Abstract
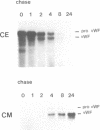
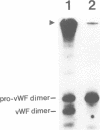
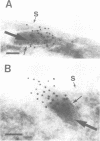
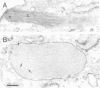
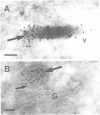
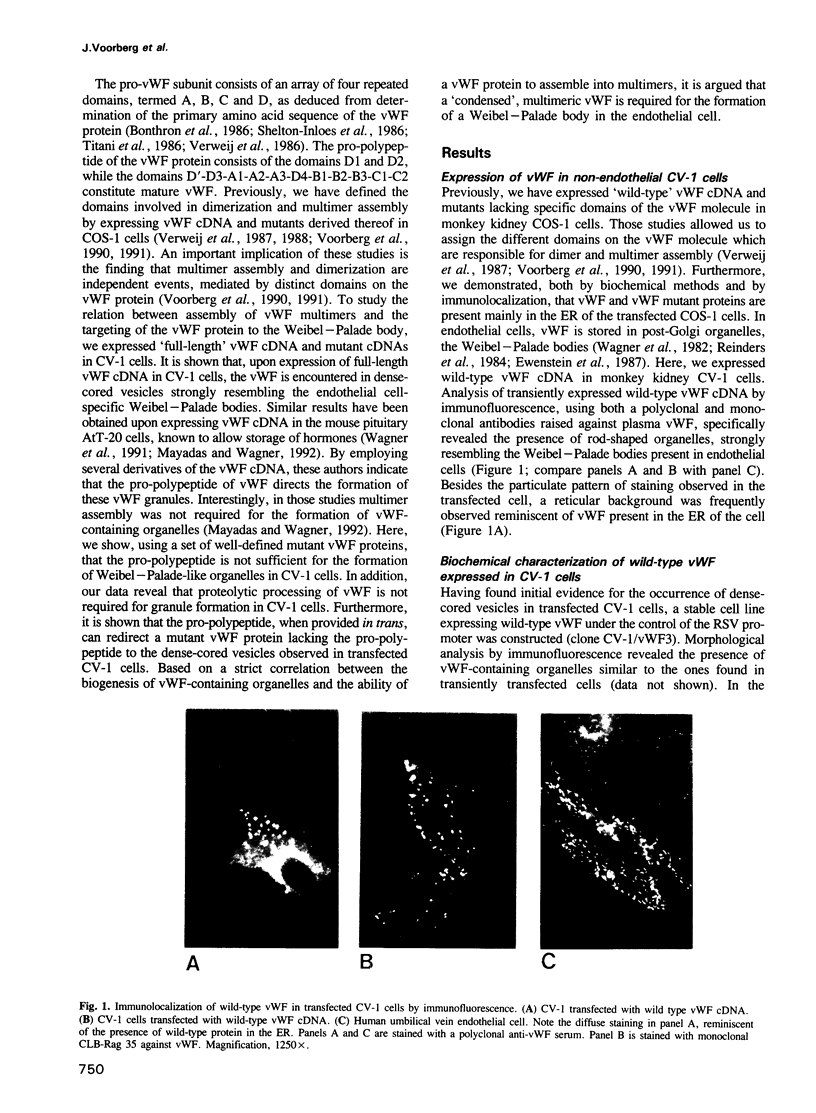
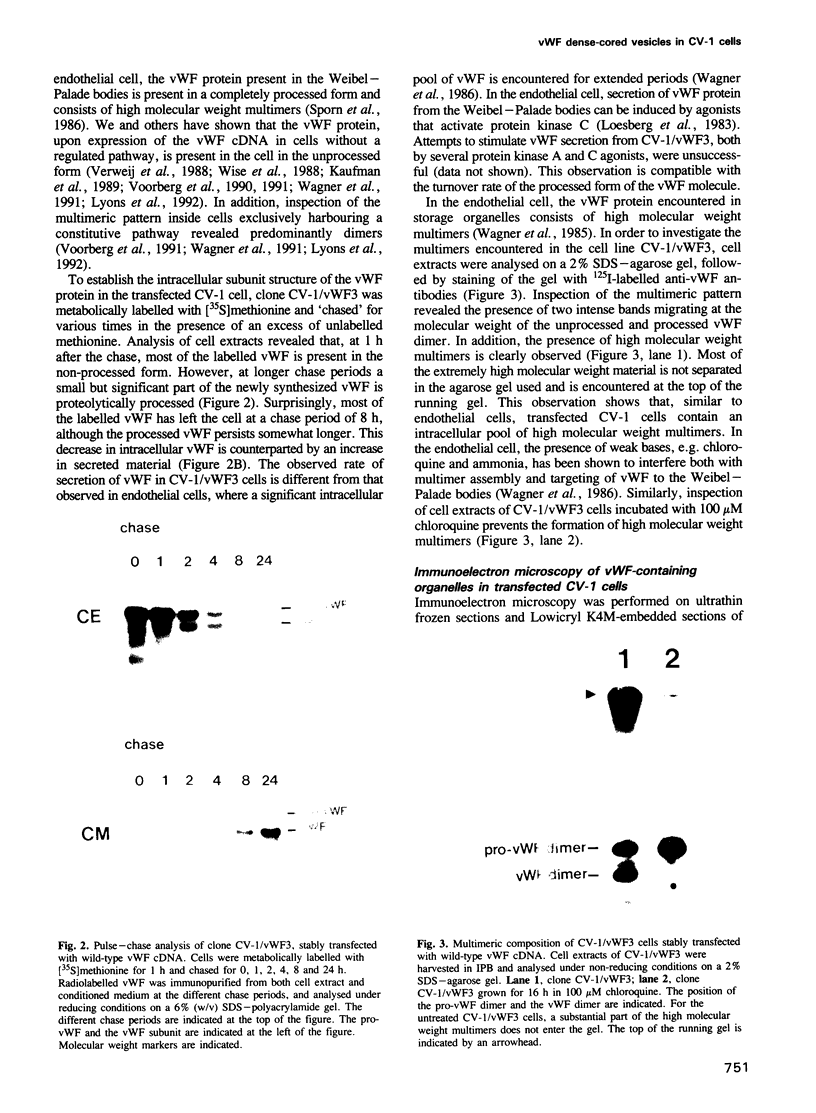
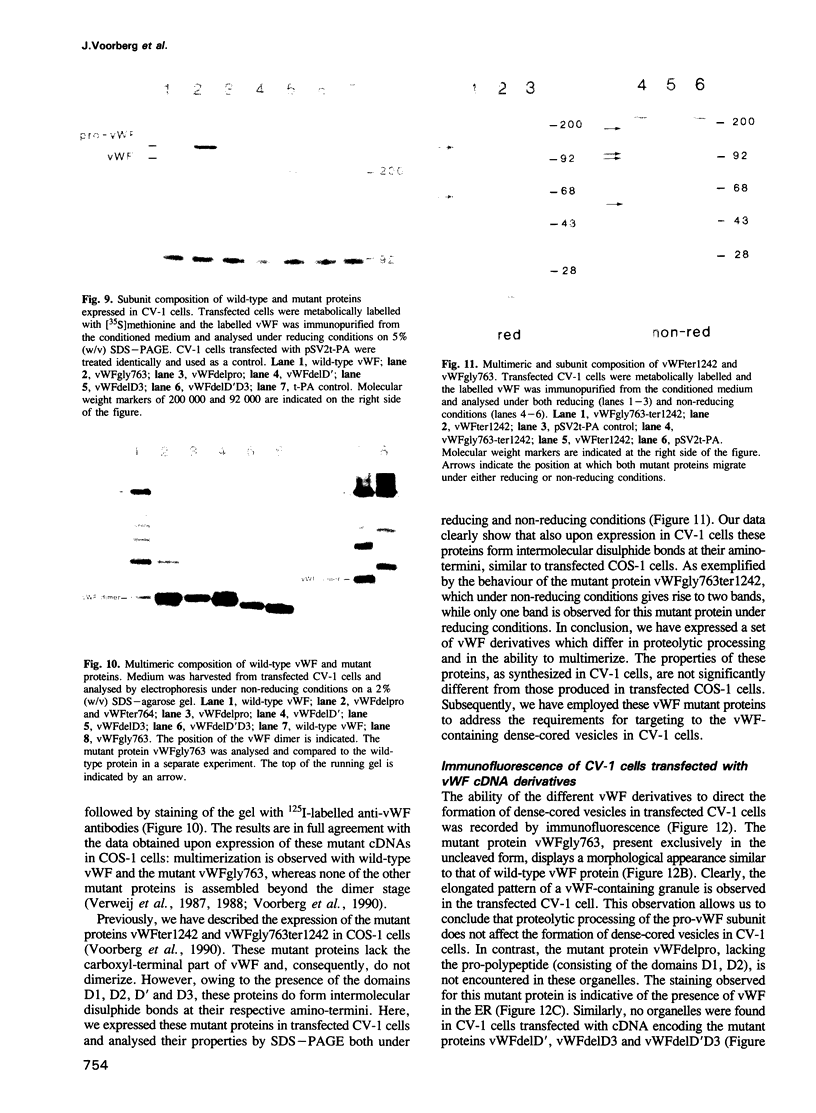
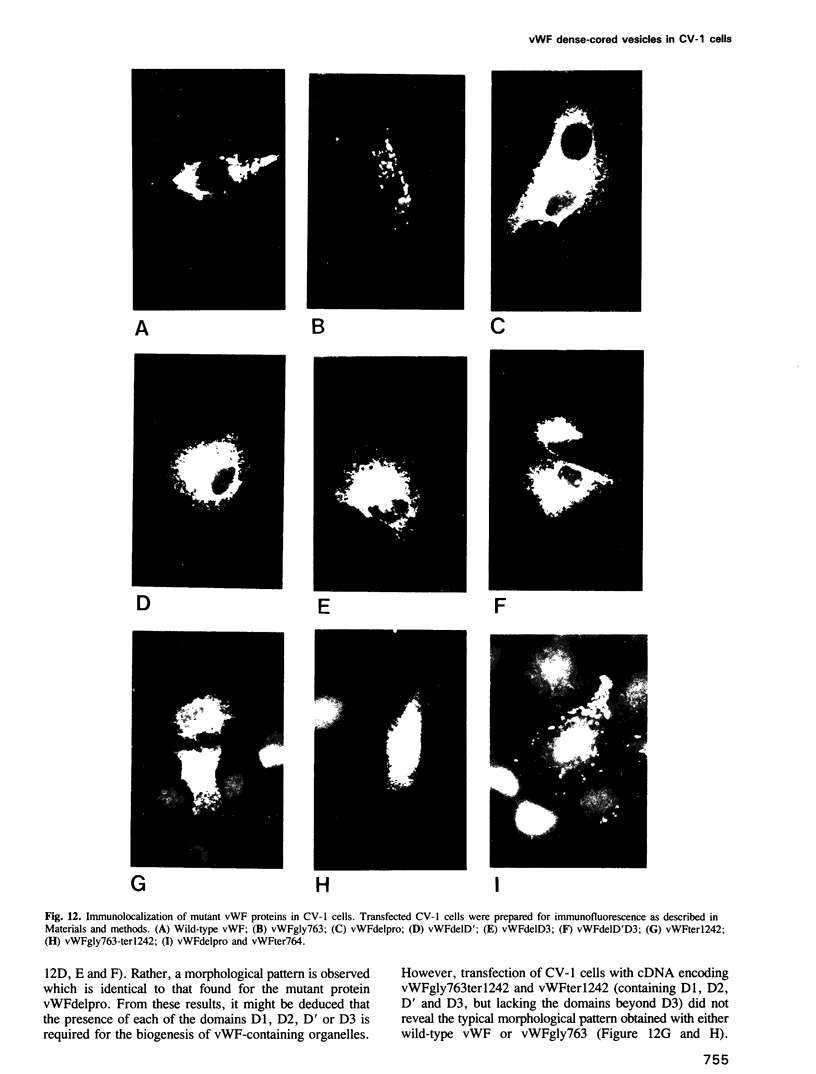
Von Willebrand factor (vWF) is a multimeric protein involved in the adhesion of platelets to an injured vessel wall. vWF is synthesized by the endothelial cell and the megakaryocyte as a precursor protein (pro-vWF) that consists of four repeated domains, denoted D1-D2-D'-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2. Previously, we have defined the domains on the pro-vWF molecule involved in dimerization as well as the domains involved in multimer assembly of vWF dimers. In the endothelial cell, part of the vWF multimers is stored in specialized organelles, the Weibel-Palade bodies. By using immunoelectron microscopy, we demonstrate that upon expression of full-length vWF cDNA, vWF-containing organelles are encountered in monkey kidney CV-1 cells that are morphologically similar to the endothelial-specific Weibel-Palade bodies. Expression in CV-1 cells of a series of vWF cDNA deletion mutants, lacking one or more domains, revealed that only those vWF mutant proteins that are able to assemble into multimers are encountered in dense-cored vesicles. Our data show that this process is independent of a particular domain on vWF and indicate that a 'condensed', multimeric vWF is required for targeting to the Weibel-Palade body.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991 Dec;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewenstein B. M., Warhol M. J., Handin R. I., Pober J. S. