Abstract
We employed latent growth curve analysis to examine trajectories of respiratory sinus arrhythmia (RSA) from 3 to 6 years among children with varying levels of prenatal substance exposure and early adversity. Data were drawn from a prospective longitudinal study of prenatal substance exposure that included 1,121 participants. Baseline RSA and RSA reactivity to an attention-demanding task were assessed at 3, 4, 5, and 6 years. Overall, there were significant individual differences in the trajectories of RSA reactivity, but not baseline RSA, across development. Greater levels of prenatal substance exposure, and less exposure to early adversity, were associated with increased RSA reactivity at 3 years, but by 6 years, both were associated with greater RSA reactivity. Prenatal substance exposure had an indirect influence through early adversity on growth in RSA reactivity. Results are in support of and contribute to the framework of allostatic load.
Keywords: allostatic load, prenatal substance exposure, early adversity, respiratory sinus arrhythmia
INTRODUCTION
Early experienced repeated stressors can have profound consequences on developing stress response systems (Grossman & Taylor, 2007; McEwen, 1998; Shonkoff, Boyce, & McEwen, 2009). Early life stress can impact developing neuroendocrine (Bush, Obradović, Adler, & Boyce, 2011), parasympathetic (El-Sheikh & Hinnant, 2011), and brain (Tottenham et al., 2011) functioning through allostatic mechanisms. Allostasis is the process by which the body adapts to changing environmental demands in order to maintain homeostasis (McEwen & Stellar, 1993). Over time, however, exposure to chronic stress or allostatic load “costs” the system in the form of “wear and tear” on the body, subsequently increasing the likelihood of disease (Grossman & Taylor, 2007; McEwen, 1998; McEwen & Stellar, 1993) and psychopathology (El-Sheikh, 2005). Most studies of allostatic load have focused on the neuroendocrine and sympathetic stress response systems because of documented effects between chronic over-activation of these systems and later disease (Hinnant, Elmore-Staton, & El-Sheikh, 2011). However, a core tenet of allostasis is that exposure to prolonged stressors impacts the functional range of multiple biological systems (McE-wen & Stellar, 1993). In this study, we investigated relations between allostatic load and the less studied parasympathetic nervous system.
Parasympathetic activity is thought to measure mood and emotion regulation which can be affected by chronic stress, thereby increasing risk for psychopathology (El-Sheikh, 2005). Chronic stress has been shown to impact mesolimbic brain regions associated with emotion regulation in both animal and human studies (Kidwell & Barnett, 2007). Respiratory sinus arrhythmia (RSA), oscillatory increases and decreases of heart rate across the respiratory cycle (Beauchaine, 2001), is a parasympathetic measure used to study emotion regulation. RSA occurs when heart rate shows high frequency variability in time with respiration (Beauchaine, 2001). This variability is controlled by phasic influences of the vagus nerve projecting from the nucleus ambiguus (NA) to the sinoatrial pacemaker (Berntson et al., 1997; Grossman & Taylor, 2007; Porges, 2007). RSA is sensitive to early environmental experiences (Beauchaine, 2001; Conradt & Ablow, 2010; Conradt, Measelle, & Ablow, 2013; Propper, 2012) and in one study was related to allostatic load (El-Sheikh & Hinnant, 2011). Decreases in RSA in response to acute stress allow for sympathetic nervous system activation, promoting metabolic output to respond to environmental demands. Thus, it seems reasonable that effects of chronic stress on RSA could be a parasympathetic measure of allostatic load.
In this study, we measured parasympathetic activity via baseline RSA and RSA reactivity. High baseline RSA reflects the functional relationship between the central nervous system and the heart as mediated by the vagus (Berntson, Cacioppo, & Quigley, 1993; Porges, 2007) and is related to neural integrity and readiness to respond to environmental stressors (Beauchaine, 2001). In typically developing populations, RSA increases with development (Bornstein & Suess, 2000; Calkins & Keane, 2004; Porges, Doussard-Roosevelt, Portales, & Suess, 1994), and approaches adult levels by middle childhood or early adolescence (Bornstein & Suess, 2000; El-Sheikh, 2005; Hinnant et al., 2011). Higher baseline RSA is associated with lower levels of psychopathology, greater social competence, empathy, cognitive functioning, and appropriate emotion regulation (Beauchaine, 2001; Calkins, Graziano, & Keane, 2007; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996; Staton, El-Sheikh, & Buckhalt, 2009). By contrast, in at- risk children, lower baseline RSA was associated with a more positive relationship with an experimenter during an emotionally charged task, and parents reported greater internalizing and externalizing symptoms among children with higher baseline RSA in low-income African American children (Kidwell & Barnett, 2007). Gottman and Katz (1989) found that children with higher baseline RSA had parents who reported greater conflict and exhibited unresponsive, cold, and angry parenting. We (Conradt et al., 2013) found that, in a sample of infants raised in low SES environments, high baseline RSA was adaptive only when infants were raised in environments that fostered secure attachment relationships (compared to disorganized attachment relationships). Other studies have found no associations between baseline RSA and emotion regulation or competence in children (Blair, Peters, & Granger, 2004; Calkins & Keane, 2004; Donzella, Gunnar, Krueger, & Alwin, 2000; Stevenson-Hinde & Marshall, 1999). High baseline RSA, therefore, may not always be protective or adaptive, particularly in populations of risk.
RSA reactivity reflects decreases in RSA in response to task demands. In typically-developing children, RSA decreases from baseline to task and increases once the challenge has resolved (Beauchaine, 2001). Moderate RSA reactivity in response to attention-demanding tasks is functional, and may reflect “optimal” engagement with the environment. On the other hand, greater RSA reactivity may be a form of emotional lability suggestive of the “fight or flight” response that involves increased sympathetic activation (Beauchaine, 2001). There is some evidence for moderate stability during infancy and early childhood (Blandon, Calkins, Keane, & O’Brien, 2010; Bornstein & Suess, 2000; Calkins & Keane, 2004).
According to polyvagal theory, the development of RSA reactivity should follow a growth process as the central nervous system matures with increased cortical development and exerts greater control over neural pathways (Porges, 2007). Although studies of baseline RSA have spanned multiple time points, only three studies have examined the development of RSA reactivity across more than two time points, two of which were during infancy. Both showed decreases in RSA reactivity over time (Blandon et al., 2010; Propper et al., 2008). One study examined the development of baseline RSA and RSA reactivity at 6, 12, 42, and 60 months in a sample of low-income Latino children and found that baseline RSA increased across development, while RSA reactivity exhibited a significant amount of variability, with RSA reactivity highest at 5 years (Alkon, Boyce, Davis, & Eskenazi, 2011).
