Abstract
Radiation-induced bystander effects have been demonstrated in both normal and tumor cells using a variety of different radiation qualities. Literature reports are contradictory, however, on whether there is an LET dependence of the bystander effect. This study investigated the ability of DU-145 human prostate carcinoma cells irradiated with either α particles or 250 kVp X rays to cause medium-mediated bystander effects in unirradiated populations of DU-145 cells or in AG01522 human fibroblasts. The end points measured in both of the bystander cell lines were micronucleus formation, γ-H2AX focus induction, and the surviving fraction. The incidence of micronuclei increased 1.5–2.0-fold in both tumor and fibroblast bystander cells after 4 h of co-culture with DU-145 tumor cells that had been directly irradiated with either α particles or X rays. Only the AG01522 fibroblasts showed bystander effects for the γ-H2AX focus (a 1.5-fold increase) and surviving fraction (a decrease to 0.8) end points when co-cultured with X-irradiated tumor cells. Alpha-particle irradiation of DU-145 tumor cells produced no decrease in the surviving fraction and no increase in γ-H2AX focus induction in co-cultured bystander cells of either cell line. These results indicate that there are LET-dependent differences in the signal released from DU-145 human prostate carcinoma cells and that, for some end points, bystander AG01522 fibroblasts and bystander DU-145 prostate carcinoma cells respond differently to the same medium-mediated signal.
INTRODUCTION
Traditionally, it has been assumed that radiation-induced damage occurs only in cells actually traversed by the incident radiation. However, since the early 1990s there have been a number of reports describing a phenomenon known as the bystander effect in which cells not directly exposed to the radiation nevertheless show damage. Bystander effects have been demonstrated with both high- and low-LET radiation, in a variety of cell lines, and using various end points including DNA double-strand breaks, chromosome damage, mutation, genomic instability and cell survival; for recent reviews see refs. (1–5).
We have reported previously that α-particle irradiation of DU-145 human prostate carcinoma cells induced micronucleus (MN) formation in co-cultured DU-145 bystander cells (6). In that study, we also reported that MN formation in the bystander cells was observed only if the bystander DU-145 cells were present on inserts and co-cultured in the medium above the directly irradiated cells during the irradiation; no bystander effect was observed when the inserts containing the bystander cells were added 1 min after the irradiation and then co-cultured for an additional 2 h. This was interpreted as evidence for a very short apparent lifetime of the bystander signal (or some component in a signaling cascade) released by the irradiated DU-145 tumor cells (6). In the current study, we have expanded on these previous results. We now include parallel irradiations with 250 kVp X rays to provide a comparison between low-LET radiation and α particles. We have added AG01522 normal human fibroblasts as a second co-cultured bystander cell line for comparison with the bystander effects observed in the DU-145 prostate tumor cells. We have monitored three end points in each of these co-cultured bystander cell lines: MN formation, γ-H2AX focus formation and the surviving fraction. This matrix of experiments allows us to address two questions: (1) Are there LET-dependent differences in bystander effects caused by 250 kVp X rays and α particles? (2) Can irradiated DU-145 prostate carcinoma cells cause bystander effects in the AG01522 normal human fibroblasts?
MATERIALS AND METHODS
Experimental Approach
For all experiments, DU-145 prostate carcinoma cells were irradiated directly with either X rays or α particles. For each of these two radiation qualities, two co-cultured bystander cells were used (DU-145 human prostate carcinoma and AG01522 normal human fibroblasts). Sham-irradiated controls were included in all experiments. For each of the four permutations of radiation quality and bystander cell line, three experimental end points were quantified in the bystander cells (γ-H2AX focus induction, MN formation, and the surviving fraction). This yielded a matrix of 12 experiments. For each of these 12 experimental conditions, when a positive bystander effect (significantly greater than control levels) was detected, the experiments were repeated independently with a radical scavenger present. Scavenger-only controls were included in all experiments.
Cell Lines
Cells of the human diploid skin fibroblast primary cell line AG01522 were obtained from the Genetic Cell Respository at the Coriell Institute for Medical Research (Camden, NJ). AG01522 cells were grown at 37°C in a humidified atmosphere of 95% air and 5% CO2 using α-modified MEM (Sigma) supplemented with 20% fetal bovine serum (Hyclone), 100 µg/ml streptomycin and 100 U/ml penicillin. AG01522 cells were grown to confluence prior to use in experiments, and the culture was restarted from frozen stocks after 12 passages. Cells of the human prostate carcinoma (metastatic) cell line DU-145 were obtained from the American Type Culture Collection (Manassas, VA). DU-145 cells were grown at 37°C in a humidified atmosphere of 95% air and 5% CO2 using Eagle’s minimum essential medium containing Earle’s BSS (MEM/EBSS, Hyclone) supplemented with 2 mM l-glutamine, 1.0 mM sodium pyruvate, 0.1 mM non-essential amino acids, 1.5 g/liter sodium bicarbonate, and 14% fetal bovine serum (Sigma). All experiments with DU-145 cells used cultures in exponential growth. The AG01522 and DU-145 cells were harvested by trypsinization, counted and replated at the appropriate densities for each experiment, as described below.
