Abstract
Aims
Genome-wide association studies revealed an association between a locus at 10q11, downstream from CXCL12, and myocardial infarction (MI). However, the relationship among plasma CXCL12, cardiovascular disease (CVD) risk factors, incident MI, and death is unknown.
Methods and results
We analysed study-entry plasma CXCL12 levels in 3687 participants of the Chronic Renal Insufficiency Cohort (CRIC) Study, a prospective study of cardiovascular and kidney outcomes in chronic kidney disease (CKD) patients. Mean follow-up was 6 years for incident MI or death. Plasma CXCL12 levels were positively associated with several cardiovascular risk factors (age, hypertension, diabetes, hypercholesterolaemia), lower estimated glomerular filtration rate (eGFR), and higher inflammatory cytokine levels (P < 0.05). In fully adjusted models, higher study-entry CXCL12 was associated with increased odds of prevalent CVD (OR 1.23; 95% confidence interval 1.14, 1.33, P < 0.001) for one standard deviation (SD) increase in CXCL12. Similarly, one SD higher CXCL12 increased the hazard of incident MI (1.26; 1.09,1.45, P < 0.001), death (1.20; 1.09,1.33, P < 0.001), and combined MI/death (1.23; 1.13–1.34, P < 0.001) adjusting for demographic factors, known CVD risk factors, and inflammatory markers and remained significant for MI (1.19; 1.03,1.39, P = 0.01) and the combined MI/death (1.13; 1.03,1.24, P = 0.01) after further controlling for eGFR and urinary albumin:creatinine ratio.
Conclusions
In CKD, higher plasma CXCL12 was associated with CVD risk factors and prevalent CVD as well as the hazard of incident MI and death. Further studies are required to establish if plasma CXCL12 reflect causal actions at the vessel wall and is a tool for genomic and therapeutic trials.
Keywords: Atherosclerosis, Chemokines, Myocardial infarction, CXCL12
Introduction
Despite considerable advances in treatment and understanding of cardiovascular diseases (CVD), there is still substantial residual risk for cardiovascular events even after optimal management of known risk factors.1 This observation supports the need to discover novel pathways in CVD and to identify causal biomarkers to leverage opportunities for novel therapeutic targeting. Modern genomics, particularly genome-wide association studies (GWAS), have revealed a substantial list of potentially causal genes and pathways for CVD, although most remain largely unexplored.2–5 One such example is the chemokine ‘CXC Motif, Ligand 12’ (CXCL12), which encodes an inflammatory chemokine expressed in cells of relevance to CVD.6 A particular distinction of this locus is that CXCL12 has a measureable gene product circulating in blood.7 In several GWAS, the CXCL12 region was identified as a locus for myocardial infarction (MI),2–5 and single-nucleotide polymorphisms (SNPs) at this region appear to modulate plasma CXCL12 levels although findings on plasma CXCL12 are conflicting.8,9 There are several isoforms of the human CXCL12 protein, also called stromal-derived factor (SDF)-1, expressed in a tissue-specific manner adding to the complexity of studying CXCL12 biology.10 CXCL12 is a complex chemokine with putative anti-inflammatory and anti-atherogenic functions that also plays a role acutely in recruiting EPCs in response to vascular injuries. CXCL12 is highly expressed in human atherosclerotic plaque within endothelial cells (ECs) and smooth muscle cells (SMCs).6 The protein products of the gene CXCL12 are alternative spliced variants and the common isoforms, often called SDF 1α and SDF 1β, are coded from 3 exons and 4 exons, respectively.7 CXCL12 has been implicated in haematopoiesis, stem cell mobilization from the bone marrow (BM), and angiogenesis,11 and these functions contribute to its homeostatic functions and response to injuries. There are two receptors for CXCL12, CXCR4, and CXCR7. CXCR4 has been implicated in recruitment of atherogenic cells, including macrophages and SMCs,12 to the neo-intima. These biological observations suggest an important role for CXCL12 in human vascular disease.
Clinical data for CXCL12 in human atherosclerotic CVD are sparse. In one small study, Damas et al.13 reported that plasma levels of CXCL12 were decreased in symptomatic CAD cases compared with controls and, in particular, levels were lowest in patients with acute coronary syndromes. They found also that CXCL12 treatment reduced ex vivo production of inflammatory atherogenic signals, MCP-1, IL-8, and MMPs, in peripheral blood mononuclear cells (PBMCs). These results may suggest anti-inflammatory and plaque stabilization functions of CXCL12 in atherosclerosis. Kiechl et al.9 found that rs501120, an MI-related SNP in the CXCL12 region, was associated with greater carotid intimal-medial thickness (IMT) and lower plasma CXCL12 levels, whereas we have demonstrated that the same MI risk alleles were related to higher plasma CXCL12 levels.8 To date, mouse models have failed to resolve the vascular biology of Cxcl12 in part due to the distinct receptors and signalling pathways, lethality of germline gene deficiencies, and differences in vascular model employed and phenotypes examined.14–16
Patients with chronic kidney disease (CKD) are at high risk for CVD in part due to a pro-inflammatory milieu characterized by increases in circulating cytokines and chemokines.17 The Chronic Renal Insufficiency Cohort (CRIC) study is a multi-centre, prospective cohort study designed to examine risk factors for progressive CKD and CVD.18 We focused on this unique clinical population at increased risk of CVD for measurement of plasma CXCL12 to better understand the relationship of this GWAS locus with cross-sectional data and incident CVD events. Using data from the CRIC study, we performed the first prospective study of plasma CXCL12 levels with subsequent clinical outcomes and hypothesized that higher plasma CXCL12 levels at study entry would be associated with increased rates of MI and death.