We chose to study baseline RSA and RSA reactivity in a high risk sample of children with prenatal substance exposure, many of whom also experience postnatal environmental adversity. Prenatal substance exposure is a biological stressor that can alter the infant’s physiological response to the postnatal environment (Lester & Padbury, 2009). Moreover, polysubstance exposure is very common, especially in the case of illicit substances (Mayet, Groshkova, Morgan, Mac-Cormack, & Strang, 2008). The purpose of many prenatal drug exposure studies is to examine the effects of a single substance (such as cocaine), while controlling for the effects of all other substances (such as alcohol, tobacco, and marijuana). A complementary approach is to take into account recent work suggesting that prenatal substance exposure is a stressor, and as such cumulative prenatal stress models may provide an alternative way of modeling the contribution of prenatal substance exposure to relevant developmental processes. Specifically, in addition to its teratogenic properties, prenatal substance exposure is a biological stressor that can alter the infant’s physiological response to the postnatal environment (Lester, Marsit, Conradt, Bromer, & Padbury, in press). That is, exposure to substances prenatally acts as a stressor that may alter the expression of specific genes important to placental function in late gestation (Lester & Padbury, 2009), which may lead to fetal exposure to high levels of maternal cortisol (Lester et al., in press; Lester & Padbury, 2009). A similar process is thought to occur in response to prenatal alcohol (Hellemans, Sliwowska, Verma, & Weinberg, 2010) and tobacco (Knopik, Maccani, Francazio, & McGeary, 2012) exposure. Furthermore, women who use substances prenatally typically use other substances; therefore, polydrug use could be conceptualized as a cumulative prenatal stressor (Lester & Padbury, 2009). Support for this view comes from the work of Fisher et al. (2011), who found that a summative index of prenatal substance exposure predicted growth in neurobehavioral disinhibition, defined as a profile of behavioral undercontrol, poor emotion regulation, and executive cognitive dysfunction, across adolescence.
The literature linking prenatal substance exposure to RSA is small and no study that we know of has examined the effects of polysubstance exposure. Thus, we focus in this study on polysubstance exposure, with the caveat that different substances may impact RSA via different mechanisms that are unknown thus far. What is known is that prenatal substance exposure was related to increased (Mehta et al., 1993), decreased (Schuetze & Eiden, 2006; Schuetze, Eiden, & Edwards, 2009), or no effect on baseline RSA (DiPietro, Suess, Wheeler, Smouse, & Newlin, 1995) and to lack of RSA reactivity (Schuetze, Eiden, & Coles, 2007) or greater RSA reactivity (Haley, Handmaker, & Lowe, 2006; Suess, Newlin, & Porges, 1997).
Early adverse experiences among children with prenatal substance exposure tend to co-occur, and include exposure to parental psychopathology, neighborhood violence, chaotic home environments, and lack of monetary resources (Dong et al., 2004; Propper, 2012), making it extremely difficult to separate the effect of one adverse experience from another. Many researchers therefore take a cumulative risk approach to studying the impact of co-occurring risk factors on physiological functioning (Eiden, Granger, Schuetze, & Veira, 2011; Fisher et al., 2011; Sameroff, Seifer, Baldwin, & Baldwin, 1993). Early adverse experiences have been related to the sympathetic and HPA systems (Blair et al., 2011; Gunnar & Fisher, 2006; Heim, Plotsky, & Nemeroff, 2004; Lupien, King, Meaney, & McEwen, 2001; Saridjan et al., 2010), though evidence of how these adverse experiences impact the parasympathetic system is scarce (Propper, 2012), despite known associations between early adversity and emotion regulation (Pechtel & Pizzagalli, 2011; Tottenham et al., 2011). Though there are exceptions, low income (Propper, 2012), parental psychopathology and depression (Field, Pickens, Fox, Nawrocki, & Gonzalez, 1995; Pickens & Field, 1995), exposure to domestic violence (Rigterink, Fainsilber Katz, & Hessler, 2010), and marital conflict (El-Sheikh & Hinnant, 2011) have been associated with altered RSA either in the form of lower baseline RSA (Field et al., 1995; Pickens & Field, 1995; Schuetze & Eiden, 2006; Schuetze et al., 2009) or less growth in baseline RSA (Rigterink et al., 2010). In a study of allostatic load effects on RSA, El-Sheikh and Hinnant (2011) found that boys with greater RSA reactivity who experienced higher marital conflict at age 8 or increasing marital conflict from age 8 to 11 exhibited decreases in resting RSA across early adolescence, suggesting “wear and tear” on the parasympathetic system. This response could also be interpreted as adaptive in the face of chronic stress.
In this study, we sampled a variety of risk indices characteristic of drug-exposed populations by taking a cumulative risk approach, in order to evaluate whether prenatal substance exposure is a significant contributor to, or simply a marker of baseline RSA and RSA reactivity (Fisher et al., 2011). For instance, the association between prenatal substance exposure and RSA may be indirect. Some studies have shown that a supportive environment postnatally can mitigate the effects of prenatal substance exposure on child outcome (Eiden et al., 2011; Hans & Jeremy, 2001; Yumoto, Jacobson, & Jacobson, 2008). For example, using the current sample of children, Fisher et al. (2011) found that early adversity mediated the relation between prenatal substance exposure and neurobehavioral disinhibition. In this study we tested this hypothesis by examining the direct and indirect effects of prenatal substance exposure and early adversity on growth in baseline RSA and RSA reactivity.
In the present study, our first goal was to describe the growth trajectories of baseline RSA and RSA reactivity at ages 3, 4, 5, and 6 in a sample of children with prenatal substance exposure. Our second goal was to determine the role of prenatal substance exposure and early adversity on growth in baseline RSA and RSA reactivity. On the basis of prior evidence with children exposed prenatally to substances, we expected that higher baseline RSA and greater RSA reactivity were related to greater prenatal substance exposure. Our third goal was to determine if early adversity mediates the relation between prenatal substance exposure and growth in RSA. We hypothesized that early adversity would at least partially mediate this relation.
METHODS
Participants
We used data from 1,121 participants drawn from the Maternal Lifestyle Study (MLS), a multisite (Detroit, MI; Memphis, TN; Miami, FL; Providence, RI) investigation of the effects of prenatal substance exposure in a longitudinal follow-up from 1 month to 16 years. Details on the enrollment and exclusion criteria are described elsewhere (Lester et al., 2002). In brief, the families were selected to be in the exposed group (i.e., maternal report of cocaine or opiate use during pregnancy and gas chromatography–mass spectrometry confirmation of presumptive positive meconium screens for cocaine or opiate metabolites) or the comparison group (i.e., maternal denial of cocaine or opiate use during the pregnancy and a negative enzyme multiplied immunoassay meconium screen for cocaine and opiate metabolites). The exposed and comparison youths were group matched on race, gender, and gestational age within each study site. Background substances associated with cocaine use (i.e., alcohol, tobacco, and marijuana) were present in both groups. Thus, most participants were polysubstance exposed. In the present study, we use a cumulative index of prenatal substance exposure and do not rely on group based comparisons.
The study was approved by the institutional review board at each study site, and written informed consent (caregivers) was obtained for all participants. Each site had a certificate of confidentiality from the National Institute on Drug Abuse. For this study, we used data from visits to the hospital clinic at age 3, 4, 5, and 6 years. All children were seen within approximately 6 weeks of their birthday (range = 1.3 months before −1.6 months after their birthday). Examiners were blind to group status. The MLS sample includes children in the following racial and ethnic categories: African American (77%), Caucasian (16%), Hispanic (6%), and children whose parents identified other racial or ethnic backgrounds (1%). Additional demographic characteristics of the sample are included in Table 1.
Table 1.
