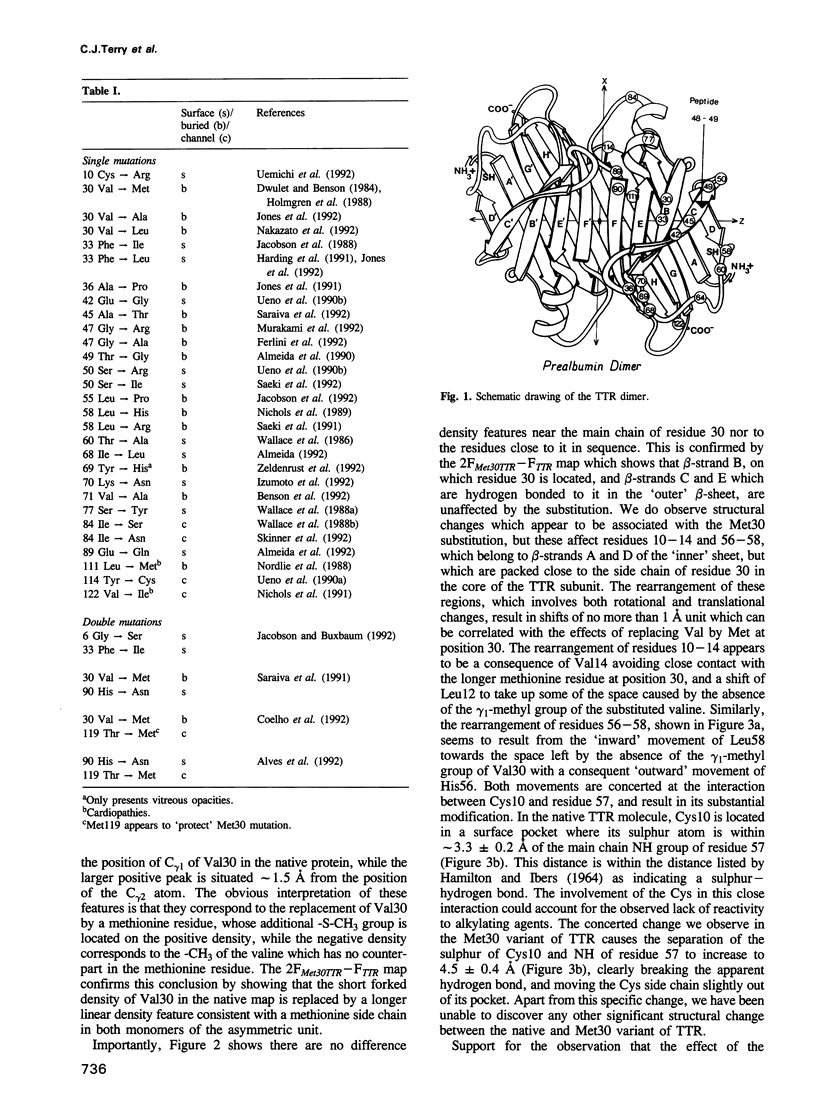
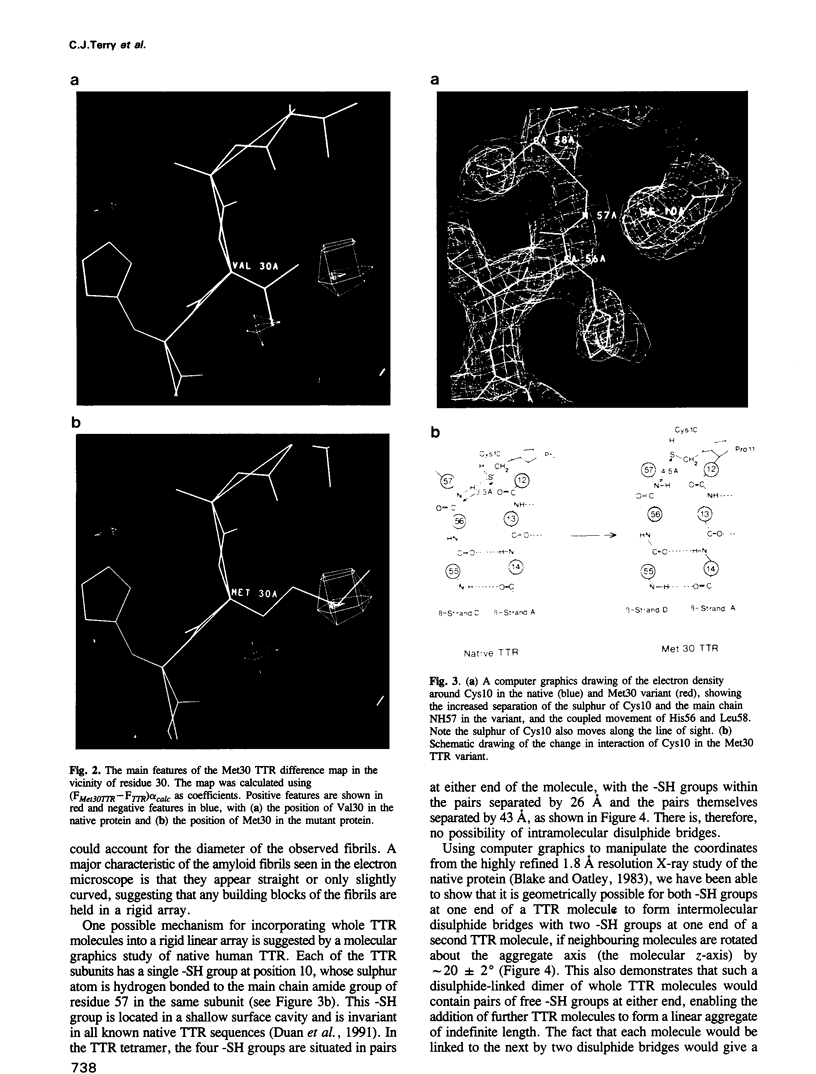
Abstract
Familial amyloidotic polyneuropathy (FAP) is an autosomal dominant hereditary type of lethal amyloidosis involving single (or double) amino acid substitutions in the amyloidogenic protein transthyretin (TTR). The most common type of FAP (Type I, or Portuguese) is characterized by a Val-->Met substitution at position 30. The Met30 variant of TTR has been produced by recombinant methods, crystallized in a form isomorphous with native TTR, subjected to X-ray analysis and compared structurally with the wild-type protein. The comparison shows that the effect of the substitution at position 30 is transmitted through the protein core to Cys10, the only thiol group in the TTR subunit, which becomes slightly more exposed. The variant TTR molecule is otherwise in a near-native state. Use of computer graphics has shown that it is possible to model a linear aggregate of TTR molecules, each linked to the next by a pair of disulphide bonds involving Cys10 residues. Formation of these disulphide bonds involves a small number of slightly short molecular contacts with native TTR molecules, most of which are relieved in the Met30 variant. We propose this model as a possible basis for a molecular description of the FAP amyloid fibrils.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida M. R., Alves I. L., Sakaki Y., Costa P. P., Saraiva M. J. Prenatal diagnosis of familial amyloidotic polyneuropathy: evidence for an early expression of the associated transthyretin methionine 30. Hum Genet. 1990 Oct;85(6):623–626. doi: 10.1007/BF00193586. [DOI] [PubMed] [Google Scholar]
- Almeida M. R., Hesse A., Steinmetz A., Maisch B., Altland K., Linke R. P., Gawinowicz M. A., Saraiva M. J. Transthyretin Leu 68 in a form of cardiac amyloidosis. Basic Res Cardiol. 1991 Nov-Dec;86(6):567–571. doi: 10.1007/BF02190707. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Swan I. D., Rerat C., Rerat B. Strjcture of human plasma prealbumin at 2-5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. J Mol Biol. 1974 Sep 5;88(1):1–12. doi: 10.1016/0022-2836(74)90291-5. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Swan I. D., Rerat C., Berthou J., Laurent A., Rerat B. An x-ray study of the subunit structure of prealbumin. J Mol Biol. 1971 Oct 14;61(1):217–224. doi: 10.1016/0022-2836(71)90218-x. [DOI] [PubMed] [Google Scholar]
- Branch W. T., Robbins J., Edelhoch H. Thyroxine-binding prealbumin. Conformation in urea and guanidine. Arch Biochem Biophys. 1972 Sep;152(1):144–151. doi: 10.1016/0003-9861(72)90202-0. [DOI] [PubMed] [Google Scholar]