Methods
Study population
The CRIC study is an ongoing National Institute of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored, prospective, observational cohort study of CKD. Participants were recruited across seven study sites in the USA between 2003 and 200618,19 and additional Hispanic participants were recruited from one site between 2005 and 2008, as the ancillary Hispanic CRIC.20 The baseline characteristics of the CRIC cohort have been reported.19 Briefly, the CRIC study recruited 3939 individuals between 2003 and 2008 and the cohort has been followed with annual in-person visits since recruitment. Subjects were aged 21–74 years, 46% female, and were recruited to be racially and ethnically diverse (45% white, 46% black, 5% Hispanic, 4% Asian/Pacific Islander/Native American), to have a broad spectrum of renal disease severity [estimated glomerular filtration rate (eGFR) ∼15–90; mean 43.4 ± 13.5 mL/min] with ∼50% having diabetes mellitus. To limit the proportion of older individuals who were recruited with age-related diminutions of GFR but otherwise non-progressive CKD, age-based eGFR entry criteria were used. Participants were excluded if unable to provide written informed consent, institutionalized, enrolled in other studies, pregnant, had New York Heart Association class III to IV heart failure, human immunodeficiency virus infection, cirrhosis, myeloma, polycystic kidney disease, renal cancer, recent chemotherapy or immunosuppressive therapy, organ transplant, or had prior treatment with dialysis for at least 1 month.18 The CRIC study protocol was approved by the Institutional Review Boards (IRB) of all participating institutions (Ann Arbor, Michigan; Baltimore, Maryland; Chicago, Illinois; Cleveland, Ohio; New Orleans, Louisiana; Philadelphia, Pennsylvania; and Oakland, California) and study participants provided written informed consent. The CRIC study has extensive data management protocols with security and privacy checks18 overseen by the CRIC study Coordinating Centre, based at the University of Pennsylvania. The study complies with the Declaration of Helsinki.
Exposures and outcomes
The primary exposure was plasma CXCL12, measured in samples at study entry in 3869 of the 3939 participants. Due to missing values for body mass index (BMI) (n = 10), interleukin-6 (IL6) and tumour necrosis factor-alpha (TNFα) (n = 37), and for urinary albumin:creatinine ratio (n = 135), the final sample for analysis consisted of 3687 CRIC participants. The 252 excluded participants differed slightly from included subjects in age (58 vs. 60 years; P < 0.001) and racial/ethnic distribution (White 29% vs. 42%, Black 51% vs. 41%, Hispanic 17% vs. 12%; P < 0.001), but otherwise had no statistically significant differences in cardiovascular and kidney parameters.
CXCL12 levels were measured in previously unthawed plasma samples using a commercial ELISA [Quantikine Immunoassay (R+D Systems, Minneapolis, MN, USA)].8,13 Blood samples were spun within an hour of collection at 1000 g at 4°C, and EDTA plasma frozen at −80°C. Aliquots were thawed and spun at 10 000 g for 15 min at 4°C prior to performing the CXCL12 ELISA. Samples were run in duplicate, averaged, and the intra- and inter-assay coefficients of variation were 4.2 and 13.8%, respectively. The specificity of this assay using western blotting and recombinant proteins confirms that the predominant isoform, isoform-alpha, but not the beta isoform, is detected (communication Chris Larson, R+D).10 As described,17 high-sensitivity plasma C reactive protein (hsCRP) was assayed by nephelometry and levels of IL6 and TNFα were measured by high-sensitivity ELISA (R+D Systems).
Demographic factors and clinical data were obtained at baseline and annually by interview and questionnaire. Additional blood and urine laboratory tests were measured centrally using standard assays. Lipids including total, high- and low-density cholesterol, as well as triglycerides were measured enzymatically (Hitachi 912, Roche Diagnostic Systems, Inc., NJ, USA). We used a CRIC-specific equation (including serum creatinine, cystatin C, age, sex, and race) for estimating glomerular filtration rate (eGFR) based on iothalamate GFR measurements as described.21 Diabetes mellitus was defined as fasting glucose ≥126 mg/dL, random glucose ≥200 mg/dL, or use of insulin or anti-diabetic medication. Participants had three seated blood pressures recorded at each visit, as previously described.18 Hypertension was defined as a systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medications. Hypercholesterolaemia was defined as the use of cholesterol-lowering medications or total serum cholesterol >200 mg/dL.
The primary outcomes were incident MI, all-cause death, and a composite of these endpoints. Incident MI was defined by review of hospitalization records and participant interviews. Participants were asked at each contact if they had been hospitalized, and if so, records were obtained if the billing codes included pre-defined MI codes. Records were reviewed by two physician adjudicators for cardiac biomarker data, including troponin and creatine kinase, electrocardiograms (ECGs), and symptoms of angina. The classification of an event as a definite, probable, or not an MI was made by each physician separately based on independent review of data and the final adjudication required the agreement of the two physician reviewers. All-cause death was confirmed by report from next of kin, a review of hospital records if death occurred in hospital or via the social security death index. Participants were followed at annual visits and interim telephone calls until occurrence of death, withdrawal from the study, loss to follow-up, or 30 June 2009 when data were locked for this analysis. More than 90% of the participants were retained during the longitudinal observation period through 2009.
Prevalent CVD at enrolment [defined as self-reported prior diagnosis of coronary heart disease (CHD), stroke or peripheral vascular disease] was analysed as a secondary outcome.