Demographic Characteristics
| Demographic Variable | Mean ± SD or % |
|---|---|
| Maternal age | 28.35 ± 5.83 |
| Birth weight (g) | 2,629.82 ± 818.53 |
| SES (continuous) | |
| Low (IV–V) | 56.9 |
| Mid-high (I–III) | 43.1 |
| Sex | |
| Male | 52.4 |
| Female | 47.6 |
| Race | |
| Caucasian | 15.9 |
| African American | 76.6 |
| Hispanic | 6.3 |
| Other | 1.2 |
| Gestational age | 36.25 ± 4.03 |
| Head circumference (cm) | 32.12 ± 3.03 |
| Nicotine exposure | 53.9 |
| Cocaine exposure | 43.7 |
| Alcohol exposure | 59.4 |
| Marijuana exposure | 23.3 |
| Opiate exposure | 8.4 |
Measures
Respiratory Sinus Arrhythmia
Baseline RSA and RSA reactivity were measured at 3, 4, 5, and 6 years. RSA was derived from the R-R time-series (or time between adjacent R-waves) collected from digitized ECG recordings using Porges algorithm from MXEdit (Delta-Biometrics, Inc., 1988–1993). ECGs were recorded via three electrodes placed on the child’s chest and abdomen. The ECG signal was sampled at 1 kHz, and stored on a computer for later scoring. Interbeat intervals were defined by detection of R-waves to the nearest ms.
Postprocessing of the data took place off-line by using a series of automated algorithms. These algorithms were written to identify R-R intervals outside of expected values. Missed or spurious R-waves were flagged and corrected by linear interpolation. A 21-point moving polynomial was then applied to remove low frequency trends in the HR signal. Next, a bandpass filter extracted the variance in heart period within the frequency band of spontaneous respiration in young children (.24–1.04 Hz). This process removes periodicities in the ECG signal that are outside the frequency range of the respiratory cycle. The resulting measure of RSA is in the frequency range of respiration. RSA was computed as the natural logarithm of heart period variance and reported in units of milliseconds squared, ln(ms)2. This method was described initially by Porges (1985), and is one of several acceptable approaches for calculating RSA (Berntson et al., 1997). RSA data were calculated in 30-s overlapping windows and then averaged within each episode (baseline and attention task). The RSA data for an individual were used as long as there was a 30-s segment with less than 20% of segments identified with artifact (Jennings et al., 1981), which is the shortest duration recommended when calculating RSA with infants. Thirty-second epochs are frequently sampled in studies of infants and young children (Blandon et al., 2010; Buss, Goldsmith, & Davidson, 2005; Huffman et al., 1998; Moore & Calkins, 2004). In addition, small amounts of artifact can be expected to have a minimal effect on measures of heart rate variability such as RSA (Berntson et al., 1993).
Baseline RSA was assessed while the children were waiting for a mastery motivation procedure (described below) to begin. Because RSA can be activated by psychomotor activity (Bazhenova & Porges, 1997; Porges, 2007), like others (Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010) we assessed baseline RSA during a non-challenge “control” task that paralleled the motor demands of our challenge task (described below). Baseline RSA values were assessed during this control task. Specifically, children played with a toy alone or with an experimenter for an average of 164.8 s (range = 135.6–199.3 s). Although this baseline required some attention, which may evoke vagal withdrawal (Suess, Porges, & Plude, 1994), the toy with which the child played was simple and no task demands were placed on the child during this time. Our estimates of baseline RSA are similar (within 1 SD) to other studies of infants and young children of similar ages (Alkon et al., 2003; Buss et al., 2005; Calkins et al., 2007; Calkins & Keane, 2004; Doussard-Roosevelt, Montgomery, & Porges, 2003), who have also calculated RSA based on Porges technique. Furthermore, the sample as a whole had RSA levels that decreased from this baseline to the attention demanding task at each age assessed (Table 3), indicating that our baseline measure was perceived as less stressful than the attention-demanding task.
Table 3.
Means and Correlations Between RSA, Prenatal Substance Exposure, and Early Adversity
| Mean RSA (SD) | % of RSA Data Present | Correlation With Prenatal Substance Exposure | Correlation With Early Adversity | |
|---|---|---|---|---|
| RSA-B, 3y | 5.64 (1.29) | 53.4 | .08* | .01 |
| RSA-B 4y | 5.78 (1.26) | 51.6 | .08* | <.001 |
| RSA-B 5y | 6.06 (1.23) | 48.1 | .09* | .07 |
| RSA-B 6y | 6.27 (1.23) | 51.9 | .13*** | .09* |
| RSA-R 3y | .60 (.56) | 51.6 | .09* | −.09* |
| RSA-R 4y | .29 (.55) | 50.6 | −.01 | −.04 |
| RSA-R 5y | .27 (.55) | 47.6 | .04 | .08* |
| RSA-R 6y | .57 (.58) | 51.4 | .08* | .11** |
RSA-B, Baseline Respiratory Sinus Arrhythmia; RSA-R, Respiratory Sinus Arrhythmia Reactivity; prenatal substance exposure and early adversity are continuous scales.
p < .05.
p < .01.
p < .001.
RSA reactivity was assessed in response to two episodes of a 9-min mastery motivation procedure (Dwek, 1991), which consisted of quiet play with a series of three toy-related tasks of increasing difficulty. This attention-demanding task comprised the first 3 min of the procedure. Children transitioned from supportive play with an experimenter to play with a moderately challenging toy. Toys included a pathfinder (3 years), tinker toys (4 and 5 years), and a puzzle (6 years). RSA reactivity was calculated using a difference score (RSAbaseline–RSAtask). Positive values reflect greater RSA reactivity (i.e., a decrease in RSA from baseline to task). Our estimates of RSA reactivity are similar (within 1 SD) to other studies of infants and young children of similar ages using attention-demanding tasks and Porges technique (Alkon et al., 2003; Buss et al., 2005; Calkins et al., 2007; Calkins & Keane, 2004).
Key Predictors
As polysubstance exposure during pregnancy appears to be the rule rather than the exception (Mayet et al., 2008), following the work of Fisher et al. (2011) with this sample, prenatal substance exposure was measured as a summative index ranging from 0 to 5 for mother-reported (or gas chromatography–mass spectrometry confirmation of presumptive positive meconium screens for cocaine or opiate metabolites) use of cocaine, opiates, marijuana, alcohol and tobacco during pregnancy. Maternal report/meconium screen of drug use (1, yes; 0, no) prenatally was computed. One point was assigned for each substance used. Dichotomous measures are frequently used in testing prenatal substance exposure effects (Fisher et al., 2011; Smith, Johnson, Pears, Fisher, & DeGarmo, 2007).
Early adversity was a summative risk index from birth to the age 3 assessments and included nine risk factors. Cumulative risk models assume that combinations of risk factors are more powerful predictors of developmental outcomes than the measurement of a single risk factor, which is less ecologically valid in substance-exposed populations (Lester et al., 2005). Each risk factor was either a continuous scale or a count score that was dichotomized to create an overall risk index (0 = no/none, or 1 = yes/one or more). Cut-offs were based on prior research findings indicating that extreme values on these risk indices represent valid indicators of risk for developing problem behavior (Fisher et al., 2011; Sheinkopf et al., 2007): (1) any maternal report of postnatal substance use of cocaine, opiates, tobacco, alcohol, or marijuana up to the Year 3 assessment; (2) chronic poverty status calculated as income below $10K for at least 75% of the visits; (3) low social status scored from the Hollingshead Index of Social Position (Hollingshead, 1975) using education and occupation averaged over annual visits and scored as 1 SD or more below the mean; (4) any primary caretaker changes assessed annually; (5) any report of sexual or physical abuse reported by caregivers; (6) annual assessments of caregiver depression of 1 SD or greater above the mean for averaged depressive symptoms on the caregiver reported Beck Depression Inventory (Beck, Steer, & Brown, 1996); (7) annual assessments of caregiver psychological distress of 1 SD or greater above the mean for total psychological symptoms on the Brief Symptom Inventory (Derogatis & Coons, 1993); (8) poor quality home environment of 1 SD or more below the mean on the Home Observation Measurement of the Environment (Caldwell & Bradley, 1984) as assessed by a home visitor when the child was 10 months old; and (9) any history of Child Protective Services Involvement for the target child assessed annually by caregiver report until the Year 3 assessment. Descriptive data for the cumulative risk index are included in Table 2.
