Mutations in the gene encoding the dopamine-synthetic enzyme GTP cyclohydrolase-1 (GCH1) cause DOPA-responsive dystonia (DRD). Mencacci et al. demonstrate that GCH1 variants are associated with an increased risk of Parkinson's disease in both DRD pedigrees and in patients with Parkinson's disease but without a family history of DRD.
Keywords: GCH1, DOPA-responsive-dystonia, Parkinson’s disease, dopamine, exome sequencing
Abstract
GTP cyclohydrolase 1, encoded by the GCH1 gene, is an essential enzyme for dopamine production in nigrostriatal cells. Loss-of-function mutations in GCH1 result in severe reduction of dopamine synthesis in nigrostriatal cells and are the most common cause of DOPA-responsive dystonia, a rare disease that classically presents in childhood with generalized dystonia and a dramatic long-lasting response to levodopa. We describe clinical, genetic and nigrostriatal dopaminergic imaging ([123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) tropane single photon computed tomography) findings of four unrelated pedigrees with DOPA-responsive dystonia in which pathogenic GCH1 variants were identified in family members with adult-onset parkinsonism. Dopamine transporter imaging was abnormal in all parkinsonian patients, indicating Parkinson’s disease-like nigrostriatal dopaminergic denervation. We subsequently explored the possibility that pathogenic GCH1 variants could contribute to the risk of developing Parkinson’s disease, even in the absence of a family history for DOPA-responsive dystonia. The frequency of GCH1 variants was evaluated in whole-exome sequencing data of 1318 cases with Parkinson’s disease and 5935 control subjects. Combining cases and controls, we identified a total of 11 different heterozygous GCH1 variants, all at low frequency. This list includes four pathogenic variants previously associated with DOPA-responsive dystonia (Q110X, V204I, K224R and M230I) and seven of undetermined clinical relevance (Q110E, T112A, A120S, D134G, I154V, R198Q and G217V). The frequency of GCH1 variants was significantly higher (Fisher’s exact test P-value 0.0001) in cases (10/1318 = 0.75%) than in controls (6/5935 = 0.1%; odds ratio 7.5; 95% confidence interval 2.4–25.3). Our results show that rare GCH1 variants are associated with an increased risk for Parkinson’s disease. These findings expand the clinical and biological relevance of GTP cycloydrolase 1 deficiency, suggesting that it not only leads to biochemical striatal dopamine depletion and DOPA-responsive dystonia, but also predisposes to nigrostriatal cell loss. Further insight into GCH1-associated pathogenetic mechanisms will shed light on the role of dopamine metabolism in nigral degeneration and Parkinson’s disease.
Introduction
Parkinson’s disease is a common neurodegenerative disease mainly characterized by severe loss of dopaminergic neurons in the substantia nigra pars compacta and by the formation of α-synuclein positive aggregates (Lees et al., 2009). Nigral neuron degeneration and consequent decrease in dopaminergic striatal innervation result in classic Parkinson’s disease motor symptoms. Symptomatic treatment with levodopa or dopamine agonists is effective in alleviating these symptoms, although, along with disease progression, levodopa-induced motor complications (e.g. dyskinesias, wearing-off, on-off fluctuations) may appear.
In recent years several Mendelian loci have been unequivocally linked to hereditary forms of Parkinson’s disease (Houlden and Singleton, 2012) and genome-wide association studies have succeeded in identifying many common, low risk variants (Plagnol et al., 2011).
The GCH1 gene (14q22.1-q22.2; OMIM 600225) encodes GTP cyclohydrolase 1, the enzyme controlling the first and rate-limiting step of the biosynthesis of tetrahydrobiopterin (BH4), the essential cofactor for the activity of tyrosine hydroxylase, and for dopamine production in nigrostriatal cells (Kurian et al., 2011). Mutations in GCH1 are the most common cause of DOPA-responsive dystonia (DYT5; OMIM#128230) (Clot et al., 2009), a rare movement disorder that presents typically in childhood with lower limb dystonia and subsequent generalization (Nygaard, 1993b). The hallmark of the disease is an excellent and sustained response to small doses of levodopa, generally without the occurrence of motor fluctuations (Trender-Gerhard et al., 2009). Reduction of CSF levels of pterins, dopamine and serotonin metabolites (Assmann et al., 2003), or an abnormal phenylalanine-loading test (Bandmann et al., 2003) are supportive findings in the diagnosis of DOPA-responsive dystonia. Inheritance is usually autosomal dominant with incomplete penetrance (Furukawa et al., 1998), though recessive cases have been described (Opladen et al., 2011). Dominant GCH1 mutations result in a significant reduction of GCH1 activity through a dominant negative effect of the mutant protein on the normal enzyme (Hwu et al., 2000).
Neuropathological examination in a limited number of cases with DOPA-responsive dystonia, revealed marked reduction of melanin pigment and dopamine content in nigrostriatal neurons, but no evidence of nigral cell loss or degeneration (Furukawa et al., 1999).
Parkinsonian features are frequently detected in patients with DOPA-responsive dystonia (Tassin et al., 2000) and family studies have shown that carriers of GCH1 mutations may develop adult-onset parkinsonism in the absence of dystonia (Nygaard et al., 1990). Based on previous studies, the prevailing hypothesis was that parkinsonism represented an atypical, age-specific, presentation of DOPA-responsive dystonia without nigral degeneration (Nygaard and Wooten, 1998).
The aim of this study was to further explore the relationship between GCH1 mutations and parkinsonism and consider whether adult GCH1 mutation carriers are at increased risk of developing neurodegenerative Parkinson’s disease.
We first describe the clinical, genetic and nigrostriatal dopaminergic imaging findings of DOPA-responsive dystonia pedigrees in which pathogenic GCH1 variants were identified in family members with adult-onset parkinsonism. We subsequently explore the hypothesis that GCH1 variants might be associated with an increased risk for Parkinson’s disease, even without a family history for DOPA-responsive dystonia, through examination of whole-exome sequencing data from a large cohort of cases and controls.
Materials and methods
Family study
Pedigrees
The clinical and demographic features of the pedigrees with GCH1 mutations involved in this study are described in the ‘Results’ section. DOPA-responsive dystonia pedigrees were included in the study, where family members affected with adult-onset parkinsonism were available for clinical and genetic examination and in whom dopaminergic studies had been performed. Local ethics committees approved the study and informed consent for genetic testing was obtained in all cases.
Genetic analysis
Genomic DNA was extracted from peripheral blood leucocytes using standard procedures. Probands were screened for GCH1 mutations (NCBI transcript NM_000161.2) by standard bi-directional Sanger sequencing of all six coding exons and exon-intron boundaries (primer sequences available on request). Dosage analysis for GCH1 exonic deletions and duplications was performed by multiplex ligation-dependent probe amplification (MLPA, MRC).
Dopamine transporter imaging studies
Dopaminergic striatal innervation was evaluated as dopamine reuptake transporter (DAT) density by means of single photon computed tomography (SPECT) and [123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) tropane (123I-FP-CIT). SPECT data acquisition and reconstruction has been described in detail elsewhere (Isaias et al., 2010). To obtain comparable measurements among different centres, 123I-FP-CIT binding values for the caudate nucleus and putamen were calculated by means of the basal ganglia matching tool (Nobili et al., 2013).
Whole-exome sequencing study
Participants and study design
The study initially involved 1337 unrelated subjects with Parkinson’s disease and 1764 control subjects of European origin or North American of European descent that underwent whole-exome sequencing. Cases, originating mainly from the USA, UK, Holland and France, were recruited by the International Parkinson Disease Genomics Consortium (IPDGC), an international collaboration to understand the genetics of Parkinson’s disease.