Cell Irradiation Procedures
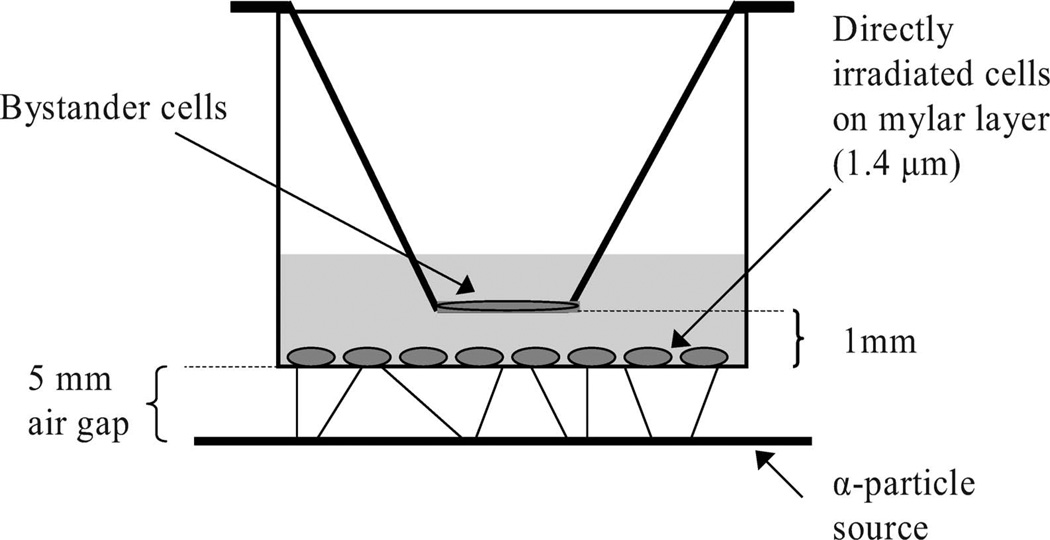
The α-particle source was described previously (6). Briefly, the source is a sealed planar, custom-manufactured 241Am foil (NRD, LLC, Grand Island, NY). The 241Am foil emits α particles at an average energy of 3.98 MeV and an average LET of 127 keV/µm. For α-particle irradiations, a custom-made cell culture dish with a replaceable mylar bottom was used. This culture dish has been described previously (6, 7). Briefly, a stainless steel cylinder was machined to allow placement of 1.4-µm-thick mylar across the bottom, creating a 3.81-cm-diameter growing surface at the bottom of the dish. The mylar was held in place by a secondary outer stainless steel cylinder fitted with a Vinton rubber o-ring between the two cylinders. The mylar dishes were sterilized in an autoclave and covered with standard 60-mm-diameter plastic petri dish covers during use. The mylar membrane was treated with FNC Coating Mix (BRFF AF-10, AthenaES, Baltimore, MD) to help cell adhesion. The cell irradiation apparatus comprises the 241Am foil, an electronic shutter, and a machined collar to position the stainless steel mylar dish above the shutter. The air gap between the 241Am foil and the mylar layer is 5 mm. DU-145 tumor cells (3–5 × 105) were plated 24 h prior to irradiation on the coated mylar membrane and were allowed to attach overnight. These served as the directly irradiated cells. The dose rate at the cell position on the mylar membrane is 1.2 Gy/min (6). At the cell position on the mylar membrane, the dose rate from the 60 keV γ rays emitted during 241Am decay was negligible (6). The doses used for all α-particle irradiation experiments were 0.1, 0.6, 1.2 and 6.0 Gy, with irradiation times of 5 s, 30 s, 1 min and 5 min, respectively. All α-particle irradiations were performed at room temperature.
X irradiations were performed using a Philips RT250 unit operating at 250 kVp and 12 mA with 0.4 mm tin plus 0.25 mm copper filtration and a focus-to-target distance of 32 cm. For the X irradiations, 1.0–1.3 × 105 DU-145 tumor cells per well were plated in six-well plates (Falcon) 24 h prior to irradiation. The X-ray dose rate in the six-well plates was 1.0 ± 0.03 Gy/min. The doses used for the X irradiations were 0.06, 0.1, 0.5, 1.0 and 2.0 Gy, with irradiation times of 3.6 s, 6 s, 30 s, 1 min and 2 min, respectively. All X irradiations were performed at room temperature.
Co-culture Procedures
Tumor cells plated on the mylar membrane or in six-well plates served as the directly irradiated cells for the α-particle and X-ray experiments, respectively. Bystander cells were present on cover slips held in inserts above the mylar membrane (see Fig. 1) or in the six-well plates. For α-particle irradiations, 3–5 × 105 DU-145 cells were plated on the coated mylar membrane 24 h prior to irradiation. Similarly, for the X irradiations, 1.0–1.3 × 105 DU-145 tumor cells were plated per well in six-well plates (Falcon) 24 h prior to irradiation. Bystander populations of DU-145 or AG01522 cells were prepared on 18-mm-diameter glass cover slips (VWR International) by plating 1 × 105 cells per cover slip in 12-well plates (Falcon) and allowing the cells to attach overnight. On the day of irradiation, the medium was changed in the mylar dish or the six-well plates as well as for the bystander cells growing on cover slips in 12-well plates. When AG01522 fibroblasts were to be co-cultured with irradiated DU-145 tumor cells, both cell lines were allowed to attach in their medium, but the medium for the tumor cells was changed to AG01522 fibroblast medium just prior to irradiation. Thus, for co-culture experiments with AG01522 fibroblasts, DU-145 cells were irradiated in AG01522 fibroblast medium. It was confirmed that the AG01522 fibroblast medium caused no changes in the rate or growth fraction of DU-145 cells. The bystander cells were then co-cultured with the directly irradiated cells for 4 h (except as noted below), removed and then processed for the specific end points, as described below.
FIG. 1.
Schematic view of the insert co-culture system for α-particle irradiations.
Radical scavenger experiments used either the nitric oxide scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, MP Biomedicals, Inc.) at a concentration of 20 µM or dimethyl sulfoxide (DMSO, Sigma) at 0.5% vol/vol. The scavengers were added 5 min prior to irradiation and were present in the medium during the entire co-culture period.
The geometry of the two irradiation approaches produced a limitation and an opportunity. For X irradiations, the co-cultured bystander cells could not be present in the medium during the irradiation (because they could not be shielded) and were always added within 5 min after the irradiation. During α-particle irradiations, the range of the α particles is so short that the bystander cells could be present in the same medium above the irradiated cells but still receive no direct dose. Alternatively, the bystander cells on the inserts could be added after the α-particle irradiations (within 5 min) to parallel the X-ray conditions. This provided the opportunity to investigate the nature of the signal released by α-particle- irradiated DU-145 cells, i.e., the lifetime of the medium-mediated signal, or the nature of the bystander cell response to the signaling pathway initiated by the α-particle irradiation, by comparing the results when the bystander cells were added just after the irradiation (6).
In all X-ray experiments, bystander cells and directly irradiated cells were combined for co-culturing ~5 min after irradiation. In α-particle experiments, the bystander cells could be added to the medium above the irradiated cells either before or after irradiation. The experimental conditions used are specified in the results sections below.
γ-H2AX Assay
Immunofluorescence staining of γ-H2AX foci was carried out as described previously by Yang et al. (8). At the end of the co-culture period, the bystander cells were rinsed with PBS, then fixed in 3% (v/v) paraformaldehyde in PBS for 30 min at 4°C. After a 5-min rinse with 50 mM NH4Cl and two rinses with PBS, the cells were then permeabilized for 15 min in ice-cold Triton X-100 buffer (50 mM NaCl, 3 mM MgCl2, 200 mM sucrose, 10 mM Hepes, pH 7.4, and 0.5% Triton-100). Cells were incubated with 10% goat serum for 1 h at 37°C, then incubated for 1 h at room temperature with antibodies against phosphorylated histone γ-H2AX (Trevigen) using a 1:100 dilution of the antibody in PBS supplemented with 3% goat serum and 0.1% Triton X-100. After three 10-min washes with PBS, cells were incubated in 1% BSA for 1 h at 37°C. After blocking, the cells were stained with Alexa Fluor 488 goat antirabbit IgG secondary antibody (Molecular Probes) for 45 min at room temperature. Cells were washed twice with PBS, stained with the nuclear stain 4,6-diamidimo-2-phenylindole (DAPI, Sigma) at a concentration of 10 µg/ml in water for 2 min, followed by two more washes with PBS. The cells were treated with FluoroGuard Antifade reagent (Bio-Rad) to preserve the fluorescence. A cell was counted positive for γ-H2AX focus induction if it exhibited more than five foci in the nucleus. At least 500 cells were scored from at least 10 fields of view per sample.
