Abstract
Germinal centers (GC) are sites of intense B cell proliferation, central for T cell dependent antibody responses. However, the role of MYC, a key cell cycle regulator, in this process has been questioned. Here, we identified MYC positive B cell subpopulations in immature and mature GCs, and show through genetic ablation of Myc that they play indispensable roles in GC formation and maintenance. The identification of these functionally critical cellular subsets has important implications for human B cell lymphomagenesis, which mostly originates from GC B cells and frequently involves MYC chromosomal translocations. As these translocations are generally dependent on transcription of the recombining partner loci, the MYC positive GC subpopulations may be at a particularly high risk for malignant transformation.
In response to T cell dependent antigens, antigen-specific B cells are driven into the germinal center (GC) reaction, which is critical for the generation and selection of memory B and plasma cells expressing somatically mutated high-affinity antibodies1. GCs are sites of massive B cell proliferation2. However, despite extensive research on the GC reaction, the mechanisms driving GC B cell proliferation have remained elusive. An issue of particular importance is the notion that MYC, a master regulator of cellular proliferation both in nonhematopoietic and hematopoietic cells including B cells3, 4, does not play a role in this context5, 6.
The MYC transcription factor was first identified as the cellular homolog of the transforming determinant carried by the avian myelocytomatosis virus MC297. The conservation of cellular homologs of viral oncogenes across evolutionary time and species suggests important roles for these genes in normal cellular physiology7. Indeed, germline ablation of Myc leads to early (E9-10) embryonic lethality due to widespread failure in organ and tissue growth8. In the hematopoietic compartment MYC is required at early developmental stages of both B and T cells in the bone marrow and thymus, respectively4, 9. Experimental evidence demonstrates that MYC plays a crucial role in regulating cellular proliferation, apoptosis and differentiation of mammalian cells8. During cell cycle progression, MYC promotes G0/G1-S transition through the activation of genes encoding cyclin-dependent kinase (CDK) complex proteins, including Cyclin D2 (Ccnd2), as well as by the repression of CDK inhibitors10, 11.
Although early studies suggested high levels of MYC transcripts and protein in human and mouse GC B cells12-14, later work refuted these observations, detecting only low MYC expression levels in these cells5, 6. According to these studies MYC levels were comparable or even lower than the ones observed in quiescent follicular (FO) B cells where MYC does not seem to play a major function3, suggesting a dispensable role for MYC in GC B cell proliferation. Further support for this hypothesis came from the observation that BCL-6, a master regulator of GC B cell development15, interacts with the transcription factor MIZ1 (ZBTB17), a known partner of MYC, to suppress CDK inhibitors16, and that BCL-6 itself can repress MYC transcription17. Moreover, in line with the ability of BCL-6 to inhibit Ccnd2 expression17, GC B cells predominantly express Cyclin D3 (Ccnd3), which, although dispensable for GC initiation18, 19, is required for GC maturation and is not controlled by MYC10, 11.
MYC plays a critical role in the proliferation of most, if not all human B cell lymphomas, the vast majority of which originate from GC or post-GC B cells as demonstrated by their somatically mutated immunoglobulin (Ig) genes20-22. Indeed, in the GC microenvironments rapidly dividing B cells undergo somatic hypermutation (SHM) and class switch recombination (CSR) in their Ig genes, both of which involve DNA strand breaks22. Infidelity in these processes increases the probability of oncogenic events such as chromosomal rearrangements22. The MYC gene itself is frequently involved in chromosomal translocations in human GC-derived B cell lymphomas22. Such translocations, seen in roughly 10% of diffuse large B cell lymphomas (DLBCLs) and almost all cases of sporadic Burkitt lymphoma, juxtapose MYC and enhancers in the Ig loci, leading to deregulated MYC expression22. However, clear evidence supports the dependence on gene transcription for the introduction of somatic mutations by activation-induced cytidine deaminase (AICDA)23, 24. Thus currently there is an apparent contradiction between the absence of MYC transcription in GC B cells and the recurrent MYC translocations observed in the human lymphomas originating from these cells. An attractive hypothesis is that the association of MYC deregulated expression with GC B cell lymphomagenesis reflects its role in the regulation of cell proliferation at some stage of the GC reaction.
Here, by performing genetic experiments in the mouse, we found that MYC is expressed in subsets of GC B cells in both immature and mature GCs, and that these cells play an essential role in GC formation and maintenance.
RESULTS
MYC target genes are enriched in GC B cells
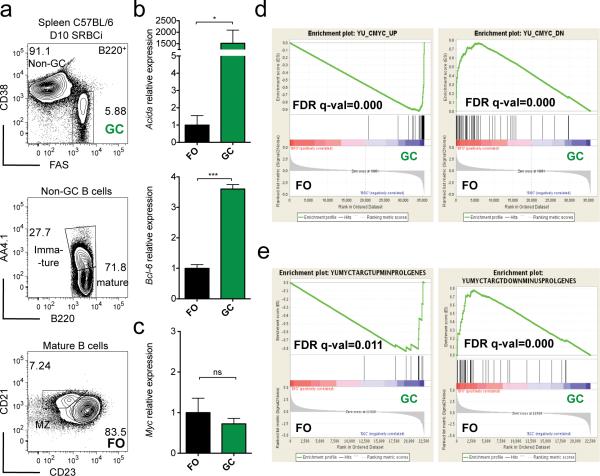
Conflicting observations were made with respect to MYC expression levels between FO and GC B cells5, 6, 12-14. We re-evaluated this question by performing gene expression analysis by quantitative PCR (qPCR) in flow purified splenic FO and GC B cells derived from previously immunized wild-type mice (Fig. 1a). First, we compared the transcript levels of genes predominantly expressed in GC versus FO B cells, providing an internal control for our sorting strategy. Consistent with previous findings15, 22, expression of both Aicda (Aid) and Bcl6 was significantly increased in GC B cells compared to FO B cells (Fig. 1b). In contrast, Myc transcripts levels were identical in GC and FO B cells (Fig. 1c), in agreement with previous studies and supporting the hypothesis that MYC is dispensable in GC B cells5, 6. However, Myc mRNA is highly unstable25, therefore transcript levels might not correlate with protein activity25. For that reason we decided to perform gene set enrichment analysis (GSEA)26 on published gene expression profiles of FO and GC B cells27, evaluating MYC activity through the enrichment of its target genes, (see Methods for details). Using a MYC target gene list derived from mouse B cells28, we observed a highly significant enrichment of MYC induced genes in GC B cells, whereas the converse was true for genes downregulated by MYC. (Fig. 1d). These observations remained statistically significant when genes associated with the GO terms proliferation, cell cycle and growth function were removed from the analysis, arguing against the possibility that the results simply reflected the high proliferative activity of GC B cells (Fig. 1e). Similar observations were made using lists of genes containing MYC binding motifs in their promoter, and a gene set of MYC up-regulated genes obtained through the intersection of MYC ChIP-Seq and MYC siRNA knockdown data in (GC B cell derived) Burkitt lymphoma lines29 (Supplementary Fig. 1). Thus, although on average Myc mRNA is not expressed at higher levels in GC than FO B cells, GC B cells display an enrichment of MYC target genes.