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987 May;104(5):1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gross D. J., Halban P. A., Kahn C. R., Weir G. C., Villa-Komaroff L. Partial diversion of a mutant proinsulin (B10 aspartic acid) from the regulated to the constitutive secretory pathway in transfected AtT-20 cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4107–4111. doi: 10.1073/pnas.86.11.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Davies M. V., Wise R. J., Israel D. I., Dorner A. J. Effect of von Willebrand factor coexpression on the synthesis and secretion of factor VIII in Chinese hamster ovary cells. Mol Cell Biol. 1989 Mar;9(3):1233–1242. doi: 10.1128/mcb.9.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loesberg C., Gonsalves M. D., Zandbergen J., Willems C., van Aken W. G., Stel H. V., Van Mourik J. A., De Groot P. G. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta. 1983 Sep 22;763(2):160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- Lyons S. E., Bruck M. E., Bowie E. J., Ginsburg D. Impaired intracellular transport produced by a subset of type IIA von Willebrand disease mutations. J Biol Chem. 1992 Mar 5;267(7):4424–4430. [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Kelly R. B. Constitutive and basal secretion from the endocrine cell line, AtT-20. J Cell Biol. 1991 Mar;112(5):843–852. doi: 10.1083/jcb.112.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. In vitro multimerization of von Willebrand factor is triggered by low pH. Importance of the propolypeptide and free sulfhydryls. J Biol Chem. 1989 Aug 15;264(23):13497–13503. [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3531–3535. doi: 10.1073/pnas.89.