hurdles faced when performing this procedure is the possibility of incomplete radical excision of the tumor, regardless of the surgical technique [i.e. open, laparoscopic or robotic–assisted laparoscopic partial nephrectomy (RALPN)], and it has been reported that positive margins can vary from anywhere between 2.5% to 5.7% [19–21]. The main challenge for radical removal of the tumor occurs when larger and more invasive changes penetrate deep into the kidney parenchyma. Currently, intraoperative US has been employed to assess the boundary between healthy and pathologic kidney tissue. However, the number of limitations does not allow this technique to always be utilized. In addition, the variability of renal vasculature which occurs in up to 47% of cases [22], can lead to further complications.
Again, NIR–guided ICG in this form of surgery has shown potential. To date, it has been demonstrated in both open and partial nephrectomy [23] and in RALPN [24]. When ICG is administered intravenously, it remains almost exclusively in the vasculature by binding to plasma proteins. Therefore, the NIR camera can very accurately depict organ vascularity, as well as visualize the border between healthy and cancerous tissue. It has been found that after administration, ICG then becomes transported in association with plasma proteins to the proximal tubule cells, where using bilitranslocase, it becomes transported into the cells of the kidney. Renal cortical tumor cells have reduced expression of the bilitranslocase protein, resulting in a lower amount of ICG transported into those cells than in normal proximal tubule cells [25, 26]. As tumors have reduced fluorescence in the NIR–guided image, borders can be well differentiated. The intensity of fluorescence depends on the type of change in the tissue [27]. All malignant tumors, regardless of the type – i.e. clear cell, papillary, chromophobe, and cystic renal cell carcinoma – are characteristically hypo–fluorescent and are well differentiated from the surrounding normal parenchyma. Although oncocytoma was reported to have hypo–fluorescent properties, this is to be expected as it differed minimally from normal tissues. To demonstrate the difference, benign cysts show increased fluorescence and a simple cyst with thick walls exhibits similar fluorescence intensity to the surrounding healthy parenchyma, as it was associated with varying bilitranslocase protein expression [25]. Decreased bilitranslocase expression also occurs in oncocytoma and shows fluorescence using NIR intraoperatively.
Furthermore, differentiation by ICG has the potential to provide additional benefits. First, improving the identification of the boundary between healthy and tumor tissue allows for a smaller percentage of positive margins on final histopathology, as well as greater preservation of healthy kidney tissue. Second, visualization of the vasculature allows for selective resection of the blood supply, preventing ischemia of the entire kidney during resection of the tumor, and may also prevent excessive bleeding and further related complications. Third, if selective renal ischemia of the area was present before its resection, there would be decreased perfusion of blood to the kidneys; which would in turn cause hypo–fluorescence intraoperatively, indicating the hypoperfusion in real–time. These properties and benefits of NIR–guided ICG were demonstrated by the works of Tobis et al. [24], who carried out RALPN guided tumor resection.
Intraoperative assessment of margins during tumor removal also plays an important role in prostate cancer. NIR–guided prostate cancer resection was performed by Nakajima et al. [28], using ICG conjugated to prostate specific membrane antigen (PSMA). Using this in mice, the authors were able to detect extracapsular tumor extension, SLNs with metastases, residual tumor after resections, and to visualize the cancer cells present in the resected tissue [28]. An interesting phenomenon of ICG conjugated to PSMA was the loss of fluorescence in this combination. However, it was shown that after uptake by the cell, the conjugation was destroyed and fluorescence was regained (Figures 8 and 9).
Figure 8.
Kidney tumor seen in white light during surgery.
Figure 9.
Kidney tumor in NIR light after intravenous ICG during surgery.
RESULTS
Although NIR–guided ICG and MB are still in their initial phases, there have been significant developments in uro–oncological surgery like sentinel lymph node Biopsy, detection and imaging of tumors. NIR–guided methods provide a pioneering alternative to current uro–oncological image–guided surgery.
CONCLUSIONS
Usage of NIR technique seems to be very helpful during SLNB and tumor imaging in case of renal masses. It should be, in the near future, an important tool that provides more accurate and more precise diagnoses and treatment in oncological urology, as new options of oncological treatment, occurring not only during surgery, are sought [29].