Statistical analysis
One-way analysis of variance (continuous variables) and Pearson's χ2 tests (categorical variables) were used to compare clinical characteristics across baseline quartiles of plasma CXCL12 levels. We used logistic regression to examine unadjusted and multivariable-adjusted relationships between plasma CXCL12 level and prevalent CVD at enrolment. CXCL12 values were standardized by subtracting the sample mean and dividing by the standard deviation (SD). Odds ratios (ORs) are reported per one SD increment of standardized CXCL12. Because we lacked prior evidence of linear associations of CXCL12 with outcomes, we also report ORs for above the median plasma CXCL12 value (median is 2.432 ng/mL). Incremental models were fitted adjusting for: (Model 1) demographic factors (age, sex, and race); (Model 2) demographic and traditional CVD risk factors (BMI and binary indicators for diabetes, hypertension, hypercholesterolaemia, and tobacco use); (Model 3) demographic, traditional risk factors, and inflammatory biomarkers (log-transformed IL6, TNFα, and high-sensitivity C reactive protein levels); and (Model 4) demographic, traditional risk factors, inflammatory biomarkers, and measures of kidney function (CRIC-defined eGFR and urinary albumin:creatinine ratio). Cox proportional hazards regression was used to examine unadjusted and multivariable-adjusted relationships between CXCL12, per 1 SD increment of standardized CXCL12 and for above the median plasma CXCL12, and each of the following incident endpoints: (1) probable MI, (2) definite MI, (3) death, and (4) a composite outcome of MI and death (MI/death). A similar modelling approach was applied as outlined above with adjustment for additional factors as detailed in results. Kaplan–Meier plots were generated using quartiles of CXCL12 data to illustrate findings. The impact of plasma CXCL12 on the prognostic performance in risk prediction models was examined with change in the area under the curve (AUC) for probable MI, death and their combined outcome using approaches described by Pencina et al.,22 and then using the Net Reclassification Index (NRI) from simple cross-tabulation of 10-year risk categories (e.g. ‘low risk’ 0%–10%, ‘medium risk’ 10%–20%, ‘high risk’ >20%) based on predicted probabilities obtained using models with and without CXCL12 (included as a continuous variable).22,23 Analyses were performed using the R platform for statistical computing, version 2.15.2. AUC in receiver operator curve (ROC) analysis is performed using the concordance.index() and cindex.comp() functions in the survcomp package in R version 3.0.1., and the NRI analysis was performed using the nricens () function in the nricens package in R version. All statistical tests were 2-sided, and P values < 0.05 were considered statistically significant.
Results
Association of plasma CXCL12 levels with demographic and clinical factors
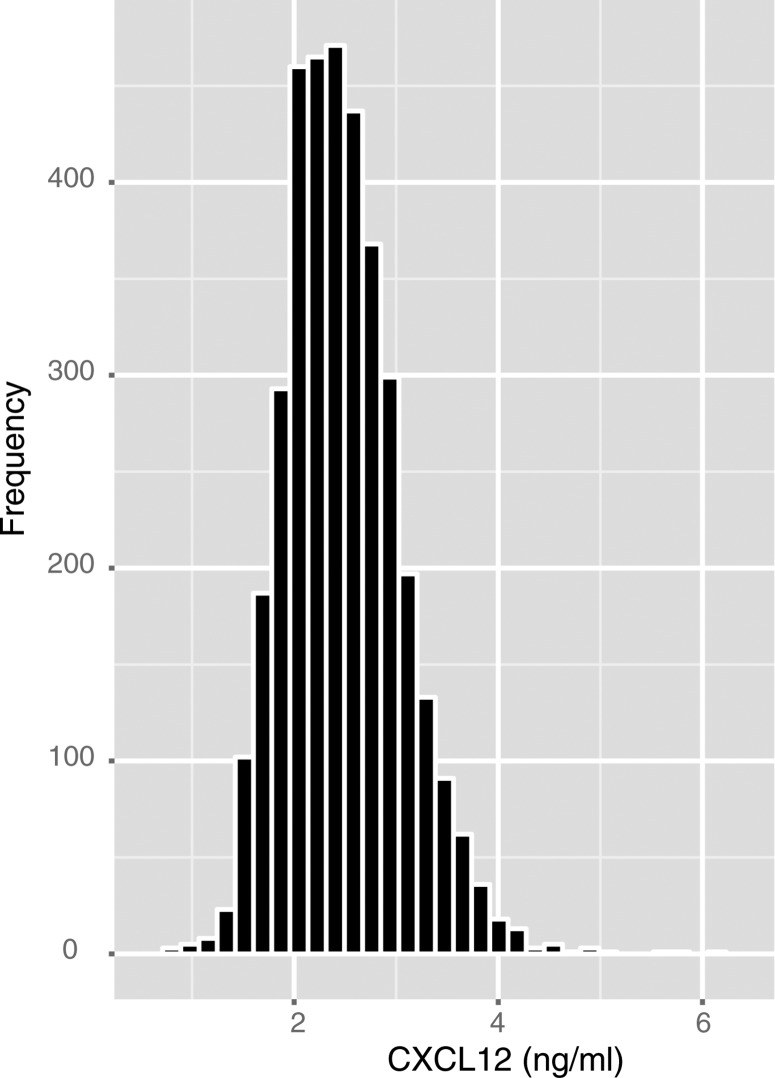
The distribution of plasma levels of CXCL12 in the full cohort is shown in Figure 1. Baseline characteristics of study participants by CXCL12 quartiles are presented in Table 1 and Supplementary material online, Table S1. There were significant associations of higher plasma CXCL12 with increasing age, Black and Hispanic race/ethnicity, prior CVD, diabetes, hypertension, hypercholesterolaemia, BMI, and plasma levels of inflammatory markers IL6, TNFα, and high-sensitivity C reactive protein. Reduced kidney function, estimated by lower eGFR, higher plasma cystatin C, and higher urinary albumin:creatinine ratio were also associated with higher CXCL12.
Figure 1.
Distribution of plasma CXCL12 levels in the study sample.
Table 1.