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiff J. M., Gil J. Secretion of Weibel-Palade bodies observed in extra-alveolar vessels of rabbit lung. J Appl Physiol Respir Environ Exerc Physiol. 1983 May;54(5):1284–1286. doi: 10.1152/jappl.1983.54.5.1284. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meulien P., Nishino M., Mazurier C., Dott K., Piétu G., Jorieux S., Pavirani A., Girma J. P., Oufkir D., Courtney M. Processing and characterization of recombinant von Willebrand factor expressed in different cell types using a vaccinia virus vector. Thromb Haemost. 1992 Jan 23;67(1):154–160. [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Perrelet A., Powell S. K., Quinn D. L., Moore H. P. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987 Dec 24;51(6):1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990 Dec;15(12):483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Quinn D., Orci L., Ravazzola M., Moore H. P. Intracellular transport and sorting of mutant human proinsulins that fail to form hexamers. J Cell Biol. 1991 Jun;113(5):987–996. doi: 10.1083/jcb.113.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J. H., de Groot P. G., Sixma J. J., van Mourik J. A. Storage and secretion of von Willebrand factor by endothelial cells. Haemostasis. 1988;18(4-6):246–261. doi: 10.1159/000215811. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986 Sep;103(3):839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A., Fuller S. D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987 Sep;105(3):1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Flatmark T., Tooze J., Huttner W. B. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991 Dec;115(6):1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Proteolytic cleavage of the precursor of von Willebrand factor is not essential for multimer formation. J Biol Chem. 1988 Jun 15;263(17):7921–7924. [PubMed] [Google Scholar]