- Christmanson L., Betsholtz C., Gustavsson A., Johansson B., Sletten K., Westermark P. The transthyretin cDNA sequence is normal in transthyretin-derived senile systemic amyloidosis. FEBS Lett. 1991 Apr 9;281(1-2):177–180. doi: 10.1016/0014-5793(91)80387-i. [DOI] [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Sletten K., Johansson B., Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988 Jul 29;154(2):648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Achen M. G., Richardson S. J., Lawrence M. C., Wettenhall R. E., Jaworowski A., Schreiber G. Isolation, characterization, cDNA cloning and gene expression of an avian transthyretin. Implications for the evolution of structure and function of transthyretin in vertebrates. Eur J Biochem. 1991 Sep 15;200(3):679–687. doi: 10.1111/j.1432-1033.1991.tb16232.x. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Primary structure of an amyloid prealbumin and its plasma precursor in a heredofamilial polyneuropathy of Swedish origin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):694–698. doi: 10.1073/pnas.81.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eanes E. D., Glenner G. G. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968 Nov;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- Felding P., Fex G., Westermark P., Olofsson B. O., Pitkänen P., Benson L. Prealbumin in Swedish patients with senile systemic amyloidosis and familial amyloidotic polyneuropathy. Scand J Immunol. 1985 Feb;21(2):133–140. doi: 10.1111/j.1365-3083.1985.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Furuya H., Saraiva M. J., Gawinowicz M. A., Alves I. L., Costa P. P., Sasaki H., Goto I., Sakaki Y. Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP). Biochemistry. 1991 Mar 5;30(9):2415–2421. doi: 10.1021/bi00223a017. [DOI] [PubMed] [Google Scholar]
- Gustavsson A., Engström U., Westermark P. Normal transthyretin and synthetic transthyretin fragments form amyloid-like fibrils in vitro. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1159–1164. doi: 10.1016/0006-291x(91)91687-8. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Steinrauf L. K., Liepnieks J., Benson M. D., Holmgren G., Sandgren O., Steen L. Alteration in molecular structure which results in disease: the Met-30 variant of human plasma transthyretin. Biochim Biophys Acta. 1992 Jun 9;1139(1-2):9–16. doi: 10.1016/0925-4439(92)90075-x. [DOI] [PubMed] [Google Scholar]
- Harding J., Skare J., Skinner M. A second transthyretin mutation at position 33 (Leu/Phe) associated with familial amyloidotic polyneuropathy. Biochim Biophys Acta. 1991 Oct 21;1097(3):183–186. doi: 10.1016/0925-4439(91)90033-6. [DOI] [PubMed] [Google Scholar]