Micronucleus Assay
Micronucleus formation in the bystander cells was measured using the cytokinesis-block technique (9). After the directly irradiated cells and the bystander cells had been co-cultured for 4 h, the cover slips with the bystander cells were removed from the mylar dish or the six-well plates and placed in individual wells of a 12-well plate (Falcon). The bystander cells received 2 ml of fresh medium (specific to each cell line) containing cytochalasin B (Sigma) at a final concentration of 1.5 µg/ml for AG01522 cells and 3.0 µg/ml for DU-145 cells. After 48 h for the DU-145 cells (6) and 72 h for AG01522 fibroblasts (8), the cells were fixed in methanol: acetic acid (3:1 v/v). After the samples were allowed to dry, the cells were stained with DAPI. The stained bystander cells, still attached to the cover slips, were treated with FluoroGuard Antifade reagent; the cover slips were then inverted and applied to microscope slides. Micronuclei present in binucleated cells were scored using a fluorescence microscope. At least 500 binucleated cells were scored, using at least 10 fields of view, from each cover slip.
Colony Formation Assay
A colony formation assay was used to quantify the surviving fraction of bystander cells after co-culture with directly irradiated DU-145 tumor cells. For α-particle experiments, the bystander cells (either AG01522 or DU-145; 1 × 105 cells per cover slip) were added to the medium above the irradiated cells either just prior to irradiation or shortly after irradiation depending on the experiment. For X-ray experiments, the bystander cells were added within 5 min after the irradiation. The bystander cells were co-cultured for 4 h, removed, trypsinized and plated in 100-mm petri dishes at a density of 300 cells per dish. The cells were then incubated for 9 additional days to allow colony formation.
To determine the effect of added scavengers on the X-ray-induced bystander effect, 1 × 105 DU-145 cells were plated in 1.0-µm porous membrane inserts (Falcon) to allow passage of small molecules. Bystander fibroblast cells were plated in the six-well companion plates (Falcon) at a density of 100 cells/well. After the cells were allowed to attach for 24 h, the medium was replaced in both the wells and the companion inserts. For experiments with scavengers, either of the two radical scavengers was added to both the inserts and the six-well plates prior to irradiation and the inserts holding the DU-145 cells were then irradiated with X rays. Within 5 min after irradiation, the inserts were placed into the companion wells holding the bystander cells and the cells were co-cultured for 24 h. After 24 h, the inserts were removed and discarded, and the bystander cells in the wells were incubated for 8 additional days.
For all survival experiments, colonies were fixed with methanol and stained with methylene blue. Colonies, defined as containing greater than 50 cells, were scored per well or per dish, and the surviving fraction was calculated.
Statistical Analysis
All data are from at least three separate experiments. The results of the MN and γ-H2AX experiments are expressed as the means of all replicate measurements and are plotted as means ± 1 SD. The results of the colony formation surviving fraction experiments are the means of three to five replicate dishes or wells per experiment; results are expressed as the means ± 1 SD of all replicate dishes or wells for all experiments. Student’s t test was used to evaluate differences in the effects measured in the bystander cells from the nonirradiated control conditions and the bystander cells co-cultured with directly irradiated cells. In scavenger experiments, statistical significance was judged relative to the same absorbed dose delivered to the directly targeted cells but without the scavenger present.
RESULTS
γ-H2AX Focus Induction in Bystander AG01522 Fibroblasts
Preliminary experiments were carried out with bystander AG01522 and DU-145 cells to determine the optimum coculture time for measurement of γ-H2AX focus induction. The times evaluated ranged from 30 min to 8 h. The X-ray dose was 2.0 Gy and the α-particle dose was 1.2 Gy for these time course studies.
X rays
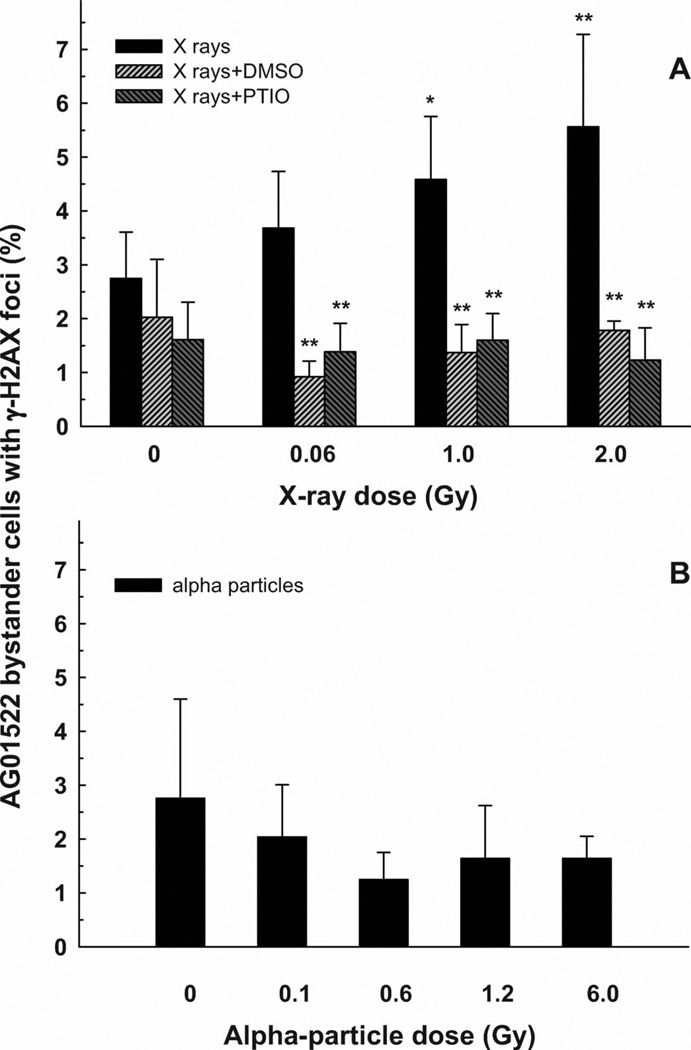
Bystander AG01522 cells were co-cultured with X irradiated (2.0 Gy) DU-145 cells for 0.5–4 h, after which the γ-H2AX foci were scored. The control population showed 4.5% of cells with greater than five foci per nucleus, whereas the cells co-cultured with irradiated DU-145 cells showed relative increases of 1.2, 1.4, 1.5 and 2.0 at co-culture times of 0.5, 1, 2 and 4 h, respectively (average of two experiments). Four hours was chosen as the coculture time for the subsequent dose–response study. The percentage of bystander AG01522 fibroblasts showing an increase in the incidence of γ-H2AX foci as a function of the direct X-ray dose delivered to the DU-145 cells is shown in Fig. 2A. The magnitude of the incidence of γ-H2AX foci in the bystander cells in the medium above X-irradiated DU-145 cells increased linearly up to a level that was twofold greater than the control. When either of the free radical scavengers DMSO or PTIO was present in the medium during the irradiation of the DU-145 cells with X rays and during the 4-h postirradiation co-culture period with the bystander AG01522 cells, the bystander effect was completely blocked at all X-ray doses (Fig. 2A).