Figure 1. MYC target genes are enriched in GC B cells.
(a) Flow cytometry analysis and gating strategy for flow sorting of splenic GC B cells (B220posCD38lowCD95high) and FO B cells (B220posCD38highAA4.1negCD21posCD23high) of 10 day SRBC immunized (D10 SRBCi) C57BL/6 wild-type mice. (b) Real-time PCR analysis of Bcl-6 and Aicda mRNA of flow sorted GC (green) and FO (black) B cells. Data are presented relative to Hprt and normalized to FO B cells, (mean and s.e.m. of triplicates). (c) Real-time PCR analysis of Myc mRNA as in (b). Data are presented as in (b). (d) GSEA of FO and GC B cell gene expression profiles for the MYC up-regulated (YU_MYC_TARGETS_UP, left panel) and downregulated (YU_MYC_TARGETS_DOWN, right panel) gene sets. (e) GSEA of FO and GC B cell gene expression profiles for MYC up-regulated (left panel) and downregulated (right panel) gene sets, as in (d) upon removal of genes, from those gene sets, whose GO-terms contain, ‘proliferation’, ‘cell cycle’ or ‘growth’, MINUSPROLGENES. FO and GC B cell gene expression profiles (GEO:GSE15907, ImmGen). See Methods for GSEA analysis details. Data are representative of 3 independent experiments (a-c). ns, not statistically significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
Some immature and mature GC B cells express MYC
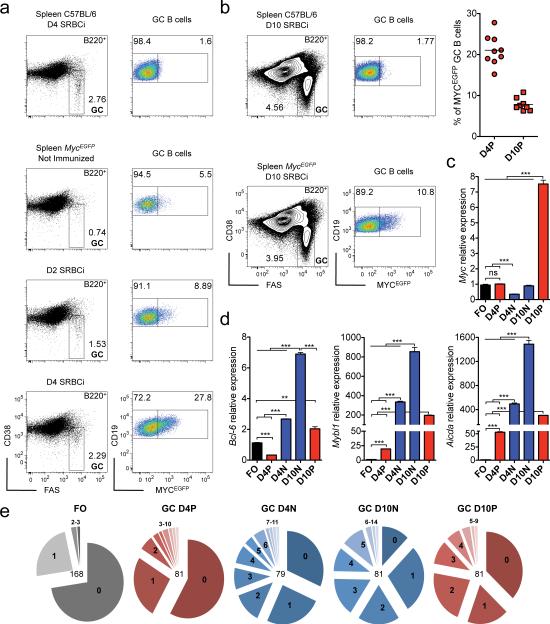
The enrichment of MYC target genes in the absence of increased Myc mRNA levels in the overall population of GC B cells led us to hypothesize that MYC activity may be restricted to a fraction of GC B cells. To address this issue, we used a Myc reporter allele, (MycEGFP)30, in which a MYC-EGFP fusion protein (MYCEGFP) is expressed from the endogenous Myc locus. By flow cytometry, we observed the appearance of MYC positive GC B cells early after immunization, reaching roughly 20% of GC B cells by day 4 (Fig. 2a,b), an early time-point of GC development before the onset of strong GC B cell expansion, (Supplementary Fig. 2). We also observed a population of GC B cells positive for MYC (~10%) at day 10 after immunization, a time-point that reflects the peak of the GC reaction (Fig. 2b and Supplementary Fig. 2). Small subsets of non-GC B cells, but not of plasma cells, expressing low amounts of MYC protein were also present in the immunized animals (Supplementary Fig. 3 and data not shown). Using the MycEGFP reporter allele and GC B cell markers, we purified the MYC positive GC B cells as well as their MYC negative counterparts by flow cytometry on day 4 and day 10 after immunization, and performed gene expression analysis by qPCR. MYC positive GC B cells isolated on day 10 displayed increased levels of Myc transcripts compared to FO B cells, whereas this was not the case for the MYC positive GC B cells on day 4 after immunization (Fig. 2c). Yet, the amount of MYCEGFP in day 4 MYC positive GC B cells were higher than in their day 10 counterparts, likely reflecting the impact of post-transcriptional control on MYC protein8 (Supplementary Fig. 3). A further analysis of the day 4 MYC positive GC B cells revealed a three-fold decrease of Bcl615 mRNA compared to FO B cells (Fig. 2d), whereas the transcript expression of other GC B cell genes, namely Mybl1 (A-myb)31 and Aicda22 was strongly increased (~20 and 50-fold respectively; Fig. 2d). Compared to FO B cells there was also an increase in the load of somatic mutations in the Ig heavy chain (IgH) variable (V) region gene rearrangements in day 4 MYC positive GC B cells (Fig. 2e). The load of somatic mutations and the fraction of cells carrying these mutations in their IgH V region genes was further increased in day 4 MYC negative GC B cells as well as both MYC positive and negative GC B cells from day 10 immunized mice (Fig. 2e). In addition, these GC B cell subpopulations demonstrated markedly elevated transcript expression of Bcl6, Mybl1 and Aicda in comparison to FO and day 4 MYC positive GC B cells (Fig. 2d,e). Hence, using a MycEGFP reporter allele that allows the visualization of MYC protein in single cells, we identified distinct subpopulations of MYC positive and negative GC B cells in both immature and mature GCs.
Figure 2. A fraction of both newly formed and mature GC B cells is positive for MYC.
