Summary
Bacterial pathogens must overcome host sequestration of zinc (Zn2+), an essential micronutrient, during the infectious disease process. While the mechanisms to acquire chelated Zn2+ by bacteria are largely undefined, many pathogens rely upon the ZnuABC family of ABC transporters. Here we show that in Yersinia pestis, irp2, a gene encoding the synthetase (HMWP2) for the siderophore yersiniabactin (Ybt) is required for growth under Zn2+-deficient conditions in a strain lacking ZnuABC. Moreover, growth stimulation with exogenous, purified apo-Ybt provides evidence that Ybt may serve as a zincophore for Zn2+ acquisition. Studies with the Zn2+-dependent transcriptional reporter znuA∷lacZ indicate that the ability to synthesize Ybt affects the levels of intracellular Zn2+. However, the outer membrane receptor Psn and TonB as well as the inner membrane (IM) ABC transporter YbtPQ, that are required for Fe3+ acquisition by Ybt, are not needed for Ybt-dependent Zn2+ uptake. In contrast, the predicted IM protein YbtX, a member of the Major Facilitator Superfamily, was essential for Ybt-dependent Zn2+ uptake. Finally, we show that the ZnuABC system and the Ybt synthetase HMWP2, presumably by Ybt synthesis, both contribute to the development of a lethal infection in a septicemic plague mouse model.
Introduction
Zinc (Zn2+) is an essential trace metal which has structural and/or catalytic functions in an estimated 3-8% of bacterial proteins (Andreini et al., 2006; Hantke, 2005; Katayama et al., 2002; Vallee and Falchuk, 1993). Similar to iron, mammalian hosts restrict the availability of Zn2+ to invading pathogens by a number of mechanisms. Zn2+ serum levels are in the micromolar range with the metal tightly chelated by proteins such as transferrin, albumin and α2-macrogloubin (Foote and Delves, 1984; Rahuel-Claremont and Dunn, 1998; Rink and Haase, 2007; Weinberg, 1972). Upon infection, mammals sequester Zn2+ systemically and locally in an attempt to further reduce access to this critical micronutrient; one example being calprotectin (Corbin et al., 2008; Foote and Delves, 1984; Hood and Skaar, 2012; Kehl-Fie and Skaar, 2010; Liuzzi et al., 2005; Rahuel-Claremont and Dunn, 1998; Rink and Haase, 2007; Sohnle et al., 2000; Weinberg, 1972). Consequently high affinity Zn2+ uptake systems should be needed to acquire this transition metal during the infectious process.
In contrast to the variety of bacterial iron uptake systems, relatively few bacterial high-affinity Zn2+ uptake systems have been identified. The ABC transporter ZnuABC (and its cluster 9 [C9] family orthologs) remains the most widespread mechanism for high affinity Zn2+ uptake. In some bacteria, a second periplasmic Zn2+-binding protein, ZinT, works in conjunction with ZnuA, the primary periplasmic Zn2+-binding protein of the ZnuABC system (Graham et al., 2009; Hantke, 2005; Kehl-Fie and Skaar, 2010; Petrarca et al., 2010). ZupT, a member of the ZIP family of proton motive force-dependent transporters, is described as a low-affinity Zn2+ transporter but contributes to Zn2+ uptake at a concentration of 10 μM Zn2+ in vitro, and in infection models, at least for E. coli UPEC and Salmonella (Cerasi et al., 2014; Grass et al., 2002; Karlinsey et al., 2010; Sabri et al., 2009). Additional inner membrane (IM) transporters ZevAB and ZurAM have recently been implicated in Zn2+ acquisition by Haemophilus influenzae and Listeria monocytogenes, respectively (Corbett et al., 2012; Rosadini et al., 2011).
Little is known about Zn2+ transport across the outer membrane (OM). In Neisseria meningitidis, a TonB-dependent OM receptor (ZnuD), with high similarity to heme OM receptors, functions in zinc and heme uptake (Kumar et al., 2012; Stork et al., 2010). In Acinetobacter baumannii, two genes encoding TonB-dependent OM receptors with similarities to ZnuD are repressed by Zn2+ (Hood et al., 2012). The cyanobacterium Anabaena encodes two TonB-dependent OM receptors whose transcription is highly repressed by the Zn2+-responsive transcriptional regulator Zur (Napolitano et al., 2012). However the substrate(s) for these receptors has not been investigated.
Secreted Zn2+-chelating compounds (zincophores), analogous to siderophores which chelate Fe3+, have been proposed as a Zn2+ acquisition mechanism for pathogens (Hood and Skaar, 2012) and confirmed in several organisms. The fungal pathogen Candida albicans secretes an ∼33 kDa Zn2+-binding protein, Pra1, involved in Zn2+ scavenging (Citiulo et al., 2012). Pseudomonas putida produces a small siderophore (198 daltons), pyridine-2,6-bis(thiocarboxylic acid) (PDTC), that binds Fe3+ and Zn2+ and delivers these cations to the bacterial cell (Cortese et al., 2002; Leach et al., 2007). Finally, Streptomyces coelicolor produces a siderophore-like molecule, coelibactin, which has been proposed, but not proven, as a zincophore (Hesketh et al., 2009; Zhao et al., 2012).
A role for Zn2+ acquisition during infections has been demonstrated in a number of bacterial pathogens: Acinetobacter baumannii, Brucella abortus, Campylobacter jejuni, pathogenic E. coli strains, Haemophilus ducreyi, H. influenzae, L. monocytogenes, Moraxella catarrhalis, Neisseria gonorrhoeae, Pasteurella multocida, Proteus mirabilis, Salmonella enterica serovar Typhimurium, Streptococcus pneumoniae, Streptococcus pyogenes, Vibrio parahaemolyticus, and Yersinia ruckeri (Ammendola et al., 2007; Bayle et al., 2011; Campoy et al., 2002; Cerasi et al., 2013; Cerasi et al., 2014; Corbett et al., 2012; Dahiya and Stevenson, 2010; Davis et al., 2009; Gabbianelli et al., 2011; Garrido et al., 2003; Hood et al., 2012; Karlinsey et al., 2010; Kim et al., 2004; Lewis et al., 1999; Lim et al., 2008; Liu et al., 2012; Liu et al., 2013; Murphy et al., 2013; Nielubowicz et al., 2010; Plumptre et al., 2014; Rosadini et al., 2011; Sabri et al., 2009; Weston et al., 2009; Yang et al., 2006).
Yersinia pestis is a Gram-negative bacterium that causes a flea-borne zoonotic disease, bubonic plague. After the infected flea bite, Y. pestis typically spreads from the site of the mammalian skin wound to the regional lymph node where multiplying bacteria cause necrosis resulting in a swollen and painful lymph node or bubo. Bubonic plague progresses rapidly to an often terminal systemic stage of disease or septicemic plague. A small proportion of mammals can develop septicemia without a prior lymphatic stage when plague bacteria are directly injected into the blood vessel by the flea bite or other means. Septicemia is required to infect naïve fleas, completing the zoonotic disease cycle. During the course of mammalian disease, the lungs may become infected leading to the development of secondary pneumonic plague. In humans that have developed secondary pneumonic plague, Y. pestis can then spread via respiratory droplets from person to person causing primary pneumonic plague (Perry and Fetherston, 1997; Sebbane et al., 2010; Sebbane et al., 2006).
We previously showed that ZnuABC is required during in vitro growth of Y. pestis in Chelex-100-treated PMH2 medium (cPMH2) which contains submicromolar Zn2+ levels (Desrosiers et al., 2010; Perry et al., 2012). Both the znuA and znuCB promoters are regulated by Zn2+ via the transcriptional regulator Zur that is common to many bacteria; a mutation in the Y. pestis znu system increases expression under low Zn2+ conditions. However, the growth response of the Y. pestis znu mutant to supplementation of cPMH2 with 0.4 μM ZnCl2, suggests that Y. pestis has a second high-affinity Zn2+ transporter. In addition, the znu mutant is highly virulent in mouse models of bubonic and pneumonic plague lending further support to the existence of another unidentified Y. pestis Zn2+ transporter that can effectively substitute for ZnuABC during mammalian infections. Deletion or overexpression of nine genetic loci that encode divalent cation transporters or cation transporters repressed by Zn2+ -Zur showed that none of these systems are needed by a znu mutant for in vitro growth under Zn2+ -deficient conditions (Desrosiers et al., 2010; Li et al., 2009; Perry et al., 2012). Thus one or more Zn2+ transporters of Y. pestis remained to be identified.
Siderophores are low-molecular weight compounds produced and secreted by bacteria, fungi and plants and are typically associated with the chelation of ferric iron (Fe3+) for acquisition by the producing organism. Siderophores are often vital for bacteria in different environments, including the mammalian host where some can remove iron from lactoferrin and transferrin (Hood and Skaar, 2012; Schaible and Kaufmann, 2004). While siderophores have a high affinity for Fe3+, some, including pyochelin which is structurally similar to the yersiniabactin (Ybt) siderophore produced by Yersinia and other bacteria (Fig. 1), have been shown to bind other transition metals as well (Braud et al., 2010; Braud et al., 2009a; Braud et al., 2009b; Chaturvedi et al., 2012). In addition, micacocidin, a compound with a chemical structure similar to Ybt made by Pseudomonas spp and Ralstonia solanacearum (Fig. 1), has been crystallized with Zn2+, Mn2+, and Fe3+ (Kreutzer et al., 2011; Nakai et al., 1999). However, there is no evidence that siderophore-dependent acquisition of transition metals other than iron plays any role in bacterial pathogenesis.
Fig. 1.
The structures of Ybt, micacocidin (A) and pyochelin (B). The asterisks indicate Fe3+ chelation sites in Ybt. The pentyl chain (in grey) of micacocidin is the only structural difference with Ybt (A). Pyochelin (B) lacks the malonyl linker and second thiazoline ring present in YbtA (A).
In Y. pestis, the Ybt siderophore-dependent iron uptake system has been shown to have a primary role in iron acquisition in vitro and to be essential for the development of bubonic plague and critical in pneumonic plague (Fetherston et al., 2010; Perry and Fetherston, 2011). Iron uptake via the Ybt system requires the TonB-dependent OM receptor Psn, TonB and the IM ABC transporter YbtPQ (Fetherston et al., 1999; Fetherston et al., 1995; Perry et al., 2003b) Here, we demonstrate that the Ybt siderophore and the IM protein YbtX play a role in Zn2+ accumulation and that both ZnuABC and Ybt synthetase HMWP2 are critical for lethal infections in a mouse model of septicemic plague.
Results
The pgm locus that encodes the Ybt system is required for growth under metal-deficient conditions and for the virulence of a znu mutant
Previously we failed to identify a putative second Zn2+ uptake system that allowed Zn2+ use from low concentrations in vitro and full virulence of a Y. pestis znu mutant (Desrosiers et al., 2010; Perry et al., 2012). Since then two previously unknown bacterial Zn2+ transporters, ZurAM (not encoded in Y. pestis) and ZevAB, have been shown to be important for Zn2+ uptake and virulence by L. monocytogenes and H. influenzae, respectively (Corbett et al., 2012; Rosadini et al., 2011). Y1329-1330 are ZevAB homologues encoded in Y. pestis KIM10+. Y1329 and ZevA each have a COG3683 domain which is annotated as a periplasmic component of an ABC transporter. Like H. influenza zevB and the Y. pestis Zn2+-repressible y1245 gene (Li et al., 2009), y1330 encodes a NicO domain that is annotated as a proton motive force-dependent nickel transport permease. While some NicO domain proteins have demonstrated Ni2+ uptake activity, including the Y1245 homolog in Yersinia pseudotuberculosis (Sebbane et al., 2002), the evidence in H. influenzae suggests they may also participate in Zn2+ transport (Rosadini et al., 2011). Previously, we showed that a znu y1245 double mutant grew as well as a znu mutant in cPMH2 medium (Desrosiers et al., 2010). Since Y1245 and Y1330 might serve redundant Zn2+ uptake functions, we constructed and tested a ΔznuBC Δy1245∷cam Δy1329-y1330∷kan triple mutant. Growth of the ΔznuBC single mutant and the triple mutant at 37°C did not differ significantly in cPMH2 with or without ZnCl2 added to 0.6 μM (Fig. S1in supporting information). Thus neither of these NicO domain proteins nor Y1329 appears to play a critical role in Zn2+ uptake in Y. pestis.