- Voorberg J., Fontijn R., Calafat J., Janssen H., van Mourik J. A., Pannekoek H. Assembly and routing of von Willebrand factor variants: the requirements for disulfide-linked dimerization reside within the carboxy-terminal 151 amino acids. J Cell Biol. 1991 Apr;113(1):195–205. doi: 10.1083/jcb.113.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg J., Fontijn R., van Mourik J. A., Pannekoek H. Domains involved in multimer assembly of von willebrand factor (vWF): multimerization is independent of dimerization. EMBO J. 1990 Mar;9(3):797–803. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBEL E. R., PALADE G. E. NEW CYTOPLASMIC COMPONENTS IN ARTERIAL ENDOTHELIA. J Cell Biol. 1964 Oct;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Mayadas T., Marder V. J. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J Cell Biol. 1986 Apr;102(4):1320–1324. doi: 10.1083/jcb.102.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Mayadas T., Urban-Pickering M., Lewis B. H., Marder V. J. Inhibition of disulfide bonding of von Willebrand protein by monensin results in small, functionally defective multimers. J Cell Biol. 1985 Jul;101(1):112–120. doi: 10.1083/jcb.101.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Saffaripour S., Bonfanti R., Sadler J. E., Cramer E. M., Chapman B., Mayadas T. N. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991 Jan 25;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- van de Ven W. J., Voorberg J., Fontijn R., Pannekoek H., van den Ouweland A. M., van Duijnhoven H. L., Roebroek A. J., Siezen R. J. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990 Nov;14(4):265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]