(a) Flow cytometry analysis of splenic GC B cells for MYCEGFP expression of 4 day SRBC immunized (D4 SRBCi) C57BL/6 wild-type mice and of MycEGFP mice either not-immunized, 2 day (D2 SRBCi) and 4 day (D4 SRBCi) SRBC immunized. (b) Flow cytometry as in (a) of 10 day SRBC immunized (D10 SRBCi) C57BL/6 wild-type and MycEGFP mice. Right panel, graphical representation of the frequency of MYCEGFP positive cells within GC B cells of D4 SRBCi and D10 SRBCi MycEGFP mice (c) Real-time PCR analysis of Myc mRNA of flow sorted FO and of day 4 MYCEGFP positive (D4P) and negative (D4N) GC Bcells, and day 10 MYCEGFP positive (D10P) and negative (D10N) GC B cells. Data are presented relative to Hprt and normalized to FO B cells, (mean and s.e.m. of triplicates). (d) Real-time PCR analysis of Bcl6, Mybl1 and Aicda mRNA as in (c). Data are presented as in (c). (e) Igh somatic mutation of FO B cells and D4P, D4N, D10N, D10P GC B cells. Numbers in the center of the pie-chart represent sequences analyzed, and in each pie-chart section the number of mutations. Data are representative of at least 3 (a,b), 5 independent experiments (c,d), and of 3 mice (f). ns, not statistically significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
Early GC B cells express MYC and BCL-6
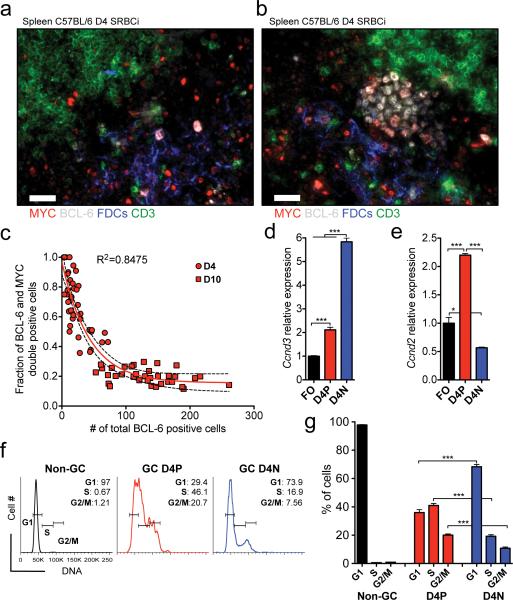
Following antigen binding and activation via the B cell receptor, B cells migrate to the T-B cell border where they are co-stimulated through interaction with cognate T helper cells2, 32. Upon productive T-B cell interaction, B cells migrate back to the intra-follicular B cell area (~4 days after initial antigen encounter) where they undergo massive expansion in the environment of follicular dendritic cells (FDCs), forming clusters of proliferating GC B cells33. BCL-6 is an essential transcription factor for GC B cell formation15, and is required already at the stage where B cells migrate back into the intra-follicular B cell area34. Given the fact that early in the GC response MYC positive GC B cells display lower transcript levels of the GC B cell genes Bcl6, Mybl1 and Aicda and fewer somatic mutations compared to their MYC negative counterparts (Fig. 2d,e), we considered the possibility that these early MYC positive GC B cells represent precursors of the latter. Using immunofluorescence histology we found through the analysis of spleens of mice 4 days after immunization that at the very initial stages of GC B cell cluster formation (≤20 cells) the cells in the clusters are positive for both MYC and BCL-6 (Fig. 3a, and Supplementary Fig.4a), and that as the number of GC B cells in the cluster increases (>20 cells), B cells gradually lose MYC expression (Fig. 3b,c, and Supplementary Fig.4b). While both MYC positive and negative GC B cells expressed the GC B cell typical Ccnd318, 19 (Fig. 3d), the MYC positive cells also expressed Ccnd2, a direct target of MYC10, 11 (Fig. 3e). Both Ccnd2 and Ccnd3 are known positive regulators of the cell cycle G0/G1-S transition in B cells10, 11, 18, 19. Consistently, we found that more than 40% of the MYC positive GC B cells were at the S-phase of the cell cycle, compared to ~20% of MYC negative GC B cells (Fig. 3f,g). Taken together these results suggest that early GC B cells are positive for both MYC and BCL-6, display hyperproliferative properties and give rise to GC B cells positive only for BCL-6.
Figure 3. Early GC B cells express both MYC and BCL-6 and are hyperproliferative.
(a) Representative image of histological immunofluorescence analysis of spleen of 4 day SRBC immunized (D4 SRBCi) C57BL/6 wild-type mice. T cells (CD3, green), FDCs (CD35, Blue), BCL-6 (white), MYC (red), cut-off of ≤20 BCL-6 positive cells per FDC area. (b) Representative image as in (a), cut-off of >20 BCL-6 positive cells per FDC area (c) Graphical representation of the fraction of BCL-6 and MYC double positive cells as function of BCL-6 positive cell count per FDC area. Data of GCs of D4 SRBCi (D4) and D10 SRBCi (D10) C57BL/6 wild-type mice. Each red symbol represents the analysis of one FDC area. Red line represents non-linear regression curve fit assuming an one-phase decay. Dashed line represents 95% confidence interval. (d) Real-time PCR analysis of Ccnd3 mRNA, of flow sorted FO and of day 4 MYCEGFP positive (D4P) and negative (D4N) GC B cells. Data are presented relative to Hprt and normalized to FO B cells, (mean and s.e.m. of triplicates). (e) Real-time PCR analysis of Ccnd2 mRNA, as in (d). Data are presented as in (d). (f) Flow cytometry analysis of the DNA content of non-GC B cells, and of D4P and D4N GC B cells. (g) Graphical representation of the data presented in (f), (mean and s.e.m.). Scale bar 25μm (a, b). Data are representative of at least 3 (a-c,f,g) and 5 (d,e) independent experiments. ns, not statistically significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