We noted that the Ybt siderophore, which has a high affinity for Fe3+ vs ferrous (Fe2+) iron, has recently been shown to chelate Cu2+ (Chaturvedi et al., 2012; Perry et al., 1999) and that the structurally similar micacocidin (Fig. 1A) has been crystallized with Zn2+ (Kreutzer et al., 2011; Nakai et al., 1999). Therefore, we hypothesized that the Ybt system might be involved in Zn2+ uptake with the Ybt siderophore serving as a zincophore. In preliminary studies, we used Y. pestis KIM6 strains with a spontaneous deletion of the chromosomal pgm locus that eliminates four ybt operons encoding siderophore biosynthetic, uptake, and regulatory functions (Bearden et al., 1997; Fetherston and Perry, 1994; Perry and Fetherston, 2011). Strikingly, we found that a combination of pgm and znuBC deletions completely abrogated growth of this mutant in cPMH2 supplemented with 0.6 μM ZnCl2 compared to the parent Pgm+ ΔznuBC mutant (Fig. 2A). This growth defect was rescued by integration of the znuA and znuCB operons into the att Tn7 site on the chromosome of the double Δpgm ΔznuBC mutant (data not shown). Thus, it appears that gene(s) within the pgm locus are involved in Zn2+ acquisition by Y. pestis.
Fig. 2.
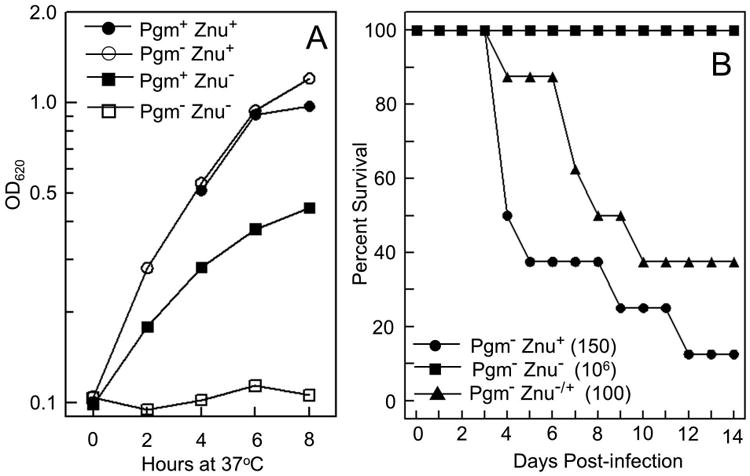
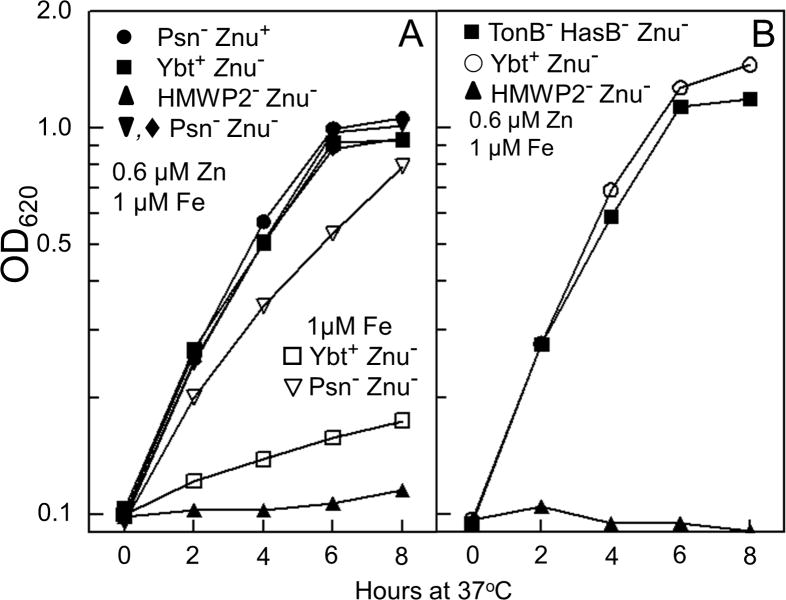
Growth and virulence of a Y. pestis Pgm- Znu- mutant. A. Cells were grown at 37°C in cPMH2 supplemented with 0.6 μM ZnCl2; strains: KIM6+ (Pgm+ Znu+); KIM6 (Pgm- [Δpgm] Znu+); KIM6-2077+ (Pgm+ Znu- [ΔznuBC]); KIM6-2077 (Pgm- [Δpgm] Znu- [ΔznuBC]). Growth curves shown are from one experiment that is representative of two or more independent experiments. B. Average time-to-death (percent survival) analyses for two independent septicemic Y. pestis infections. Strains: KIM5-2077(pCD1Ap) (Pgm- [Δpgm] Znu- [ΔznuBC]); KIM5(pCD1Ap) (Pgm- [Δpgm] Znu+); KIM5-2077.10(pCD1Ap) (Pgm- [Δpgm] Znu-/+ [ΔznuBC/znuABC+). The average infectious doses are indicated in parentheses.
A Y. pestis KIM Δpgm mutant is completely avirulent by subcutaneous injection (bubonic plague) and highly attenuated by intranasal installation (pneumonic plague). However, various Y. pestis Δpgm strains, including KIM, are fully virulent via an intravenous route of infection (septicemic plague) (Bearden and Perry, 1999; Fetherston et al., 2010; Jackson and Burrows, 1956; Une and Brubaker, 1984). Consequently, we used a mouse model of septicemic plague to compare Δpgm and Δpgm ΔznuBC mutants. Unlike our in vitro studies, these strains carry pCD1Ap, a plasmid encoding a type three secretion system and effector proteins that is essential for virulence (Gong et al., 2001; Perry et al., 1998). The Δpgm ΔznuBC mutant had an ∼ 106-fold loss of virulence compared to the Δpgm strain. The virulence of the Δpgm ΔznuBC mutant was restored by complementation with the znuABC+ locus integrated into the chromosome (Table 1). A time-to-death analysis comparing these three strains (Δpgm, Δpgm ΔznuBC, Δpgm znuBC-/+) strikingly illustrates these virulence differences (Fig. 2B). Thus, the Zn2+ uptake system, ZnuABC, and components within the pgm locus are critical for the development of lethal infection in a mouse model of septicemic plague.
Table 1. Virulence of Y. pestis strains in a mouse model of septicemic plague.
| Strain or genotypea | LD50 valuesb | Virulence loss |
| Δpgm | <6 ± 2.8 (<8; 4) | -- |
| Δpgm Δznu | 5.7 × 106 ± 4.2 × 106 | >9.5 × 105-fold |
| Δpgm ΔznuBC attTn7∷znuABC+ | <10; >101; 25 | -- |
| Wild type | <14 ± 1.4 (<13; <15) | -- |
| Δznu | ∼11 ± 5 (<10; >16; <8; <18) | none |
| irp2∷kan | ∼ 48 (<11; 84.5) | none |
| irp2 Δznu | ∼6.0 × 106 ± 3.5 × 106 (5.1 × 106; 2.7 × 106; 5.4 × 106; >1.1 × 107) | >4.3 × 105-fold |
| Δpsn∷kan | <14 | none |
| Δpsn∷kan ΔznuBC | ∼40 ± 14 (30; <50) | none |
Strains: Δpgm – KIM5(pCD1Ap); Δpgm Δznu – KIM5-2077(pCD1Ap) [Δpgm ΔznuBC] and KIM5-2197(pCD1Ap) [Δpgm ΔznuA]; Δpgm ΔznuBC attTn7∷znuABC – KIM5-2077.10(pCD1Ap); wild type - KIM5(pCD1Ap)+; Δznu – KIM5-2077(pCD1Ap)+ [ΔznuBC] and KIM5-2197(pCD1Ap)+ [ΔznuA]; irp2∷kan – KIM5-2046.1(pCD1Ap); irp2 Δznu – KIM5-2077.7(pCD1Ap) [irp2∷kan ΔznuBC], KIM5-2077.8(pCD1Ap) [Δirp2 ΔznuBC] and KIM5-2197.1(pCD1Ap) [Δirp2 ΔznuA]; Δpsn∷kan – KIM5-2045.6(pCD1Ap); Δpsn∷kan ΔznuBC – KIM5-2077.9(pCD1Ap). In all backgrounds tested, an in-frame ΔznuA mutation and the ΔznuBC mutation caused similar LD50s. In addition, irp2∷kan and Δirp2 mutations in all backgrounds tested yielded similar LD50s. These LD50s were combined and genotypes for these strains are listed in the table as Δznu, Δpgm Δznu, and irp2 Δznu.
Where the LD50 was lower than the lowest dose of the trial (e.g., <8) or higher than the highest dose (e.g., >16), these doses and the calculated LD50s are given in parentheses (e.g., <8; 4) and were used in calculating the mean LD50. The means ± standard deviations from two to four separate trials are given, except for the Δpgm ΔznuBC attTn7∷znuABC+ strain were the LD50s of three separate trials are listed.
The irp2 gene, encoding the Ybt synthase HMWP2, is involved in Zn2+ acquisition
To specifically address the role of the Ybt system in Zn2+ acquisition, we constructed an irp2∷kan mutation in the ΔznuBC strain. The irp2-encoded HMWP2 protein is essential for the synthesis of Ybt – condensing two cysteines and linking them to salicylate to form three of the first four heterocylic rings of Ybt (Fig. 1A) (Perry and Fetherston, 2011). We previously demonstrated that the kan insertion in irp2 has a polar effect on downstream genes in the irp2-irp1-ybtUTE operon, completely abrogating Ybt biosynthesis (Bearden et al., 1997; Miller et al., 2010; Perry et al., 1999). For growth studies of this mutant, cPMH2 was supplemented with 1 μM FeCl3 and 0.6 μM ZnCl2. Iron was added to compensate for the loss of iron uptake via the Ybt system while submicromolar Zn2+ was added to enhance the growth of the single ΔznuBC mutant. This single mutant showed the expected growth defect compared to the Ybt+ Znu+ parent strain while the irp2∷kan ΔznuBC double mutant exhibited no growth in cPMH2 despite FeCl3 and ZnCl2 supplementation (Fig. 3A). Moreover, a similar severe growth defect was caused by an in-frame deletion of irp2 in the ΔznuBC background. This defect was alleviated by complementation of the Δirp2 mutation with a plasmid expressing the cloned irp2 gene (Fig. 3B). Thus, our data indicate that, in addition to Zn2+ uptake by the ZnuABC system, the Ybt system is needed for Y. pestis growth under low Zn2+ conditions in the absence of ZnuABC and that Ybt may serve as a zincophore.
Fig. 3.
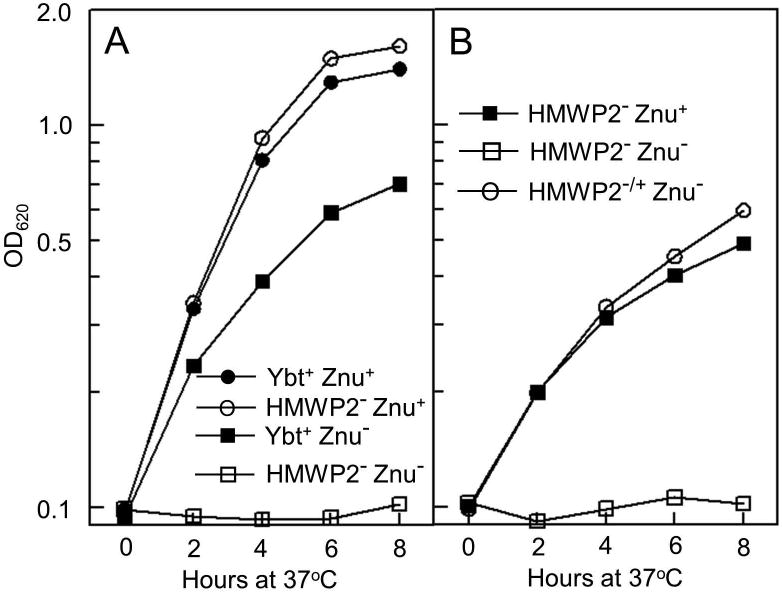
The Y. pestis HMWP2- Znu- mutant has a severe growth defect at 37°C in cPMH2 supplemented with 0.6 μM ZnCl2 and 1.0 μM FeCl3. A: Strains – KIM6+ (Ybt+ Znu+); KIM6-2046.1 (HMWP2- [irp2∷kan] Znu+); KIM6-2077+ (Ybt+ Znu- [ΔznuBC]); KIM6-2077.7 (HMWP2- [irp2∷kan] Znu- [ΔznuBC]). B: strains – KIM6-2046.3(pBGL2) (HMWP2- [Δirp2] Znu+); KIM6-2077.8 (Δirp2 ΔznuBC) carrying pBGL2 (HMWP2- Znu-) or pIrp2 (HMWP2-/+ Znu-). An irp2 mutant cannot express the Ybt synthetase HMWP2 and thus cannot synthesize the Ybt siderophore (Perry and Fetherston, 2011). To complement the in-frame Δirp2 mutation (KIM6-2077.8), the WT irp2 gene with its native promoter was cloned into the pBGL2 vector plasmid generating pIrp2. Growth curves shown are from one experiment that is representative of two or more independent experiments.