FIG. 2.
Percentage of the bystander AG01522 fibroblast cells showing induction of γ-H2AX foci after 4 h co-culture with DU-145 tumor cells irradiated with X rays (panel A) or α particles (panel B). X-ray experiments were carried out with and without the addition of DMSO or PTIO scavengers. Results are the average of at least four independent experiments. Error bars represent ±1 SD. **P < 0.01, *P < 0.05.
Alpha particles
Bystander AG01522 cells were co-cultured with α-particle-irradiated (1.2 Gy) DU-145 cells for 1–8 h, after which the γ-H2AX foci were scored. There was no significant increase in the percentage of bystander cells with greater than five foci per nucleus at any co-culture time. The control group showed 1.4%; the values for co-culture times of 1, 2, 4, 6 and 8 h were 1.1, 0.9, 1.0, 1.6 and 0.9%, respectively. A co-culture time of 8 h was used for the subsequent dose–response study. Figure 2B shows that there was no increase in γ-H2AX focus formation in bystander AG01522 fibroblasts as the α-particle dose delivered to the directly irradiated DU-145 cells was increased from 0.1 to 6.0 Gy. For all of the α -particle irradiations, the bystander AG01522 cells were added within 5 min after the direct irradiation of the DU-145 cells.
The data presented in Fig. 2A and B show an example of an LET-dependent bystander effect. X irradiation of DU-145 tumor cells produced a significant γ-H2AX bystander effect in AG01522 fibroblasts, but α-particle irradiation did not. In both cases, co-culture of the bystander AG01522 cells with the directly irradiated DU-145 cells began within 5 min after irradiation.
γ-H2AX Focus Induction in Bystander DU-145 Prostate Carcinoma Cells
Bystander DU-145 tumor cells did not show increased γ-H2AX focus formation under any conditions. In the preliminary time course studies, the baseline level of cells with more than five γ-H2AX foci per nucleus was 1.4% (sham irradiation, 2 h co-culture). Bystander DU-145 cells co-cultured with X irradiated (2.0 Gy) DU-145 cells for 5 min, 30 min, 1 h, 2 h and 4 h showed levels of 0.6, 1.3, 1.4, 1.8 and 0.6%, respectively. Similarly, no significant increase in γ-H2AX focus formation was seen in bystander DU-145 tumor cells co-cultured for up to 8 h with α-particle-irradiated (1.2 Gy) DU-145 tumor cells. For the α-particle irradiations, the DU-145 bystander cells were present during the irradiation; i.e., the co-culture began just prior to the irradiation.
The fact that the bystander DU-145 cells showed no increase in γ-H2AX foci under any conditions led us to examine whether direct irradiation of these tumor cells resulted in a significant increase in γ-H2AX focus induction. DU-145 tumor cells directly irradiated with 2 or 10 Gy of X rays or 6 Gy of α particles showed increases in γ-H2AX foci in 2.3, 10.8 and 17.3% of the cells, respectively. The baseline level of cells with greater than five γ-H2AX foci per nucleus was 0.8% in this experiment. In comparison, AG01522 fibroblasts directly irradiated with 2 Gy of X rays have been reported to show 70% of the population with increased γ-H2AX focus induction (8). Together, these data suggest that there is a difference in the doses (level of damage) required to initiate γ-H2AX focus formation between AG01522 fibroblasts and DU-145 tumor cells.
Micronucleus Formation in Bystander AG01522 Fibroblasts
X rays
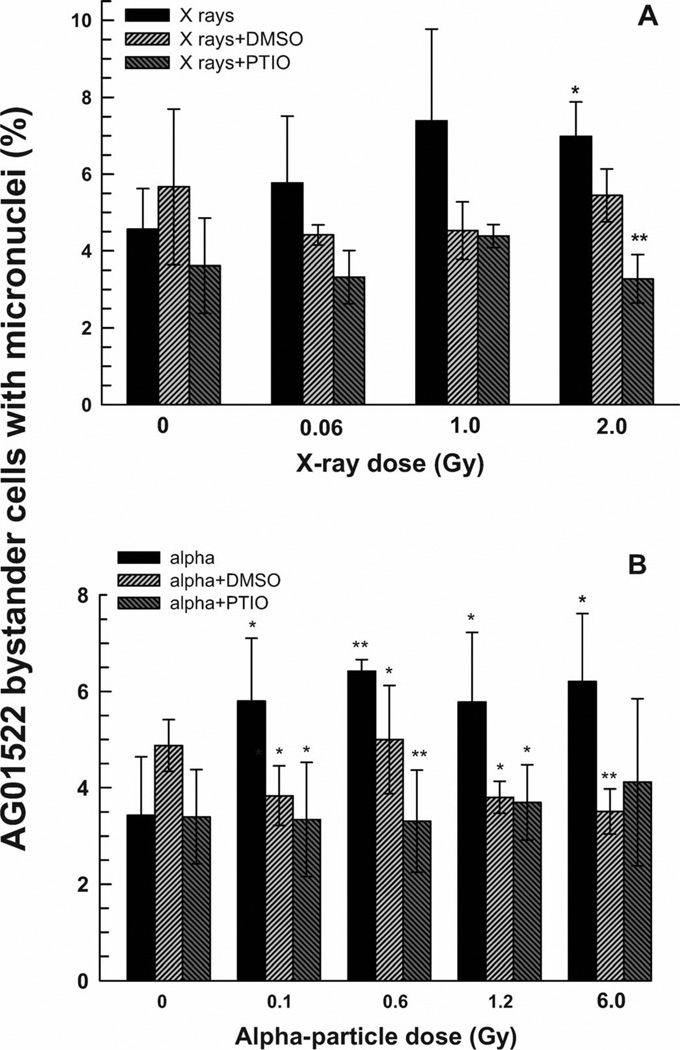
An increased incidence of micronuclei formation was observed in bystander AG01522 cells co-cultured for 4 h with X-irradiated DU-145 cells that showed saturation above 1 Gy (Fig. 3A). A 1.5-fold bystander response (P < 0.05) was measured when an X-ray absorbed dose of 2 Gy was delivered to the directly irradiated DU-145 cells (Fig. 3A). DMSO appeared to reduce this bystander effect, but the differences from the corresponding data points for cells with no scavenger added did not reach statistical significance (P > 0.05). PTIO blocked the MN bystander effect, but only at the 2-Gy dose was the effect statistically significant (P < 0.01).