Demographic, traditional risk factors and renal parameters stratified by plasma CXCL12 quartiles
| CXCL12 (ng/mL) |
|||||
|---|---|---|---|---|---|
| Quartile 1, 0.83–2.07 ng/mL | Quartile 2, 2.07–2.43 ng/mL | Quartile 3, 2.43–2.82 ng/mL | Quartile 4, 2.82–6.17 ng/mL | P value | |
| Characteristic (n = 3687) | n = 923 | n = 922 | n = 921 | n = 921 | |
| Demographics | |||||
| Age (years) | 59 (50, 65) | 60 (53, 66) | 60 (53, 66) | 61 (54, 68) | <0.001 |
| Female sex (n = 1655, 44.9%) | 422 (11.5%) | 378 (10.3%) | 402 (10.9%) | 453 (12.3%) | <0.01 |
| Race | |||||
| White (n = 1564, 42.4%) | 423 (27.1%) | 417 (26.7%) | 369 (23.6%) | 355 (22.7%) | <0.001 |
| Black (n = 1521, 41.3%) | 365 (24.0%) | 375 (24.7%) | 388 (25.5%) | 393 (25.8%) | |
| Hispanic (n = 455, 12.3%) | 86 (18.9%) | 94 (20.7%) | 128 (28.1%) | 147 (32.3%) | |
| Other (n = 147, 4%) | 49 (33.3%) | 36 (24.5%) | 36 (24.5%) | 26 (17.7%) | |
| Traditional CV risk factors | |||||
| Prior CVD (n = 1232, 33.4%) | 226 (18.3%) | 265 (21.5%) | 339 (27.5%) | 402 (32.6%) | <0.001 |
| Diabetes (n = 1781, 48.3%) | 358 (20.1%) | 416 (23.4%) | 482 (27.1%) | 525 (29.5%) | <0.001 |
| Tobacco use (n = 473, 12.8%) | 106 (22.4%) | 108 (22.8%) | 124 (26.2%) | 135 (28.5%) | 0.13 |
| Body mass index (kg/m2)a | 30.6 (26.9, 35.1) | 30.8 (27.0, 35.9) | 30.9 (26.8, 36.2) | 31.20 (26.8, 37.1) | 0.01 |
| Hypertension (n = 3171, 86.0%) | 750 (23.7%) | 787 (24.8%) | 811 (25.6%) | 823 (26.0%) | <0.001 |
| Hyperlipidaemia (n = 3034, 82.3%) | 732 (24.1%) | 754 (24.9%) | 769 (25.4%) | 779 (25.7%) | 0.02 |
| Kidney function measuresa | |||||
| Estimated GFR (mL/min/1.73 m2) | 52.3 (41.3, 64.2) | 46.3 (35.8, 58.5) | 39.9 (31.1, 51.2) | 34.2 (25.6, 44.0) | <0.001 |
| Cystatin C (mg/L) | 1.16 (0.95, 1.45) | 1.30 (1.07, 1.66) | 1.50 (1.20, 1.88) | 1.75 (1.42, 2.18) | <0.001 |
| Urinary ACR (µg/mg)* | 20.1 (5.6, 217.3) | 35.1 (7.2, 344.9) | 84.0 (9.8, 622.4) | 137.5 (21.1, 842.7) | <0.001 |
| Mineral metabolism parametersa | |||||
| Serum calcium (mg/dL) | 9.2 (8.9, 9.5) | 9.2 (8.9, 9.5) | 9.2 (8.9, 9.4) | 9.2 (8.8, 9.5) | 0.27 |
| Serum phosphate level (mg/dL) | 3.5 (3.2, 3.9) | 3.6 (3.2, 4.0) | 3.7 (3.3, 4.2) | 3.9 (3.4, 4.3) | <0.001 |
| Fibroblast growth factor 23 (RU/mL) | 111 (81, 172) | 130 (86, 201) | 157 (107, 252) | 208 (128, 339) | <0.001 |
| Serum inflammatory biomarkersa | |||||
| IL6 (pg/mL)* | 1.53 (0.93, 2.48) | 1.78 (1.07, 2.73) | 2.00 (1.26, 3.42) | 2.37 (1.49, 3.98) | <.001 |
| TNFα (pg/mL)* | 1.80 (1.20, 2.60) | 2.00 (1.40, 3.00) | 2.30 (1.70, 3.30) | 2.80 (2.00, 3.80) | <.001 |
| High-sensitivity C-reactive protein (pg/mL)* | 2.37 (1.02, 5.55) | 2.58 (1.09, 6.70) | 2.58 (1.04, 6.25) | 2.71 (1.06, 7.13) | 0.03 |
Values shown as median (IQR) or n (%).
CVD, cardiovascular disease; GFR, glomerular filtration rate; ACR, albumin:creatinine ratio; tobacco use defined as former or current smoker.
P-value by ANOVA (continuous variables; those marked with * were log-transformed to meet normality assumption) and χ2 test (categorical variables).
aPresented as median (interquartile range).
Plasma CXCL12 and prevalent cardiovascular disease
Our primary focus was to understand the effect of plasma CXCL12 on incident events. First, however, we analysed cross-sectional associations to understand how plasma CXCL12 levels at enrolment related to prevalent CVD and its risk factors. Study entry CXCL12 levels, whether considered as a 1 SD increase in standardized CXCL12 or greater than the median CXCL12, were associated with higher prevalence of CVD in models adjusting for demographic factors, known CVD risk factors, and inflammatory biomarkers (IL6, TNFα, and high-sensitivity C reactive protein) (OR 1.30; P < 0.001 and OR 1.53; P < 0.001, respectively) and after controlling further for study entry eGFR and urinary albumin:creatinine ratio (OR 1.23; P < 0.001 and OR 1.38; P < 0.001, respectively) (Table 2).
Table 2.
Association of plasma CXCL12 with prevalent cardiovascular diseasea (CVD) at baseline
| Model (total n = 3687) | Odds ratio (95% CI)b | P value | Odds ratio (95% CI)c | P value |
|---|---|---|---|---|
| Model 1 | 1.42 (1.32, 1.53) | <0.001 | 1.81 (1.56, 2.09) | <0.001 |
| Model 2 | 1.36 (1.26, 1.46) | <0.001 | 1.66 (1.43, 1.93) | <0.001 |
| Model 3 | 1.30 (1.20, 1.40) | <0.001 | 1.53 (1.31, 1.78) | <0.001 |
| Model 4 | 1.23 (1.14, 1.33) | <0.001 | 1.38 (1.18, 1.62) | <0.001 |
Model 1: CXCL12 + demographic factors (age, sex, race).