Our original ΔznuBC mutation eliminates the entire znuC ORF, most of znuB and the promoter regions between znuA and znuCB (Desrosiers et al., 2010). The y2248 gene that encodes a member of the M23 family of endopeptidases lies only 20 bp downstream of znuA (Fig. S2). Thus, it is possible that znuA and y2248 are transcribed from the same promoter, in which case the expression of y2248 would be abrogated in the znuBC mutant. Consequently, we constructed an in-frame znuA deletion (Fig. S2) in KIM6+ and the Δirp2 mutant and tested their growth under low Zn2+ conditions. Both the ΔznuA and Δirp2 ΔznuA mutants had identical in vitro and in vivo phenotypes compared to the ΔznuBC and Δirp2 ΔznuBC mutants, respectively (data not shown; Table 1). These data together with the results showing that the ΔznuBC mutation is complemented by integration of znuABC+ into the att Tn7 site confirm that the znuBC mutation is responsible for the Y. pestis growth defect under in vitro low Zn2+ conditions.
The intracellular levels of Zn2+ in Y. pestis and other bacteria are tightly regulated by the Zn2+-responsive transcriptional regulator Zur that represses znuA and znuCB promoters under Zn2+-sufficient conditions (Desrosiers et al., 2010; Hantke, 2005; Li et al., 2009). Using a znuA∷lacZ reporter we have previously shown that transcription from the znuA promoter during growth in cPMH2 in the ΔznuBC mutant is higher than in the parent Znu+ strain (Desrosiers et al., 2010). We used the same reporter to determine whether the Ybt system plays any role in Zn2+ acquisition when ZnuABC is functional. In the single irp2∷kan mutant, growth in unsupplemented cPMH2 showed a significant (∼2-fold) increase in transcription from znuA∷lacZ compared to the Ybt+ parent strain (Fig. 4A). Thus the Ybt system contributes to intracellular Zn2+ levels even when ZnuABC is functional.
Fig. 4.
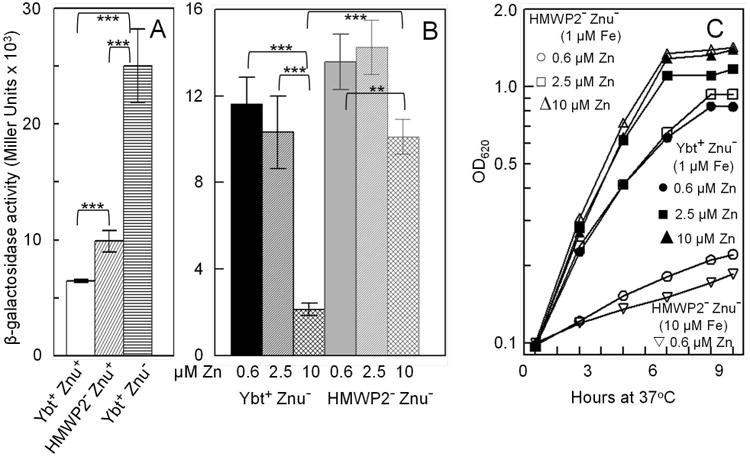
An irp2 mutation in Y. pestis results in lower intracellular Zn2+ levels (A and B) and increases the concentration of exogenous Zn2+ required to stimulate growth (C). The β-galactosidase activities shown (A and B) are averages (with standard deviations) of replicate samples from two independent cultures. Statistical significances were calculated using the Student's two tailed t-test; p = <0.001 - ***; p = < 0.005 - **. Growth curves (C) shown are from one experiment that is representative of two or more independent experiments. Strains were grown in cPMH2 at 37°C unsupplemented (A) or with increasing levels of ZnCl2 (B and C). cPMH was also supplemented with 1.0 μM FeCl3 (B) or indicated FeCl3 concentrations (C). Strains: KIM6+ (Ybt+ Znu+); KIM6-2046.1 (HMWP2- [irp2∷kan] Znu+); KIM6-2077+ (Ybt+ Znu- [ΔznuBC]); KIM6-2077.7 (HMWP2- [irp2∷kan] Znu- [ΔznuBC]). In A and B, all strains carry pEUZnu1 which encodes the znuA∷lacZ transcriptional reporter.
We again used the znuA∷lacZ reporter to determine if the double irp2∷kan ΔznuBC mutant is more Zn2+ starved than the single ΔznuBC mutant. In the single mutant, 10 μM ZnCl2 caused a 5-fold loss of β-galactosidase activity from znuA∷lacZ compared with 0.6 μM ZnCl2 while the double irp2∷kan ΔznuBC mutant showed only a 1.3-fold loss of β-galactosidase activity under the same growth conditions. At 10 μM ZnCl2, znuA∷lacZ activity in the single mutant was repressed 4.7-fold compared to the double mutant (Fig. 4B). These data indicate that the Ybt system supplies Zn2+ that represses the znuA∷lacZ reporter under these growth conditions.
These differences in intracellular Zn2+ levels due to loss of uptake functions translate into growth defects. Zn2+ titration studies demonstrated that the ΔznuBC mutant in cPMH2 with 0.6 μM ZnCl2 grew as well as the irp2∷kan ΔznuBC mutant only when the double mutant was grown with 2.5 μM ZnCl2 (Fig. 4C). In addition, supplementation of cPMH2 with 0.6 μM ZnCl2 and 10 μM FeCl3 showed no alleviation of the growth defect (Fig. 4C). Thus, our data confirm that growth of the double mutant is Zn2+-dependent and not iron-dependent.
Expression of HMWP2 from the irp2-irp1-ybtUTE promoter is normally repressed by iron (Perry et al., 2003a), to determine if it is also affected by Zn2+ we examined the activity of an irp2∷lacZ transcriptional reporter (pEUIrp2) in Ybt+ Znu+ and Ybt+ Znu- strains. Cells were grown in cPMH with or without ZnCl2 or FeCl3 added to 10μM. In a Znu+ background, transcription of the irp2 promoter was repressed by iron but not by Zn2+ (Fig. 5). This is in agreement with the microarray finding of Li et al that ybt operons are not regulated by Zn2+ or Zur (Li et al., 2009). However, the znu mutation resulted in an ∼1.7 fold increase in transcription from the irp2 promoter compared to the Znu+ parent, which was repressed by Zn2+ (∼1.6-fold) as well as Fe3+ (Fig.5). Iron repressed transcription of the irp2 reporter ∼10-12 fold in a Znu+ background but only ∼4 fold in the Znu- strain. These results show that extreme Zn2+ starvation can affect expression of the Ybt system; perhaps, the low intracellular Zn2+ levels affect Fur activity. At least in vitro, E. coli and Bacillus subtilis Fur proteins bind Fe2+ as well as other divalent metals, including Zn2+. In addition, Fur has been shown to bind Zn2+ as a structural component (Althaus et al., 1999; Ma et al., 2012; Mills and Marletta, 2005; Sheikh and Taylor, 2009). Perhaps, in the znu mutant, there is insufficient Zn2+ for maximal Fur activity. Thus, in the absence of Zn2+ the irp2∷lacZ reporter is not fully repressed in this strain. The finding that the activity of this reporter in the presence of Zn2+ is the same in both Znu+ and Znu- strains lends credence to this hypothesis.
Fig. 5.
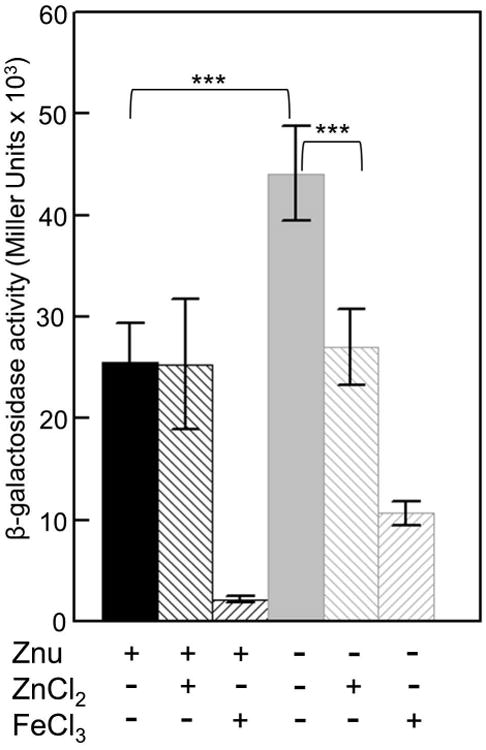
Transcription from the irp2 promoter is affected by Zn2+. The β-galactosidase activities from the irp2∷lacZ transcriptional reporter carried by KIM6+ (Znu +) or KIM6-2077+ (Znu -) are averages (with standard deviations) of replicate samples from two independent cultures. Strains were grown in cPMH2 at 37°C unsupplemented or with ZnCl2 or FeCl3 added to 10 μM. Statistical significances were calculated using the Student's two tailed t-test; p = <0.001 - ***. As previously demonstrated (Perry et al., 2003a), transcription from the irp2∷lacZ reporter in both strains was repressed by FeCl3 supplementation (p = <3 × 10-7; not shown on the graph for clarity).
TonB-dependent OM receptors are not required for Zn2+ acquisition in Y. pestis
The OM receptor Psn is required for Fe3+ acquisition by the Ybt siderophore and its function is dependent upon TonB, an IM anchored, periplasm spanning protein that is required for active transport across the OM via TonB-dependent receptors. Y. pestis strains with mutations in either psn or tonB exhibit a severe growth defect due to increased chelation of residual Fe3+ from the medium by Ybt that is unable to enter the cells. These growth defects can be rescued by supplementation with 1μM FeCl3. Y. pestis has a second TonB-like gene (hasB) that is not required for Fe3+-Ybt utilization (Fetherston et al., 1995; Perry et al., 2003b; Perry and Fetherston, 2011).
In Pseudomonas species, TonB-dependent receptors required for Fe3+-siderophore uptake are also needed for the uptake of siderophores complexed with other metals (Braud et al., 2010; Hannauer et al., 2012; Leach et al., 2007; Schalk et al., 2011). To test if the Psn receptor is also involved in Zn2+ acquisition via Ybt, we constructed either Δpsn or Δpsn∷kan mutations in the Znu- strain. Surprisingly, in contrast to the Δirp2 ΔznuBC and irp2∷kan ΔznuBC double mutants, both the Δpsn ΔznuBC and Δpsn∷kan ΔznuBC mutants grew as well as the ΔznuBC and psn single mutants in cPMH2 supplemented with 1.0 μM FeCl3 and 0.6 μM ZnCl2 (Fig. 6A). Curiously, the Δpsn∷kan ΔznuBC strain grew better than the Δznu mutant when Zn2+ was omitted from the growth medium (Fig. 6A), suggesting that the Psn receptor might hinder Ybt-dependent Zn2+ uptake in Y. pestis.
Fig. 6.
Ybt-dependent Zn2+ acquisition does not require the OM Ybt receptor Psn or any other TonB-dependent OM receptor. Cells were grown at 37°C in cPMH2 supplemented with 0.6 μM ZnCl2 and 1.0 μM FeCl3 or 1.0 μM FeCl3 alone. Strains: KIM6-2045.1 (Psn- [Δpsn] Znu+); KIM6-2077+ (Ybt+ Znu- [ΔznuBC]); KIM6-2077.7 (HMWP2- [irp2∷kan] Znu- [ΔznuBC]); KIM6-2077.12+ (Znu- [ΔznuBC] TonB-[tonB∷kan] HasB- [ΔhasB]); Psn- Znu- (KIM6-2077.9 [psn∷kan ΔznuBC], ▼ and KIM6-2077.14 [Δpsn ΔznuBC];◆). Psn is the OM receptor for Ybt and HasB is a second TonB-like protein in Y. pestis (Perry and Fetherston, 2011; Perry et al., 2003b). Growth curves shown are from one experiment that is representative of two or more independent experiments.