A further 190 cases with Parkinson’s disease were recruited through a community-based epidemiological study of Parkinson’s disease in Estonia (University of Tartu, Estonia). Cases with Parkinson’s disease were clinically diagnosed according to the UK Parkinson’s Disease Society Brain Bank (UKPDSBB) criteria (Hughes et al., 1992).
Control samples were collected by the UCL-exomes, a consortium of researchers within University College London (London, UK) designed to share raw read level data from multiple exome sequencing projects. Control subjects had no diagnosis of Parkinson’s disease, DOPA-responsive dystonia or any other movement disorder. Whole-exome sequencing data from an additional 4300 North American individuals of European descent were analysed from the publicly available NHLBI Exome Sequencing Project Exome Variant Server (EVS) database (http://evs.gs.washington.edu/EVS/).
Procedures
Paired-end sequence reads (TruSeq chemistry sequenced on the Illumina HiSeq 2000) were aligned with Burrows-Wheeler Aligner (for IPDGC) and novoalign (for UCL-exomes) against the reference human genome (UCSC hg19). Duplicate read removal, format conversion, and indexing were performed with Picard (http://picard.sourceforge.net/). The Genome Analysis Toolkit was used to recalibrate base quality scores, perform local realignments around possible indels, and to call and filter the variants. ANNOVAR software was used to annotate the variants (Wang et al., 2010).
Pathogenicity of the identified missense variants was predicted using the following bioinformatics tools: HumVar-trained PolyPhen-2 model (http://genetics.bwh.harvard.edu/pph2/), SIFT (http://sift.jcvi.org/), LRT (s.wustl.edu/jflab/lrt_query.html) and MutationTaster (http://www.mutationtaster.org/). Evolutionary conservation of the mutated amino acids was evaluated using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Statistical analysis
Frequencies of coding and splice-site GCH1 variants in cases and controls were compared by means of Fisher’s exact (statistical significance set at P-value < 0.05 using a two-tailed test) and odds ratios (OR) and 95% confidence intervals (CI) were calculated. Analyses were performed using the statistical analysis program R (http://www.r-project.org/).
Results
Family study
Family A
The proband (Case III-1, Fig. 1A) is a British 18-year-old male who had a difficult caesarean birth, with perinatal distress and subsequent developmental delay. At 18 months he developed inward turning of his feet with walking difficulties and frequent falls. He was diagnosed clinically with DOPA-responsive dystonia at the age of 3 years and administration of levodopa (300 mg/day) markedly improved his symptoms. [123I]FP-CIT SPECT, performed at age 17, was normal (data not shown).
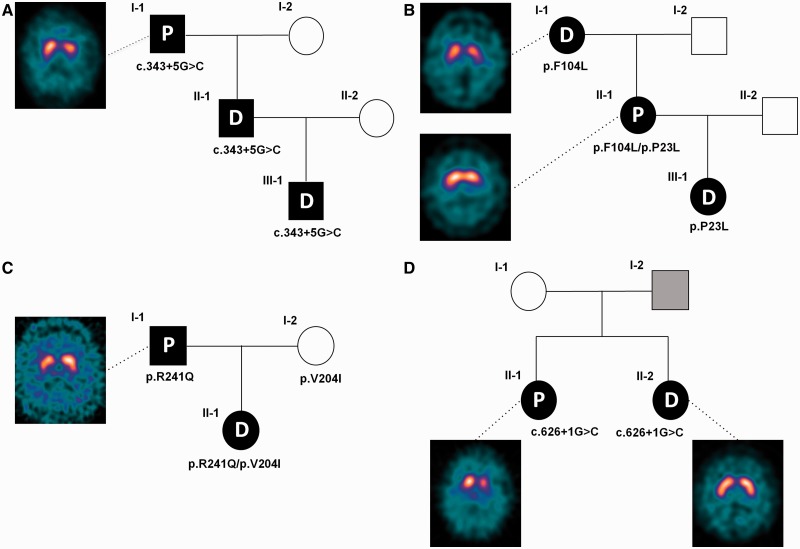
Figure 1.
Pedigrees and 123I-FP-CIT SPECT scan images of the four families with GCH1 mutations involved in this study. Subject I-2 of Family D was reported to be affected by a movement disorder (hand tremor) but was not available for clinical or genetic assessment. P = Parkinson’s disease; D = DOPA-responsive dystonia.
The proband’s father (Case II-1), who was initially thought to have cerebral palsy due to a birth injury, was subsequently diagnosed, at the age of 42, with DOPA-responsive dystonia. The proband’s grandfather (Case I-1) is a 65 year-old male with a 6-year history of progressive asymmetric rest tremor in the right upper limb. Examination showed signs of typical Parkinson’s disease with hypomimia, unilateral rest tremor and asymmetric bradykinesia. He did not present signs of dystonia. 123I-FP-CIT SPECT showed bilateral reduced tracer uptake more marked on the left (Fig. 1A), consistent with nigrostriatal dopaminergic denervation. He responded well to levodopa therapy (300 mg/day).
GCH1 analysis revealed a heterozygous splice site mutation (c.343+5G>C) in the three affected individuals. We previously detected c.343+5G>C in a recessive pedigree, carried by the unaffected mother of two very severely affected children who also inherited the K224R mutation from their unaffected father (Bandmann et al., 1996b; Trender-Gerhard et al., 2009). However the c.343+5G>C mutation has not been previously described in DOPA-responsive dystonia dominant pedigrees, making its pathogenicity uncertain. Complimentary DNA analysis showed aberrant splicing resulting in a premature stop codon and retention of intron 1 in a proportion of mutant transcripts, confirming the loss-of-function effect of the variant. See Supplementary material for details of the RNA analysis.
Family B
The proband (Case III-1; see Fig. 1B) is a 12-year-old right-handed female of German origin with DOPA-responsive dystonia, with an onset at age 11, with writing and foot dystonia. Her mother (Case II-1) presented at age 39 with progressive loss of dexterity and slowness in her right arm and dystonic posturing of the right foot. Examination showed an asymmetric rigid-akinetic parkinsonian syndrome without tremor and severe right foot fixed dystonia. Levodopa therapy resulted in marked improvement of both dystonic and parkinsonian symptoms. 123I-FP-CIT SPECT revealed an asymmetric bilateral reduced tracer uptake, more marked in the left striatum. There was sustained response to levodopa therapy although there was an increase in dose requirement (up to 800 mg/day). Levodopa-induced dyskinesias developed 6 years after initiation of levodopa. Examination of the proband’s 66-year-old grandmother (Case I-1) revealed oromandibular dyskinesias and upper limb dystonic features. She declined a trial of levodopa. Her 123I-FP-CIT SPECT displayed border-line reduced DAT values in both putamens.
GCH1 screening in this family revealed two variants: c.68C>T;p.P23L (carried by Cases III-1 and II-1) and c.312C>A;p.F104L (carried by Cases II-1 and I-1). There were no GCH1 exonic rearrangements. F104L is absent in public control data sets and has been previously reported in association with DOPA-responsive dystonia (Clot et al., 2009). P23L (rs41298432) is a benign polymorphism present in population controls at a frequency of 1–2% (Jarman et al., 1997; Hauf et al., 2000).
To confirm GCH1 deficiency, phenylalanine-loading test (100 mg/kg) was performed in Cases I-I and II-I and showed pathologically elevated phenylalanine/tyrosine ratios in both (Supplementary Fig. 2). CSF analysis, performed in Case III-I, displayed low levels of BH4 (13 nmol/l; 18–53 nmol/l) and neopterin (6 nmol/l; 10–31 nmol/l), consistent with GCH1 deficiency. Given the benign nature of P23L, we hypothesize that the GCH1 deficiency confirmed in this patient may be the result of an—as yet—unidentified non-coding causative mutation.