Table 2.
Descriptives for Total Cumulative Risk Score and Individual Items on Cumulative Risk Index
| Risk Score Items | M | SD | Range | Risk Present (Proportion)a |
|---|---|---|---|---|
| 1. Postnatal substance use | NA | .81 | ||
| 2. Chronic poverty status | NA | .37 | ||
| 3. Low social status | NA | .14 | ||
| 4. Primary caretaker changes | NA | .22 | ||
| 5. Sexual or physical abuse | NA | .02 | ||
| 6. Caregiver depression (BDI) | 7.45 | 6.35 | 0–45 | .15 |
| 7. Caregiver psychological distress | .56 | .48 | 0–3.10 | .15 |
| 8. Poor quality home environment | 33.83 | 5.16 | 13.50–46.00 | .16 |
| 9. History of child protective services involvement | NA | .28 | ||
| Total risk score (sum of items) | 2.24 | 1.41 | 0–7 | NA |
NA, not available; BDI, Beck Depression Inventory.
Families that met this criteria received one point on their cumulative risk score.
Analytic Strategy
A multilevel modeling strategy was used because of the dependency in the data (e.g., physiology nested within child over time; Raudenbush & Bryk, 2002). A major advantage of multi-level modeling is the simultaneous estimation of both within-person effects and between-person effects. At Level 1, within-person variation in RSA over time is modeled with individual-specific growth parameters (i.e., intercept and linear slope) that may vary across people. Level 2 covariates (e.g., prenatal substance exposure, early adversity) were used to predict inter-individual differences in RSA intercept and linear slope. Latent growth curves were used to test a series of models examining the level and shape, from 3 to 6 years of age, of children’s: (1) baseline RSA and (2) RSA reactivity to an attention-demanding task. All analyses were conducted using Mplus 5.1 (Muthén & Muthén, 2007).
Since standardization of coefficients should be used with caution because they yield sample-specific beta weights (Eisinga, Scheepers, & Van Snippenburg, 1991), we report both the standardized and unstandardized coefficients in our models. The unstandardized coefficients are represented in parentheses in the text and in figures.
Missing Data
In the present study, analyses were restricted to the 1,121 families whose mothers had prenatal substance exposure scores and there were RSA data for the youths. The MLS study originally enrolled 1,388 mothers with prenatal substance exposure scores. There were no significant differences in prenatal substance exposure or early adversity between children with and without baseline RSA and RSA reactivity data within each age (all p’s > .14). We then examined whether there were differences in demographic variables between comparison groups. There were no significant differences in birth weight (p’s > .56), poverty status as measured using the average Hollingshead from birth to 6 years (p’s > .07), and sex (p’s > .09) between children with and without RSA data within each age. There were no differences in prenatal substance exposure or RSA among children with or without missing early adversity data (all p’s > .15).
RESULTS
Correlations
Correlations between baseline RSA, RSA reactivity, prenatal substance exposure, and early adversity are presented in Table 3. There was a statistically significant, positive association between prenatal substance exposure and baseline RSA from age 3–6 years. Baseline RSA increased with increasing levels of prenatal substance exposure. There was also a significant positive association between baseline RSA and early adversity at age 6. The relations between RSA reactivity and our primary predictors are less clear. At 3 and 6 years, greater exposure to substances prenatally was associated with greater RSA reactivity. At 3 years, lower levels of early adversity were associated with greater RSA reactivity, but by 5 and 6 years, as with prenatal substance exposure, higher levels of early adversity were associated with greater RSA reactivity.
Unconditional Growth Models
Latent growth models were fit to describe the level and change in children’s RSA across four waves of data collection spanning approximately 3 years. We first examined unconditional growth models (i.e., with no predictors or covariates included in the model) for baseline RSA and RSA reactivity. Missing data were accounted for using the full-information maximum likelihood (FIML) feature of Mplus, which uses all available information from the observed data to provide statistically appropriate standard errors (Brown et al., 2008).
Baseline RSA
First, models were tested examining the level and shape of children’s RSA trajectories during the baseline episode. Individual, within-person variation in RSA over time was allowed to vary across persons. On average and consistent with typically developing children, children in this sample showed a significant linear increase in baseline RSA over time. Children’s baseline RSA at 3 years was 5.63 ln(msec)2, and the average rate of change in baseline RSA was .19 ln (msec)2. Significant variability was found for children’s intercepts (σ2 = .79, p < .001), but not slopes (σ2 = .003, p = .86). This suggests that the initial level for baseline RSA varied across children, but the rate of change was relatively constant. The model fit the data well, χ2(5) = 4.40, p = .35; comparative fit index (CFI) = 1.00 (Bentler, 1990), root mean square error of approximation (RMSEA) = .01 (Browne & Cudeck, 1993).
RSA Reactivity
Identical models also tested the RSA reactivity scores in response to the attention-demanding task. A model that constrained the variances to be equal fit the data best. A model that included a curvilinear growth term (quadratic) provided the excellent fit to the data, χ2(4) = 4.45, p = .35; CFI = .99, RMSEA = .01, with reactivity highest at 3 years, decreasing at 4 and 5 years, and then increasing again at 6 years. Children’s average linear slope was decreasing, suggesting that children were becoming less reactive over time, Mlinear slope = −1.24, p < .001, from an intercept of 2.12 ln(msec)2 at age 3 years. Significant variability in these parameters demonstrated that these patterns differed across children (σ2intercept = .08, p < .001; σ2linear slope = .14, p = .03). Children, on average, also exhibited a significant quadratic trend representing the decreases at ages 4 and 5 and the increase at age 6, Mquadratic = .15, p < .001, with significant variability suggesting differences across children in this pattern over time (σ2quadratic = .01, p = .05).
Effects of Prenatal Substance Exposure and Early Adversity on Growth in RSA
The following analyses were conducted using a standard set of covariates: child birth weight, child sex, and study site1. Primary predictors were grand-mean centered prior to analyses.
Baseline RSA
In the following analyses, we only report on predictors of baseline RSA intercept (3 years) because there was not enough variability in RSA linear slopes. The main effect of prenatal substance exposure was first included as a predictor of initial level of baseline RSA. There was a significant effect of prenatal substance exposure on baseline RSA at 3 years, β = .13 (.07), p < .001. Greater levels of prenatal substance exposure were associated with greater baseline RSA at 3 years. Early adversity was then added as an additional predictor of initial level of baseline RSA. Early adversity was not a significant predictor of baseline RSA intercept (β = .001 (−.05), p = .99). Prenatal substance exposure remained a significant predictor of initial levels of baseline RSA. The interaction between prenatal substance exposure and early adversity was also tested but was not significant.