While these results suggest that Ybt synthesized by HMWP2 provides Zn2+ by a route independent of the Fe3+-Ybt uptake system, a different OM receptor might be used for Zn2+ uptake. Y. pestis has a second locus encoding Ybt-like proteins with Y3404 showing significant similarity to Psn (21% identity and 39% similarity) (Forman et al., 2010; Perry and Fetherston, 2004). In addition, the TonB-dependent OM receptor ZnuD in Neisseria meningitidis has similarities to the family of heme receptors and functions in heme and Zn2+ uptake (Kumar et al., 2012; Stork et al., 2010). To determine if TonB is required for Zn2+ acquisition, we constructed a mutant lacking both of the Y. pestis tonB genes in a ΔznuBC background. Again, the ΔtonB∷kan ΔhasB ΔznuBC and single ΔznuBC mutants had similar growth defects in cPMH2 supplemented with 1.0 μM FeCl3 and 0.6 μM ZnCl2 (Fig. 6B). This indicates that Ybt-dependent Zn2+ uptake does not require any TonB-dependent OM receptor in Y. pestis.
The Ybt synthetase but not the Ybt OM receptor is critical for virulence in a Znu- background during septicemic plague
Based on studies using Y. pestis Δpgm strains, it is clear that the Ybt system is not essential for the virulence of Y. pestis by an intravenous route of infection (Bearden and Perry, 1999; Jackson and Burrows, 1956; Une and Brubaker, 1984). However, specific mutations in ybt genes have not been tested in a mouse model of septicemic plague. LD50s for the Ybt+ strain as well as the Δpsn∷kan and irp2∷kan mutants were similar and confirm that Y. pestis does not require the Ybt system to cause lethal septicemic plague in mice (Table 1).
However, the irp2∷kan mutant, which had an LD50 similar to that of the Ybt+ parent strain, showed some attenuation at low infectious doses. While all 8 mice infected with the parent strain at infectious doses of 13 and 15 quickly succumbed to infection, 5 out 8 mice infected with doses of 11 and 16 cells of the irp2∷kan mutant survived (p = 0.01). Thus, while the Ybt system is not required for septicemic plague it may play a modest role during the systemic stage of the infection resulting from low infectious doses.
To assess the importance of the Ybt-dependent Zn2+ acquisition during septicemic plague, we tested the effect of irp2 mutations in Δznu backgrounds. All three double mutants, Δirp2 ΔznuBC, irp2∷kan ΔznuBC, Δirp2 ΔznuA, showed drastic attenuation (∼105-fold virulence losses) compared to the Ybt+ Znu+ parent strain as well as single ΔznuBC and znuA mutants (Table 1). Thus HMWP2 is required for virulence in the septicemic plague model in strains lacking a functional ZnuABC system.
To demonstrate that HMWP2 contributes to virulence of znu mutants due to Zn2+ and not Fe3+ uptake, we tested the Δpsn∷kan ΔznuBC strain in the septicemic plague model. The Psn receptor, that is required for Fe3+ uptake via the Ybt siderophore, is critical for lethal infection during bubonic and pneumonic plague (Fetherston et al., 2010). Unlike HMWP2, Psn is not involved in Zn2+ uptake in vitro. Thus, we reasoned that a Δpsn∷kan ΔznuBC mutant would not show a drastic virulence defect in the septicemic plague model. Indeed, this double mutant remained highly virulent indicating that Fe3+ uptake via the Ybt siderophore is not needed for virulence of the ΔznuBC mutant during septicemic plague and that the Psn receptor is not involved in Zn2+ acquisition in vivo. Thus the combination of data from in vitro growth and animal experiments strongly suggest that the virulence defect observed for the irp2 znu mutants in mice is not due to defects in the acquisition of both Zn2+ and Fe3+ but rather attributable to the roles of the ZnuABC system and Ybt in Zn2+ uptake.
The IM protein YbtX, but not YbtQ, is required for Zn2+ acquisition via the Ybt system in vitro
Two genes, ybtP and ybtQ, encoded within a putative ybtPQXS operon, are required for Ybt-dependent Fe3+ transport into the cell. YbtP and YbtQ are putative IM proteins with both permease and ATPase domains (Fetherston et al., 1999). We tested if these components are also involved in Zn2+ transport using a ΔybtQX ΔznuBC double mutant. Previous studies have shown that ybtX is not required for Fe3+ uptake via Ybt (Fetherston et al., 1999). In cPMH2 supplemented with 1.0 μM FeCl3 and 0.6 μM ZnCl2, the growth of this double mutant was defective compared to that of the ΔznuBC and ΔybtQX single mutants. A higher level of ZnCl2 supplementation was required to significantly enhance the growth of the ΔybtQX ΔznuBC mutant compared to the ΔznuBC mutant (Fig. 7). Addition of FeCl3 to 10 μM did not stimulate growth of the double mutant (data not shown). This demonstrates that the growth defect was due to a defect in Zn2+ uptake.
Fig. 7.
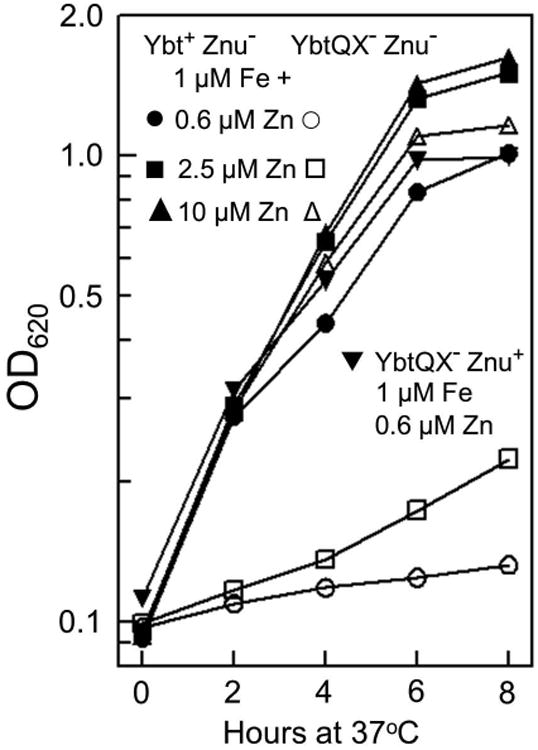
A YbtQX- Znu- mutant (open symbols) has a severe growth defect and requires additional Zn2+ supplementation to restore growth, compared to single Znu- and YbtQX- mutants (closed symbols). Cells were grown at 37°C in cPMH2 with 1.0 μM FeCl3 and increasing levels of ZnCl2. Strains: KIM6-2066 (YbtQX- [ΔybtQX]); KIM6-2077+ (Znu- [ΔznuBC]); KIM6-2077.13 (YbtQX- [ΔybtQX] Znu- [ΔznuBC]). YbtQ, but not YbtX, is part of the IM ABC transporter (YbtPQ) required for the use of Fe3+ from Ybt (Perry and Fetherston, 2011). Growth curves shown are from one experiment that is representative of two or more independent experiments.
This growth defect was alleviated by complementation with the ybtPQX locus expressed from its native promoter on a recombinant plasmid (Fig. 8A). However, a plasmid expressing ybtPQ did not complement the growth defect of the ΔybtQX ΔznuBC mutant (Fig. 8A) while expression of only ybtX did restore the growth of this mutant (Fig. 8B). Based on bioinformatics, YbtX is an IM protein that belongs to the Major Facilitator Superfamily (MFS). Several members of MFS have been shown to be involved in siderophore utilization (Chatfield et al., 2012; Ó Cuív et al., 2004). Our data suggest that YbtX, but not YbtQ, is involved in Zn2+ uptake. To further confirm this we constructed and tested an in frame ΔybtX ΔznuA mutant. This mutant showed a growth defect similar to that of the double ΔybtQX ΔznuBC mutant (data not shown) indicating that YbtX is required for Ybt-dependent Zn2+ utilization.
Fig. 8.
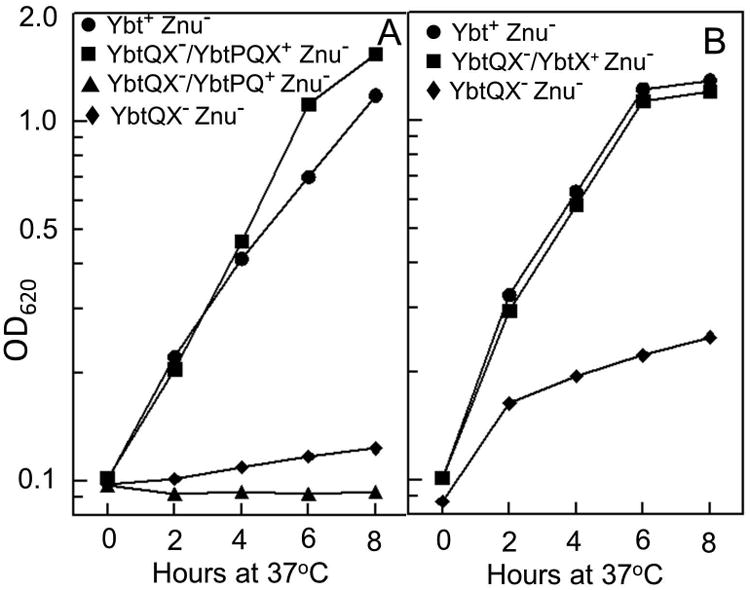
YbtX is required for Ybt-dependent Zn2+ uptake. Cells were grown at 37°C in cPMH2 with 1.0 μM FeCl3 and 0.6 μM ZnCl2. A. The growth of KIM6-2077.13 (YbtQX- [ΔybtQX] Znu- [ΔznuBC] is restored when carrying pYbtPQX, which expresses ybtPQX+ (YbtQX-/YbtPQX+) but not when carrying pYbtPQ, which expresses only ybtPQ+ (YbtQX-/YbtPQ+). B. A plasmid (pYbtX) expressing only ybtX+ restored the growth of KIM6-2077.13. KIM6-2077+ (Ybt+ Znu- [ΔznuBC]) and strains carrying the vector plasmid pACYC184 were used as controls. Growth curves shown are from one experiment that is representative of two or more independent experiments.
We also used feeding assays with culture supernatants from a Ybt-producing strain and an irp2∷kan mutant to determine whether secreted siderophore/zincophore supported the growth of strains lacking the Fe3+-Ybt receptor Psn but not the IM protein YbtX (Fig. 9A). Indeed, the growth of a triple Δpsn irp2∷kan ΔznuBC mutant was supported by Ybt-containing supernatant from KIM6+ but not by the Ybt-negative supernatant from the irp2∷kan mutant KIM6-2046.1. Supernatant from a ybtU mutant, KIM6-2071, also failed to stimulate the growth of the Δpsn irp2∷kan ΔznuBC mutant (data not shown). YbtU reduces the middle thiazoline ring to thiazolidine (Fig. 1) and thus it is required for Ybt synthesis (Miller et al., 2002). Finally, we tested purified apo-Ybt for its ability to stimulate the growth of Δpsn irp2∷kan ΔznuBC mutant cells in cPMH2 supplemented with 1.0 μM FeCl3 and 0.6 μM ZnCl2. As expected, addition of apo-Ybt stimulated growth of this mutant similar to the addition of culture supernatants containing Ybt (Fig. 9B). This provides evidence that Ybt in the external environment likely serves as a zincophore. Moreover, Ybt-dependent Zn2+ translocation across the OM is independent of TonB and the OM receptor Psn.
Fig. 9.