- Holmgren G., Haettner E., Nordenson I., Sandgren O., Steen L., Lundgren E. Homozygosity for the transthyretin-met30-gene in two Swedish sibs with familial amyloidotic polyneuropathy. Clin Genet. 1988 Nov;34(5):333–338. doi: 10.1111/j.1399-0004.1988.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Jacobson D. R., Gorevic P. D., Buxbaum J. N. A homozygous transthyretin variant associated with senile systemic amyloidosis: evidence for a late-onset disease of genetic etiology. Am J Hum Genet. 1990 Jul;47(1):127–136. [PMC free article] [PubMed] [Google Scholar]
- Jacobson D. R., McFarlin D. E., Kane I., Buxbaum J. N. Transthyretin Pro55, a variant associated with early-onset, aggressive, diffuse amyloidosis with cardiac and neurologic involvement. Hum Genet. 1992 May;89(3):353–356. doi: 10.1007/BF00220559. [DOI] [PubMed] [Google Scholar]
- Jacobson D. R., Santiago-Schwartz F., Buxbaum J. N. Restriction fragment analysis confirms the position 33 mutation in transthyretin from an Israeli patient (SKO) with familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1988 May 31;153(1):198–202. doi: 10.1016/s0006-291x(88)81208-7. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Skare J. C., Cohen A. S., Harding J. A., Milunsky A., Skinner M. Familial amyloidotic polyneuropathy: a new transthyretin position 30 mutation (alanine for valine) in a family of German descent. Clin Genet. 1992 Feb;41(2):70–73. doi: 10.1111/j.1399-0004.1992.tb03635.x. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Skare J. C., Harding J. A., Cohen A. S., Milunsky A., Skinner M. Proline at position 36: a new transthyretin mutation associated with familial amyloidotic polyneuropathy. Am J Hum Genet. 1991 May;48(5):979–982. [PMC free article] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Moore D. H., Landow H. AN ELECTROPHORETIC STUDY OF THE PROTEIN COMPONENTS IN CEREBROSPINAL FLUID AND THEIR RELATIONSHIP TO THE SERUM PROTEINS. J Clin Invest. 1942 Sep;21(5):571–577. doi: 10.1172/JCI101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Murakami T., Maeda S., Yi S., Ikegawa S., Kawashima E., Onodera S., Shimada K., Araki S. A novel transthyretin mutation associated with familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 1992 Jan 31;182(2):520–526. doi: 10.1016/0006-291x(92)91763-g. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Ikeda S., Shiomi K., Matsukura S., Yoshida K., Shimizu H., Atsumi T., Kangawa K., Matsuo H. Identification of a novel transthyretin variant (Val30----Leu) associated with familial amyloidotic polyneuropathy. FEBS Lett. 1992 Jul 20;306(2-3):206–208. doi: 10.1016/0014-5793(92)81001-3. [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., Snyder E. L., Benson M. D. Senile cardiac amyloidosis associated with homozygosity for a transthyretin variant (ILE-122). J Lab Clin Med. 1991 Mar;117(3):175–180. [PubMed] [Google Scholar]