Model 2: CXCL12 + demographic factors + traditional risk factors (diabetes, hypertension, hypercholesterolaemia, tobacco use, body mass index).
Model 3: CXCL12 + demographic factors + traditional risk factors + plasma inflammatory biomarkers (log transformed IL6, TNFα, and high-sensitivity C-reactive protein).
Model 4: CXCL12 + demographic factors + traditional risk factors + plasma inflammatory biomarkers + kidney function measures (CRIC-defined estimated glomerular filtration rate21 and log-transformed urinary albumin:creatinine ratio).
aPrevalent CVD is defined as prior MI or coronary revascularization (n with any CVD = 1232; n without CVD = 2455).
bFor one standard deviation increase in a plasma CXCL12.
cFor plasma CXCL12 levels above the median cut point value of 2.432 ng/mL.
Plasma CXCL12 and occurrence of myocardial infarction and death
During a median follow-up of 6 years, a total of 174 participants experienced a probable MI; of these participants, 127 experienced a definite MI. A total of 355 participants died and 485 experienced the composite outcome of MI or death. Compared with those without the respective event, median CXCL12 levels were significantly higher in those who had probable MI [2.63 ng/mL; IQR (2.29, 2.96) vs. 2.42 ng/mL (2.07, 2.81)], definite MI [2.63 ng/mL (2.30, 2.95) vs. 2.42 ng/mL (2.07, 2.81)], died [2.63 ng/mL (2.26, 3.09) vs. 2.41 ng/mL (2.06, 2.79)] or experienced the composite of MI/death [2.63 ng/mL (2.26, 3.03) vs. 2.41 ng/mL (2.06, 2.79)].
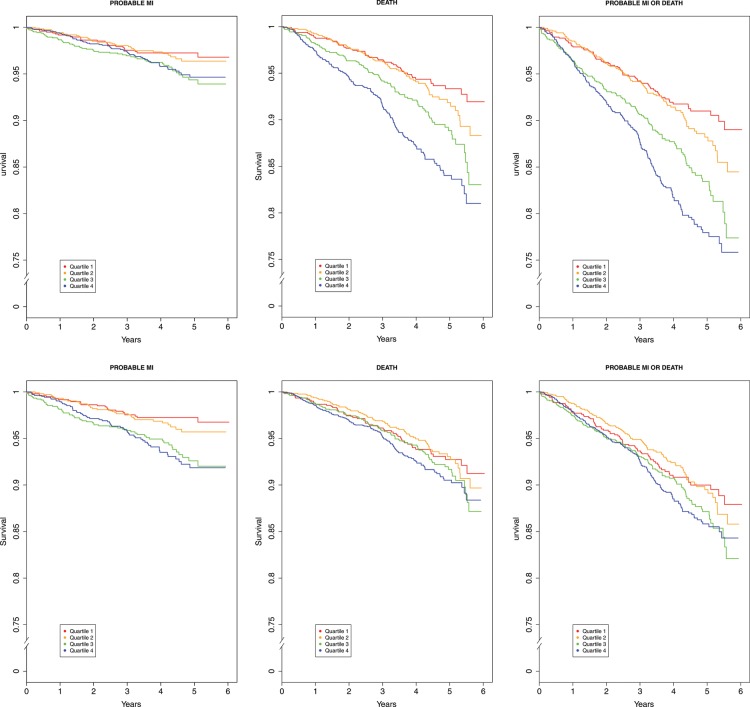
Table 3 presents multivariable-adjusted hazard ratios (HRs) for probable MI, death, or the composite of MI/death according to 1 SD increase in the standardized CXCL12 and for those with above-median plasma CXCL12. Study entry CXCL12 levels predicted MI (HR 1.26; P = 0.001), death (HR 1.20; P < 0.001), and the combined event (HR 1.23; P < 0.001) in models adjusted for demographic factors, known risk factors, and inflammatory biomarkers (IL6, TNFα, and high-sensitivity C reactive protein) and remained significant for MI (1.19; P = 0.01) and the composite of MI/death (1.13; P = 0.01) outcomes after further adjusting for study entry eGFR and urinary albumin:creatinine ratio (Table 3). Analyses of CXCL12 levels above the median had similar patterns (e.g. in fully adjusted models HR for probable MI 1.52; P < 0.001, HR for death 1.28; P = 0.03, HR for composite of MI/death 1.30; P = 0.008) (Table 3). Further adjustment for prevalent CVD slightly attenuated the estimates for incident events (HR for MI 1.43; P = 0.034; HR for death 1.21, P = 0.097; HR for composite of MI/death 1.23, P = 0.033). Kaplan–Meier plots for age, gender, and race adjusted as well as fully adjusted associations between quartiles of plasma CXCL12 and probable MI, death, or composite of MI/death are shown in Figure 2.
Table 3.
Multivariable association of plasma CXCL12 with incident clinical events
| Failure event (total n = 3687) | Hazard Ratioa (95% CI) | P value | Hazard ratiob (95% CI) | P value |
|---|---|---|---|---|
| Probable Myocardial Infarction (n = 174) | ||||
| Model 1 | 1.37 (1.20, 1.56) | <0.001 | 2.01 (1.46, 2.75) | <0.001 |
| Model 2 | 1.34 (1.17, 1.53) | <0.001 | 1.89 (1.38, 2.59) | <0.001 |
| Model 3 | 1.26 (1.09, 1.45) | 0.001 | 1.69 (1.22, 2.33) | 0.001 |
| Model 4 | 1.19 (1.03, 1.39) | 0.02 | 1.52 (1.09, 2.13) | 0.01 |
| Death (n = 355) | ||||
| Model 1 | 1.36 (1.24, 1.49) | <0.001 | 1.90 (1.53, 2.37) | <0.001 |
| Model 2 | 1.32 (1.20, 1.44) | <0.001 | 1.77 (1.42, 2.21) | <0.001 |
| Model 3 | 1.20 (1.09, 1.33) | <0.001 | 1.51 (1.21, 1.88) | <0.001 |
| Model 4 | 1.10 (0.99, 1.22) | 0.09 | 1.28 (1.02, 1.61) | 0.04 |
| Any MI or death (n = 485) | ||||
| Model 1 | 1.37 (1.27, 1.49) | <0.001 | 1.90 (1.58, 2.30) | <0.001 |
| Model 2 | 1.34 (1.23, 1.45) | <0.001 | 1.78 (1.47, 2.15) | <0.001 |
| Model 3 | 1.23 (1.13, 1.34) | <0.001 | 1.53 (1.26, 1.85) | <0.001 |
| Model 4 | 1.13 (1.03, 1.24) | 0.01 | 1.30 (1.07, 1.59) | 0.008 |
Model 1: CXCL12 + demographic factors (age, sex, race).