Family C
The proband (Case II-1, Fig. 1C) is a German 41-year-old female, affected by DOPA-responsive dystonia, who presented at age 4 years with bilateral foot inversion on walking. Her father (Case I-1) is a 67-year old male with a 1-year history of typical Parkinson’s disease with left hand rest tremor, bilateral rigidity and bradykinesia and mild gait difficulties. There was no dystonia. 123I-FP-CIT SPECT examination revealed asymmetrically reduced DAT-density in the striatum. Rasagiline and pramipexole were started with good response. The mother (Case I-2), aged 62 years, had a normal neurological examination.
The proband was compound heterozygous for two GCH1 missense variants, c.610G>A;p.V204I, inherited from the asymptomatic mother, and the novel variant c.722G>A;p.R241Q, which was paternally inherited. R241Q is absent in public control data sets, is predicted deleterious by all in silico prediction tools and involves an amino acid residue conserved down to invertebrate species. Furthermore a pathogenic mutation at the same residue has already been reported (Bandmann et al., 1998).
CSF analysis in the parkinsonian case supported a pathogenic effect of the R241Q mutation on GCH1 activity: pterin analysis revealed low BH4 (8 nmol/l; 18–53), but normal neopterin (24 nmol/l; 10–31); neurotransmitter analysis showed low homovanillic acid (95 nmol/l; 115–455) and 5-hydroxyindolacetic acid (59 nmol/l; 61–204), which are metabolites of dopamine and serotonin, respectively.
Family D
The proband is an Italian 58-year-old female (Case II-1, Fig. 1D), who developed progressive tremor and clumsiness in the right arm at age 44 years. Clinical examination showed typical Parkinson’s disease with hypomimia, hypophonia and asymmetrical bradykinesia and rigidity. Action dystonic tremor (right > left), poor postural reflexes and slow gait were also evident and there was a sustained response to levodopa. The dose was gradually increased up to 400 mg/day, after which rotigotine 4 mg/day was added. Dyskinesias and wearing-off symptoms developed 6 years after levodopa initiation. 123I-FP-CIT SPECT revealed asymmetrically reduced DAT binding values in the striatum.
Her sister (Case II-2; Fig. 1D), aged 60, had a childhood onset of mild walking difficulties. At age 55, she developed exercise-induced left foot dystonia and dystonic tremor in both arms. She had no bradykinesia or other parkinsonian signs. Low-dose levodopa (100 mg alternate days) was started with excellent symptom control. 123I-FP-CIT SPECT was normal. Their father was reported to have a tremulous condition, but was not available for clinical or genetic examination. GCH1 sequencing revealed that both sisters were heterozygous for the previously reported pathogenic mutation c.626+1G>C (Garavaglia et al., 2004).
The main clinical features of the GCH1 mutation carriers with adult-onset parkinsonism and abnormal 123I-FP-CIT SPECT imaging are summarized in Table 1. Their clinical features fully met the UKPDSBB criteria for definite Parkinson’s disease diagnosis. None of these cases presented significant diurnal fluctuations, worsening of symptoms in the evening or substantial sleep benefit, features often recognized in cases with DOPA-responsive dystonia (Kurian et al., 2011). DAT binding values are reported in Supplementary Table 1.
Table 1.
Characteristics of parkinsonian cases with GCH1 pathogenic variants and abnormal dopaminergic imaging described in this study and present in the literature
| Origin | Sex/age at scan/age at onset (y) | Mutation | Relatives with DRD | Age at levodopa start (y) | Current treatment dose (mg/day) | Parkinsonian features | H&Y score | Dystonic features | Levodopa-induced complications | Scan result | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UK | M/65/59 | c.343+5G>C/w | Son and grandson | 60 | l-DOPA 300 | Hypomimia, R hand rest and re-emergent postural tremor, and bilateral rigidity and bradykinesia (R>L) | 2 | No | No | Bilateral (L>R) reduced DAT density | Present study (Family A) |
| Germany | F/47/39 | F104L/ P23L | Daughter and mother | 41 | l-DOPA 800 | Hypomimia, bilateral rigidity, bradykinesia, reduced arm swinging (R>L), and mild gait difficulties | 2 | R foot dystonia |
|
Bilateral (L>R) reduced DAT density | Present study (Family B) |
| Germany | M/67/66 | R241Q/w | Daughter | / | Rasagiline 1 Pramipexole 0.375 | Hypomimia, L hand rest tremor, bilateral bradykinesia and rigidity (L>R), and mild gait difficulties | 2 | No | No | Bilateral (R>L) reduced DAT-density | Present study (Family C) |
| Italy | F/58/44 | c.626+1 G>C/w | Sister | 53 |
|
Hypomimia, bilateral rigidity and bradykinesia (R>L), mild postural instability, and gait difficulties | 2 | Bilateral (R>L) upper limb dystonic tremor |
|
Bilateral (L>R) reduced DAT density | Present study (Family D) |
| Japan | M/54/39 | R184H/w | No | 40 | l-DOPA 600 | Cogwheel rigidity, akinesia, and postural instability | NA | Dystonic posture in the four limbs (R>L) |
|
Bilateral reduced FD intake | Kikuchi et al., 2004 |
| Denmark | M/38/28 | P199S/w | Brother | 33 |
|
Bradykinesia and rigidity in the L arm | NA | Dystonia of neck, trunk and four limbs, action tremor (L>R) |
|
Bilateral (R>L) reduced DAT density | Hjermind et al., 2006 |
| Germany | F/65/50 | Complete deletion of the GCH1 gene/w | Daughter | 60 (for 10 y on dopamine agonist only) |
|
Tremor in the R hand, reduced dexterity and mild gait disturbance | NA | No | No | Bilateral (L>R) reduced DAT density | Eggers et al., 2012 |
| Italy | M/59/NA | Deletion of exons 5-6/w | Son with DRD, sister with MSA | NA | NA | Hypomimia, L hand rest tremor. bradykinesia (L>R), mild gait difficulties | NA | No | Dyskinesias after 10 y of therapy | Bilateral reduced DAT density | Ceravolo et al., 2013 |
NA = not available; DRD = DOPA-responsive dystonia; H&Y = Hoehn and Yahr; F = female; M = male L = left; R = right; MSA = multiple system atrophy; y = years; w = wild-type.
Whole-exome sequencing study
We hypothesized that pathogenic variants in GCH1 could be found in subjects with Parkinson’s disease without a family history for DOPA-responsive dystonia. To investigate this we examined whole-exome sequencing data of a large cohort of patients predominantly affected by early-onset or familial Parkinson’s disease and controls. After quality control checks (removal of gender mismatches, duplicate, related and non-Caucasian samples, samples with low call rate or excess of heterozygosity), 1318 cases with Parkinson’s disease and 1635 controls remained. Additional control data (n = 4300) were obtained from the publically available Exome Variant Server (EVS) data set.
In total 1318 cases and 5935 controls were analysed for the presence of GCH1 coding (including small insertions/deletions, missense and stop-gain changes) or splice-site variants (± 5 base pairs from the coding exons). The mean age of subjects with Parkinson’s disease was 55.7 ± 13.9 years (range 17–101; data available for 970 cases) and the mean age at onset was 46.7 ± 13.8 years (range 6–98; data available for 1194 cases). Four hundred and twenty-three of 1194 (35.4%) were early-onset cases (age at onset ≤ 40 years) and ∼630 were familial cases (positive family history for Parkinson’s disease in a first or second-degree relative).
Coverage of the six GCH1 coding exons (NCBI transcript NM_000161.2) was comparable in the three data sets (IPDGC, UCL-ex and EVS; Supplementary Table 2). No common variants (frequency >1%) were identified. The benign polymorphisms P23L (rs41298432) and P69L (rs56127440), detected at similar frequencies in cases and controls, were not included in the analysis.