FIG. 3.
Percentage of binucleated bystander AG01522 cells containing micronuclei after 4 h co-culture with DU-145 cells that had been irradiated with X rays (panel A) or α particles (panel B) in the presence or absence of DMSO and PTIO scavengers. Results are the average of at least three independent experiments. Error bars represent ±1 SD. **P < 0.01, *P < 0.05, relative to X rays only (panel A) or α particles only (panel B).
Alpha particles
A twofold increase in the fraction of binucleated cells with micronuclei was seen in bystander AG01522 fibroblasts that were co-cultured for 4 h with α-particle-irradiated DU-145 tumor cells (Fig. 3B). This bystander effect showed saturation at doses of 0.6 Gy and above delivered to the directly irradiated DU-145 cells. Both PTIO and DMSO reduced the bystander response to control levels. The MN bystander effect in the AG01522 fibroblasts was observed regardless of whether the bystander cells on the cover slip were added prior to or after the α-particle irradiation of the tumor cells, indicating a long apparent lifetime of the signal. The data shown in Fig. 3B are for addition of the inserts containing the bystander cells within 5 min after the irradiations.
Micronucleus Formation in Bystander DU-145 Prostate Carcinoma Cells
X rays
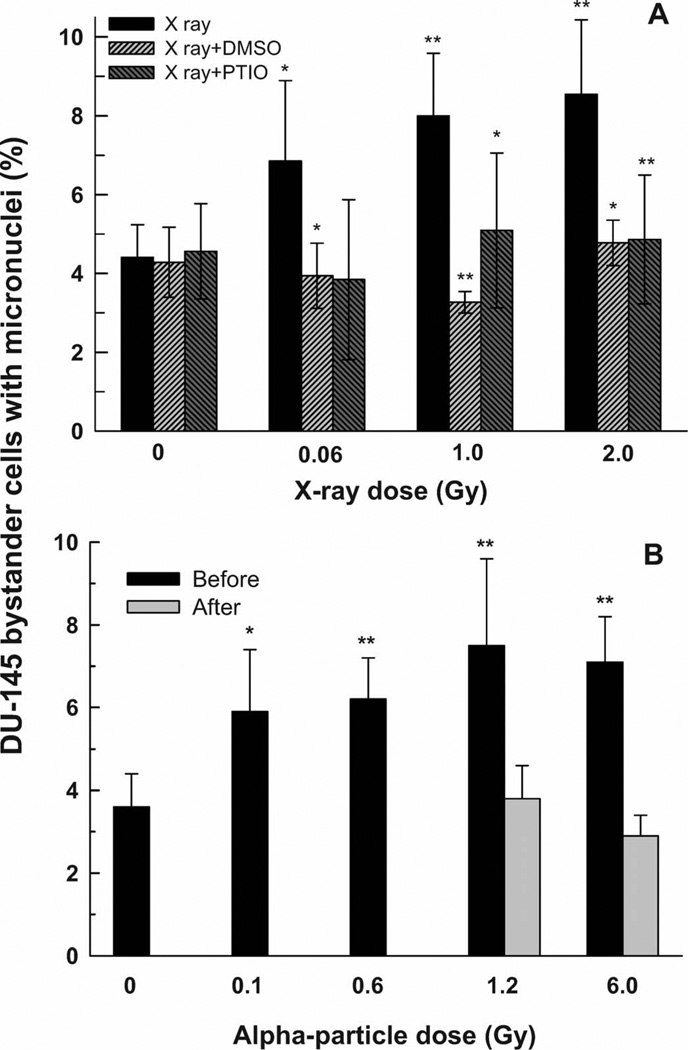
The incidence of micronuclei in bystander DU-145 tumor cells that were co-cultured for 4 h with X-irradiated DU-145 tumor cells is shown in Fig. 4A. The percentage of binucleated cells containing micronuclei increased as a function of increasing X-ray dose to the directly irradiated DU-145 cells, reaching a plateau of about twofold greater than the control level at ~1–2 Gy. The presence of either DMSO or PTIO during the irradiation and the co-culture period reduced the bystander effect to control levels (Fig. 4A).
FIG. 4.
Percentage of binucleated bystander DU-145 cells containing micronuclei after 4 h co-culture with DU-145 cells that had been irradiated with X rays (panel A) or α particles (panel B). The X-ray experiments were carried out in the presence or absence of DMSO and PTIO scavengers. The α-particle experiments were carried out with the bystander DU-145 cells present during the irradiation (Before) or added within 5 min after the irradiation (After). Results are the average of at least three independent experiments. Error bars represent ±1 SD. **P < 0.01, *P < 0.05 relative to X rays only (panel A) or α particles only (panel B).
Alpha particles
The increased incidence of micronuclei in bystander DU-145 cells that were co-cultured for 4 h with α-particle-irradiated DU-145 cells (Fig. 4B) reached a plateau at a level about twofold greater than the controls (P < 0.01). The bystander effect was observed only when the insert was added just before the irradiation (i.e., the bystander cells were present during the irradiation) and not when the insert was added within 5 min after the irradiation, indicating a very short apparent lifetime of the bystander signal.
The data shown in Fig. 4A and B suggest that there is an LET-dependent difference in the signal released from (or signaling pathway initiated by) X- and α-particle-irradiated DU-145 prostate carcinoma cells. In the X-ray experiment (Fig. 4A), the bystander DU-145 cells were added within 5 min after the irradiation; a significant MN formation bystander effect was observed. However, when α particles were used (Fig. 4B), the MN formation bystander effect was observed only when the bystander DU-145 cells were added before the irradiation. No bystander effect was seen when the bystander DU-145 cells are added within 5 min after the α-particle irradiation.