Model 2: CXCL12 + demographic factors + traditional risk factors (diabetes, hypertension, hypercholesterolaemia, tobacco use, body mass index).
Model 3: CXCL12 + demographic factors + traditional risk factors + plasma inflammatory biomarkers (log transformed IL6, TNFα, and high-sensitivity C-reactive protein).
Model 4: CXCL12 + demographic factors + traditional risk factors + plasma inflammatory biomarkers + kidney function measures (CRIC-defined estimated glomerular filtration rate21 and log-transformed urinary albumin:creatinine ratio).
aFor one standard deviation increase in plasma CXCL12.
bFor plasma CXCL12 levels above the median cut point value of 2.432 ng/mL.
Figure 2.
Multivariable-adjusted Kaplan–Meier plots for effect of plasma CXCL12 quartiles on incident events. Plasma CXCL12 level within the lowest CXCL12 quartile (0.83–2.07) served as the referent value (hazard = 1.0). The top panel presents Kaplan–Meier plots for models adjusted for age, sex, and race. The bottom panel presents plots for models adjusted for age, sex, race, traditional cardiovascular risk factors, plasma inflammatory biomarkers (IL6, TNFα, and high-sensitivity C-reactive protein), and kidney function measures (CRIC-defined estimated glomerular filtration rate and urinary albumin:creatinine ratio).Tick marks on the x-axis indicate individual observations at corresponding levels of CXCL12. The solid black line represents the multivariable-adjusted hazard of outcomes as a function of the CXCL12 level. Traditional risk factors include body mass index and binary indicators for diabetes, hypertension, hypercholesterolaemia, and tobacco use.
When using the more stringent outcome definition of definite MI, similar effect estimates as those for probable MI were observed (Supplementary material online, Table S2); however, statistical significance was slightly attenuated, likely due to the smaller number of events (127 definite MIs vs. 174 probable MIs). The HR for definite MI was 1.14, CI 0.95, 1.36, P = 0.16 in adjusted model (demographics, CV risk factors, inflammatory biomarkers, eGFR, urinary albumin:creatinine ratio) for 1 SD increase in standardized plasma CXCL12 and 1.53, CI 1.04–2.25, P = 0.03 in the same adjusted model for values above the median CXCL12.
Finally, we evaluated the AUC and the NRI in order to explore the predictive performance of plasma CXCL12 when added to traditional and novel risk factors. Change in AUC for probable MI, death, and their combined outcome was evaluated in incrementally adjusted models (Table 4). Plasma CXCL12 significantly improved the AUC when added to demographic and traditional risk factor for all three outcomes and modestly increased the AUC for all outcomes when added to demographic factors, known risk factors and eGFR (Table 4A). In comparison, we found that eGFR significantly improved the AUC when added to demographic and traditional risk factor for all three outcomes as well as improving AUCs for all outcomes when added to demographic factors, known risk factors, and CXCL12 (Table 4B). In reclassification analyses, there was small improvement in the NRI for probable MI and death when CXCL12 was added to a model containing traditional risk factors (Supplementary material online, Table S3).
Table 4.
Change in the area under the curve for probable myocardial infarction, death and the composite of myocardial infarction or death with addition of (A) CXCL12 dataa and (B) estimated glomerular filtration rate data
| (A) Add CXCL12 to model | ||||||
| ROC AUC in Model 2 | ROC AUC in Model 2 + eGFR | |||||
| Without CXCL12 | With CXCL12 | P-value | Without CXCL12 | With CXCL12 | P-value | |
| Probable MI | 0.664 | 0.685 | 0.003 | 0.690 | 0.698 | 0.023 |
| Death | 0.674 | 0.695 | <0.001 | 0.723 | 0.727 | 0.049 |
| MI or death | 0.660 | 0.680 | <0.001 | 0.703 | 0.707 | 0.057 |
| (B) Add eGFR to model | ||||||
| ROC AUC in Model 2 | ROC AUC in Model 2 + CXCL12 | |||||
| Without eGFR | With eGFR | P-value | Without eGFR | With eGFR | P-value | |
| Probable MI | 0.664 | 0.690 | 0.002 | 0.685 | 0.698 | 0.017 |
| Death | 0.674 | 0.723 | <0.001 | 0.695 | 0.727 | <0.001 |
| MI or death | 0.660 | 0.703 | <0.001 | 0.680 | 0.707 | <0.001 |
aStandardized plasma CXCL12 values.
Model 2: CXCL12 + demographic factors + traditional risk factors (diabetes, hypertension, hypercholesterolaemia, tobacco use, body mass index).
Discussion
In cross-sectional analyses and the first prospective study of plasma CXCL12 with longitudinal outcomes, we found that study entry levels were associated with prevalent CVD as well as incident MI and death over a 6-year period in a high-risk CKD population. Although plasma CXCL12 levels were associated with measures of kidney function as well as multiple demographic and CVD risk factors, significant associations persisted, albeit with some attenuation, even after adjusting for risk factors and measures of kidney function.