The main results of GCH1 analysis are summarized in Table 2. Combining cases and controls, 11 unique heterozygous GCH1 variants (10 missense and one stop-gain mutation) were identified in 16 individuals. Six variants were found only in cases with Parkinson’s disease (Q110X, Q110E, A120S, D134G, G217V and M230I), three in controls alone (T112A, I154V and R198Q) and two were detected in both groups (V204I, K224R). The frequency of GCH1 variants was significantly higher in cases with Parkinson’s disease (10/1318; 0.75%) than in individual (UCL-ex controls 1/1635; 0.06%; P = 0.003; OR 12.4 95% CI 1.7–541.1; EVS database 5/4300; 0.11%; P = 0.0004; OR 6.5, 95% CI 2.0–24.5) and combined data sets of controls (6/5935; 0.1%; P = 0.0001; OR 7.5, 95% CI 2.4–25.3).
Table 2.
List of GCH1 variants identified by exome sequencing in patients with Parkinson disease and controlsa
| Mutation | Exon | dbSNP | Prediction scoreb | Previously described in DRD? | PD patients (n = 1318) | UCL-ex controls (n = 1635) | OR (95% CI) | P-value | EVS controls (n = 4300) | OR (95% CI) | P-value | Total controls (n = 5935) | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All variants | 10 (0.75%) | 1 (0.06%) | 12.4 (1.7–541.1) | 0.003 | 5 (0.11%) | 6.5 (2.0–24.5) | 0.0004 | 6 (0.1%) | 7.5 (2.4–25.3) | 0.0001 | ||||
| c.328C>T; p.Q110X | 1 | NA | Yes, in dominant and recessive pedigrees | 1 | 0 | 0 | 0 | |||||||
| c.328C>G; p.Q110E | 1 | 2 | No | 1 | 0 | 0 | 0 | |||||||
| c.334A>G; p.T112A | 2 | rs199990434 | 2 | No | 0 | 0 | 1 | 1 | ||||||
| c.358G>T; p.A120S | 2 | 4 | No | 1 | 0 | 0 | 0 | |||||||
| c.401A>G; p.D134G | 2 | 4 | No | 1 | 0 | 0 | 0 | |||||||
| c.460A>G; p.I154V | 3 | 2 | No | 0 | 1 | 0 | 1 | |||||||
| c.593G>A; p.R198Q | 5 | rs201238926 | 0 | No | 0 | 0 | 1 | 1 | ||||||
| c.610G>A; p.V204I | 5 | rs200891969 | 4 | Yes, in sporadic and recessive cases | 3 | 0 | 1 | 1 | ||||||
| c.650G>T; p.G217V | 6 | 4 | No | 1 | 0 | 0 | 0 | |||||||
| c.671A>G; p.K224R | 6 | rs41298442 | 2 | Yes, in dominant and recessive pedigrees | 1 | 0 | 2 | 2 | ||||||
| c.690G>A; p.M230I | 6 | 4 | Yes, in a sporadic case | 1 | 0 | 0 | 0 |
NA = not applicable; DRD = DOPA-responsive dystonia; PD = Parkinson disease; UCL-ex = University College of London exomes consortium; EVS = Exome Variant Server.
P-values were calculated by means of Fisher’s exact test.
a NCBI transcript NM_000161.2..This count includes all detected coding and splice-site variants at any frequency, but the two benign variants P23L and P69L.
b This score, ranging from 0 to 4, indicates the number of tools (Polyphen-2, SIFT, LRT and MutationTaster) predicting a pathogenic effect on the protein function.
All carriers of variants in GCH1 were negative for pathogenic mutations in the known genes associated with Mendelian forms of parkinsonism (SNCA, LRRK2, VPS35, PARK2, PARK7, PINK1, ATP13A2, PLA2G6 and FBXO7). The presence of copy number variants in the SNCA, PARK2, PARK7, and PINK1 genes was excluded by MLPA in all cases.
One case was heterozygous for the GBA mutation E326K. This is a relatively common variant (∼1–2% Caucasians) that was recently shown to be associated with a modest but significant increase in the disease risk (Duran et al., 2013). The main features of the 10 cases with Parkinson’s disease with pathogenic or possibly pathogenic GCH1 variants are listed in Table 3.
Table 3.
Clinical features of Parkinson disease cases with GCH1 variants identified in the exome-sequencing study
| Case | Origin | Sex/age/age at onset (y) | GCH1 mutation | Family history of PD | Age at l-DOPA start (y) | Current treatment (mg/day) | Parkinsonian features | l-DOPA responsi-veness | H&Y score | Cognitive symptoms | Other non-motor features | Dystonia | Levodopa-induced complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | USA | F/47/43 | M230I/w | No | / | Pramipexole 0.75 | Asymmetric onset, bilateral involvement with rest and postural tremor, bradykinesia and rigidity, mild gait difficulties | Good | 2 | No | No | No | No |
| 2 | USA | M/55/37 | K224R/w | Yes (father) | NA | NA | Asymmetric onset, moderate bilateral involvement with rest tremor, bradykinesia and rigidity, postural instability and gait difficulties | Good | 3 | No | Fatigue | No | Dyskinesias and wearing-off |
| 3 | Holland | M/49/35 | G217V/w | No | 43 |
|
Asymmetric onset, slurred speech, mild L arm rest and postural tremor, moderate bilateral bradykinesia and rigidity, postural instability | Good | 3 | Subjective loss of memory (MMSE 29/30) | Hyposmia, ICD | No | Initial dyskinesias and wearing-off |
| 4 | UK | M/63/32 | V204I/w | Yes (1st degree cousin) | 36 |
|
Asymmetric onset, hypomimia, slurred speech, hypophonia, marked bilateral rest and postural tremor, moderate bilateral rigidity and bradykinesia, postural instability | Good | 4 | No | Hyposmia, constipation, RBD | Right foot exercise-induced dystonia at onset | Disabling dyskinesias and on-off fluctuations |
| 5 | Estonia | M/75/61 | V204I/w | Yes (mother) | 61 |
|
Asymmetric onset, rest and postural tremor (R>L), bradykinesia and rigidity, mild gait disorder, hypomimia | Good | 3 | Mild cognitive impairment | Hyposmia, fatigue, sleep and bladder disorder | Lower limb off-dystonia | On-off fluctuations (30% of waking day in off-state) |
| 6 | Estonia | M/72/59 | V204I/w | Yes (mother) | 65 |
|
Asymmetric onset, bilateral bradykinesia and rigidity (L>R), no tremor. Mild gait difficulties and postural instability | Good | 3 | No | Hyposmia, sleep and bladder disorder | No | Dyskinesias (30-40% of waking day), wearing-off |
| 7 | USA | M/57/52 | D134G/w | Yes (father and paternal aunt) | / | Ropinirole 14 | Asymmetric onset, unilateral left arm rest tremor, bradykinesia and rigidity. Reduced arm swing | Good | 1 | No | NA | No | No |
| 8 | USA | F/59/51 | A120S/wa | Yes (mother) | NA | NA | Asymmetric onset, bilateral bradykinesia and rigidity. No tremor. Mild gait difficulties | Good | 2 | No | NA | No | NA |
| 9 | Portugal | F/73/17 | Q110X/w | Yes (sister; father had tremor) | 49 | l-DOPA 600 Trihexyphenidyl 6 | Bilateral rest and postural tremor (L>R), bilateral rigidity and bradykinesia. Some postural instability | Good | 3 | No | Urinary urgency | Lower limb dystonia at onset | Marked limb and truncal dyskinesias, off phases in the morning |
| 10 | Estonia | M/58/45 | Q110E/w | No | 55 |
|
Bilateral severe bradykinesia and rigidity, postural instability, mild tremor, hypomimia | Good | 3 | No | Hyposmia, constipation, fatigue, sleep disorder | No | Mild dyskinesias and wearing-off |
NA = information not available; M = male; F = female; PD = Parkinson disease, y = years, ICD = Impulse control disorder, DBS = deep brain stimulation, RBD = REM behavioural sleep disorder, H&Y = Hoehn and Yahr; MMSE = Mini-Mental State Examination.
aThis case also carries in the heterozygous state the GBA E326K variant.