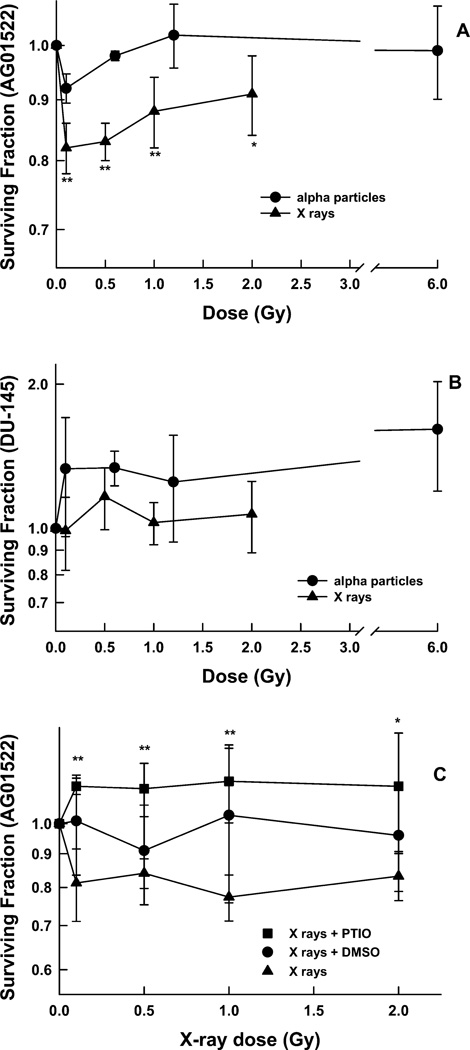
Surviving Fraction in Bystander AG01522 Fibroblasts
X rays
A reduction to a surviving fraction of ~0.8 was seen in bystander AG01522 fibroblasts that had been co-cultured for 4 h with X-irradiated DU-145 cells (Fig. 5A). The ability of scavengers to block this bystander effect is shown in Fig. 5C. When PTIO was present in the medium during the irradiation and the co-culture period, there was no significant reduction in the surviving fraction; i.e., the nitric oxide scavenger PTIO was able to block this bystander effect.
FIG. 5.
Surviving fraction of bystander AG01522 cells (panel A) and DU-145 cells (panel B) after 4 h co-culture with DU-145 cells that had been irradiated with either X rays or α particles. Points are the means ± 1 SD from at least three independent experiments. **P < 0.01, *P < 0.05 relative to the nonirradiated controls. Panel C: Surviving fraction of bystander AG01522 cells after 24 h co-culture with DU-145 cells that had been irradiated with X rays in the presence or absence of DMSO and PTIO scavengers. Points are the means ± 1 SD from four independent experiments with X rays alone and five experiments with scavengers. **P < 0.01, *P < 0.05 relative to the same absorbed dose delivered to the directly targeted cells in the absence of scavenger.
Alpha particles
No decrease in the surviving fraction was seen in bystander AG01522 fibroblasts that were co-cultured for 4 h with α-particle-irradiated DU-145 cells (Fig. 5A). In these survival experiments, the inserts containing the AG01522 cells were added within 5 min after the α-particle irradiation.
This is another example of an LET-dependent bystander effect. When X rays were used to irradiate the DU-145 cells, the bystander AG01522 fibroblasts showed a decrease in the surviving fraction. When α particles were used, however, there was no decrease in the surviving fraction of the bystander AG01522 fibroblasts.
Surviving Fraction in Bystander DU-145 Prostate Carcinoma Cells
No decrease in the surviving fraction was seen in bystander DU-145 cells after 4 h co-culture with DU-145 cells that had been directly irradiated with increasing doses of either X rays or α particles (Fig. 5B). Because of our previous experience with a very short-lived signal in bystander effects with DU-145 cells (6), the inserts containing the DU-145 cells were added before the α-particle irradiation.
Summary of LET-Dependent Bystander Effects
The matrix of experiments described in this report has allowed us to detect three examples of LET-dependent differences in the bystander effect. In all cases, all experimental variables were constant; only the radiation quality used to irradiate the DU-145 cells was changed. Together, these data suggest that a different signal is released depending on whether the DU-145 cells were irradiated with X rays or with α particles.
Bystander AG01522 fibroblasts showed a significant increase in the fraction of cells with greater than five γ-H2AX foci per nucleus when the target DU-145 cells were irradiated with X rays (Fig. 2A), but no increase in γ -H2AX focus induction was observed with α particles (Fig. 2B).
Bystander DU-145 cells showed a significant increase in MN formation after 4 h co-culture with X-irradiated DU-145 cells. The co-culture period started within 5 min after the irradiation (Fig. 4A). Bystander DU-145 cells showed a significant increase in MN formation when co-cultured with α-particle-irradiated DU-145 cells. This bystander effect was present when the co-culture period began just before the irradiation but was absent when the co-culture period began within 5 min after the irradiation (Fig. 4B).
Bystander AG01522 fibroblasts showed a significant decrease in the surviving fraction after 4 h co-culture with X-irradiated DU-145 cells but no decrease when α particles were used (Fig. 5A).
Summary of Instances where AG01522 Cells and DU-145 Cells Respond Differently to the Same Signal
Our data indicate three experiments in which the two different bystander cell lines have responded differently to the same signal. In these cases, all variables are constant; only the cell line on the co-cultured inserts was changed.
Bystander AG01522 fibroblasts showed a significant increase in the fraction of cells with greater than five γ-H2AX foci per nucleus when the target DU-145 cells were irradiated with X rays (Fig. 2A), but bystander DU-145 cells showed no increase (data not shown).
Bystander AG01522 fibroblasts showed a significant increase in MN formation after 4 h co-culture with α-particle- irradiated DU-145 cells. The same effect was observed regardless of whether the co-culture period started before or within 5 min after the irradiation (Fig. 3B). When bystander DU-145 cells were exposed to the same signal (originating from α particle-irradiated DU-145 cells), no effect was seen when the co-culture period began within 5 min after the irradiation (Fig. 4B).
Bystander AG01522 fibroblasts showed a significant decrease in the surviving fraction after co-culture with X-irradiated DU-145 cells (Fig. 5A). Bystander DU-145 cells exposed to the same signal showed no decrease in the surviving fraction (Fig. 5B).
DISCUSSION
We have shown that irradiated DU-145 prostate carcinoma cells can produce medium-mediated bystander effects in nonirradiated, co-cultured populations of either DU-145 tumor cells or AG01522 normal human fibroblasts. Further, we present evidence that the signal released from irradiated DU-145 tumor cells was different depending on whether the tumor cells were exposed to X rays or to α particles. The data also indicate that, in some situations, the bystander tumor cells and the bystander fibroblasts responded differently to the same signal.