MYC is essential for GC formation
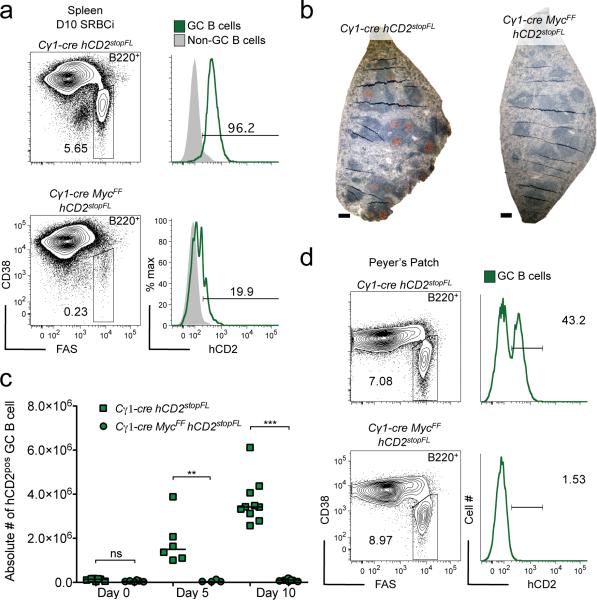
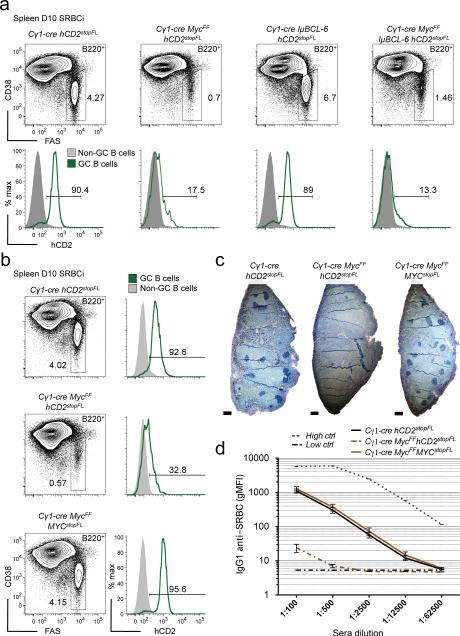
To test whether MYC is critical for GC formation, we genetically deleted Myc in GC B cells at an early stage of the GC reaction3, 35, 36. This resulted in a virtually complete ablation of GCs in response to T cell-dependent immunization (Fig. 4a, left panel). Analysis of the few remaining GC B cells present in the Cγ1-cre MycFF hCD2stopFL mice for expression of the hCD2 reporter showed that these cells had largely escaped cre-mediated recombination (Fig. 4a, right panel, and Supplementary Fig. 5). Impairment of GC B cell formation upon Myc ablation was further confirmed analyzing additional GC B cell markers by histology and immunohistochemistry (Fig. 4b). Ablation of Myc in GC B cells interfered with the GC reaction from early on after immunization, consistent with the observed expression of MYC at the initiation of the GC response (Fig. 4c). It also prevented B cells from participating in GC responses in Peyer's patches in the absence of intentional immunization, as evident from the strong counter-selection of GC B cells expressing the hCD2 reporter in Peyer's patch GCs, in which the Cγ1-cre transgene induces cre-mediated recombination in approximately 30 to 40% of the GC B cells in control animals35 (Fig. 4d). We conclude that MYC plays an essential role in both induced and spontaneous GC formation.
Figure 4. MYC is essential for GC formation.
(a) Flow cytometry analysis of splenic GC B cells of 10 day SRBC immunized (D10 SRBCi) mice of the indicated genotypes, right panel analysis of reporter (hCD2) positive GC B cells. (b) Representative images of histological immunohistochemical analysis of spleen of D10 SRBCi mice of the indicated genotypes. PNA (brown) and hematoxylin as counterstain. (c) Graphical representation of flow cytometry data of splenic hCD2 positive GC B cells of mice of the indicated genotypes, either not immunized or 5 and 10 days after SRBC immunization. (d) Flow cytometry analysis of peyer's patch GC B cells of mice of the indicated genotypes, right panel analysis of hCD2 positive GC B cells. Scale bar 25μm (b). Data are representative of at least 3 independent experiments (a-d). ns, not statistically significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
BCL-6 fails to rescue GC formation upon MYC ablation
It has been proposed that BCL-6 plays a role in GC B cell proliferation through transcriptional repression of CDK inhibitors via the interaction with MIZ1, a known partner of MYC16. We therefore decided to test whether prematurely enhanced Bcl6 expression could rescue the formation of GCs upon Myc ablation. To address this issue, we ectopically expressed Bcl6 using the IμBCL6 allele, in which a mouse Bcl6 cDNA is inserted downstream of the Iμ promoter in the IgH locus, leading to transgenic Bcl6 expression from early stages of B cell development onwards37. Although expression of Bcl6 from the IμBCL6 allele was shown to be sufficient to rescue the differentiation of GC B cells in the spleens of Bcl6 deficient mice37, this was not the case upon conditional ablation of Myc (Fig. 5a, and Supplementary Fig. 6a). Similarly, ectopic Bcl6 expression was unable to rescue spontaneous GC formation in Peyer's patches of Cγ1-cre MycFF hCD2stopFL mice (Supplementary Fig. 6b,c). The deficiency in induced and spontaneous GC formation upon Myc ablation could also not be rescued by ectopic Bcl2 expression38, emphasizing an essential role for MYC in the development of GC B cells (Supplementary Fig. 6a,b,d,e). In contrast, concomitant ablation of the endogenous Myc gene and enforced expression of MYC from a new ROSA26 allele, termed MYCstopFL, was able to fully rescue GC formation (Fig. 5b,c and Supplementary Fig. 5 and 6a,b) as well as IgG1 production (Fig. 5d and Supplementary Fig. 6f). These results demonstrate that MYC plays a non-redundant role in the initiation of GC formation.
Figure 5. Enforced Bcl6 expression fails to rescue GC formation upon Myc ablation.
(a) Flow cytometry analysis of splenic GC B cells of 10 day SRBC immunized (D10 SRBCi) mice of the indicated genotypes, lower panel, analysis of reporter (hCD2) positive GC B cells. (b) Flow cytometry analysis as in (a), of mice of the indicated genotypes, right panel as in (a). (c) Representative images of histological immunohistochemical analysis of spleen of D10 SRBCi mice of the indicated genotypes. BCL-6 (dark blue). (d) Graphical representation of flow cytometry measured anti-SRBC specific IgG1 serum antibodies at D10 SRBCi, (mean and s.e.m.), Low ctrl, unimmunized mice, High ctrl, serum from mice hyperimmunized. Scale bar 25μm (c). Data are representative of at least 3 independent experiments (a-c) and of 4 mice (d).