The age at onset of GCH1-mutated cases was 43.2 ± 13.4 years (range 17–61). Seven had a positive family history of Parkinson’s disease. DNA of other family members was available for only one case and we showed segregation of the same GCH1 mutation (Q110X) in the affected sister of the index case. All cases exhibited a variable combination of asymmetrical bradykinesia, rigidity, rest and postural tremor, walking difficulties, postural instability and excellent response to dopaminergic treatment, consistent with a clinical diagnosis of Parkinson’s disease.
The two subjects with the youngest age at onset of symptoms (Cases 4 and 9, who developed symptoms at age 32 and 17, respectively) presented with dystonic features in the lower limbs at onset, a well recognized characteristic of young-onset Parkinson’s disease cases (Bozi and Bhatia, 2003). Case 5 developed lower limb dystonia in off periods over the course of the disease. The remainder did not present with any symptoms or signs of dystonia.
Detailed information about treatment was available for eight cases: the two cases (Cases 1 and 7) with the shortest disease duration (≤5 years) were treated only with a dopamine-agonist, whereas the other cases were taking a combination of levodopa and other anti-parkinsonian drugs. Mean disease duration was 17.6 ± 15.4 years (range 4–56). Cases with longer disease duration displayed a more severe clinical picture with some degree of postural instability (Hoehn and Yahr score ≥ 3), indicating disease progression in spite of the dopaminergic treatment.
In those patients taking levodopa and for whom follow-up information was available (n = 7), all developed clinically relevant motor complications of chronic levodopa treatment, including wearing off, motor fluctuations and dyskinesias. Dyskinesias in Case 4 were so disabling that he required treatment with deep brain stimulation of the subthalamic nuclei at age 60.
Most cases exhibited some of the typical non-motor features often recognized in Parkinson’s disease (Lees et al., 2009), such as cognitive difficulties (Case 5), hyposmia (Cases 3–6 and 10) constipation (Cases 4 and 10), urinary problems (Cases 5, 6 and 9), fatigue (Cases 2 and 5) and sleep disturbances (Cases 4–6 and 10).
Discussion
Family study
We report here four unrelated DOPA-responsive dystonia pedigrees in which loss-of-function GCH1 mutations (two splice-site mutations and two missense mutations, confirmed to be pathogenic by metabolic or CSF studies) were found in individuals, asymptomatic for DOPA-responsive dystonia during childhood, who developed adult-onset parkinsonism. They all met the UKPDSBB clinical criteria for a definite diagnosis of Parkinson’s disease and had imaging evidence of a Parkinson’s disease-like nigrostriatal dopaminergic denervation.
A parkinsonian syndrome in the absence of dystonia has been reported in adults who are first-degree relatives of children with DOPA-responsive dystonia. In a series of 21 families, Nygaard showed that 7/50 (14%) individuals older than 40 years had parkinsonism (Nygaard, 1993a) and Hagenah et al. (2005) reported that 8/23 (34.7%) patients of their series had a positive family history for Parkinson’s disease. GCH1 mutations have also been shown to segregate in pedigrees with multiple individuals affected by isolated parkinsonism (Irie et al., 2011).
Our study provides evidence that in most of the cases the parkinsonian phenotype in adult GCH1 mutation carriers is likely due to nigrostriatal degeneration, rather than being simply part of the phenotypic spectrum of metabolic GCH1-related striatal dopamine deficiency. This is consistent with other previous isolated reports of adult-onset parkinsonism in GCH1 mutation carriers with abnormal nigrostriatal imaging (features summarized in Table 1) (Kikuchi et al., 2004; Hjermind et al., 2006; Eggers et al., 2012; Ceravolo et al., 2013).
Our imaging findings are, however, in apparent contrast to a previous report by Nygaard et al. (1992). The authors described a large DOPA-responsive dystonia pedigree, in which three subjects had a late-onset benign parkinsonism, two of which had normal nigrostriatal dopaminergic function determined by means of 18F-fluorodopa PET.
Compensatory mechanisms at the presynaptic level (e.g. increased dopamine-intake and dopamine-decarboxylation activity) may result in relatively higher striatal 18F-fluorodopa uptake in the initial phase of Parkinson’s disease, underestimating the degree of nigral cell decrease (Nandhagopal et al., 2011). DAT values are therefore a more precise indicator of dopaminergic innervation loss (Ito et al., 1999). We speculate that GCH1-parkinsonian cases with normal 18F-fluorodopa-PET scan could have upregulated compensatory dopaminergic activity at the presynaptic level, possibly masking the presence of striatal denervation.
In agreement with our findings, Gibb and Lees reported in 1991 a case that presented with juvenile-onset parkinsonism and dystonia with good response to levodopa (commenced at the age of 30) and occurrence of disabling dyskinesias after 1 year of treatment. The patient died at 39 years and pathological examination showed a striking combination of low melanin content in nigral neurons and devastating neuronal loss with reactive gliosis. Furthermore, Lewy bodies were found in surviving nigral cells and in the locus coeruleus (Gibb et al., 1991). This case was subsequently demonstrated to be carrier of a heterozygous mutation in GCH1 (c.276delC) (Segawa et al., 2004).
Whole-exome sequencing study
We subsequently showed, in a large cohort of patients with Parkinson’s disease without family history of DOPA-responsive dystonia, that rare GCH1 coding variants are associated with Parkinson’s disease and increase the disease risk by 7-fold on average.
Among the GCH1 variants identified by exome sequencing, two (Q110X and K224R) have been shown to cause GCH1 deficiency and DOPA-responsive dystonia in dominant pedigrees (Leuzzi et al., 2002; Saunders-Pullman et al., 2004) and two (V204I and M230I) have been reported in heterozygous sporadic or in recessive cases with DOPA-responsive dystonia (Segawa et al., 2004; Trender-Gerhard et al., 2009; Opladen et al., 2011).
It was not possible to functionally investigate (e.g. phenylalanine-loading test or CSF analysis) the other heterozygous variants identified in this study, therefore their effect on GCH1 activity remains undetermined. However, three of the four novel variants (A120S, D134G and G217V) detected in cases with Parkinson’s disease were located at amino acid positions that are fully conserved through species down to invertebrates and were predicted to be pathogenic by all in silico prediction tools, whereas this was not the case for any of the novel mutations present in controls.
Nevertheless, the limitations of prediction tools in reliably distinguishing benign from pathogenic missense changes are well known and therefore we did not exclude any variant from the association test based on predictions scores, possibly underestimating the effect size of GCH1 pathogenic variants.
Previous studies investigating the contribution of rare coding GCH1 variants in small cohorts of cases with Parkinson’s disease have reported negative results although these were insufficiently powered to draw conclusions (Bandmann et al., 1996a; Hertz et al., 2006; Cobb et al., 2009). An as-yet unpublished meta-analysis of existing genome-wide association study data has, however, identified GCH1 as a common low-risk locus (Singleton, personal communication), consistent with the hypothesis of a causal role for GCH1 in Parkinson’s disease.
The mechanism whereby GCH1 mutations could predispose to nigral cell degeneration is uncertain. Biochemical evidence of GCH1 deficiency and reduced dopamine production has been reported in asymptomatic carriers of GCH1 mutations (Takahashi et al., 1994; Furukawa et al., 2002). We speculate that GCH1 deficiency and the consequent chronic dopamine deficiency could directly predispose to nigral cell death. This would suggest that normal levels of dopamine exert a protective role on the survival of nigral neurons. There is increasing evidence that levodopa is not toxic to nigral neurons as was previously thought (Parkkinen et al., 2011). Furthermore, activation of dopamine receptors may have a strong anti-apoptotic effect and increase survival of dopaminergic neurons (Nair et al., 2003; Vaarmann et al., 2013). In animal models, levodopa has been shown to promote recovery of nigrostriatal denervation (Datla et al., 2001).