Bystander effects have been well documented with γ rays, α particles and heavier ions, but studies on the LET dependence of bystander effects carried out under conditions where all parameters remain the same and only the LET of the radiation incident on the targeted cells is varied are limited. Shao et al. used a broad beam of high-LET carbon ions to irradiate human salivary gland tumor cells and then co-cultured the irradiated cells with a bystander population of the same type of cells for 24 h (10). They reported an LET-dependent increase in micronuclei in the bystander cells, with a greater effect produced by 100 keV/µm carbon ions than 13 keV/µm carbon ions. There was no low-LET photon control in those experiments, however, to provide a true low-LET comparison. The same group used a microbeam to individually target a subset of confluent AG01522 human fibroblast cells with either 40Ar (1260 keV/µm) or 20Ne (380 keV/µm) ions (11). The increase in micronuclei observed in the total cell population was independent of the LET (particle) used or the number of particles delivered to the targeted cells (one to four particles per cell). This was reported as no LET dependence of the bystander effect, but both of these particles have very high-LET properties, and the ability of the cells to produce a bystander effect signal may have been saturated. Lyng et al. found no LET-dependent differences in bystander effects in a study intended to directly compare medium transfer experiments to a microbeam experiment (12). This group had previously measured mitochondrial membrane potential, expression of BCL2, cytochrome c release, induction of ROS, and apoptosis in bystander cells (HPV-G human keratinocytes) exposed to medium harvested from HPV-G cells irradiated with 60Co γ rays (0.2 keV/um) (13–15). When a proton microbeam (3.2 MeV protons; ~11 keV/um) was used to target a population of these human keratinocytes growing on a mylar membrane in the same medium as a separate unirradiated population, the magnitude of the response in the co-cultured bystander cells was similar to that observed in the previous γ-irradiated medium transfer experiments. The authors concluded that there was no LET dependence of the bystander effect and that the mechanisms involved were common across different radiation qualities; however, the range of radiation quality tested was limited (0.2 and 11 keV/µm) and much lower than typical α-particle LET values (~100–150 keV/µm), where the majority of the bystander effect literature has been generated. Boyd et al. reported LET-dependent differences in the survival of bystander cells exposed to medium from tumor cells that had been irradiated with either externalbeam low-LET 60Co γ rays or with one of several different intracellularly incorporated radionuclides being evaluated for targeted therapy (16). Medium from human glioma cells or human bladder carcinoma cells exposed to increasing activity concentrations of the incorporated radionuclides (the high-LET α-particle emitter 211At, the low-LET α-particle emitter 131I, or the high-LET-like Auger-electron emitter 123I) produced decreases in the surviving fraction of nonirradiated tumor cells that were distinctly different from the effect observed with medium from cells irradiated with increasing doses of 60Co γ rays. The authors suggested that exposure of the tumor cells to high activity concentrations of the high-LET radionuclides (123I, 211At) could inhibit the ability of the cells to generate bystander signals. No LET-dependent differences were reported by Baskar et al. (17) between 137Cs γ rays and α particles in a medium transfer experiment. In this report, conditioned medium from either irradiated normal human fetal lung fibroblasts or irradiated ataxia telangiectasia mutated fibroblasts was transferred to unirradiated normal fibroblast cells. An enhancement of 10–30% in colony formation efficiency was observed when the recipient cells were treated with the conditioned medium from either irradiated cell line and with either α particles or γ rays. These results were similar to the increased proliferation in bystander cells reported by others for γ rays (18) and for α particles (19). No LET dependence of the bystander effect was reported by Yang et al. (20, 21) in studies that used the same bystander cells and end points as we used here, but they used a different high-LET radiation and different directly irradiated cells. Absorbed doses of 0.5 and 2.0 Gy from 250 kVp X rays (2 keV/µm) or 1 GeV/nucleon iron ions (151 keV/µm) were used to directly irradiate AG01522 human fibroblasts. Bystander effects were then monitored in a separate population of AG01522 fibroblasts co-cultured on an insert in the same medium for various times ranging from 1–24 h (20, 21). These authors reported no differences in γ-H2AX formation, MN induction or the surviving fraction of the bystander cells between the 1 GeV/nucleon iron ions and the 250 kVp X rays and concluded that the bystander effect was independent of LET. In the current study, we detected significant LET-dependent differences in the responses of the bystander AG01522 cells to the signals coming from DU-145 cells irradiated with 250 kVp X rays or α particles (127 keV/µm) for both γ-H2AX foci and the surviving fraction. One possible explanation for the different results seen in the current study and those reported by Yang et al. is that there are differences in the signals released into the medium by irradiated AG01522 fibroblasts and by irradiated DU-145 prostate tumor cells.
Bystander effects involve two discrete steps: the production of a signal by (or the initiation of a signaling pathway in) the directly irradiated cells, and the response of the bystander cells to that signal. The nature of the signal released from the irradiated cells remains unclear due at least in part to the fact that the signal may differ with the different experimental conditions, cell lines and end points that have been studied (22). The mechanism(s) by which radiations of different LET cause the DU-145 cells to release different signals remains unknown. The deposition of energy by high-LET radiation is highly localized. The type of signal released (or the type of signaling pathway initiated) may depend on the severity of damage in the directly irradiated cells. Some of the responses we observe (i.e., the two bystander cell lines responding differently to the same signal) could be due to the two bystander cell lines responding differently to various components of one signaling pathway. For medium-mediated bystander effects, a number of reports have shown that reactive oxygen species (ROS) play a role in the bystander effect either by direct detection of cells undergoing oxidative stress using colorimetric dyes (8) or by introduction of scavengers and monitoring the decrease in the observed bystander effect (23–26). We have used both the non-specific radical scavenger DMSO as well as the nitric oxide scavenger PTIO in these studies. For both γ-H2AX foci and MN formation, DMSO blocked all bystander effects; however, DMSO did not block the decrease in the surviving fraction in the bystander fibroblasts co-cultured with X-irradiated DU-145 cells. This suggests that, whereas ROS are critically involved in the pathways leading to MN formation and γ-H2AX focus formation in bystander cells, an additional signal is involved in the pathway leading to cell death. Similar results have been reported by Yang et al. on the involvement of ROS in bystander effects in AG01522 cells. They showed that addition of superoxide dismutase and catalase reduced the incidence of micronucleus formation, reduced the induction of p21Waf1, and reduced the number of γ-H2AX foci in bystander AG01522 cells co-cultured with X-irradiated AG01522 cells, but that SOD and catalase did not have any effect on the decrease in the surviving fraction of the co-cultured cells (8). In our study, the nitric oxide scavenger PTIO was effective in blocking all bystander effects in the AG01522 fibroblasts including the decrease in the surviving fraction. Nitric oxide serves as a signaling agent in a wide variety of pathways, and the induction of inducible nitric oxide synthase has been shown to be an early signaling event in response to ionizing radiation (27). Nitric oxide has been identified as a component of the signaling pathway leading to bystander effects in a number of reports, in particular, the bystander effects reported after irradiation of tumor cells (10, 28–30).