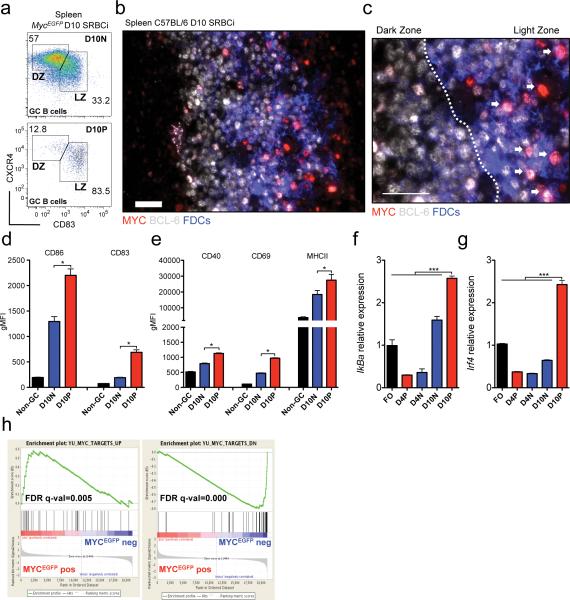
Mature MYC+ GC B cells localize to the light zone
Early histological observations using light microscopy suggested the compartmentalization of mature GCs in two morphologically distinct areas: a ‘dark’ zone (DZ), adjacent to the T cell zone, and a ‘light’ zone (LZ) contiguous to the splenic marginal zone or to the lymph node capsule39. The ‘lighter’ and ‘darker’ appearances of the GC LZ and DZ respectively, derive from the fact that B cells in the LZ are scattered amongst a network of FDCs, which are largely absent from the DZ2. In an attempt to better characterize the population of mature MYC positive GC B cells, we performed antibody staining for markers that allow the discrimination of B cells in the DZ and LZ of the GC2, 40. Using flow cytometry we found that the vast majority of MYC positive GC B cells had lower levels of CXCR4 and higher levels of CD83, compared to their MYC negative counterparts (Fig. 6a). In agreement we found, by histology and immunofluorescence, MYC positive GC B cells to be interspersed amongst FDCs (Fig. 6b,c and Supplementary Fig.7a-c). These observations lead us to conclude that the majority of the MYC positive GC B cells in mature GCs localize to the LZ. Consistent with the notion that in the LZ a fraction of GC B cells undergoes T cell-mediated activation40, the MYC positive mature GC B cells displayed a phenotype characteristic of recently activated lymphocytes. This phenotype included increased surface expression of the activation markers CD86, CD83, CD40, CD69 and MHCII (Fig. 6d,e and Supplementary Fig.7d) and increased transcripts for the ‘immediate early’ genes IκBa (Nfkbia, indicating NF-κβ activity) and Myc itself (Fig. 6f and 2c). Consistent with increased NF-κβ activity41, Irf4 expression was also elevated in the MYC positive GC B cells (Fig. 6g), whereas Bcl6 was downregulated (Fig. 2d). This is in agreement with the idea that CD40 activation signals delivered to B cells by T cells in the LZ direct them to class-switching42 and inhibit Bcl6 expression43. The MYC positive GC B cells also displayed an enrichment of MYC target genes compared to their MYC negative counterparts (Fig. 6h and Supplementary Fig. 7e). Taken together, MYC positive GC B cells in mature GCs mainly localize to the LZ and display an activated phenotype.
Figure 6. MYC positive GC B cells in mature GCs localize to the light zone and display an activated phenotype.
(a) Flow cytometry analysis of splenic MYCEGFP negative (D10N) and positive (D10P) GC B cells of 10 day SRBC immunized (D10 SRBCi) MycEGFP mice. DZ, dark zone; LZ, light zone. (b) Representative image of histological immunofluorescence analysis of spleen of D10 SRBCi C57BL/6 wild-type mice. FDCs (CD35, Blue), BCL-6 (white), MYC (red). (c) Inset, dark zone and light zone boundary, white dashed line. White arrows, MYC positive cells. (d) Graphical representation of flow cytometry analysis of CD86, CD83 expression of non-GC B cells, and of D10N and D10P GC B cells. gMFI, geometrical mean fluorescence intensity, (mean and s.e.m.) (e) Graphical representation as in (d) of CD40, CD69 and MHCII expression. Data are presented as in (d). (f) Real-time PCR analysis of IγBa mRNA of flow sorted FO B cells and of D10N and D10P GC B cells. Data are presented relative to Hprt and normalized to FO B cells, (mean and s.e.m. of triplicates). (g) Real-time PCR analysis of Irf4 mRNA as in (f). Data are presented as in (f). (h) GSEA of D10 MYCEGFP positive and negative GC B cell gene expression profiles, for the MYC up-regulated (YU_MYC_TARGETS_UP, left panel) and downregulated (YU_MYC_TARGETS_DOWN, right panel) gene sets. See Methods for GSEA analysis details. Scale bar 25μm (c,d). Data are representative 3 (a-c, d,e) and 5 (f,g) independent experiments. ns, not statistically significant. * p≤0.05, ** p≤0.01, *** p≤0.001.
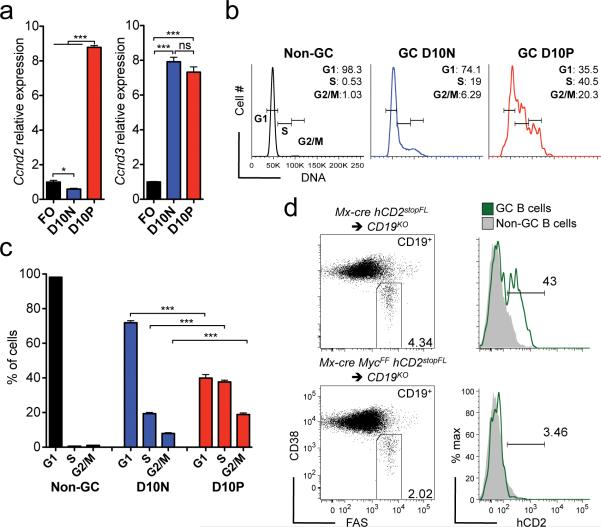
MYC positive GC B cells are required for GC maintenance
As a key function of MYC is to promote G0/G1-S transition10, 11, we assessed whether mature GC B cells positive for MYC display a proliferative phenotype. Indeed, we found increased mRNA levels for both Ccnd2 and Ccnd3 in these cells, compared to FO B cells (Fig. 7a). In contrast, MYC negative GC B cells showed increased levels only of Ccdn3 (Fig. 7a). DNA labeling and flow cytometry demonstrated that a considerable fraction (~38%) of the MYC positive GC B cells were in the S phase of the cell cycle, while this was the case for ~20% of their MYC negative counterparts (Fig. 7b,c). Given the proliferative phenotype of MYC positive GC B cells in mature GCs we decided to assess whether MYC also plays an essential role in the GC maintenance. To this end we devised an experimental cell transfer system, which allowed us to conditionally ablate Myc in mature GC B cells. For that we used CD19 knockout mice (CD19KO), in which GC B cells do not develop44, as recipients for mature B cells. Donor cells were derived from mice carrying a hCD2stopFL reporter allele either alone or together with a conditional Myc allele (MycF), in combination with the Mx-cre transgene45. Following cell transplantation, recipient mice were immunized and subsequently treated with Poly(I:C) at the peak of GC B cell formation to achieve cre-mediated recombination in the progeny of the transferred B cells. In control mice we observed a robust GC response following immunization, and cre-mediated recombination was detected in 40 to 50% of mature GC B cells derived from B cells of mice carrying the hCD2stopFL allele alone, as measured by hCD2 surface expression (Fig. 7d, upper panel). In contrast, there was a reduction of ~50% in the total fraction of GC B cells when the donor cells were derived from mice carrying the MycF allele, and only ~3% of the GC B cells were positive for hCD2 (Fig. 7d, lower panel). These results indicate massive counter-selection of MYC deficient mature GC B cells and suggest that Myc expression in mature GC B cells is required for the perpetuation of the GC reaction. We conclude that mature GC B cells go through a stage in the LZ at which MYC is essential for their maintenance and that of the GC.