Another possibility is that GCH1 mutation carriers who do not develop symptoms of DOPA-responsive dystonia in childhood may have compensatory mechanisms that allow for normal nigrostriatal dopaminergic transmission. The maintenance of these mechanisms may increase nigral cell vulnerability to ageing or other environmental and genetic factors, favouring degeneration.
It is also possible that the reduced striatal basal dopamine levels found in GCH1 mutation carriers may simply lower the threshold of nigral cell loss before parkinsonian symptoms are exhibited. Lastly, we cannot exclude that other yet unrecognized cellular pathways, not related to dopamine synthesis, may be disrupted by GCH1 and BH4 deficiency. However, the observation that no DOPA-responsive dystonia cases, treated with levodopa since childhood, have been shown to develop nigral cell loss (Snow et al., 1993; Turjanski et al., 1993; Jeon et al., 1998), supports the notion that levodopa may indeed have a role in reducing the risk of degeneration.
Limitations of the study
First, dopamine transporter imaging was not available for the cases with Parkinson’s disease with GCH1 variants identified in the exome sequencing study. It remains a possibility therefore that some of these cases (in particular Case 9, who presented at age 17, with lower limb dystonia and parkinsonism) may represent DOPA-responsive dystonia cases with a parkinsonian phenotype, which may have been misdiagnosed as Parkinson’s disease.
However, removal of the aforementioned case from the statistical analysis did not change substantially the significance of the association (P = 0.0003). Furthermore, most of the patients for whom clinical follow-up data were available showed a progressive disease course with increasing levodopa requirements, emergence of motor complications due to chronic treatment with levodopa and presence of classic non-motor features of Parkinson’s disease, strongly supporting nigrostriatal cell loss as the underlying pathology.
Although dyskinesias have been rarely described also in DOPA-responsive dystonia cases, these are significantly different from the ones generally observed in Parkinson’s disease. Indeed they tend to appear at the beginning of the treatment and subside after dose reduction without reoccurring with subsequent slow dose increase (Furukawa et al., 2004; Lee et al., 2013). Second, we could not determine at the individual level the effect on pterin and dopamine metabolism of the GCH1 variants detected in the exome sequencing study. Reduced penetrance of GCH1 pathogenic variants for the DOPA-responsive dystonia phenotype is a well-established feature. Nevertheless it has been repeatedly reported, through analysis of brain tissue (Furukawa et al., 2002), CSF (Takahashi et al., 1994) and urine (Leuzzi et al., 2013), that even completely asymptomatic carriers of GCH1 mutations have abnormal metabolism of biopterins and dopamine, although to a lesser extent than DOPA-responsive dystonia cases. This indicates the existence of a metabolic endophenotype, which we speculate could be the pathogenic mechanism underlying the increased risk for Parkinson’s disease.
Third, we evaluated a cohort enriched with early-onset and familial Parkinson’s disease cases. Thus the frequency of detected GCH1 variants may not reflect the frequency in late-onset sporadic cases. Finally, we did not assess our samples for the presence of GCH1 copy number variants, possibly underestimating the frequency of GCH1 mutations.
Conclusion
We provide evidence that rare GCH1 coding variants should be considered as a risk factor for Parkinson’s disease. This is derived both from imaging evidence of striatal dopaminergic denervation in GCH1 pathogenic variant carriers with a clinical diagnosis of definite Parkinson’s disease (in DOPA-responsive dystonia pedigrees) and from exome sequencing data that show a significant association between GCH1 coding variants and an increased risk for the disease.
These findings expand the clinical and biological relevance of GCH1 deficiency, suggesting a role not only in biochemical dopamine depletion and DOPA-responsive dystonia, but also in nigrostriatal degeneration. The question as to how the same variants known to cause a Mendelian disease may also exist as risk alleles in Parkinson’s disease may be explained by the well-known reduced penetrance of GCH1 pathogenic variants. Whether additional genetic or epigenetic factors play a role in determining the clinical phenotype of GCH1 variant carriers should be addressed by future studies. A better understanding of the relationship between GCH1 deficiency and Parkinson’s disease will shed light on the role of dopamine metabolism on nigral neuron survival, with potential therapeutic implications for patients.
Supplementary Material
Acknowledgements
We used DNA panels, samples, and clinical data from the National Institute of Neurological Disorders and Stroke Human Genetics Resource Centre DNA and Cell Line Repository. People who contributed samples are acknowledged in descriptions of every panel on the repository website. We would like to thank the NINDS sponsored Neurogenetics Repository hosted by Coriell Cell Repositories for the use of both case and control samples. The authors also thank the French Parkinson’s Disease Genetics Study Group: Y. Agid, M. Anheim, A-M. Bonnet, M. Borg, A. Brice, E. Broussolle, J-C. Corvol, P. Damier, A. Destée, A. Dürr, F. Durif, S. Klebe, E. Lohmann, M. Martinez, P. Pollak, O. Rascol, F. Tison, C. Tranchant, M. Vérin, F. Viallet, and M. Vidailhet.
Glossary
Abbreviations
- BH4
tetrahydrobiopterin
- DAT
dopamine-transporter
- 123I-FP-CIT
[123I]N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) tropane
- SPECT
single photon computed tomography
Funding
This study was supported by the Wellcome Trust/Medical Research Council (MRC) Joint Call in Neurodegeneration award (WT089698) to the UK Parkinson's Disease Consortium whose members are from the UCL/Institute of Neurology, the University of Sheffield, and the MRC Protein Phosphorylation Unit at the University of Dundee. This project was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and the Grigioni Foundation for Parkinson Disease. This work was also supported in part by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Aging (NIA), and the National Institute of Environmental Health Sciences both part of the National Institutes of Health, Department of Health and Human Services; project numbers Z01-AG000949-02 and Z01-ES101986. In addition this work was supported by the Department of Defense (award W81XWH-09-2-0128), and the Michael J Fox Foundation for Parkinson’s Disease Research. This work was supported by National Institutes of Health grants R01NS037167, R01CA141668, American Parkinson Disease Association (APDA); Barnes Jewish Hospital Foundation; Greater St Louis Chapter of the APDA; Hersenstichting Nederland; Neuroscience Campus Amsterdam; the Deutsche Forschungsgemeinschaft (SFB 936); and the section of medical genomics, the Prinses Beatrix Fonds. The KORA (Cooperative Research in the Region of Augsburg) research platform was started and financed by the Forschungszentrum für Umwelt und Gesundheit, which is funded by the German Federal Ministry of Education, Science, Research, and Technology and by the State of Bavaria. This study was also funded by the German National Genome Network (NGFNplus number 01GS08134, German Ministry for Education and Research); by the German Federal Ministry of Education and Research (NGFN 01GR0468, PopGen); and 01EW0908 in the frame of ERA-NET NEURON and Helmholtz Alliance Mental Health in an Ageing Society (HA-215), which was funded by the Initiative and Networking Fund of the Helmholtz Association. As with previous IPDGC efforts, this study makes use of data generated by the Wellcome Trust Case-Control Consortium. A full list of the investigators who contributed to the generation of the data is available from www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 076113, 085475 and 090355. The work was also funded in part by Parkinson's UK (Grants 8047 and J-1101) and the Medical Research Council UK (G0700943, G1100643) for H.R.M and S.J.L. DNA extraction work that was done in the UK was undertaken at University College London Hospitals, University College London, who received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding. This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Md. (http://biowulf.nih.gov). This work was also supported by the France-Parkinson Association and the French program “Investissements d’avenir” funding (ANR-10-IAIHU-06). This study was also partly supported by the Grant 3.2.1001.11-0017 of the EU European Regional Development Fund and the Grant IUT2-4 of the Estonian Research Council. The study was also partially supported by European Regional Development Fund in the frame of Centre of Excellence for Translational Medicine.