CXCL12 was identified as a potential gene for CHD through GWAS of MI.4 Most recently, this GWAS locus association with CHD was confirmed in GWAS meta-analyses of over 100 000 individuals.2,5 CXCL12 is biologically plausible in CVD as it plays a role in recruiting leucocytes in response to vascular injuries14 and has been implicated in atherosclerosis in rodent models.15,16 CXCL12 is also expressed in human atherosclerosis within endothelial cells and SMCs.12 Findings, however, are conflicting with respect to the direction of effect of CXCL12 in human disease perhaps reflecting its complex biology with six known CXCL12 coding splice variants7,10 and two distinct signalling receptors (CXCR4 and CXCR7)24 as well as a dearth of prospective clinical data. Two small cross-sectional human studies reported that plasma CXCL12 was altered in human CAD,13 with lower levels associated with acute MI. It is possible, however, that plasma CXCL12 levels drop during the acute inflammation of MI. Indeed, we have observed a drop in plasma CXCL12 levels following intravenous lipopolysaccharide administration in humans (NNM and MPR, unpublished data). A fall in plasma CXCL12 during inflammatory stress could reflect active signalling and sequestration of CXCL12 at its receptors25,26 confounding interpretation of plasma level measurement in acute MI. Kiechl et al.9 found in the Bruneck study that increased carotid IMT was associated with lower plasma CXCL12 levels. In contrast, our group has shown that the GWAS allele for increased MI was associated with increased CXCL12 mRNA levels in the liver and natural killer cells, as well as higher plasma CXCL12 levels, suggesting that this GWAS locus might increase risk of MI through increased CXCL2 expression.8 Importantly, our new data in stable CKD patients recruited to a prospective cohort show that higher plasma CXCL12 relate to future MI and death over a 6-year follow-up.
Animal studies of this pathway are challenging to interpret due to contrasting effects of Cxcl12 depending on the vascular phenotype, the acuity of the model, or the receptor pathway studied. For example, in mice with established atherosclerosis, acute blockade of Cxcr4, one of the Cxcl12 receptors, accelerated atherosclerosis with expansion of plaque neutrophils and macrophages suggesting an anti-atherogenic function for Cxcl12.15 In contrast, atherosclerotic apoE−/− mice had smaller plaque areas and SMC content after repopulation with Cxcr4−/− bone marrow or transfer of a lentivirus encoding a Cxcl12-alpha antagonist, suggesting that CXCL12-alpha might be pro-atherogenic.16 Knockout of Cxcl12 and its receptors is lethal in mice and studies of conditional deletion in atherosclerosis have not yet been published. Thus, current experimental data suggest a complex cell-specific, context- and time-dependent role for the CXCL12 system in cardiovascular biology and disease.
In this setting, ours is the first examination of the relationship of plasma CXCL12 with prospective outcomes in humans. We found that higher study-entry plasma CXCL12 related to incident MI and death in a high-risk CKD population. The most straightforward interpretation of our findings is that CXCL12 accelerates CHD and that plasma levels provide a surrogate of CXCL12 actions in the vasculature and for use in causal genomic interrogation (e.g. Mendelian randomization) and therapeutic trials. However, several caveats require consideration. First, plasma CXCL12 levels were correlated with several cardio-metabolic risk factors, including measures of kidney function. Although plasma CXCL12 predicted incident events even in adjusted models, it is possible that residual confounding (by estimates of renal-metabolic dysfunction and more precise measures of cardiac injury) might have resulted in a spurious CXCL12 association. Second, the association might be driven by reverse causation whereby vascular disease leads to increased CXCL12 secretion in response to vascular injury, as suggested.14 Rodent studies using conditional deletion of Cxcl12 coupled to specific dissection of its actions via its two cognate receptors (Cxcr4 and Cxcr7) will help to clarify the in vivo biology and causality. However, the possibility that human mechanisms are distinct from rodent models needs consideration given precedent for species difference in chemokine expression, splicing, and signalling.27
Human studies, employing a Mendelian randomization design,28,29 should provide insights into the causality of CXCL12 in human disease. Such work is planned but is also challenging. To date, tool SNPs in the CXCL12 region that relate to both plasma levels of CXCL12 and CHD that could serve as instrumental variables are poorly characterized. Indeed, the SNPs associated with MI through GWAS have only a minor impact on circulating CXCL12. Further, tool variants that also lack impact on other biomarkers or causal intermediates have not been defined. Last, circulating CXCL12 levels may be poor surrogates of cell-specific actions of CXCL12 isoforms in the vasculature rendering Mendelian randomization using plasma CXCL12 assays non-informative of the underlying vascular biology.
Our study has several strengths. The CRIC study is a unique, well-phenotyped, prospective cohort study of CKD in participants at high-risk for CVD. In CRIC, we can gain insight into how plasma CXCL12 relates to multiple CVD and kidney biomarkers. Further, we are able to simultaneously evaluate multiple cross-sectional CVD phenotypes as well as adjudicated incident events. In future work, repeated-measures biomarker data as well as whole-genome data can be interrogated contributing greater insight into the relation of CXCL12 and its signalling receptors with CHD in CRIC.
This work also has limitations. In CRIC, death events have not been characterized as CV or non-CV in origin. However, for MI there were careful review and adjudication by two blinded investigators ensuring high-quality data. Current assays of plasma CXCL12 may not capture CXCL12 isoform activity in vasculature, but emerging data do suggest that the alpha isoform, which circulates in plasma, is abundantly expressed in macrophages and vascular cells.30 Residual confounding and reverse causation are not excluded and, in the absence of additional very large datasets with genomic data, plasma CXCL12 data, and prospective events, human Mendelian randomization studies are not yet feasible. Future analyses will also need to address whether CXCL12 levels predict CVD after controlling for circulating natriuretic peptides and high-sensitivity cardiac troponins, powerful predictors of CVD in CKD. Our exploration of performance in risk prediction suggests that plasma CXCL12 improves prediction of MI and death when added to traditional risk factors and eGFR and also enhances modestly risk reclassification in patients with CKD. However, our goal in this work was not to suggest development plasma CXCL12 as a new biomarker of CVD risk for use clinically. Additional large studies are needed in diverse populations to define clinical utility in risk prediction using both plasma CXCL12 and genetic data from the CXCL12 region in the context of evolving evidence for CXCL12 in CVD.