Figure 7. A large fraction of MYC positive mature GC B cells are in cell cycle, and these cells are essential for GC maintenance.
(a) Real-time PCR analysis of Ccdn2 and Ccdn3 mRNA, of flow sorted FO and of day 10 MYCEGFP negative (D10N) and positive (D10P) GC B cells. Data are presented relative to Hprt and normalized to FO B cells, (mean and s.e.m. of triplicates). (b) Representative analysis by flow cytometry of the DNA of non-GC B cells and of D10N and D10P GC B cells. (c) Graphical representation of data presented in (b), (mean and s.e.m.) (d) Flow cytometry analysis of splenic GC B cells 13 days after SRBC immunization in mice of the indicated genotypes. Right panel analysis of reporter (hCD2) positive GC B cells. Data are representative of 5 (a), 3 (b, c) and 2 (d) independent experiments.
DISCUSSION
In the present work we re-evaluated the role of the transcription factor MYC, a master regulator of cellular proliferation, in GC B cell physiology. This unresolved problem derives from the conflicting observations that GC B cells have one of the fastest rates of cell division known in mammals2, while the average level of MYC transcripts in these cells is similar to those of quiescent FO B cells3, 5, 6. We found that, whereas the analysis of bulk GC B cells indeed did not show an increase in Myc transcript levels versus FO B cells, analysis of MYC protein at the single cell level revealed the presence of hyperproliferative MYC positive subpopulations of GC B cells in both immature and mature GCs, essential for the formation and maintenance of GCs.
Using a conditional knockout approach, we show that ablation of Myc at an early stage of GC B cell development leads to a complete absence of GC formation. Furthermore, MYC protein is expressed at the initiation of GC B cell cluster formation, preceding the phase of massive GC B cell expansion in the intra-follicular B cell area. At this stage we did not detect increased Myc transcript expression compared to FO B cells. This discrepancy likely reflects mechanisms of post-transcriptional control of MYC protein8, and the fact that enhanced Myc transcription is an early transitory event peaking at ~2 hours after the activation of B cells, preceding the increase in RNA synthesis and DNA replication25. Repression by BCL-6 may further restrain Myc transcription17, 46. This may be desirable, because AICDA, essential in GC B cells for antibody repertoire diversification, can target transcriptionally active genes outside the Ig loci22-24 and promote their translocation24.
In mature GCs, however, we identified another subset of GC B cells displaying increased MYC transcripts and protein levels and localizing predominantly to the LZ. Identical observations were made in human GCs46. These cells show features of cellular activation and may represent the fraction of GC B cells in the LZ undergoing T cell mediated activation to reenter the DZ. In agreement, we detected in MYC positive mature GC B cells activation of the NF-κB pathway and elevated transcript levels of target genes of this pathway, namely Myc47 itself and Irf442, whereas Bcl643 was downregulated. In addition, the hyperproliferative phenotype of the MYC positive mature GC B cells, many of which at the G2/M stage of the cell cycle which is known to largely segregate to DZ GC B cells40, together with the strong impact of acute Myc ablation on the total fraction of GC B cells suggests that MYC positive mature GC B cells are poised for DZ re-entry and perpetuation of the GC reaction, an hypothesis further supported by the experiments of Dominguez-Sola et al.46.
The transcription factor BCL-6 is essential for the development of GC B cells15. Detailed examination of the relation between BCL-6 and cellular proliferation led the hypothesis that GC B cells display an alternative, MYC-independent cell cycle program16. Along these lines, BCL-6 interacts with the transcription factor MIZ1, a MYC partner, to suppress CDK inhibitors, and MYC transcription is repressed by BCL-616, 17, 48. In addition BCL-6 inhibits the expression of Ccnd217, a MYC target, and Ccnd3, which is not controlled by MYC10, 11, is prominently expressed in GC B cells18, 19. Our studies reveal that at early stages of GC formation MYC positive GC B cells express both Ccnd2 and Ccnd3, which possibly contributes to their hyperproliferative phenotype, and that a similar pattern of expression of these genes is observed in mature GC B cells when they upregulate MYC. The cyclic dependence of GC B cell proliferation on MYC activity likely reflects a process termed “cyclic re-entry”, in which step-wise antibody affinity maturation takes place through multiple rounds of selection40, 49. In this setting, the function of MYC in the GC reaction may be to induce ‘competence’25 for GC B cell division to occur, i.e. to decide whether a GC B cell enters a phase of proliferative expansion.
Our work has direct implications for the understanding of the pathogenesis of human GC B cell derived lymphomas carrying MYC and Ig chromosomal translocations, seen in almost all sporadic Burkitt lymphomas and ~10% of DLBCLs22. B cell lymphomas, the most common type of human lymphoid malignancies overall, mostly originate either from GC B cells or B cells that have passed through the GC reaction22. In the GC microenvironment, B cells undergo processes of DNA mutation (SHM) and recombination (CSR) in their immunoglobulin genes while rapidly dividing, and infidelity in these processes leads to an increased frequency of oncogenic genetic lesions20-22. AICDA, the enzyme responsible for both SHM and CSR, participates in the formation of chromosomal breaks in the MYC gene locus that lead to MYC and Ig translocations, and MYC transcription is required for this process23, 24. In this study we identified in the mouse subpopulations of GC B cells for which Myc transcription and activity is functionally critical. Identical MYC positive GC B cell subsets were also found in the human46. Given the mechanism of MYC and Ig chromosomal translocations, these GC B cell subsets may be prone to undergo the latter event and thus represent a stage of GC differentiation with a particularly high risk for malignant transformation.
ONLINE METHODS
Ethics statement
All animal care and procedures followed NIH guidelines and were approved by the IACUC (03341) of Harvard University and the Immune Disease Institute, and of the Max Delbrück Center for Molecular Medicine.