Supplementary material
Supplementary material is available at Brain online.
References
- Assmann B, Surtees R, Hoffmann GF. Approach to the diagnosis of neurotransmitter diseases exemplified by the differential diagnosis of childhood-onset dystonia. Ann Neurol. 2003;54(Suppl 6):S18–24. doi: 10.1002/ana.10628. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Daniel S, Marsden CD, Wood NW, Harding AE. The GTP-cyclohydrolase I gene in atypical parkinsonian patients: a clinico-genetic study. J Neurol Sci. 1996a;141:27–32. doi: 10.1016/0022-510x(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Goertz M, Zschocke J, Deuschl G, Jost W, Hefter H, et al. The phenylalanine loading test in the differential diagnosis of dystonia. Neurology. 2003;60:700–2. doi: 10.1212/01.wnl.0000048205.18445.98. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Nygaard TG, Surtees R, Marsden CD, Wood NW, Harding AE. Dopa-responsive dystonia in British patients: new mutations of the GTP-cyclohydrolase I gene and evidence for genetic heterogeneity. Hum Mol Genet. 1996b;5:403–6. doi: 10.1093/hmg/5.3.403. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Valente EM, Holmans P, Surtees RA, Walters JH, Wevers RA, et al. Dopa-responsive dystonia: a clinical and molecular genetic study. Ann Neurol. 1998;44:649–56. doi: 10.1002/ana.410440411. [DOI] [PubMed] [Google Scholar]
- Bozi M, Bhatia KP. Paroxysmal exercise-induced dystonia as a presenting feature of young-onset Parkinson's disease. Mov Disord. 2003;18:1545–7. doi: 10.1002/mds.10597. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Nicoletti V, Garavaglia B, Reale C, Kiferle L, Bonuccelli U. Expanding the clinical phenotype of DYT5 mutations: is multiple system atrophy a possible one? Neurology. 2013;81:301–2. doi: 10.1212/WNL.0b013e31829bfd7c. [DOI] [PubMed] [Google Scholar]
- Clot F, Grabli D, Cazeneuve C, Roze E, Castelnau P, Chabrol B, et al. Exhaustive analysis of BH4 and dopamine biosynthesis genes in patients with Dopa-responsive dystonia. Brain. 2009;132:1753–63. doi: 10.1093/brain/awp084. [DOI] [PubMed] [Google Scholar]
- Cobb SA, Wider C, Ross OA, Mata IF, Adler CH, Rajput A, et al. GCH1 in early-onset Parkinson's disease. Mov Disord. 2009;24:2070–5. doi: 10.1002/mds.22729. [DOI] [PubMed] [Google Scholar]
- Datla KP, Blunt SB, Dexter DT. Chronic L-DOPA administration is not toxic to the remaining dopaminergic nigrostriatal neurons, but instead may promote their functional recovery, in rats with partial 6-OHDA or FeCl(3) nigrostriatal lesions. Mov Disord. 2001;16:424–34. doi: 10.1002/mds.1091. [DOI] [PubMed] [Google Scholar]
- Duran R, Mencacci NE, Angeli AV, Shoai M, Deas E, Houlden H, et al. The glucocerobrosidase E326K variant predisposes to Parkinson's disease, but does not cause Gaucher's disease. Mov Disord. 2013;28:232–6. doi: 10.1002/mds.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C, Volk AE, Kahraman D, Fink GR, Leube B, Schmidt M, et al. Are Dopa-responsive dystonia and Parkinson's disease related disorders? A case report. Parkinsonism Relat Disord. 2012;18:666–8. doi: 10.1016/j.parkreldis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Filiano JJ, Kish SJ. Amantadine for levodopa-induced choreic dyskinesia in compound heterozygotes for GCH1 mutations. Mov Disord. 2004;19:1256–8. doi: 10.1002/mds.20194. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Kapatos G, Haycock JW, Worsley J, Wong H, Kish SJ, et al. Brain biopterin and tyrosine hydroxylase in asymptomatic dopa-responsive dystonia. Ann Neurol. 2002;51:637–41. doi: 10.1002/ana.10175. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Lang AE, Trugman JM, Bird TD, Hunter A, Sadeh M, et al. Gender-related penetrance and de novo GTP-cyclohydrolase I gene mutations in dopa-responsive dystonia. Neurology. 1998;50:1015–20. doi: 10.1212/wnl.50.4.1015. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Nygaard TG, Gutlich M, Rajput AH, Pifl C, DiStefano L, et al. Striatal biopterin and tyrosine hydroxylase protein reduction in dopa-responsive dystonia. Neurology. 1999;53:1032–41. doi: 10.1212/wnl.53.5.1032. [DOI] [PubMed] [Google Scholar]
- Garavaglia B, Invernizzi F, Carbone ML, Viscardi V, Saracino F, Ghezzi D, et al. GTP-cyclohydrolase I gene mutations in patients with autosomal dominant and recessive GTP-CH1 deficiency: identification and functional characterization of four novel mutations. J Inherit Metab Dis. 2004;27:455–63. doi: 10.1023/B:BOLI.0000037349.08483.96. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Narabayashi H, Yokochi M, Iizuka R, Lees AJ. New pathologic observations in juvenile onset parkinsonism with dystonia. Neurology. 1991;41:820–2. doi: 10.1212/wnl.41.6.820. [DOI] [PubMed] [Google Scholar]
- Hagenah J, Saunders-Pullman R, Hedrich K, Kabakci K, Habermann K, Wiegers K, et al. High mutation rate in dopa-responsive dystonia: detection with comprehensive GCHI screening. Neurology. 2005;64:908–11. doi: 10.1212/01.WNL.0000152839.50258.A2. [DOI] [PubMed] [Google Scholar]
- Hauf M, Cousin P, Solida A, Albanese A, Ghika J, Schorderet D. A family with segmental dystonia: evidence for polymorphism in GTP cyclohydrolase I gene. Movement Disorders. 2000;15(Suppl.3):154–5. [Google Scholar]
- Hertz JM, Ostergaard K, Juncker I, Pedersen S, Romstad A, Moller LB, et al. Low frequency of Parkin, Tyrosine Hydroxylase, and GTP Cyclohydrolase I gene mutations in a Danish population of early-onset Parkinson's Disease. Eur J Neurol. 2006;13:385–90. doi: 10.1111/j.1468-1331.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- Hjermind LE, Johannsen LG, Blau N, Wevers RA, Lucking CB, Hertz JM, et al. Dopa-responsive dystonia and early-onset Parkinson's disease in a patient with GTP cyclohydrolase I deficiency? Mov Disord. 2006;21:679–82. doi: 10.1002/mds.20773. [DOI] [PubMed] [Google Scholar]
- Houlden H, Singleton AB. The genetics and neuropathology of Parkinson's disease. Acta Neuropathol. 2012;124:325–38. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwu WL, Chiou YW, Lai SY, Lee YM. Dopa-responsive dystonia is induced by a dominant-negative mechanism. Ann Neurol. 2000;48:609–13. [PubMed] [Google Scholar]
- Irie S, Kanazawa N, Ryoh M, Mochizuki H, Nomura Y, Segawa M. A case of parkinsonism and dopa-induced severe dyskinesia associated with novel mutation in the GTP cyclohydrolase I gene. Parkinsonism Relat Disord. 2011;17:769–70. doi: 10.1016/j.parkreldis.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Isaias IU, Marotta G, Hirano S, Canesi M, Benti R, Righini A, et al. Imaging essential tremor. Mov Disord. 2010;25:679–86. doi: 10.1002/mds.22870. [DOI] [PubMed] [Google Scholar]