In summary, we have shown that there were LET-dependent differences in the signal released from directly irradiated DU-145 prostate carcinoma cells that caused medium- mediated bystander effects in both AG01522 normal human fibroblasts and in DU-145 human prostate carcinoma cells. In some situations, the DU-145 tumor cells and the AG01522 fibroblasts, exhibited different bystander effect responses to the same initial signal. Clinically, the vast majority of tumors are treated with low-LET radiation; however, given the increasing applications of high-LET particle beams in radiation therapy (31) and the continuing development of targeted therapy using high-LET radionuclides (16, 32, 33), investigation of the LET dependence of bystander effects after tumor irradiation takes on increased relevance. The results presented here from a single tumor cell line and a single fibroblast cell line should not be considered predictive of tumor therapy in general. Understanding the mechanisms of bystander signal production by irradiated tumor cells and the responses of bystander cells to those signals may enable manipulation of these effects during tumor therapy.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Hongying Yang, Dr. Kathryn D. Held and Dr. Jacquelyn Yanch for their helpful guidance and discussions. VA was supported in part by the U.S. Department of Energy Nuclear Engineering and Health Physics Fellowship. This work was supported in part by NIH Grant CA103146, and the MIT Center for Environmental Health Sciences NIEHS P30 ES002109.
REFERENCES
- 1.Hall EJ. The bystander effect. Health Phys. 2003;85:31–35. doi: 10.1097/00004032-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528. doi: 10.1016/S1470-2045(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 3.Little JB. Cellular radiation effects and the bystander response. Mutat. Res. 2006;597:113–118. doi: 10.1016/j.mrfmmm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Morgan WF, Sowa MB. Non-targeted bystander effects induced by ionizing radiation. Mutat. Res. 2007;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: an “out of field” perspective. Mutat. Res. 2006;597:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Coderre JA. A bystander effect in alpha-particle irradiations of human prostate tumor cells. Radiat. Res. 2005;164:711–722. doi: 10.1667/3475.1. [DOI] [PubMed] [Google Scholar]
- 7.Metting NF, Koehler AM, Nagasawa H, Nelson JM, Little JB. Design of a benchtop alpha particle irradiator. Health Phys. 1995;68:710–715. doi: 10.1097/00004032-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–2103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 9.Fenech M, Morley AA. Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutat. Res. 1986;161:193–198. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 10.Shao C, Furusawa Y, Aoki M, Matsumoto H, Ando K. Nitric oxide-mediated bystander effect induced by heavy-ions in human salivary gland tumour cells. Int. J. Radiat. Biol. 2002;78:837–844. doi: 10.1080/09553000210149786. [DOI] [PubMed] [Google Scholar]
- 11.Shao C, Furusawa Y, Kobayashi Y, Funayama T, Wada S. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: a mechanistic study. FASEB J. 2003;17:1422–1427. doi: 10.1096/fj.02-1115com. [DOI] [PubMed] [Google Scholar]
- 12.Lyng FM, Maguire P, Kilmurray N, Mothersill C, Shao C, Folkard M, Prise KM. Apoptosis is initiated in human keratinocytes exposed to signalling factors from microbeam irradiated cells. Int. J. Radiat. Biol. 2006;82:393–399. doi: 10.1080/09553000600803904. [DOI] [PubMed] [Google Scholar]
- 13.Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyng FM, Seymour CB, Mothersill C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: a possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002;157:365–370. doi: 10.1667/0033-7587(2002)157[0365:ioaice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Maguire P, Mothersill C, Seymour C, Lyng FM. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat. Res. 2005;163:384–390. doi: 10.1667/rr3325. [DOI] [PubMed] [Google Scholar]
- 16.Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, Mairs RJ. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and Auger electron-emitting radionuclides. J. Nucl. Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- 17.Baskar R, Balajee AS, Geard CR. Effects of low and high LET radiations on bystander human lung fibroblast cell survival. Int. J. Radiat. Biol. 2007;83:551–559. doi: 10.1080/09553000701384499. [DOI] [PubMed] [Google Scholar]
- 18.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int. J. Radiat. Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 19.Iyer R, Lehnert BE. Effects of ionizing radiation in targeted and nontargeted cells. Arch. Biochem. Biophys. 2000;376:14–25. doi: 10.1006/abbi.1999.1684. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Anzenberg V, Held KD. Effects of heavy ions and energetic protons on normal human fibroblasts. Radiat. Biol. Radioecol. 2007;47:302–306. [PubMed] [Google Scholar]
- 21.Yang H, Anzenberg V, Held KD. The time dependence of bystander responses induced by iron-ion radiation in normal human skin fibroblasts. Radiat. Res. 2007;168:292–298. doi: 10.1667/RR0864.1. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz JL. Variability: the common factor linking low doseinduced genomic instability, adaptation and bystander effects. Mutat. Res. 2007;616:196–200. doi: 10.1016/j.mrfmmm.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Wu L, Han W, Zhang L, Chen S, Xu A, Hei TK, Yu Z. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis. 2006;27:245–251. doi: 10.1093/carcin/bgi224. [DOI] [PubMed] [Google Scholar]
- 24.Shao C, Folkard M, Michael BD, Prise KM. Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int. J. Cancer. 2005;116:45–51. doi: 10.1002/ijc.21003. [DOI] [PubMed] [Google Scholar]
- 25.Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double- strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–7265. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 26.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 27.Leach JK, Black SM, Schmidt-Ullrich RK, Mikkelsen RB. Activation of constitutive nitric-oxide synthase activity is an early signaling event induced by ionizing radiation. J. Biol. Chem. 2002;277:15400–15406. doi: 10.1074/jbc.M110309200. [DOI] [PubMed] [Google Scholar]
- 28.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res. 2003;63:8437–8442. [PubMed] [Google Scholar]
- 29.Shao C, Folkard M, Prise KM. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2007;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao C, Prise KM, Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat. Res. 2008;638:139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J. Clin. Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 32.Brechbiel MW. Targeted alpha-therapy: past, present, future? Dalton Trans. 2007:4918–4928. doi: 10.1039/b704726f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl. Med. Biol. 2007;34:779–785. doi: 10.1016/j.nucmedbio.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]