In conclusion, we demonstrate that in CKD, higher levels of plasma CXCL12 were associated with known cardiovascular risk factors and prevalent CVD and also predicted incident MI and death even after adjustment for traditional risk factors and measures of kidney dysfunction. While these findings suggest that higher plasma CXCL12 may be atherogenic, further mechanistic, genomic and interventional studies are required to establish if plasma CXCL12 levels reflect causal actions at the vessel wall and to determine whether CXCL12 leads to increased risk of MI and CVD in humans.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgement
We acknowledge the commitment of the participants, investigators and staff of the CRIC study.
Appendix: Chronic Renal Insufficiency Cohort (CRIC) study principal investigators
†Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, John W. Kusek, James P. Lash, Akinlolu Ojo, Mahboob Rahman, Raymond R. Townsend.
Funding
This work was supported under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This work was supported in part by: the University of Pennsylvania (CTRC CTSA UL1 RR-024134), Johns Hopkins University (UL1 RR-025005), University of Maryland (GCRC M01 RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439) from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) (UL1RR024986), University of Illinois at Chicago (CTSA UL1RR029879), The Clinical and Translational Research, Education, and Commercialization Project (CTRECP), Kaiser NIH/NCRR (UCSF-CTSI UL1 RR-024131). This work was supported directly by R01-DK071224 (to M.P.R.). N.N.M. is supported by National Institutes of Health Intramural Award HL-Z0000. M.J.F. is supported by a Department of Veterans Affairs Health Services Research and Development Service Award. H.F. is supported by K24-DK002651. A.F. is supported by R01-HL107196. M.P.R. is also supported by K24-HL107643, R01-DK090505, U01-HL108636, and R01-HL113147.
Conflict of interest: none declared.
References
- 1.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schunkert H, König IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I. Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Mühleisen TW, Muhlestein JB, Münzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nöthen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schäfer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, März W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann J, editors; Samani NJ, editor. Cardiogenics. CARDIoGRAM Consortium. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O, Altshuler D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P, Piazza A, Yee J, Friedlander Y, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Meigs JB, Williams G, Nathan DM, MacRae CA, Havulinna AS, Berglund G, Hirschhorn JN, Asselta R, Duga S, Spreafico M, Daly MJ, Nemesh J, Korn JM, McCarroll SA, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall AS, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Ouwehand W, Deloukas P, Scholz M, Cambien F, Li M, Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Epstein SE, Scheffold T, Berger K, Huge A, Martinelli N, Olivieri O, Corrocher R, McKeown P, Erdmann E, Konig IR, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Do R, Xie C, Siscovick D. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consortium CD. A genome-wide association study in Europeans and south Asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 6.Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res. 2000;86:131–138. doi: 10.1161/01.res.86.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (sdf1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 8.Mehta NN, Li M, William D, Khera AV, Derohannessian S, Qu L, Ferguson JF, McLaughlin C, Shaikh LH, Shah R, Patel PN, Bradfield JP, He J, Stylianou IM, Hakonarson H, Rader DJ, Reilly MP. The novel atherosclerosis locus at 10q11 regulates plasma cxcl12 levels. Eur Heart J. 2011;32:963–971. doi: 10.1093/eurheartj/ehr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiechl S, Laxton RC, Xiao Q, Hernesniemi JA, Raitakari OT, Kahonen M, Mayosi BM, Jula A, Moilanen L, Willeit J, Watkins H, Samani NJ, Lehtimaki TJ, Keavney B, Xu Q, Ye S. Coronary artery disease-related genetic variant on chromosome 10q11 is associated with carotid intima-media thickness and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2678–2683. doi: 10.1161/ATVBAHA.110.213785. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, Su EW, Wang J. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–179. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired cxcr4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 12.Zeiffer U, Schober A, Lietz M, Liehn EA, Erl W, Emans N, Yan ZQ, Weber C. Neointimal smooth muscle cells display a proinflammatory phenotype resulting in increased leukocyte recruitment mediated by p-selectin and chemokines. Circ Res. 2004;94:776–784. doi: 10.1161/01.RES.0000121105.72718.5C. [DOI] [PubMed] [Google Scholar]
- 13.Damas JK, Waehre T, Yndestad A, Ueland T, Muller F, Eiken HG, Holm AM, Halvorsen B, Froland SS, Gullestad L, Aukrust P. Stromal cell-derived factor-1alpha in unstable angina: potential antiinflammatory and matrix-stabilizing effects. Circulation. 2002;106:36–42. doi: 10.1161/01.cir.0000020001.09990.90. [DOI] [PubMed] [Google Scholar]
- 14.Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein e-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 15.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, Krohn R, Schober A, Sperandio M, Soehnlein O, Bornemann J, Tacke F, Biessen EA, Weber C. Protective role of cxc receptor 4/cxc ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–217. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 16.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. Sdf-1alpha/cxcr4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 17.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS. Association between albuminuria, kidney function, and inflammatory biomarker profile in ckd in cric. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 19.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP. CKD in hispanics: baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and hispanic-CRIC studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 23.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 24.Luker K, Gupta M, Luker G. Bioluminescent cxcl12 fusion protein for cellular studies of cxcr4 and cxcr7. Biotechniques. 2009;47:625–632. doi: 10.2144/000113126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luker KE, Steele JM, Mihalko LA, Ray P, Luker GD. Constitutive and chemokine-dependent internalization and recycling of cxcr7 in breast cancer cells to degrade chemokine ligands. Oncogene. 2010;29:4599–4610. doi: 10.1038/onc.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, Rot A, Thelen M. Cxcr7 functions as a scavenger for cxcl12 and cxcl11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 28.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 29.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine cxcl12 regulates monocyte-macrophage differentiation and runx3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.