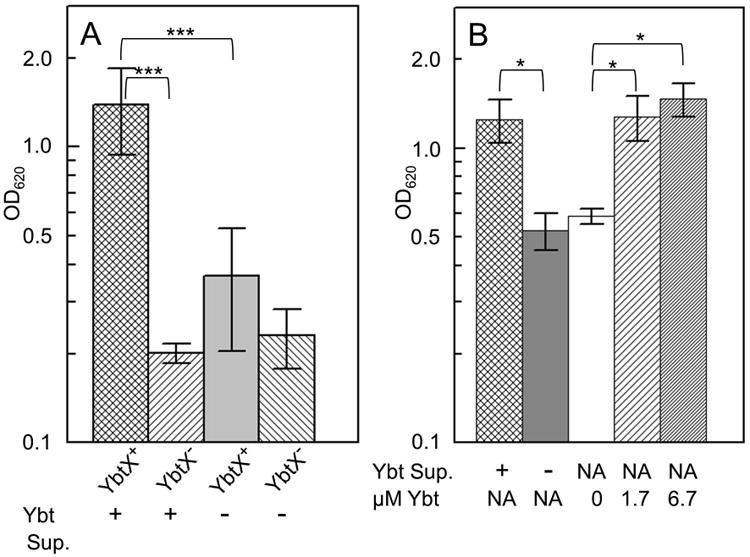
Addition of apo-Ybt or culture supernatants containing Ybt stimulates the growth of the ΔznuBC Δpsn irp2∷kan mutant. A. After acclimation to growth at 37°C in cPMH2 supplemented with 0.6 μM ZnCl2 and 1.0 μM FeCl3, cultures were then back diluted to an OD620 of ∼0.1 in a 1:1 mixture of the same medium with culture supernatants. B. Alternatively, similarly grown cultures of the ΔznuBC Δpsn irp2∷kan mutant were back diluted to an OD620 of 0.1 and incubated with apo-Ybt (μM Ybt) or ethanol solvent (0 μM Ybt). Culture optical densities were measured after overnight incubation at 37°C. Strains KIM6-2077.18 (ΔznuBC Δpsn irp2∷kan; labeled YbtX+) and KIM6-2077.19 (ΔznuBC Δpsn irp2∷kan ΔybtX; labeled YbtX-) were tested for growth with supernatants from KIM6+ (Ybt +) and KIM6-2046.1 (irp2∷kan; Ybt -) (Panel A and B) or apo-Ybt (Panel B). Addition of apo-Ybt to a final concentration of 1.7 μM is equivalent to the Ybt present in cultures with at a 1:1 mixture with supernatant from KIM6+. NA – not applicable. Data presented for the YbtX+ strain are averages from 10 independent experiments with 6 independent culture supernatants (panel A) or 3 independent experiments (panel B). Data presented for the YbtX- strain are averages from two or more independent experiments supplemented with 2 independent culture supernatants (panel A). Error bars represent standard deviations while asterisks with brackets indicate statistical significances calculated using the Student's two tailed t-test (p = <0.001 - ***; p = <0.05 - *).
In contrast, the Ybt-containing supernatant did not alleviate the growth defect of a quadruple Δpsn irp2∷kan ΔybtX ΔznuBC mutant (Fig. 9A). Thus the IM protein YbtX is essential for Ybt -dependent Zn2+ uptake demonstrating that Zn2+ uptake via this route uses a pathway completely different from that for Fe3+-Ybt utilization.
Discussion
Zinc is essential for bacterial growth and the development of disease in mammals where it is chelated as a component of nutritional immunity. While some pathogens appear to depend upon the high-affinity Zn2+ ABC transporter ZnuABC and its homologues, the mechanisms used to circumvent Zn2+ sequestration remain largely unknown. It has been suggested that pathogens may use zincophores analogous to siderophores to scavenge Zn2+ from mammalian hosts (Cerasi et al., 2013; Hood and Skaar, 2012). Previously, we found that a Y. pestis ΔznuBC mutant retained nearly full virulence in mouse models of bubonic and pneumonic plague (Desrosiers et al., 2010). Our data here provides evidence that Y. pestis likely uses the siderophore Ybt as a zincophore to acquire Zn2+.
Several lines of evidence indicate Ybt-dependent Zn2+ acquisition by Y. pestis under low Zn2+ conditions in vitro. First, in combination with the ΔznuBC mutation, two different irp2 mutations caused a more severe growth defect compared to the znu mutant in cPMH2 medium supplemented with 0.6 μM ZnCl2 and 1.0 μM FeCl3 (Fig. 3). Second, this growth defect was complemented in trans by the cloned irp2 gene or by zinc supplementation to 2.5 μM (Fig. 3 and 4C). Third, analysis of the expression of β-galactosidase from a znuA∷lacZ reporter indicates that the Y. pestis irp2∷kan mutant was more Zn2+-deficient than its Ybt+ parent (Fig. 4A) and the irp2∷kan ΔznuBC double mutant failed to accumulate significant intracellular Zn2+ in cPMH2 medium supplemented to 10 μM ZnCl2 (Fig. 4B). Finally, purified apo-Ybt or culture supernatants containing Ybt but not Ybt-negative culture supernatants supported the growth of a triple Δpsn irp2∷kan ΔznuBC mutant under low Zn2+ conditions (Fig. 9).
Recently, Chaturvedi et al have shown that Ybt binds copper and reduces copper toxicity in an E. coli strain producing Ybt and enterobactin (Chaturvedi et al., 2012). In addition, a Ybt-like compound, micacocidin, produced by some Pseudomonads and Ralstonia solanacearum chelates Zn2+ (micacocidin A), Cu2+ (micacocidin B), and Fe3+ (micacocidin C) and has anti-mycoplasma activity. A pentyl chain on the salicylate moiety of Ybt is the only structural difference between micacocidin and Ybt (Fig. 1A) (Kobayashi et al., 1998a; Kobayashi et al., 2000; Kobayashi et al., 1998b; Kreutzer et al., 2011). Moreover, two primary siderophores of Pseudomonads, Pyoverdine I (PVDI) and Pyochelin (Pch) have been shown to bind a wide range of divalent cations including Zn2+. These non-iron PVDI and Pch complexes appear to function in cation homeostasis and reduction of metal toxicities. These studies demonstrate effective chelation of non-iron metals by these two siderophores; however, acquisition of these metals, particularly Zn2+ and Mn2+, for nutritional use has not been assessed. Note that Pch (Fig. 1B) has a structure nearly identical to Ybt minus the malonyl linker group and the second thiazoline ring (Brandel et al., 2012; Braud et al., 2009a; Braud et al., 2009b; Braud et al., 2010; Schalk and Guillon, 2013; Schalk et al., 2011). In S. coelicolor, coelibactin, a non-ribosomally synthesized molecule whose expression is regulated by Zur has been proposed as a zincophore. However, Zn2+ chelation and acquisition by coelibactin has not been demonstrated. Coelibactin has a predicted structure with similarities to Ybt and micacocidin, at least prior to further putative enzymatic tailoring (Hesketh et al., 2009; Zhao et al., 2012). The secreted protein, Pra1, has a proven role in Zn2+ scavenging in C. albicans (Citiulo et al., 2012) but this system is more analogous to the bacterial hemophore system (Cescau et al., 2007) – both are ribosomally synthesized small proteins.
Pyridine-2,6-bis(thiocarboxylic acid) (PDTC), a small siderophore (198 daltons) produced by some Pseudomonads forms 1:1 or 1:2 complexes with a number of biometals with the highest affinity for Fe3+. Growth of P. putida is stimulated by supplementation with micromolar Zn2+ only if the strain produces PDTC. However, Zn2+ acquisition by PDTC appears to be much less efficient than Fe3+ acquisition (Cortese et al., 2002; Leach et al., 2007). P. putida PDTC and Y. pestis Ybt are the only two examples of siderophores/zincophores with proven roles in Zn2+ acquisition. Whether siderophore/zincophore-dependent Zn2+ uptake is wide-spread in bacteria remains to be determined.
Although the Y. pestis OM receptor Psn and the TonB system are required for Fe3+ uptake via the Ybt siderophore, neither of these are needed for Ybt-dependent Zn2+ acquisition (Fig. 6). In Gram-negative bacteria, involvement of the TonB-ExbBD complex in siderophore-Fe3+ uptake via an OM receptor is a paradigm for iron acquisition via siderophores (Miethke and Marahiel, 2007). TonB-dependent receptors also appear to be needed for uptake of Zn2+-siderophore complexes in Pseudomonas species. In P. putida, the TonB-dependent OM receptor PdtK is required for efficient growth stimulation by Zn2+-PDTC complexes (Leach et al., 2007). Hannauer et al. observed accumulation of Zn2+-PVD complexes into the periplasm of Peusdomonas aeruginosa cells only when the TonB-dependent OM FpvA receptor was present. In this instance unwanted metals were then effluxed from the periplasm (Hannauer et al., 2012). However, there are several examples of siderophore uptake by TonB-independent receptors. Legionella pneumophila and Fransicella tularensis each lack the TonB-ExbBD system. However, the OM receptor proteins LbtU and FslE are involved in Fe3+-siderophore utilization by these two pathogens (Chatfield et al., 2011; Ramakrishnan et al., 2008; Ramakrishnan et al., 2012). Use of ferrioxamines B and E are dependent upon TonB and the TonB-dependent OM receptor FoxA in Salmonella enterica; however a tonB mutant still exhibited reduced utilization of these two siderophores (Kingsley et al., 1999). How these ferrisiderophores or Zn2+-siderophore/zincophore complexes are transported through the OM remains unanswered. In Y. pestis, a putative OM receptor for Ybt-dependent Zn2+ utilization could rely upon the TolABQR system (Lloubès et al., 2012) for providing energy. While this is a possibility, the mechanism for translocation across the OM remains to be elucidated.
Transport of ferrisiderophores through the cytoplasmic membrane of bacteria typically requires ABC permeases (Miethke and Marahiel, 2007). In Y. pestis, the putative IM ABC transporter YbtPQ is essential for Fe3+-Ybt utilization (Fetherston et al., 1999). Unlike Fe3+-Ybt uptake, this study indicates that a predicted MFS member, YbtX (with no known role in Fe3+ uptake), not YbtPQ, is critical for Ybt-dependent Zn2+ import (Fig. 7 and 8). Strikingly, another MFS member, PdtE, was required for utilization of Zn2+-PDTC complexes in P. putida (Leach et al., 2007). MFS type proteins have been shown to be involved in both siderophore uptake and efflux (Chatfield et al., 2012). Several MFS members including RhtX, FptX (orthologs of YbtX) and LbtC are needed for Fe3+ uptake via the rhizobactin, pyochelin and legiobactin siderophores in Sinorhizobium meliloti, P. aeruginosa and L. pneumophila, respectively (Chatfield et al., 2012; Michel et al., 2007; Ó Cuív et al., 2004). However, in our experiments, inactivation of ybtX did not prevent secretion of the Ybt siderophore or cause any growth defects in metal-chelated medium, traits generally found in bacteria with mutations in genes encoding siderophore efflux pumps (Crouch et al., 2008; Fetherston et al., 1999; Furrer et al., 2002) suggesting that YbtX is not required for Ybt export. Thus, Ybt-dependent uptake of Fe3+ and Zn2+ appear to require completely different transport routes through the OM and IM (Fig. 10). This suggests that Ybt complexes with Fe3+ and Zn2+ may have sufficiently different structural conformations to require separate transport systems. Two isomers of the Pch siderophore, which has a similar structure to Ybt, use different OM receptors and IM transporters. In P. aeruginosa, Fe3+-Pch translocates through the OM and IM using the OM receptor FptA and MFS permease FptX, respectively, while transport of its isoform ferri-enantio-Pch occurs via the OM receptor FetA and IM ABC transporter FetDE in P. fluorescens. These transport components are not interchangeable – FptA and FptX transports Fe3+-Pch not ferri-enantio-Pch (Youard et al., 2011).
Fig. 10.
A proposed model of Zn2+ uptake in Y. pestis. Fe3+ uptake via the Ybt system as well as Zn2+ uptake is shown. Dashed arrows represent putative steps. Our results suggest passage through both the OM and IM when exogenous Ybt is supplied.
Here, we propose a model for Zn2+ acquisition in Y. pestis by the ZnuABC and Ybt siderophore/zincophore systems using two independent uptake routes (Fig. 10). Under in vitro conditions, Zn2+ may enter the cell passively through OM porins. In the periplasm, ZnuA chaperones Zn2+ to the ZnuBC ABC transporter required for the active transport of Zn2+ across the IM. Although ZnuABC is a major Zn2+ uptake system under the in vitro growth conditions we have used (Fig. 3A; (Desrosiers et al., 2010), both Zn2+ transport systems appear to be equally important in vivo. Strains with a mutation in either system retain nearly full virulence in the septicemic plague model while a double mutant is highly attenuated. We propose that Ybt is secreted to the external environment where it chelates Zn2+ and serves as a zincophore to bring this essential metal into the bacterial cell using a TonB-independent receptor or porin. How Zn2+ translocates through the OM remains to be determined but it is likely to be an energy dependent process. Subsequent transport of Zn2+ or Zn2+-Ybt from the periplasm into the cytoplasm requires YbtX in the IM. Finally, the mechanism for removing Zn2 + from Ybt is uncharacterized.(Fig. 10).