References
- 1.Krajka K, Tatarkiewicz–Suchorzewska J. Przydatność próby z zielenią indocyaninową w rozpoznaniu chorób wątroby [Usefulness of tests with Indocyanine green in the diagnosis of liver disease] Pol Przegl Chir. 1971;43:11. [PubMed] [Google Scholar]
- 2.Krajka K, Kopacz A. Effect of decompression of billiary tract on the course of elimination curve of indocyanine green from the blood. Przegl Lek. 1973;30:5. [PubMed] [Google Scholar]
- 3.Marshall MV, Rasmussen JC, Tan IC, Aldrich MB, Adams KE, Wang X, et al. Near–Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polom K, Murawa D, Rho YS, Nowaczyk P, Hünerbein M, Murawa P. Current trends and emerging future of indocyanine green usage in surgery and oncology. Cancer. 2011;117:4812–4822. doi: 10.1002/cncr.26087. [DOI] [PubMed] [Google Scholar]
- 5.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, et al. The clinical use of indocyanine green as a near–infrared fluorescent contrast agent for image–guided oncologic surgery. J Surg Oncol. 2011;104:323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevick– Muraca EM. Translation of Near–Infrared Fluorescence Imaging Technologies: Emerging Clinical Applications. Annu Rev Med. 2012;63:18.1–18.15. doi: 10.1146/annurev-med-070910-083323. [DOI] [PubMed] [Google Scholar]
- 7.Wawroschek F, Wagner T, Hamm M, Weckermann D, Vogt H, Märkl B, et al. The influence of serial sections, immunohistochemistry and extension of pelvic lymph node dissection on the lymph node status in clinically localized prostate cancer. Eur Urol. 2003;43:132–137. doi: 10.1016/s0302-2838(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 8.Motomura K, Inaji H, Komoike Y, Kasugai T, Noguchi S, Koyama H. Sentinel lymph node biopsy guided by indocyanine green dye in breast cancer patients. Jpn J Clin Oncol. 1999;29:604–607. doi: 10.1093/jjco/29.12.604. [DOI] [PubMed] [Google Scholar]
- 9.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 10.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 11.Ohdaira H1, Nimura H, Fujita T, Mitsumori N, Takahashi N, Kashiwagi H, et al. Tailoring treatment for early gastric cancer after endoscopic resection using sentinel lymph node navigation with infrared ray electronic endoscopy combined with indocyanine green injection. Dig Surg. 2009;26:276–281. doi: 10.1159/000227766. [DOI] [PubMed] [Google Scholar]
- 12.Troyan SL, Kianzad V, Gibbs–Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The FLARE intraoperative near–infrared fluorescence imaging system: a first–in–human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapp DW, Adams LG, Degrand AM, Niles JD, Ramos–Vara JA, Weil AB, et al. Sentinel Lymph Node Mapping of Invasive Urinary Bladder Cancer in Animal Models Using Invisible Light. Eur Urol. 2007;52:1700–1708. doi: 10.1016/j.eururo.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real–time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–2404. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 15.van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur Urol. 2011;60:826–833. doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Polom K, Murawa D, Nowaczyk P, Rho YS, Murawa P. Breast Cancer Sentinel Lymph Node Mapping Near Infrared Guided Indocyanine Green and Indocyanine Green–Human Serum Albumin in Comparison with Gamma Emitting Radioactive Colloid Tracer. Eur J Sug Oncol. 2012;38:137–142. doi: 10.1016/j.ejso.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Polom K, Murawa D, Rho YS, Spychala A, Murawa P. Skin melanoma sentinel node biopsy using real time fluorescence navigation with indocyanine green and indocyanine green with human serum albumin. Br J Dermatol. 2012;166:682–683. doi: 10.1111/j.1365-2133.2011.10634.x. [DOI] [PubMed] [Google Scholar]
- 18.Hutteman M, Mieog JS, van der Vorst JR, Liefers GJ, Putter H, Löwik CW, et al. Randomized, double–blind comparison of indocyanine green with or without albumin premixing for near–infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127:163–170. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scoll BJ, Uzzo RG, Chen DY, Boorjian SA, Kutikov A, Manley BJ, Viterbo R. Robotic–assisted partial nephrectomy: a large single institutional experience. Urology. 2010;75:1328–1334. doi: 10.1016/j.urology.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multiinstitutional analysis of perioperative outcomes. J Urol. 2009;182:866–872. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Wang AJ, Bhayani SB. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi–institutional analysis of perioperative outcomes. J Urol. 2009;182:866–872. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Sampaio FJ, Passos MA. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992;14:113–117. doi: 10.1007/BF01794885. [DOI] [PubMed] [Google Scholar]
- 23.Golijanin DJ, Madeb RR, Singer EA, Marshall JS, Wood RW, Reeder JE, et al. Intraoperative imaging of renal cortical tumors using near infrared fluorescence of intravenous indocyanine green. Can Urol Assoc J. 2007;1:305–332. [Google Scholar]
- 24.Tobis S, Knopf J, Silvers C, Yao J, Rashid H, Wu G, Golijanin D. Near Infrared Fluorescence Imaging with robotic assisted laparoscopic partial nephrectomy: Initial Clinical Experience for Renal Cortical Tumors. J Urol. 2011;186:47–52. doi: 10.1016/j.juro.2011.02.2701. [DOI] [PubMed] [Google Scholar]
- 25.Golijanin DJ, Marshall J, Cardin A, Singer EA, Wood RW, Reeder Bilitranslocase (BLT) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumors accurately corresponding to intraoperative near infrared fluorescence (NIRF) expression of renal cortical tumors using intravenous indocyanine green (ICG) J Urol. 2008;(suppl 179) abst 386. [Google Scholar]
- 26.Elias MM, Lunazzi GC, Passamonti S, Gazzin B, Miccio M, Stanta G, Gazzin B, Miccio M, Stanta G, et al. Bilitranslocase localization and function in basolateral plasma membrane of renal proximal tubule in rat. Am J Physiol. 1990;259:F559. doi: 10.1152/ajprenal.1990.259.4.F559. [DOI] [PubMed] [Google Scholar]
- 27.Golijanin DJ, Marshall J, Cardin A, Singer EA, Wood RW, Reeder JE, et al. Near infrared fluorescence of intravenous indocyanine green for intraoperative imaging of renal cortical tumors: a completed feasibility study. J Urol. 2008;(suppl 179) abst 598. [Google Scholar]
- 28.Nakajima T, Mitsunaga M, Bander N, Heston W, Choyke P, Kobayashi H. Targeted, Activatable, In Vivo Fluorescence Imaging of Prostate–Specific Membrane Antigen (PSMA) Positive Tumors Using the Quenched Humanized J591 Antibody Indocyanine Green (ICG) Conjugate. Bioconjugate Chem. 2011;22:1700–1705. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banyra O, Tarchynets M, Shulyak A. Renal cell carcinoma: how to hit the targets? Cent European J Urol. 2013;66:394–404. doi: 10.5173/ceju.2013.04.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]