- Ito Y, Fujita M, Shimada S, Watanabe Y, Okada T, Kusuoka H, et al. Comparison between the decrease of dopamine transporter and that of L-DOPA uptake for detection of early to advanced stage of Parkinson's disease in animal models. Synapse. 1999;31:178–85. doi: 10.1002/(SICI)1098-2396(19990301)31:3<178::AID-SYN2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Jarman PR, Bandmann O, Marsden CD, Wood NW. GTP cyclohydrolase I mutations in patients with dystonia responsive to anticholinergic drugs. J Neurol Neurosurg Psychiatry. 1997;63:304–8. doi: 10.1136/jnnp.63.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BS, Jeong JM, Park SS, Kim JM, Chang YS, Song HC, et al. Dopamine transporter density measured by [123I]beta-CIT single-photon emission computed tomography is normal in dopa-responsive dystonia. Ann Neurol. 1998;43:792–800. doi: 10.1002/ana.410430614. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Takeda A, Fujihara K, Kimpara T, Shiga Y, Tanji H, et al. Arg(184)His mutant GTP cyclohydrolase I, causing recessive hyperphenylalaninemia, is responsible for dopa-responsive dystonia with parkinsonism: a case report. Mov Disord. 2004;19:590–3. doi: 10.1002/mds.10712. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Gissen P, Smith M, Heales S, Jr, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–33. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- Lee JY, Yang HJ, Kim JM, Jeon BS. Novel GCH-1 mutations and unusual long-lasting dyskinesias in Korean families with dopa-responsive dystonia. Parkinsonism Relat Disord. 2013;19:1156–9. doi: 10.1016/j.parkreldis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–66. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Leuzzi V, Carducci C, Carducci C, Cardona F, Artiola C, Antonozzi I. Autosomal dominant GTP-CH deficiency presenting as a dopa-responsive myoclonus-dystonia syndrome. Neurology. 2002;59:1241–3. doi: 10.1212/wnl.59.8.1241. [DOI] [PubMed] [Google Scholar]
- Leuzzi V, Carducci C, Chiarotti F, D'Agnano D, Giannini MT, Antonozzi I, et al. Urinary neopterin and phenylalanine loading test as tools for the biochemical diagnosis of segawa disease. JIMD Rep. 2013;7:67–75. doi: 10.1007/8904_2012_144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair VD, Olanow CW, Sealfon SC. Activation of phosphoinositide 3-kinase by D2 receptor prevents apoptosis in dopaminergic cell lines. Biochem J. 2003;373:25–32. doi: 10.1042/BJ20030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain. 2011;134:3290–8. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- Nobili F, Naseri M, De Carli F, Asenbaum S, Booij J, Darcourt J, et al. Automatic semi-quantification of [123I]FP-CIT SPECT scans in healthy volunteers using BasGan version 2: results from the ENC-DAT database. Eur J Nucl Med Mol Imaging. 2013;40:565–73. doi: 10.1007/s00259-012-2304-8. [DOI] [PubMed] [Google Scholar]
- Nygaard T. An analysis of North American families with dopa-responsive dystonia. In: Segawa M, editor. Hereditary progressive dystonia. London: Parthenon Publishing; 1993a. pp. 97–106. [Google Scholar]
- Nygaard TG. Dopa-responsive dystonia. Delineation of the clinical syndrome and clues to pathogenesis. Adv Neurol. 1993b;60:577–85. [PubMed] [Google Scholar]
- Nygaard TG, Takahashi H, Heiman GA, Snow BJ, Fahn S, Calne DB. Long-term treatment response and fluorodopa positron emission tomographic scanning of parkinsonism in a family with dopa-responsive dystonia. Ann Neurol. 1992;32:603–8. doi: 10.1002/ana.410320502. [DOI] [PubMed] [Google Scholar]
- Nygaard TG, Trugman JM, de Yebenes JG, Fahn S. Dopa-responsive dystonia: the spectrum of clinical manifestations in a large North American family. Neurology. 1990;40:66–9. doi: 10.1212/wnl.40.1.66. [DOI] [PubMed] [Google Scholar]
- Nygaard TG, Wooten GF. Dopa-responsive dystonia: some pieces of the puzzle are still missing. Neurology. 1998;50:853–5. doi: 10.1212/wnl.50.4.853. [DOI] [PubMed] [Google Scholar]
- Opladen T, Hoffmann G, Horster F, Hinz AB, Neidhardt K, Klein C, et al. Clinical and biochemical characterization of patients with early infantile onset of autosomal recessive GTP cyclohydrolase I deficiency without hyperphenylalaninemia. Mov Disord. 2011;26:157–61. doi: 10.1002/mds.23329. [DOI] [PubMed] [Google Scholar]
- Parkkinen L, O'Sullivan SS, Kuoppamaki M, Collins C, Kallis C, Holton JL, et al. Does levodopa accelerate the pathologic process in Parkinson disease brain? Neurology. 2011;77:1420–6. doi: 10.1212/WNL.0b013e318232ab4c. [DOI] [PubMed] [Google Scholar]
- Plagnol V, Nalls M, Bras JM, Hernandez DG, Sharma M, Sheerin UM, et al. A two-stage meta-analysis identifies several new loci for Parkinson's disease. PLoS Genet. 2011;7:e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders-Pullman R, Blau N, Hyland K, Zschocke J, Nygaard T, Raymond D, et al. Phenylalanine loading as a diagnostic test for DRD: interpreting the utility of the test. Mol Genet Metab. 2004;83:207–12. doi: 10.1016/j.ymgme.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Segawa M, Nomura Y, Yukishita S, Nishiyama N, Yokochi M. Is phenotypic variation of hereditary progressive dystonia with marked diurnal fluctuation/dopa-responsive dystonia (HPD/DRD) caused by the difference of the locus of mutation on the GTP cyclohydrolase 1 (GCH-1) gene? Adv Neurol. 2004;94:217–23. [PubMed] [Google Scholar]
- Snow BJ, Nygaard TG, Takahashi H, Calne DB. Positron emission tomographic studies of dopa-responsive dystonia and early-onset idiopathic parkinsonism. Ann Neurol. 1993;34:733–8. doi: 10.1002/ana.410340518. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Levine RA, Galloway MP, Snow BJ, Calne DB, Nygaard TG. Biochemical and fluorodopa positron emission tomographic findings in an asymptomatic carrier of the gene for dopa-responsive dystonia. Ann Neurol. 1994;35:354–6. doi: 10.1002/ana.410350317. [DOI] [PubMed] [Google Scholar]
- Tassin J, Durr A, Bonnet AM, Gil R, Vidailhet M, Lucking CB, et al. Levodopa-responsive dystonia. GTP cyclohydrolase I or parkin mutations? Brain. 2000;123:1112–21. doi: 10.1093/brain/123.6.1112. [DOI] [PubMed] [Google Scholar]
- Trender-Gerhard I, Sweeney MG, Schwingenschuh P, Mir P, Edwards MJ, Gerhard A, et al. Autosomal-dominant GTPCH1-deficient DRD: clinical characteristics and long-term outcome of 34 patients. J Neurol Neurosurg Psychiatry. 2009;80:839–45. doi: 10.1136/jnnp.2008.155861. [DOI] [PubMed] [Google Scholar]
- Turjanski N, Bhatia K, Burn DJ, Sawle GV, Marsden CD, Brooks DJ. Comparison of striatal 18F-dopa uptake in adult-onset dystonia-parkinsonism, Parkinson's disease, and dopa-responsive dystonia. Neurology. 1993;43:1563–8. doi: 10.1212/wnl.43.8.1563. [DOI] [PubMed] [Google Scholar]
- Vaarmann A, Kovac S, Holmstrom KM, Gandhi S, Abramov AY. Dopamine protects neurons against glutamate-induced excitotoxicity. Cell Death Dis. 2013;4:e455. doi: 10.1038/cddis.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.