- Nordlie M., Sletten K., Husby G., Ranløv P. J. A new prealbumin variant in familial amyloid cardiomyopathy of Danish origin. Scand J Immunol. 1988 Jan;27(1):119–122. doi: 10.1111/j.1365-3083.1988.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Pettersson T. M., Carlström A., Ehrenberg A., Jörnvall H. Transthyretin microheterogeneity and thyroxine binding are influenced by non-amino acid components and glutathione constituents. Biochem Biophys Res Commun. 1989 Jan 16;158(1):341–347. doi: 10.1016/s0006-291x(89)80218-9. [DOI] [PubMed] [Google Scholar]
- ROBBINS J., RALL J. E. Proteins associated with the thyroid hormones. Physiol Rev. 1960 Jul;40:415–489. doi: 10.1152/physrev.1960.40.3.415. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Dwulet F. E., Benson M. D. Reduced affinity for thyroxine in two of three structural thyroxine-binding prealbumin variants associated with familial amyloidotic polyneuropathy. J Clin Endocrinol Metab. 1986 Dec;63(6):1432–1437. doi: 10.1210/jcem-63-6-1432. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Ueno S., Takahashi N., Soga F., Yanagihara T. A novel mutant (transthyretin Ile-50) related to amyloid polyneuropathy. Single-strand conformation polymorphism as a new genetic marker. FEBS Lett. 1992 Aug 10;308(1):35–37. doi: 10.1016/0014-5793(92)81044-m. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Ueno S., Yorifuji S., Sugiyama Y., Ide Y., Matsuzawa Y. New mutant gene (transthyretin Arg 58) in cases with hereditary polyneuropathy detected by non-isotope method of single-strand conformation polymorphism analysis. Biochem Biophys Res Commun. 1991 Oct 15;180(1):380–385. doi: 10.1016/s0006-291x(05)81304-x. [DOI] [PubMed] [Google Scholar]
- Sandgren O., Holmgren G., Lundgren E. Vitreous amyloidosis associated with homozygosity for the transthyretin methionine-30 gene. Arch Ophthalmol. 1990 Nov;108(11):1584–1586. doi: 10.1001/archopht.1990.01070130086036. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J., Almeida M. R., Alves I. L., Moreira P., Gawinowicz M., Costa P. P., Rauh S., Banhzoff A., Altland K. Molecular analyses of an acidic transthyretin Asn 90 variant. Am J Hum Genet. 1991 May;48(5):1004–1008. [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Almeida M. do R., Sherman W., Gawinowicz M., Costa P., Costa P. P., Goodman D. S. A new transthyretin mutation associated with amyloid cardiomyopathy. Am J Hum Genet. 1992 May;50(5):1027–1030. [PMC free article] [PubMed] [Google Scholar]