Generation of transgenic mice
MYC transgenic mice (MYCstopFL), were generated by targeting the ROSA26 locus using a construct encoding the human MYC cDNA, preceded by a loxP flanked STOP cassette and marked by a signaling deficient truncated version of human CD2 (hCD2) under the control of a internal ribosomal entry site (IRES) downstream of the inserted cDNA. Transgene transcription is controlled by the CAG promoter. hCD2 reporter transgenic mice (hCD2stopFL), were generated following the same procedures as for MYCstopFL, whereas hCD2 cDNA was used and transgene transcription is controlled by the endogenous ROSA26 promoter. C57BL/6 mouse embryonic stem cells (Artemis pharmaceuticals) were used following described procedures50.
Genetic systems to monitor, ablate or induce MYC expression
MYC expression was monitored using the (MycEGFP)30 allele in which the endogenous Myc locus encodes a MYC fusion protein with EGFP. For conditional Myc ablation and/or enforced MYC expression we used a Myc conditional loss-of-function allele (MycF)3 and/or a newly generated ROSA26 allele, termed MYCstopFL. Conditional activation of the MYCstopFL allele by cre-mediated recombination is traced by expression of hCD2. To report cre-mediated recombination in cells carrying the MycF allele, we combined it with a novel ROSA26 reporter allele hCD2stopFL. For targeted conditional Myc mutagenesis, we used either the Cγ1-cre transgene35, or Mx-cre, where cre expression is under the control of an interferon-responsive promoter element45. For enforced Bcl6 expression, we used an allele that mimics a chromosomal translocation found in DLBCL (IμBCL6)37. EmuBCL-2 mice were used for ectopic Bcl-2 expression38. Mice carrying either the Cγ1-cre or the Mx-cre transgenes in combination with the hCD2stopFL allele served as controls.
Immunization and acute Myc deletion
For T cell dependent immunization, eight- to 10-week-old mice were injected i.v. with 1×109 SRBCs (Cedarlane) in PBS. To induce acute Myc deletion in mature GC B cells, 10×106 CD43neghCD2neg splenic B cells from either Mx-cre hCD2stopFL or Mx-cre MycFF hCD2stopFL mice were transferred into CD19KO mice by i.v. injection. Mice were immunized the next day with SRBCs and given i.p. injections of 300μg of polyinosinic-polycytidylic acid (pIpC; Amersham Biosciences), at day 7 and 10 after immunization. GCs were analyzed at day 13 after immunization.
Flow cytometry
Single-cell suspensions were stained with the antibodies described bellow. To exclude dead cells we used Topro3 (Invitrogen). Samples were acquired on a FACSCanto II (BD) using FACSDiva software (BD), and analyzed using FlowJo software (Tree Star).
| Flow cytometry antibodies | ||
|---|---|---|
| Antigen | Clone | Company |
| Mouse_CD19 | ID3 | BD pharmigen |
| Mouse_CD95 | Jo2 | BD pharmigen |
| Mouse_CXCR4 | 2B11 | BD pharmigen |
| Mouse_GL7 | GL7 | BD pharmigen |
| Mouse_CD40 | 3/23 | BD pharmigen |
| Mouse_CD38 | 90 | eBioscience |
| Mouse_CD93 | AA4.1 | eBioscience |
| Mouse_MHCII | M5/114.15.2 | eBioscience |
| Mouse_B220 | RA3-6B2 | eBioscience |
| Mouse_CD69 | H1.2F3 | eBioscience |
| Human_CD2 | TS1/8 | Biolegend |
| Mouse_CD83 | Michel-19 | Biolegend |
| Mouse_CD21 | 7E9 | Biolegend |
| Mouse_CD23 | B3B4 | Biolegend |
ELISA and SRBC-specific antibody detection
These analyses were performed following described procedures36.
Quantitative RT-PCR
These analyses were performed following described procedures36. Primer sequences are described bellow. At least 3 biological samples were assayed in triplicate.
| Primers sequences | |
|---|---|
| Bcl6 | 5′-GCCCACGTTCCCGGAGGAGA-3′ |
| 5′-CGTCTGCAGCGTGTGCCTCT-3′ | |
| IkBa | 5′-TTGGTCAGGTGAAGGGAGAC-3′ |
| 5′-GCTTTCAGAAGTGCCTCAGC-3′ | |
| Irf4 | 5′-AGGTCTGCTGAAGCCTTGGC-3′ |
| 5′-CTTCAGGGCTCGTCGTGGTC-3′ | |
| MybL1 | 5′-AGCGCCTGGGAAACCGTTGG-3′ |
| 5′-GCCCTCCTGTTCCACTTTTCTTCGC-3′ | |
| Ccnd2 | 5′-TCGCAAGCTGCCCCAGCAAA-3′ |
| 5′-GGCTGCTCCCACGCTTCCAG-3′ | |
| Aicda | 5′-TAGTGCCACCTCCTGCTCACT-3′ |
| 5′-CAACAATTCCACGTGGCAGCC-3′ | |
| Ccnd3 | 5′-CGGCGGGGTTGTGATCACTCG-3′ |
| 5′-TCCTGCGATGGCTCACGGGT-3′ | |
| Myc | 5′-GCCAGCCCTGAGCCCCTAGT-3′ |
| 5′-GGGCTGTGCGGAGGTTTGCT-3′ | |
| Hprt | 5′-GTCATGCCGACCCGCAGTC-3′ |
| 5′-GTCCTGTCCATAATCAGTCCATGAGGAATAAAC-3′ | |
Histology and immunofluorescence
Spleens were fixed with 10% formalin (Sigma), paraffin-embedded and sections stained with hematoxylin (Sigma) and PNA (Vector). For immunofluorescence spleens were embedded in OCT (Sakura) and flash frozen in liquid nitrogen. Tissue sections were cut on a CM3050s cryostat (Leica), fixed in 4% paraformaldehyde (Sigma), and stained with the antibodies described bellow. Images were acquired with a Nikon Ti-E inverted microscope (Nikon) using Slidebook (3i) and visualized with Photoshop (Adobe). We performed the scoring of MYC and BCL-6 positivity in individual GC B cell clusters using single fluorescence channels and the “Cell Counter” plug-in in ImageJ software (http://imagej.nih.gov/ij/).
| Immunofluorescence antibodies | ||
|---|---|---|
| Antigen | Clone | Company |
| Mouse/Human_BCL-6 | sc-C19 | Santa Cruz |
| Mouse/Human_MYC | Y69 | Epitomics |
| Mouse_BCL-6 | mGI191 E | eBioscience |
| Mouse_CD3 | 145-2C11 | BD pharmigen |
| Mouse_CD35 | 8C12 | BD pharmigen |
B cell DNA content and IgH somatic mutation
These analyses were performed following described procedures36.