RSA Reactivity
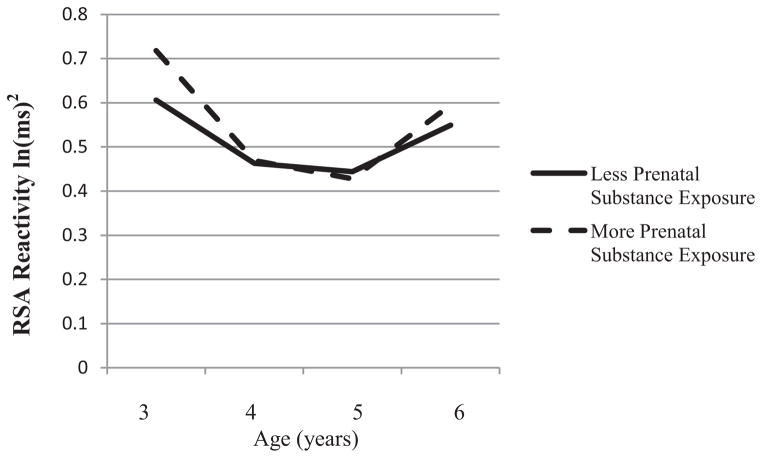
The same analyses were conducted using RSA reactivity as an outcome. Because there was a significant main effect of site on RSA reactivity, it is included in the following models. There was a significant main effect of prenatal substance exposure on RSA reactivity at 3 years, β = .16 (.03), p = .03. Children with higher levels of prenatal substance exposure exhibited more RSA reactivity (i.e., a greater decrease in RSA) at 3 years than children with lower levels of prenatal substance exposure. There was no significant effect of prenatal substance exposure on linear (β =−.17 (−.05), p = .06) growth, but there was an effect on quadratic (β = .19 (.02), p = .04) growth in RSA reactivity. Children with more prenatal substance exposure exhibited greater RSA reactivity (a greater decrease in RSA) at 3 years compared to children with less prenatal substance exposure and increased at a greater rate from 5 to 6 years. With the addition of early adversity as a predictor, the main effect of prenatal substance exposure on RSA reactivity at 3 years remained (β = .28 (.06), p < .001), and there was a main effect of early adversity (β =−.31 (−.06), p < .001). Greater levels of prenatal substance exposure, and lower levels of early adversity, were related to greater levels of RSA reactivity at 3 years. Early adversity and prenatal substance exposure were also significant predictors of linear growth in RSA reactivity, but in opposite ways. Although greater levels of prenatal substance exposure were related to greater growth in RSA reactivity (β =−.27 (−.07), p = .01), early adversity was associated with less growth in RSA reactivity (β = .25 (.07), p = .02). Prenatal substance exposure, but not early adversity (β = −.13 (−.01), p = .23), was a significant predictor of quadratic growth in RSA reactivity (β = .24 (.02), p = .03). Children with greater levels of prenatal substance exposure exhibited a more pronounced quadratic effect; specifically, a more rapid decrease in RSA reactivity from 3 to 4 years and a more rapid increase in RSA reactivity from 5 to 6 years compared with children with lower levels of prenatal substance exposure (see Fig. 1). The interaction between prenatal substance exposure and early adversity was not significant.
FIGURE 1.
The effect of prenatal substance exposure controlling for the effect of early adversity on the quadratic growth of RSA reactivity at 3, 4, 5, and 6 years. Higher levels of RSA reflect greater reactivity. Simple slopes were tested at ±1 SD from the mean.
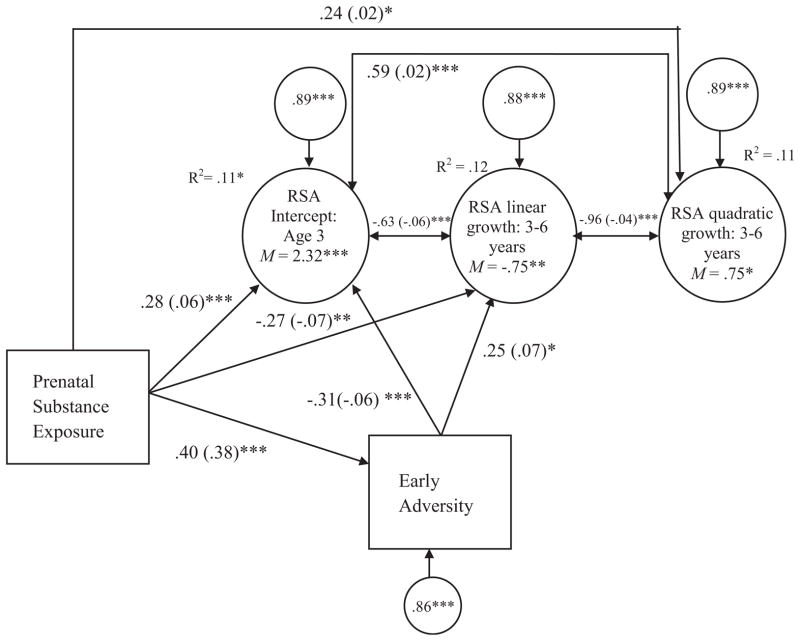
In a final model, early adversity was tested as a mediator of the effect of prenatal substance exposure on growth in RSA reactivity. We tested indirect effects using the Sobel test following MacKinnon’s (2008) conceptualization of mediation (MacKinnon, 2008). Prenatal substance exposure was a significant predictor of early adversity (see Fig. 2). Early adversity and prenatal substance exposure were significant predictors of initial levels of RSA reactivity at age 3, and linear growth in RSA reactivity. Prenatal substance exposure, but not early adversity, was a significant predictor of quadratic growth in RSA reactivity. The results of the indirect path effects for prenatal substance exposure are shown in Table 4. These results indicate that prenatal substance exposure had a significant indirect influence through early adversity on RSA reactivity at age 3 and linear growth in RSA reactivity from 3 to 6 years.
FIGURE 2.
The indirect effect of prenatal substance exposure on RSA reactivity through early adversity. Paths are standardized beta coefficients. Unstandardized beta coefficients are presented in parentheses. Covariates include site. Only significant paths are presented for ease of presentation. RSA, Respiratory sinus arrhythmia reactivity; χ2(8) = 7.90, p = .44; CFI = 1.00; RMSEA ≤.001.*p < .05.**p < .01.***p < .001.
Table 4.
Standardized Specific Indirect Path Effects of Prenatal Substance Exposure on RSA Reactivity From 3 to 6 Years
| Estimate | SE | Estimate/SE | |
|---|---|---|---|
| Prenatal substance exposure → early adversity → RSA reactivity at 3 years | −.12 | .03 | −3.57** |
| Prenatal substance exposure → early adversity → linear growth in RSA reactivity from 3 to 6 years | .10 | .04 | 2.33* |
| Prenatal substance exposure → early adversity → quadratic growth in RSA reactivity from 3 to 6 years | −.05 | .04 | −1.21 |
p < .05.
p < .001.
DISCUSSION
Our results provide insights into a developmental process by which prenatal substance exposure and early adversity become biologically embedded to impact parasympathetic stress response trajectories. We also add to the small body of work indicating that the parasympathetic nervous system may be used as an indicator of allostatic load (El-Sheikh & Hinnant, 2011). From a basic science viewpoint, we contribute to the scant literature on the development of RSA across early childhood among children with prenatal substance exposure. Below, we consider what each of these effects means, both within the context of this study and within the framework of allostatic load.