- Saraiva M. J., Costa P. P., Goodman D. S. Studies on plasma transthyretin (prealbumin) in familial amyloidotic polyneuropathy, Portuguese type. J Lab Clin Med. 1983 Oct;102(4):590–603. [PubMed] [Google Scholar]
- Sasaki H., Sakaki Y., Matsuo H., Goto I., Kuroiwa Y., Sahashi I., Takahashi A., Shinoda T., Isobe T., Takagi Y. Diagnosis of familial amyloidotic polyneuropathy by recombinant DNA techniques. Biochem Biophys Res Commun. 1984 Dec 14;125(2):636–642. doi: 10.1016/0006-291x(84)90586-2. [DOI] [PubMed] [Google Scholar]
- Steinrauf L. K., Cao Y. J., Hamilton J., Murrell J., Liepnieks J. J., Benson M. D. Preparation and crystallization of human transthyretin (prealbumin) variants. Biochem Biophys Res Commun. 1991 Sep 16;179(2):804–809. doi: 10.1016/0006-291x(91)91888-j. [DOI] [PubMed] [Google Scholar]
- Tawara S., Araki S., Toshimori K., Nakagawa H., Ohtaki S. Amyloid fibril protein in type I familial amyloidotic polyneuropathy in Japanese. J Lab Clin Med. 1981 Dec;98(6):811–822. [PubMed] [Google Scholar]
- Ueno S., Uemichi T., Takahashi N., Soga F., Yorifuji S., Tarui S. Two novel variants of transthyretin identified in Japanese cases with familial amyloidotic polyneuropathy: transthyretin (Glu42 to Gly) and transthyretin (Ser50 to Arg). Biochem Biophys Res Commun. 1990 Jun 29;169(3):1117–1121. doi: 10.1016/0006-291x(90)92011-n. [DOI] [PubMed] [Google Scholar]
- Ueno S., Uemichi T., Yorifuji S., Tarui S. A novel variant of transthyretin (Tyr114 to Cys) deduced from the nucleotide sequences of gene fragments from familial amyloidotic polyneuropathy in Japanese sibling cases. Biochem Biophys Res Commun. 1990 May 31;169(1):143–147. doi: 10.1016/0006-291x(90)91445-x. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Conneally P. M., Benson M. D. A DNA test for Indiana/Swiss hereditary amyloidosis (FAP II). Am J Hum Genet. 1988 Aug;43(2):182–187. [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Dwulet F. E., Conneally P. M., Benson M. D. Biochemical and molecular genetic characterization of a new variant prealbumin associated with hereditary amyloidosis. J Clin Invest. 1986 Jul;78(1):6–12. doi: 10.1172/JCI112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. R., Dwulet F. E., Williams E. C., Conneally P. M., Benson M. D. Identification of a new hereditary amyloidosis prealbumin variant, Tyr-77, and detection of the gene by DNA analysis. J Clin Invest. 1988 Jan;81(1):189–193. doi: 10.1172/JCI113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Sletten K., Johansson B., Cornwell G. G., 3rd Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2843–2845. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]