Under in vitro conditions when low concentrations of unchelated Zn2+ are present, the growth of many bacterial species is dependent on ZnuABC or a related C9 family ABC Zn2+ transporter. In vivo, mutations in genes encoding the Znu family of transporters cause a severe loss of virulence or colonization by B. abortus, C. jejuni, M. cararrhalis, P. multocida, N. gonorrhoeae, S. Typhimurium, S. penumoniae, S. pyogenes, and Y. ruckeri (Ammendola et al., 2007; Bayle et al., 2011; Campoy et al., 2002; Dahiya and Stevenson, 2010; Davis et al., 2009; Garrido et al., 2003; Kim et al., 2004; Lim et al., 2008; Liu et al., 2012; Murphy et al., 2013; Plumptre et al., 2014; Weston et al., 2009; Yang et al., 2006). Clearly, in the mammalian host, the Y. pestis ZnuABC transporter acquires sufficient Zn2+ to cause disease despite chelation by host proteins. In the inflamed gut, the Znu system of Salmonella overcomes Zn2+-chelation by calprotectin (Liu et al., 2012). How Zn2+ is removed from calprotectin and/or other host Zn2+-sequestering proteins is unknown. The N. meningitidis TonB-dependent OM receptor protein CbpA has been recently reported to bind calprotectin, allowing its utilization as a Zn2+ source (Stork et al., 2013). Another, possibility is that inflammation and necrosis may release Zn2+ from some host proteins.
In other bacterial pathogens, A. baumanii, uropathogenic E. coli, H. ducreyi, H. influenzae, L. monocytogenes, P. mirabilis, Vibrio parahaemoliticus, and Y. pestis, mutations in znu do not affect virulence or have a subtle effect on virulence or colonization (Desrosiers et al., 2010; Hood et al., 2012; Lewis et al., 1999; Liu et al., 2013; Nielubowicz et al., 2010; Rosadini et al., 2011; Sabri et al., 2009) suggesting that additional Zn2+ uptake systems are involved in the virulence of these bacteria. Indeed, in uropathogenic E. coli, H. influenzae, L. monocytogenes, and S. Typhimurium, mutation of a second Zn2+ transporter in a znu background further decreased virulence or colonization. Thus, in these pathogens, both ZnuABC and the 2nd Zn2+ transporter contribute to virulence (Cerasi et al., 2014; Corbett et al., 2012; Rosadini et al., 2011; Sabri et al., 2009).
A Y. pestis irp2 mutant is avirulent in the mouse model of bubonic plague and highly attenuated in the pneumonic plague model (Fetherston et al., 2010). Consequently, in this study, we have used a mouse model of septicemic plague in which a Δpgm mutant (which lacks the entire Ybt system) is known to be highly virulent (Bearden and Perry, 1999; Jackson and Burrows, 1956; Une and Brubaker, 1984). Here we show an absolute requirement for Zn2+ acquisition for Y. pestis virulence in septicemic plague. The double irp2 znu mutants had a >105-fold attenuation compared to the Ybt+ Znu+ parent strain (Table 1). Since a Y. pestis znu mutant is highly virulent in all three mouse plague models (Desrosiers et al., 2010) (Table 1), these data suggest that Ybt-dependent Zn2+ uptake compensates for the loss of Zn2+ acquisition in mice by a znu mutation in Y. pestis. Since the irp2 single mutant is highly virulent in the septicemic plague model, it appears that the ZnuABC and Ybt serve redundant functions for Zn2+ acquisition in mammals with either being sufficient to cause disease. It is also possible that Ybt may remove Zn2+ from mammalian proteins. Y. pestis is the first pathogen with interrelated siderophore/zincophore systems involved in Zn2+ and Fe3+ acquisition in vitro and in vivo.
Experimental Procedures
Bacterial strains, plasmids, primers and growth conditions
The bacterial strains, plasmids and primers used in this study are listed in Table S1 in supporting information. All bacterial strains are stored in glycerol stocks (Beesley et al., 1967) at -80°C. E. coli strains DH5α and DH5α (λpir) were used in the construction and maintenance of recombinant plasmids and were grown in Luria broth (LB) or on LB agar at 28-37°C. Select agent-exempt Y. pestis strains lacking the pCD1 virulence plasmid or the 102-kb chromosomal pgm locus were used for the construction of all mutants and in vitro studies. Y. pestis strain designations that lack a plus sign have a pgm deletion or a mutation within the pgm locus, which encodes the ∼29 kb ybt locus/high pathogenicity island. The pgm locus can be lost spontaneously in vitro at a rate of 10-5 (Brubaker, 1969; Fetherston and Perry, 1994; Perry and Fetherston, 2011). From glycerol stocks, Y. pestis strains were inoculated onto Tryptose Blood Agar Base (TBA) (Difco) or a modified Congo Red (CR) agar (Surgalla and Beesley, 1969) consisting of TBA supplemented with 0.2% (w/v) galactose, 1% (w/v) CR and ZnSO4 or ZnCl2 to a final concentration of 100 μM and grown at 32-33°C for 2 days. Formation of red colonies on CR plates indicates that the strain has retained the pgm locus and a red colony was inoculated onto TBA slants. From slants, Y. pestis strains were grown in Heart Infusion Broth (HIB) with indicated supplementations or cPMH2 at 30-37°C. Where necessary, ampicillin (Ap), kanamycin (Km), spectinomycin (Spc) or streptomycin (Sm) were used at final concentrations of 100, 50, 25 and 50 μg ml-1, respectively. TBA medium supplemented with 5% sucrose was used to cure suicide vectors as previously described (Fetherston et al., 1999).
Comparison of bacterial growth under metal-deficient conditions
All glassware used for Zn- and/or Fe-restricted growth studies was soaked overnight in a saturated chromic acid solution to remove contaminating metals and copiously rinsed in deionized water. For growth studies, Y. pestis strains were inoculated from TBA slants to an OD620 of ∼ 0.1 in the chemically defined medium, cPMH2, which had been extracted prior to use with Chelex-100 resin (Bio-Rad Laboratories), with or without ZnCl2 or FeCl3 supplementation to various concentrations. Unsupplemented cPMH2 medium has a residual Zn2+ concentration of ∼0.5 μM (Desrosiers et al., 2010; Gong et al., 2001). Cultures were aerated (250 rpm) at 37°C with culture volumes ∼10-20% of the volumes of treated glassware or plastic conical tubes (Falcon). Growth through one or two transfers (∼3-4 or 6-8 generations) was used to acclimate cells to cPMH2 and varying Zn+2 or Fe+3 conditions prior to use in all experimental studies, unless otherwise indicated. Growth of the cultures was monitored by determining the OD620 with a Genesys5 spectrophotometer (Spectronic Instruments, Inc.).
Mutant strain constructions
Suicide plasmids pSucZnu3.5, pKNGΔznuA, pKNGΔtonB2, pPSN15, pCIRP498.8 and pCVDybtX (Table S1 in supporting information) were used to introduce mutations in znuBC, znuA, hasB, psn, irp2, and ybtX, respectively, into various Y. pestis KIM strains by allelic exchange as described previously (Fetherston et al., 1995). The λ red recombinase method (Datsenko and Wanner, 2000; Lathem et al., 2007) was used to inactivate y1329-y1330 and y1245. The PCR product for replacement of y1329-1330 in KIM6-2077+ with a kan cassette was prepared using y1329red-Forw and y1330red-Rev primers and pKD4 as a template. In a double znu y1329-1330 mutant carrying pWL204, y1245 was replaced with a cat cassette from pKD3 using y1245-KMI and y1245-KMII primers. All genetic mutations were confirmed by PCR using primers listed in Table S1 in supporting information.
To construct the suicide vector pKNGΔznuA with an in-frame znuA deletion, pZnu2 (Desrosiers et al., 2010) was digested with HindIII and PstI to release a 1.9 kb fragment that was cloned into the same sites in pBluescript-KS to yield pBSZnuA. A 156 amino acid deletion within ZnuA (Fig. S2) was created by ligating a HindIII/XmnI fragment and a PstI/FspI fragment from pBSZnuA into the HindIII and PstI sites of pBluescript-KS. This plasmid, called pBSΔznuA was cut with SalI and XbaI and the 1.5 kb piece containing the deleted version of znuA was ligated into the same sites of pKNG101, resulting in pKNGΔZnuA.
Complementation of the znuBC mutation
An ∼3 kb StuI/AgeI fragment that contains the coding sequences for ZnuA, B and C, was isolated from pZnu2 and cloned into the StuI and XmaI sites of pUC18R6K-mini-Tn7T-Km to generate the Znu integration vector, pUCR6K-ZnuABC-Km. The plasmid was electroporated, along with the Tn7 transposase expression vector, pTNS2, into KIM6-2077+ and KIM6-2077. Kanamycin resistant strains containing the mini-Tn7 transposon with znuABC+ integrated at the attTn7 site were confirmed by hybridization and PCR. A 240 bp DNA fragment encompassing parts of znuA and znuC ORFs as well as the intergenic region between the znuA and znuCB operons was DIG-labeled by PCR with primers ZnuC.3 and ZnuC.5 and used as a probe against EcoRI and HindIII/BamHI digested genomic DNA from tested strains. For verification of znuABC+ integration into the chromosomal attTn7 site by PCR, ZnuA 5.3 and attTn7Yp-Fwd primers were used. The resulting strains were named KIM6-2077.10+ and KIM6-2077.10.
Construction of a YbtX expressing plasmid
To delete the ybtPQ genes (∼ 3.4 kb) from pYbtPQX, primers ybtPQdel_F and ybtPQdel_R were used in reverse splicing by overlap extension PCR. Following the reaction, DpnI was added to digest template DNA; the PCR product was precipitated with ethanol and transformed into E. coli DH5α chemically competent cells. Clones with the appropriate ybtPQ deletion were detected by PCR and confirmed by sequencing (ACGT Inc.). The resulting plasmid, pYbtX, was introduced into KIM6-2077.13 for complementation studies.
β-Galactosidase assays
The β-galactosidase activities from znuA∷lacZ or irp2∷lacZ reporter fusions were measured in Y. pestis strains carrying the reporter plasmids pEUZnu1 or pEUIrp2, respectively (Desrosiers et al., 2010; Perry et al., 2003a). To compare znuA∷lacZ reporter activity in ΔznuBC (KIM6-2077+) and irp2∷kan ΔznuBC (KIM6-2077.7) mutants, the strains were grown at 33-34°C overnight on TBA slants supplemented with Spc and ZnCl2 at a final concentrations of 25 μg ml-1 and 10 μM, respectively. The cells were resuspended in cPMH2 supplemented with Spc and various concentrations of ZnCl2 and/or FeCl3 to an OD620 ∼ 0.1, grown at 37°C for ∼ 3 generations and harvested. To compare znuA∷lacZ reporter activities in the KIM6+ parent to irp2∷kan (KIM6-2046.1) and ΔznuBC mutants, the strains were grown on TBA slants with Spc at 33-34°C overnight. The cells were grown through two transfers in cPMH2 with Spc for 5.5-6.5 generations before harvesting. To monitor irp2∷lacZ reporter activities in the KIM6+ and ΔznuBC mutant, TBA/Spc pre-grown cells were propagated through two transfers in cPMH2/Spc with or without ZnCl2 or FeCl3 for 4.5-5.5 generations before collecting samples. β-Galactosidase activities from whole-cell lysates were measured by monitoring the hydrolysis of o-nitrophenyl-β-D-galactopyranoside spectrophotometrically with the results expressed in Miller units (Miller, 1992).
Purification of apo-Ybt
Apo-Ybt was purified in a multi-step procedure modified from previous methods (Miller and DeMoll, 2011; Miller et al., 2010). No iron was added to the spent media before extraction. Eight liters of spent media were extracted in 500 ml aliquots with three washes of 200 ml ethyl acetate. The organic layers were collected, combined, and the solvent was removed by rotary evaporation at 40°C. The material was then re-dissolved in ∼5 ml of 100% ethanol. The crude material was then diluted to 50 ml with 18 MΩ water for the next step of purification.
Final Ybt purification was performed on a Combiflash Rf. Crude Ybt was dissolved in a 10:1 water-ethanol solution, loaded onto a 2.5 g pre-packed C18 reverse phase cartridge, and purified on a 4.3 g C18 reverse phase column with water and acetonitrile as the mobile phase. The separation was achieved by using the following gradient: 10% acetonitrile for 3 mins followed by a linear gradient from 20% to 60% acetonitrile over 25 minutes (Retention time ≈ 13 min). Fractions were collected every 1 minute. Fractions containing Ybt were concentrated in vacuo and the purification was repeated.