We measured baseline RSA and RSA reactivity four times across early childhood. Our finding that, across all children in this sample, baseline RSA increased over time is consistent with other work from typically developing samples (Bornstein & Suess, 2000; Calkins & Keane, 2004). That children from this high-risk sample also exhibited this normative pattern speaks to the robust nature of this process. Patterns of RSA reactivity in our sample revealed a curvilinear effect. Children’s RSA reactivity was highest at ages 3 and 6, and lowest at ages 4 and 5. This result was expected given that RSA reactivity does not appear to stabilize until middle childhood (Keller & El-Sheikh, 2009). No study that we know of has compared RSA reactivity at 3, 4, 5, and 6 years, thus, we interpret this finding in the context of what is known about behavioral development and organization in early childhood. The pre-school years are seen as a sensitive period for the development of self-regulation and executive functioning skills (Blair, 2002). At age 3, it may be that more demands are being placed on a sensitive and plastic regulatory system during the transition to preschool, and therefore greater parasympathetic resources are used to meet these attentional demands. Age 6 represents another major transition involving the consolidation and reorganization of regulatory skills, the transition to Kindergarten. Similarly, 6 year olds may also exhibit more RSA reactivity due to an over-taxed bio-behavioral regulatory system. Importantly, however, replication of this finding is needed in other high-risk samples before we can draw any firm conclusions.
We found significant variability in children’s baseline RSA at 3 years, indicating differential initial estimates of baseline RSA. Higher levels of prenatal substance exposure were related to higher baseline RSA at age 3, while there was no significant main effect of early adversity. These results should be considered in the context of a sample at high risk for problems with emotion regulation due to exposure to substances and early adversity. There is a great deal of heterogeneity in populations of risk, and while we hesitate to compare our results to others who have found similar findings in other populations of “risk”, these studies nonetheless are beginning to converge to suggest that baseline RSA may not always fit according to risk and protective terms (Conradt et al., 2013; Eisenberg et al., 2012). Our results from a large sample of children at high risk due to exposure to early adversity and prenatal substance exposure suggest that the highest levels of baseline RSA may not always be adaptive (Conradt et al., 2013; Eisenberg et al., 2012).
What accounts for the higher baseline RSA among children with greater prenatal substance exposure? Baseline RSA is thought to index an individual’s ability to respond to environmental perturbations (Beauchaine, 2001; Bornstein & Suess, 2000). In addition, increasing evidence suggests that children with prenatal substance exposure utilize greater neurobiological resources in order to meet environmental demands (Sheinkopf et al., 2009). For instance, Sheinkopf et al. (2009) found that 8- to 9-year-old children from this same sample exhibited greater activation in the frontal cortex compared to their non-exposed peers. Thus, the higher baseline RSA found among children with prenatal substance exposure might be indicative of a need for more intensive activation of their parasympathetic system in order to maintain homeostatic functioning. It may also reflect a form of hypervigilance or awareness of environmental conditions that may be adaptive given the increased likelihood these children have of living in unstable environments. That prenatal substance exposure, and not early adversity, was related to baseline RSA at 3 years suggests a biological mechanism whereby prenatal substance exposure may affect the set-point, or functional range, by which the body responds to environmental challenge. The role of early adversity in this process only became clear when examining the developmental trajectories of RSA reactivity.
Our findings highlight the importance of examining RSA reactivity across multiple developmental periods, as reactivity patterns change with development. Greater levels of prenatal substance exposure and less exposure to early adverse experiences were related to greater RSA reactivity at 3 years. By age 6, however, correlations revealed that both greater exposure to substances and greater exposure to early adverse experiences were associated with greater reactivity. With greater exposure to adverse experiences over time, there may be a re-organization that occurs in the parasympathetic system. These results support the “latent effects” hypothesis developed by Shonkoff et al. (2009), which suggests that significant stressors experienced during sensitive developmental periods may not manifest for years. These results also support the hypothesis that the effect of early adversity may be latent due to its impact on specific areas of the brain that are not on-line until childhood when the child must organize resources to respond to more complex demands (Lester et al., 2002). As children develop, they face more demands at home, school, and with peers, and it is important that they learn to organize neurobiological resources in order to achieve competence in multiple settings (Lester, LaGasse, & Seifer, 1998). Thus, in the short term, this stress response may be adaptive, as it allows for the coordination of cognitive, behavioral, and emotional systems in order to meet the demands of the environment (Bornstein & Suess, 2000; Keller & El-Sheikh, 2009).
The greater baseline RSA and RSA reactivity observed in 5- and 6-year-old children exposed to substances prenatally and high levels of early adversity may also reflect an adaptive response to stress (Boyce & Ellis, 2005; Lupien et al., 2001). In both the HPA and RSA literatures, for instance, a reactive response to stress is thought to help mobilize the organism to respond to environmental demands, though sustained elevations in the HPA and RSA systems may lead to risk for medical and psychological morbidity. In this study, perhaps the increased baseline RSA and RSA reactivity allowed children to be more aware or vigilant of their surroundings, which may be adaptive in a high-risk context (Boyce & Ellis, 2005). Follow-up is needed to determine whether this pattern of RSA responding confers risk for psychopathology later in life.
The effect of prenatal exposure on growth in RSA reactivity was mediated by early adversity. These results add to the body of work examining the independent and combined effects of prenatal substance exposure and early adversity on relevant child outcomes (Fisher et al., 2011) and suggest that one mechanism by which some children with prenatal substance exposure become physiologically reactive is through the effects of early adverse life experiences. This finding adds to the growing body of research which demonstrates that the quality of the rearing environment can exacerbate or mitigate the effects of prenatal substance exposure. For instance, Eiden et al. (2011) found that infants with prenatal cocaine exposure who experienced high levels of caregiving instability exhibited higher baseline cortisol values compared to non-exposed infants who experienced caregiving instability (Eiden et al., 2011). These findings suggest that the combination of prenatal substance exposure and adverse environmental conditions result in different patterns of physiological functioning. These results are in support of the theory of allostatic load and suggest that: (1) the parasympathetic nervous system may be used as another measure of allostatic load, and (2) the experience of prenatal substance exposure and repeated exposure to environmental risk factors early in life may be a particularly pernicious pathway by which children experience the over-activation of their parasympathetic system.
It is important that several limitations of the current study are addressed. First, like others, we used only one measure of physiology, RSA. Independent physiological indices of allostatic load, such as the sympathetic and HPA systems, should be used in future studies to corroborate our RSA findings. Second, we cannot infer causality with these data. Only through rigorous experimental design may we be able to determine whether prenatal substance exposure is a cause of growth in RSA via the effects of early adversity. Although early adversity might be one mechanism by which prenatal substance exposure impacts the development of RSA, there are certainly additional mechanisms that might be involved. For instance, prenatal substance exposure may result in disrupted physiological systems via the expression of child problem behavior (Marsh, Beauchaine, & Williams, 2008), or through insensitive or unresponsive parenting (Eiden, Veira, & Granger, 2009). In addition, we did not isolate the effects of a single substance, such as cocaine, on RSA, and instead used a more ecologically valid indicator of prenatal substance use. We acknowledge that the mechanism of action of these drugs is different, and we cannot determine/estimate the relative impact of one drug over another. Another limitation includes our measurement of baseline RSA. We did not assess RSA while the child was at “rest”, but instead chose to compare RSA activity during an attention demanding task with RSA activity when a child was playing with a simple toy and experimental demands were minimal. While our estimates of baseline RSA converge with other studies using Porges method, we nonetheless note that our baseline measurement did not measure parasympathetic activity at rest. Last, a critical next step in our study of the effects of prenatal substance exposure and early adversity on the development of RSA will be to examine behavioral outcomes. For example, when viewed through the lens of the theory of Biological Sensitivity to Context, it may be that the children with greater RSA reactivity who are raised in supportive environments emerge as resilient to the effects of their early substance exposure (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011).
Results from this study are in support of the theory of allostatic load and also extend the theory in several respects. First, we have found evidence that both prenatal substance exposure and early adversity impact the developing parasympathetic stress response system, thus extending the theory to the parasympathetic system. Furthermore, we have found evidence of allostatic load effects using a prospective, longitudinal sample with RSA assessed across early childhood, when the parasympathetic system may be most plastic (Kummer, 1992). Previous research has shown that prenatal substance exposure impacts RSA in infancy. We have documented that this effect persists into early childhood, and in fact changes with development. Thus, we have evidence that the timing of early life insults impacts the developing parasympathetic stress response system.
In order to best target populations in need of intervention, it is important to understand when in development disruptions are most likely to occur (Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2008; Cicchetti & Gunnar, 2008). Though in need of replication, our findings suggest that the effects of prenatal substance exposure and early adversity manifest during preschool. This is also the time when children must rely on more independent methods of self-regulation, and thus may rely more heavily on physiological regulatory systems. Our findings suggest that pre-schoolers with prenatal substance exposure and who grow up in contexts of early adversity are most in need of developmental support. Research suggesting that the parasympathetic system is malleable to intervention (Bagner et al., 2012), is promising given the effects of allostatic load on parasympathetic nervous system functioning.
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network and an interinstitute agreement with the National Institute on Drug Abuse (NIDA) through cooperative agreements: U10-DA-024117-01, U10-HD-21385 (to S.S.), I10-DA-024128-06, U10-HD-2786 (to H.S.B.), U10-DA-024119-01, U10-HD-27904 (to B.M.L.), and U10-DA-024118-01, U10-HD-21397 (to C.R.B.); NICHD contract N01-HD-2-3159 (to B.M.L.); and a National Research Service Award from the National Institute on Drug Abuse F32DA032175 (to E.C.).
Footnotes
With one exception (reported in the main text), including the standard set of covariates did not change the pattern of results, so we report only the main effects of prenatal drug exposure and early adversity. We also examined interaction terms between covariates and prenatal drug exposure, such as drug exposure × birth weight, drug exposure × sex, and drug exposure × site. None of these interaction terms were significantly related to initial levels or growth in baseline RSA, or RSA reactivity; thus, they were not included in the model tests.
Conflict of Interest: None of the authors have conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, or the National Institutes of Health.
References
- Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. Journal of Developmental and Behavioral Pediatrics. 2011;32(9):668–677. doi: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42(1):64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Bagner DM, Graziano PA, Jaccard J, Sheinkopf SJ, Vohr BR, Lester BM. An initial investigation of baseline respiratory sinus arrhythmia as a moderator of treatment outcome for young children born premature with externalizing behavior problems. Behavior Therapy. 2012;43(3):652–665. doi: 10.1016/j.beth.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenova OV, Porges S. The integrative neurobiology of affiliation. Annals of the New York Academy of Science. 1997;807:469. doi: 10.1111/j.1749-6632.1997.tb51940.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2008;20(3):745–774. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory—II. Psychological Corporation; 1996. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:885–908. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34 (6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. The American Psychologist. 2002;57(2):111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Investigators FLP. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Peters R, Granger D. Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: Further examination of the behavioral inhibition system/behavioral activation system scales for young children. Developmental Psychobiology. 2004;45(3):113–124. doi: 10.1002/dev.20022. [DOI] [PubMed] [Google Scholar]
- Blandon AY, Calkins SD, Keane SP, O’Brien M. Contributions of child’s physiology and maternal behavior to children’s trajectories of temperamental reactivity. Developmental Psychology. 2010;46(5):1089–1102. doi: 10.1037/a0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: Continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36(1):54–65. doi: 10.1037/0012-1649.36.1.54. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brown HC, Wang W, Kellam SG, Muthen BO, Petras H, Toyinbo P, et al. Methods for testing theory and evaluating impact in randomized field trials: Intent-to-treat analyses for integrating the perspectives of person, place, and time. Drug and Alcohol Dependence. 2008;95(Suppl 1):S74–S104. doi: 10.1016/j.drugalcdep.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociological Methods & Research. 1993;21:230–258. [Google Scholar]
- Bush NR, Obradović J, Adler N, Boyce WT. Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Development and Psychopathology. 2011;23(4):1089. doi: 10.1017/S0954579411000514. [DOI] [PubMed] [Google Scholar]
- Buss KA, Goldsmith HH, Davidson RJ. Cardiac reactivity is associated with changes in negative emotion in 24-month-olds. Developmental Psychobiology. 2005;46(2):118–132. doi: 10.1002/dev.20048. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home observation for measurement of the environment. Little Rock, AR: University of Arkansas at Little Rock Press; 1984. [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Gunnar MR. Integrating biological measures into the design and evaluation of preventive interventions. Development and Psychopathology. 2008;20(3):737. doi: 10.1017/S0954579408000357. [DOI] [PubMed] [Google Scholar]
- Conradt E, Ablow J. Infant physiological response to the still-face paradigm: Contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development. 2010;33(3):251–265. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Conradt E, Measelle J, Ablow JC. Poverty, problem behavior and promise: Differential susceptibility among infants reared in poverty. Psychological Science. 2013;24:235–242. doi: 10.1177/0956797612457381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Coons HL. Self-report measures of stress. 2. New York: Free Press; 1993. [Google Scholar]
- DiPietro JA, Suess PE, Wheeler JS, Smouse PH, Newlin DB. Reactivity and regulation in cocaine-exposed neonates. Infant Behavior and Development. 1995;18(4):407–414. doi: 10.1016/0163-6383(95)90030-6. [DOI] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse and Neglect. 2004;28(7):771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Donzella B, Gunnar MR, Krueger WK, Alwin J. Cortisol and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology. 2000;37(4):209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43(3):230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Dwek CS. Self theories and goals: Their role in motivation, personality and development. In: Dienstbeir RA, editor. Nebraska symposium on motivation. Lincoln: University of Nebraska Press; 1991. pp. 213–221. [PubMed] [Google Scholar]
- Eiden RD, Granger DA, Schuetze P, Veira Y. Child behavior problems among cocaine-exposed toddlers: Indirect and interactive effects. Development and Psychopathology. 2011;23(02):539–550. doi: 10.1017/S0954579411000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009;80(2):528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Sulik MJ, Spinrad TL, Edwards A, Eggum ND, Liew J, Hart D. Differential susceptibility and the early development of aggression: Interactive effects of respiratory sinus arrhythmia and environmental quality. Developmental Psychology. 2012;48(3):755–768. doi: 10.1037/a0026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinga R, Scheepers P, Van Snippenburg L. The standardized effect of a compound of dummy variables or polynomial terms. Quality and Quantity. 1991;25 (1):103–114. [Google Scholar]