Final fractions were then collected and concentrated to dryness via rotary evaporation at 40°C. The identity and purity of authentic Ybt in each stage of purification was confirmed via mass spectrophotometry using an Agilent 6224 TOF LC/MS equipped with the Agilent 1260 HPLC system and MassHunter software. The instrument was equipped with an Agilent Extend C-18 column (1.8 μm, 2.1 × 50 mm) with mobile phase consisting of mass spectrophotometry grade water (with 0.1% formic acid and 0.1% methanol) and acetonitrile (with 0.1% formic acid) and operated in positive ion mode (3500V Vcap, 750V OctRF Vpp, 65V skimmer, 135V fragmenter, 40 psi Nebulizer gas, 12 liter/min drying gas, and 325 C gas temperature). Samples were eluted from with a linear gradient of 5 to 100% acetonitrile at 0.3 ml/min over 15 minutes. Authentic Ybt eluted at approximately 7.66 minutes with an M+1 mass of 482.1250 and an M+2 mass 241.5643 of in agreement with the predicted M+1 and M+2 masses of 482.1236 and 241.5655 respectively.
Feeding assays with culture supernatants and purified apo-Ybt
Ybt-containing and Ybt-negative culture supernatants were obtained from KIM6+ and the irp2∷kan mutant, respectively, grown for two transfers in cPMH2 at 37°C and filter sterilized as previously described (Miller et al., 2010). For Ybt supernatant assays, Y. pestis recipient strains, Δpsn irp2∷kan ΔznuBC (KIM6-2077.18) and Δpsn irp2∷kan ΔybtX ΔznuBC (KIM6-2077.19) were grown in cPMH2 supplemented with 0.6 μM ZnCl2 and 1μM FeCl3 at 37°C for about 4-5 generations before they were back diluted to an OD620 of ∼ 0.1 in the same medium containing 50% (v/v) of culture supernatants from either KIM6+, the irp2∷kan or ybtU mutants. For apo-Ybt utilization assays, the Δpsn irp2∷kan ΔznuBC recipient strain was grown overnight as above and back diluted to an OD620 of ∼ 0.1 in the same medium with the addition of purified apo-Ybt in ethanol (0.25 or 1 μl of a 10 mM concentration in ethanol per 1.5 ml) or ethanol alone (1 μl per 1.5 ml). Bacteria were cultivated at 37°C overnight and final OD620s were measured.
Virulence testing
Construction and testing of potentially virulent strains was performed in a CDC-approved BSL3 laboratory following Select Agent regulations using procedures approved by the University of Kentucky Institutional Biosafety Committee. All animal care and experimental procedures were conducted in accordance with the Animal Welfare Act, Guide for the Care and Use of Laboratory Animals, PHS Policy and the U.S. Government Principals for the Utilization of and Care for Vertebrate Animals in Teaching, Research, and Training and approved by the University of Kentucky Institutional Animal Care and Use Committee. The University of Kentucky Animal Care Program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, Inc.
Y. pestis strains were transformed with pCD1Ap by electroporation (Fetherston et al., 1995), plasmid profiles analyzed, and transformant phenotypes determined on CR agar (Surgalla and Beesley, 1969) and magnesium-oxalate plates (Higuchi and Smith, 1961). After growth at 37°C in cPMH2 with or without 2.5 mM CaCl2, culture supernatants were tested for LcrV secretion by Western blot using polyclonal antisera against LcrV (provided by R.R. Brubaker).
For mouse infections, several colonies of Y. pestis from an agar plate were streaked onto a TBA slant and grown overnight at 30°C. Cells were washed off the slant and inoculated to an OD620 of ∼0.1 in HIB supplemented with 50 μg Ap/ml, 0.2% xylose, 2.5 mM CaCl2 and 10μM ZnCl2 and grown at 37°C overnight. These cultures were diluted to an OD620 of ∼0.1 into the same medium but without zinc supplementation and grown at 37°C for ∼2 generations. The cultures were centrifuged and cell pellets were resuspended in mouse isotonic phosphate-buffered saline (PBS; 149 mM NaCl, 16 mM Na2HPO4, 4 mM NaH2PO4 [pH 7.0]). 0.1 ml of 10-fold serially diluted bacterial suspensions was injected retro-orbitally (via the retro-orbital plexus) into groups of four 6- to 8-week old female Swiss Webster mice (Hsd∷ND4) sedated by inhalation of isoflurane-oxygen or by intraperitoneally injecting a mixture of 100 μg of ketamine and 10 μg of xylazine per kg of body weight. Appropriate serial dilutions of the cultures used for injections were inoculated onto TBA plates containing Ap (30-50 μg ml-1), 2.5 mM CaCl2 and 10 or 100 μM ZnCl2 and colonies were counted after 2-3 days of incubation at 30-33°C. Mice were observed daily for 2 weeks and LD50 values were calculated according to the method of Reed and Muench (Reed and Muench, 1938).
Supplementary Material
Acknowledgments
This work was supported by Public Health Services grant AI33481 from the U.S. National Institutes of Health. We thank Will Arnold for help with the construction of some mutants and some preliminary experiments and Bob Brubaker for providing antisera against LcrV.
Footnotes
The authors have no conflicts of interest to declare.
References
- Althaus EW, Outten CE, Olson KE, Cao H, O'Halloran TV. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6559–6569. doi: 10.1021/bi982788s. [DOI] [PubMed] [Google Scholar]
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 2011;82:904–916. doi: 10.1111/j.1365-2958.2011.07862.x. [DOI] [PubMed] [Google Scholar]
- Bearden SW, Fetherston JD, Perry RD. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden SW, Perry RD. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999;32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- Beesley ED, Brubaker RR, Janssen WA, Surgalla MJ. Pesticins. III. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967;94:19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GLA, Albrecht-Gary AM. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(iii), copper(ii) and zinc(ii) complexes. Dalton Trans. 2012;41:2820–2834. doi: 10.1039/c1dt11804h. [DOI] [PubMed] [Google Scholar]
- Braud A, Geoffroy V, Hoegy F, Mislin GLA, Schalk IJ. Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ Microbiol Rep. 2010;2:419–425. doi: 10.1111/j.1758-2229.2009.00126.x. [DOI] [PubMed] [Google Scholar]
- Braud A, Hannauer M, Mislin GLA, Schalk IJ. The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J Bacteriol. 2009a;191:3517–3525. doi: 10.1128/JB.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine - iron uptake pathway. Environ Microbiol. 2009b;11:1079–1091. doi: 10.1111/j.1462-2920.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- Brubaker RR. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969;98:1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoy S, Jara M, Busquets N, Pérez de Rozas AM, Badiola I, Barbé J. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar typhimurium virulence. Infect Immun. 2002;70:4721–4725. doi: 10.1128/IAI.70.8.4721-4725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi M, Ammendola S, Battistoni A. Competition for zinc binding in the host-pathogen interaction. Front Cell Infect Microbiol. 2013;3:108. doi: 10.3389/fcimb.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi M, Liu JZ, Ammendola S, Poe AJ, Petrarca P, Pesciaroli M, et al. The ZupT transporter plays an important role in zinc homeostasis and contributes to Salmonella enterica virulence. Metallomics. 2014;6:845–853. doi: 10.1039/c3mt00352c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescau S, Cwerman H, Létoffé S, Delepelaire P, Wandersman C, Biville F. Heme acquisition by hemophores. BioMetals. 2007;20:603–613. doi: 10.1007/s10534-006-9050-y. [DOI] [PubMed] [Google Scholar]
- Chatfield CH, Mulhern BJ, Burnside DM, Cianciotto NP. Legionella pneumophila LbtU acts as a novel, TonB-independent receptor for the legiobactin siderophore. J Bacteriol. 2011;193:1563–1575. doi: 10.1128/JB.01111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield CH, Mulhern BJ, Viswanathan VK, Cianciotto NP. The major facilitator superfamily-type protein LbtC promotes the utilization of the legiobactin siderophore by Legionella pneumophila. Microbiology. 2012;158:721–735. doi: 10.1099/mic.0.055533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol. 2012;8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citiulo F, Jacobsen ID, Miramón P, Schild L, Brunke S, Zipfel P, et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012;8:e1002777. doi: 10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett D, Wang J, Schuler S, Lopez-Castejon G, Glenn S, Brough D, et al. Two zinc uptake systems contribute to the full virulence of Listeria monocytogenes during growth in vitro and in vivo. Infect Immun. 2012;80:14–21. doi: 10.1128/IAI.05904-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Cortese MS, Paszczynski A, Lewis TA, Sebat JL, Borek V, Crawford RL. Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. and the biological activities of the formed complexes. BioMetals. 2002;15:103–120. doi: 10.1023/a:1015241925322. [DOI] [PubMed] [Google Scholar]
- Crouch MLV, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- Dahiya I, Stevenson RMW. The ZnuABC operon is important for Yersinia ruckeri infections of rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2010;33:331–340. doi: 10.1111/j.1365-2761.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LM, Kakuda T, DiRita VJ. A Campylobacter jejuni znuA orthologue is essential for growth in low-zinc environments and chick colonization. J Bacteriol. 2009;191:1631–1640. doi: 10.1128/JB.01394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers DC, Bearden SW, Mier I, Jr, Abney J, Paulley JT, Fetherston JD, et al. Znu is the predominant zinc importer in Yersinia pestis during in vitro growth but is not essential for virulence. Infect Immun. 2010;78:5163–5177. doi: 10.1128/IAI.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston JD, Bertolino VJ, Perry RD. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol. 1999;32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- Fetherston JD, Kirillina O, Bobrov AG, Paulley JT, Perry RD. The yersiniabactin transport system is critical for the pathogenesis of bubonic and pneumonic plague. Infect Immun. 2010;78:2045–2052. doi: 10.1128/IAI.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston JD, Lillard JW, Jr, Perry RD. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetherston JD, Perry RD. The pigmentation locus of Yersinia pestis KIM6+ is flanked by an insertion sequence and includes the structural genes for pesticin sensitivity and HMWP2. Mol Microbiol. 1994;13:697–708. doi: 10.1111/j.1365-2958.1994.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Foote JW, Delves HT. Albumin bound and a2 -macroglobulin bound zinc concentrations in the sera of healthy adults. J Clin Pathol. 1984;37:1050–1054. doi: 10.1136/jcp.37.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S, Paulley JT, Fetherston JD, Cheng YQ, Perry RD. Yersinia ironomics: comparison of iron transporters among Yersinia pestis biotypes and its nearest neighbor, Yersinia pseudotuberculosis. BioMetals. 2010;23:275–294. doi: 10.1007/s10534-009-9286-4. [DOI] [PubMed] [Google Scholar]
- Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol. 2002;44:1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- Gabbianelli R, Scotti R, Ammendola S, Petrarca P, Nicolini L, Battistoni A. Role of ZnuABC and ZinT in Escherichia coli O157:H7 zinc acquisition and interaction with epithelial cells. BMC Microbiol. 2011;11:36. doi: 10.1186/1471-2180-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido ME, Bosch M, Medina R, Llagostera M, Pérez de Rozas AM, Badiola I, Barbé J. The high-affinity zinc-uptake system znuACB is under control of the iron-uptake regulator (fur) gene in the animal pathogen Pasteurella multocida. FEMS Microbiol Lett. 2003;221:31–37. doi: 10.1016/S0378-1097(03)00131-9. [DOI] [PubMed] [Google Scholar]
- Gong S, Bearden SW, Geoffroy VA, Fetherston JD, Perry RD. Characterization of the Yersinia pestis Yfu ABC iron transport system. Infect Immun. 2001;67:2829–2837. doi: 10.1128/IAI.67.5.2829-2837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AI, Hunt S, Stokes SL, Bramall N, Bunch J, Cox AG, et al. Severe zinc depletion of Escherichia coli: Roles for high-affinity zinc binding by ZinT, zinc transport and zinc-independent proteins. J Biol Chem. 2009;284:18377–18389. doi: 10.1074/jbc.M109.001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Wong MD, Rosen BP, Smith RL, Rensing C. ZupT Is a Zn(II) uptake system in Escherichia coli. J Bacteriol. 2002;184:864–866. doi: 10.1128/JB.184.3.864-866.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannauer M, Braud A, Hoegy F, Ronot P, Boos A, Schalk IJ. The PvdRT-OpmQ efflux pump controls the metal selectivity of the iron uptake pathway mediated by the siderophore pyoverdine in Pseudomonas aeruginosa. Environ Microbiol. 2012;14:1696–1708. doi: 10.1111/j.1462-2920.2011.02674.x. [DOI] [PubMed] [Google Scholar]
- Hantke K. Bacterial zinc uptake and regulators. Curr Opin Microbiol. 2005;8:196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hesketh A, Kock H, Mootien S, Bibb M. The role of absC, a novel regulatory gene for secondary metabolism, in zinc-dependent antibiotic production in Streptomyces coelicolor A3(2) Mol Microbiol. 2009;74:1427–1444. doi: 10.1111/j.1365-2958.2009.06941.x. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Smith JL. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Burrows TW. The virulence-enhancing effect of iron on non-pigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- Karlinsey JE, Maguire ME, Becker LA, Crouch MLV, Fang FC. The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol. 2010;78:669–685. doi: 10.1111/j.1365-2958.2010.07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A, Tsujii A, Wada A, Nishino T, Ishihama A. Systematic search for zinc-binding proteins in Escherichia coli. Eur J Biochem. 2002;269:2403–2413. doi: 10.1046/j.1432-1033.2002.02900.x. [DOI] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J Vet Med Sci. 2004;66:1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- Kingsley RA, Reissbrodt R, Rabsch W, Ketley JM, Tsolis RM, Everest P, et al. Ferrioxamine-mediated iron(III) utilization by Salmonella enterica. Appl Environ Microbiol. 1999;65:1610–1618. doi: 10.1128/aem.65.4.1610-1618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Hidaka S, Kawamura Y, Ozaki M, Hayase Y. Micacocidin A, B and C, novel antimycoplasma agents from Pseudomonas sp. I. taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J Antibiot (Tokyo) 1998a;51:323–327. doi: 10.7164/antibiotics.51.323. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ikenishi Y, Takinami Y, Takema M, Sun WY, Ino A, Hayase Y. Preparation and antimicrobial activity of micacocidin. J Antibiot (Tokyo) 2000;53:532–539. doi: 10.7164/antibiotics.53.532. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Nakai H, Ikenishi Y, Sun WY, Ozaki M, Hayase Y, Takeda R. Micacocidin A, B and C, novel antimycoplasma agents from Pseudomonas sp. II. Structure elucidation. J Antibiot (Tokyo) 1998b;51:328–332. doi: 10.7164/antibiotics.51.328. [DOI] [PubMed] [Google Scholar]
- Kreutzer MF, Kage H, Gebhardt P, Wackler B, Saluz HP, Hoffmeister D, Nett M. Biosynthesis of a complex yersiniabactin-like natural product via the mic Locus in phytopathogen Ralstonia solanacearum. Appl Environ Microbiol. 2011;77:6117–6124. doi: 10.1128/AEM.05198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sannigrahi S, Tzeng YL. The Neisseria meningitidis ZnuD zinc receptor contributes to interactions with epithelial cells and supports heme utilization when expressed in Escherichia coli. Infect Immun. 2012;80:657–667. doi: 10.1128/IAI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007;315:509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- Leach LH, Morris JC, Lewis TA. The role of the siderophore pyridine-2,6-bis (thiocarboxylic acid) (PDTC) in zinc utilization by Pseudomonas putida DSM 3601. BioMetals. 2007;20:717–726. doi: 10.1007/s10534-006-9035-x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Klesney-Tait J, Lumbley SR, Ward CK, Latimer JL, Ison CA, Hansen EJ. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect Immun. 1999;67:5060–5068. doi: 10.1128/iai.67.10.5060-5068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qiu Y, Gao H, Guo Z, Han Y, Song Y, et al. Characterization of Zur-dependent genes and direct Zur targets in Yersinia pestis. BMC Microbiol. 2009;9:128. doi: 10.1186/1471-2180-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KHL, Jones CE, vanden Hoven RN, Edwards JL, Falsetta ML, Apicella MA, et al. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun. 2008;76:3569–3576. doi: 10.1128/IAI.01725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Janet Z, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, Kehl-Fie TE, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yan M, Liu L, Chen S. Characterization of a novel zinc transporter ZnuA acquired by Vibrio parahaemolyticus through horizontal gene transfer. Front Cell Infect Microbiol. 2013;3:61. doi: 10.3389/fcimb.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloubès R, Goemaere E, Zhang X, Cascales E, D D. Energetics of colicin import revealed by genetic cross-complementation between the Tol and Ton systems. Biochem Soc Trans. 2012;40:1480–1485. doi: 10.1042/BST20120181. [DOI] [PubMed] [Google Scholar]
- Ma Z, Faulkner MJ, Helmann JD. Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Mol Microbiol. 2012;86:1144–1155. doi: 10.1111/mmi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel L, Bachelard A, Reimmann C. Ferripyochelin uptake genes are involved in pyochelin-mediated signalling in Pseudomonas aeruginosa. Microbiology. 2007;153:1508–1518. doi: 10.1099/mic.0.2006/002915-0. [DOI] [PubMed] [Google Scholar]
- Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DA, Luo L, Hillson N, Keating TA, Walsh CT. Yersiniabactin synthetase: a four-protein assembly line producing the nonribosomal peptide/polyketide hybrid siderophore of Yersinia pestis. Chem Biol. 2002;9:333–449. doi: 10.1016/s1074-5521(02)00115-1. [DOI] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N. Y: 1992. [Google Scholar]
- Miller MC, DeMoll E. Extraction, purification, and Identification of Yersiniabactin, the siderophore of Yersinia pestis. Curr Prot Microbiol. 2011;23:5B.3.1–5B.3.22. doi: 10.1002/9780471729259.mc05b03s23. [DOI] [PubMed] [Google Scholar]
- Miller MC, Fetherston JD, Pickett CL, Bobrov AG, Weaver RH, DeMoll E, Perry RD. Reduced synthesis of the Ybt siderophore or production of aberrant Ybt-like molecules activates transcription of yersiniabactin genes in Yersinia pestis. Microbiology. 2010;156:2226–2238. doi: 10.1099/mic.0.037945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SA, Marletta MA. Metal binding characteristics and role of iron oxidation in the ferric uptake regulator from Escherichia coli. Biochemistry. 2005;44:13553–13559. doi: 10.1021/bi0507579. [DOI] [PubMed] [Google Scholar]
- Murphy TF, Brauer AL, Kirkham C, Johnson A, Koszelak-Rosenblum M, Malkowski MG. Role of the zinc uptake ABC transporter of Moraxella catarrhalis in persistence in the respiratory tract. Infect Immun. 2013;81:3406–3413. doi: 10.1128/IAI.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Kobayashi S, Ozaki M, Hayase Y, Takeda R. Micacocidin A. Acta Crystallogr. 1999;C55:54–56. [Google Scholar]
- Napolitano M, Rubio MÁ, Santamaría-Gómez J, Olmedo-Verd E, Robinson NJ, Luque I. Characterization of the response to zinc deficiency in the cyanobacterium Anabaena sp. Strain PCC 7120. J Bacteriol. 2012;194:2426–2436. doi: 10.1128/JB.00090-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielubowicz GR, Smith SN, Mobley HLT. Zinc uptake contributes to motility and provides a competitive advantage to Proteus mirabilis during experimental urinary tract infection. Infect Immun. 2010;78:2823–2833. doi: 10.1128/IAI.01220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Cuív P, Clarke P, Lynch D, O'Connell M. Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J Bacteriol. 2004;186:2996–3005. doi: 10.1128/JB.186.10.2996-3005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Abney J, Mier I, Jr, Lee Y, Bearden SW, Fetherston JD. Regulation of the Yersinia pestis Yfe and Ybt iron transport systems. Adv Exp Med Biol. 2003a;529:275–283. doi: 10.1007/0-306-48416-1_53. [DOI] [PubMed] [Google Scholar]
- Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology. 1999;145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- Perry RD, Bobrov AG, Kirillina O, Rhodes ER, Actis LA, Fetherston JD. Yersinia pestis transition metal divalent cation transporters. Adv Exp Med Biol. 2012;954:267–279. doi: 10.1007/978-1-4614-3561-7_34. [DOI] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Iron and heme uptake systems. In: Carniel E, Hinnebusch BJ, editors. Yersinia Molecular and Cellular Biology. Norfolk, U.K.: Horizon Bioscience; 2004. pp. 257–283. [Google Scholar]
- Perry RD, Fetherston JD. Yersiniabactin iron uptake: mechanisms and role in Yersinia pestis pathogenesis. Microbes Infect. 2011;13:808–817. doi: 10.1016/j.micinf.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Shah J, Bearden SW, Thompson JM, Fetherston JD. Yersinia pestis TonB: role in iron, heme and hemoprotein utilization. Infect Immun. 2003b;71:4159–4162. doi: 10.1128/IAI.71.7.4159-4162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Straley SC, Fetherston JD, Rose DJ, Gregor J, Blattner FR. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarca P, Ammendola S, Pasquali P, Battistoni A. The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage. J Bacteriol. 2010;192:1553–1564. doi: 10.1128/JB.01310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumptre CD, Eijkelkamp BA, Morey JR, Behr F, Couñago RM, Ogunniyi AD, et al. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol Microbiol. 2014;91:834–851. doi: 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- Rahuel-Claremont S, Dunn MF. The biological chemistry of zinc. In: Rainsford KD, Milanino R, Sorenson RJ, Velo GP, editors. Copper and Zinc in Inflammatory and Degenerative Diseases. Kluwer Academic Publisher; 1998. pp. 47–59. [Google Scholar]
- Ramakrishnan G, Meeker A, Dragulev B. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J Bacteriol. 2008;190:5353–5361. doi: 10.1128/JB.00181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G, Sen B, Johnson R. Paralogous outer membrane proteins mediate uptake of different forms of iron and synergistically govern virulence in Francisella tularensis tularensis. J Biol Chem. 2012;287:25191–25202. doi: 10.1074/jbc.M112.371856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Rosadini CV, Gawronski JD, Raimunda D, Argüello JM, Akerley BJ. A novel zinc binding system, ZevAB, is critical for survival of nontypeable Haemophilus influenzae in a murine lung infection model. Infect Immun. 2011;79:3366–3376. doi: 10.1128/IAI.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Houle S, Dozois CM. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect Immun. 2009;77:1155–1164. doi: 10.1128/IAI.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Schalk IJ, Guillon L. Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: implications for metal homeostasis. Environ Microbiol. 2013;15:1661–1673. doi: 10.1111/1462-2920.12013. [DOI] [PubMed] [Google Scholar]
- Schalk IJ, Hannauer M, Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol. 2011;13:2844–2854. doi: 10.1111/j.1462-2920.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- Sebbane F, Jarrett C, Gardner D, Long D, Hinnebusch BJ. Role of the Yersinia pestis yersiniabactin iron acquisition system in the incidence of flea-borne plague. PLoS ONE. 2010;5:e14379. doi: 10.1371/journal.pone.0014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F, Jarrett CO, Gardner D, Long D, Hinnebusch BJ. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc Natl Acad Sci U S A. 2006;103:5526–5530. doi: 10.1073/pnas.0509544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbane F, Mandrand-Berthelot MA, Simonet M. Genes encoding specific nickel transport systems flank the chromosomal urease locus of pathogenic Yersiniae. J Bacteriol. 2002;184:5706–5713. doi: 10.1128/JB.184.20.5706-5713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MA, Taylor GL. Crystal structure of the Vibrio cholerae ferric uptake regulator (Fur) reveals insights into metal co-ordination. Mol Microbiol. 2009;72:1208–1220. doi: 10.1111/j.1365-2958.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- Sohnle PG, Hunter Michael J, Hahn B, Chazin Walter J. Zinc reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor - related proteins 8 and 14) J Infect Dis. 2000;182:1272–1275. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- Stork M, Bos MP, Jongerius I, de Kok N, Schilders I, Weynants VE, et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 2010;6:e1000969. doi: 10.1371/journal.ppat.1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork M, Grijpstra J, Bos MP, Mañas Torres C, Devos N, Poolman JT, et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla MJ, Beesley ED. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969;18:834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T, Brubaker RR. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect Immun. 1984;43:895–900. doi: 10.1128/iai.43.3.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Infectious diseases influenced by trace element enviroment. Ann NY Acad Sci. 1972;199:274–284. [PubMed] [Google Scholar]
- Weston BF, Brenot A, Caparon MG. The metal homeostasis protein, Lsp, of Streptococcus pyogenes is necessary for acquisition of zinc and virulence. Infect Immun. 2009;77:2840–2848. doi: 10.1128/IAI.01299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Becker T, Walters N, Pascual DW. Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infect Immun. 2006;74:3874–3879. doi: 10.1128/IAI.01957-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youard Z, Wenner N, Reimmann C. Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. BioMetals. 2011;24:513–522. doi: 10.1007/s10534-010-9399-9. [DOI] [PubMed] [Google Scholar]
- Zhao B, Moody SC, Hider RC, Lei L, Kelly SL, Waterman MR, Lamb DC. Structural analysis of cytochrome P450 105N1 involved in the biosynthesis of the zincophore, coelibactin. Int J Mol Sci. 2012;13:8500–8513. doi: 10.3390/ijms13078500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.