- El-Sheikh M. Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology. 2005;46(1):66–74. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Hinnant JB. Marital conflict, respiratory sinus arrhythmia, and allostatic load: Interrelations and associations with the development of children’s externalizing behavior. Development and Psychopathology. 2011;23(3):815–829. doi: 10.1017/S0954579411000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Field T, Pickens J, Fox NA, Nawrocki T, Gonzalez J. Vagal tone in infants of depressed mothers. Development and Psychopathology. 1995;7(02):227–231. doi: 10.1017/S0954579400006465. [DOI] [Google Scholar]
- Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, Higgins R. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Development and Psychopathology. 2011;23 (3):777–788. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM, Katz LF. Effects of marital discord on young children’s peer interaction and health. Developmental Psychology. 1989;25(3):373–381. doi: 10.1037/0012-1649.25.3.373. [DOI] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74(2):263–285. doi: 10.1016/j.biopsy-cho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA. Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Development and Psychopathology. 2006;18(3):651–677. [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30(12):2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hans SL, Jeremy RJ. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Mental Health Journal. 2001;22(3):300–315. [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: Fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience and Biobehavioral Reviews. 2010;34 (6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnant JB, Elmore-Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology. 2011;53(1):59–68. doi: 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Huffman LC, Bryan YE, Carmen R, Pedersen FA, Doussard-Roosevelt JA, Forges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69(3):624–635. [PubMed] [Google Scholar]
- Jennings JR, Berg WK, Hutcheson JS, Obrist P, Porges S, Turpin G. Committee report. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18(3):226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Keller PS, El-Sheikh M. Salivary alpha-amylase as a longitudinal predictor of children’s externalizing symptoms: Respiratory sinus arrhythmia as a moderator of effects. Psychoneuroendocrinology. 2009;34(5):633. doi: 10.1016/j.psyneuen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Kidwell SL, Barnett D. Adaptive emotion regulation among low-income African American children. Merrill-Palmer Quarterly. 2007;53(2):155–183. [Google Scholar]
- Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Development and Psychopathology. 2012;24(4):1377–1390. doi: 10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W. Neuronal specificity and plasticity in the autonomic nervous system. Annals of Anatomy. 1992;174(5):409–417. doi: 10.1016/s0940-9602(11)80263-3. [DOI] [PubMed] [Google Scholar]
- Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: The meaning of subtle effects. Science. 1998;282(5389):633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- Lester BM, Liu J, LaGasse L, Seifer R, Bauer C, Shankaran S. Neurobiological dysregulation model of prenatal cocaine exposure and behavior problems at age 7. Pediatric Research. 2005;57:1625. [Google Scholar]
- Lester BM, Marsit C, Conradt E, Bromer C, Padbury J. Behavioral epigenetics and the developmental origins of child mental health disorders. Journal of Developmental Origins of Health and Disease. 2012;3:395–408. doi: 10.1017/S2040174412000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31(1–2):23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Maza PL. The maternal lifestyle study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13(3):653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. New York: Erlbaum and Taylor Francis Group; 2008. [Google Scholar]
- Marsh P, Beauchaine TP, Williams B. Dissociation of sad facial expressions and autonomic nervous system responding in boys with disruptive behavior disorders. Psychophysiology. 2008;45(1):100–110. doi: 10.1111/j.1469-8986.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayet S, Groshkova T, Morgan L, MacCormack T, Strang J. Drugs and pregnancy–outcomes of women engaged with a specialist perinatal outreach addictions service. Drug and Alcohol Review. 2008;27(5):497–503. doi: 10.1080/09595230802245261. [DOI] [PubMed] [Google Scholar]
- McEwen ??? Stress, adaptation, disease: allostasis, allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen ???, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- Mehta SK, Finkelhor RS, Anderson RL, Harcar-Sevcik RA, Wasser TE, Bahler RC. Transient myocardial ischemia in infants prenatally exposed to cocaine. The Journal of Pediatrics. 1993;122(6):945–949. doi: 10.1016/s0022-3476(09)90025-7. [DOI] [PubMed] [Google Scholar]
- Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Developmental Psychology. 2004;40(6):1068. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 5. Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl) 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens JN, Field T. Facial expressions and vagal tone of infants of depressed and non-depressed mothers. Early Development and Parenting. 1995;4(2):83–89. [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. US: 1985. [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsy-cho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29(8):697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8.<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and 3-year-olds. Developmental Psychobiology. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Propper ??? The early development of vagal tone: Effects of poverty and elevated contextual risk. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, Cox M. Gene–environment contributions to the development of infant vagal reactivity: The interaction of dopamine and maternal sensitivity. Child Development. 2008;79(5):1377–1394. doi: 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Thousand Oaks, California: SAGE Publications, Incorporated; 2002. [Google Scholar]
- Rigterink T, Fainsilber Katz L, Hessler DM. Domestic violence and longitudinal associations with children’s physiological regulation abilities. Journal of Interpersonal Violence. 2010;25(9):1669–1683. doi: 10.1177/0886260509354589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child Development. 1993;64(1):80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Saridjan NS, Huizink AC, Koetsier JA, Jaddoe VW, Mackenbach JP, Hofman A, Tiemeier H. Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The Generation R Study. Hormones and Behavior. 2010;57(2):247–254. doi: 10.1016/j.yhbeh.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: An examination of possible mediators and moderators. Journal of Pediatric Psychology. 2006;31(1):15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: Effects on infant autonomic regulation at 7 months of age. Developmental Psychobiology. 2007;49(3):276–289. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Edwards EP. A longitudinal examination of physiological regulation in cocaine-exposed infants across the first 7 months of life. Infancy. 2009;14(1):19–43. doi: 10.1080/15250000802569660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lagasse LL, Lester BM, Liu J, Seifer R, Bauer CR, Das A. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Development and Psychopathology. 2007;19(3):649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Developmental Neuroscience. 2009;31(1–2):159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS. Child maltreatment and foster care: Unpacking the effects of prenatal and postnatal parental substance use. Child Maltreat. 2007;12(2):150–160. doi: 10.1177/1077559507300129. [DOI] [PubMed] [Google Scholar]
- Staton L, El-Sheikh M, Buckhalt JA. Respiratory sinus arrhythmia and cognitive functioning in children. Developmental Psychobiology. 2009;51(3):249–258. doi: 10.1002/dev.20361. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Marshall PJ. Behavioral inhibition, heart period, and respiratory sinus arrhythmia: An attachment perspective. Child Development. 1999;70(4):805–816. doi: 10.1111/1467-8624.00058. [DOI] [PubMed] [Google Scholar]
- Suess PE, Newlin DB, Porges SW. Motivation, sustained attention, and autonomic regulation in school-age boys exposed in utero to opiates and alcohol. Experimental and Clinical Psychopharmacology. 1997;5(4):375–387. doi: 10.1037//1064-1297.5.4.375. [DOI] [PubMed] [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31(1):17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto C, Jacobson SW, Jacobson JL. Fetal substance exposure and cumulative environmental risk in an African American cohort. Child Development. 2008;79(6):1761–1776. doi: 10.1111/j.1467-8624.2008.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]