Microarray
Gene expression profiling was performed for 2 biological replicates of flow sorted MYCEGFP positive and negative GC B cells of 10 day SRBC immunized MycEGFP mice using the mouse WG-6 v2.0 Expression BeadChip array (illumina).
Statistical analysis
Data were analyzed using Wilcoxon-Mann-Whitney test; a p value ≤0.05 was considered significant. A single asterisk (*) in the graphs of figures represents a p value ≤0.05, double asterisks (**) a p value ≤0.01, and triple asterisks (***) a p value ≤0.001, and “ns” stands for not statistically significant, i.e., a p value >0.05. Data in figures are represented as mean ± standard error of the mean (SEM). Prism (GraphPad) was used for statistical analysis.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA)26 was done using the Broad Institute Java implementation code. MYC associated gene-sets YU_CMYC_UP, YU_CMYC_DN, V$MYC_Q2 and V$MYCMAX_B from the Broad Institute MSigDB were analyzed using the signal-to-noise ratio as ranking measure, and gene labels were permuted 1000 times for NES and FDR estimation. Array data for FO and GC B cells was from GEO:GSE15907 (ImmGen), raw data was processed with RMA background correction and quantile normalization. To create a high-confidence set of MYC up-regulated genes in the Burkitt Lymphoma context we curated the MYC ChIP-Seq peak regions from Seitz et al.29 as follows: only peak regions with mus-musculus orthologous regions and a FDR <1e-4 where considered. After gene annotation of the filtered peak regions, only genes showing significant downregulation (adjusted p value ≤0.05 and Log2(FC) ≤-0.5) in three Burkitt Lymphoma cell lines (via MYC siRNA29) were considered to be functional targets of MYC. This gene set was used for GSEA testing of the FO and GC B cell (GEO:GSE15907) and of MYCEGFP positive and negative GC B cells (GEO:GSE39443) gene expression profiles. The latter was also used for GSEA testing for the MYC gene sets YU_CMYC_UP, YU_CMYC_DN.
Supplementary Material
ACKNOWLEDGEMENTS
We thank D. Ghitza, J. Wang, J. Grundy, J. Xia, C. Grosse, B. Wollert-Wulff and M. Bamberg for technical assistance, M. Ottaviano and M. Bezohra for administrative assistance, the Rajewsky laboratory members for critical comments and suggestions, and D. Dominguez-Sola and R. Dalla-Favera for sharing with us unpublished results. K.R. was supported by the National Cancer Institute grant PO1CA092625 and an LLS SCOR grant, and is recipient of an Advanced Grant from the European Research Council. D.P.C. was supported by a Leukemia and Lymphoma Society special fellow award.
Footnotes
AUTHOR CONTRIBUTIONS
D.P.C. and K.R. conceived the work and designed experiments, D.P.C., Y.S., S.A.G., A.P., K.K., M.J. and S.R. did experiments and/or analyzed data, B.P.S. and I.M.A. contributed with reagents, D.P.C. and K.R. supervised all aspects of the project, D.P.C. and K.R. wrote the manuscript. All authors discussed results and edited the manuscript.
Accession codes
Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo), microarray data, GSE39443.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 3.de Alboran IM, et al. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 4.Vallespinos M, et al. B Lymphocyte commitment program is driven by the proto-oncogene c-Myc. J Immunol. 2011;186:6726–6736. doi: 10.4049/jimmunol.1002753. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer AL, et al. Signatures of the immune response. Immunity. 2001;15:375–385. doi: 10.1016/s1074-7613(01)00194-7. [DOI] [PubMed] [Google Scholar]
- 6.Klein U, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A. 2003;100:2639–2644. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop JM. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- 8.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 9.Douglas NC, Jacobs H, Bothwell AL, Hayday AC. Defining the specific physiological requirements for c-Myc in T cell development. Nat Immunol. 2001;2:307–315. doi: 10.1038/86308. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Roger I, Kim SH, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1). EMBO J. 1999;18:5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard C, et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Valdez H, et al. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutrona G, et al. c-myc proto-oncogene expression by germinal center B cells isolated from human tonsils. Ann N Y Acad Sci. 1997;815:436–439. doi: 10.1111/j.1749-6632.1997.tb52096.x. [DOI] [PubMed] [Google Scholar]
- 14.Cutrona G, et al. The propensity to apoptosis of centrocytes and centroblasts correlates with elevated levels of intracellular myc protein. Eur J Immunol. 1997;27:234–238. doi: 10.1002/eji.1830270135. [DOI] [PubMed] [Google Scholar]
- 15.Basso K, Dalla-Favera R. BCL-6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- 16.Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL-6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6:1054–1060. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer AL, et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 18.Peled JU, et al. Requirement for cyclin D3 in germinal center formation and function. Cell Res. 2010;20:631–646. doi: 10.1038/cr.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cato MH, Chintalapati SK, Yau IW, Omori SA, Rickert RC. Cyclin D3 is selectively required for proliferative expansion of germinal center B cells. Mol Cell Biol. 2010;31:127–137. doi: 10.1128/MCB.00650-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson F, et al. Insight into the origin and clonal history of B cell tumors as revealed by analysis of immunoglobulin variable region genes. Immunol Rev. 1998;162:247–259. doi: 10.1111/j.1600-065x.1998.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 22.Klein U, Dalla-Favera R. Germinal centres: role in B cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 23.Yamane A, et al. Deep-sequencing identification of the genomic targets of the cytidine deaminase AID and its cofactor RPA in B lymphocytes. Nat Immunol. 2011;12:62–69. doi: 10.1038/ni.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nussenzweig A, Nussenzweig MC. Origin of chromosomal translocations in lymphoid cancer. Cell. 2010;141:27–38. doi: 10.1016/j.cell.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly K, Cochran BH, Stiles CD, Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983;35:603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Cozma D, Park A, Thomas-Tikhonenko A. Functional validation of genes implicated in lymphomagenesis: an in vivo selection assay using a Myc-induced B cell tumor. Ann N Y Acad Sci. 2005;1059:145–159. doi: 10.1196/annals.1339.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seitz V, et al. Deep sequencing of MYC DNA-binding sites in Burkitt lymphoma. PLoS One. 2011;6:e26837. doi: 10.1371/journal.pone.0026837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CY, Bredemeyer AL, Walker LM, Bassing CH, Sleckman BP. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. Eur J Immunol. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
- 31.Golay J, et al. The A-Myb transcription factor is a marker of centroblasts in vivo. J Immunol. 1998;160:2786–2793. [PubMed] [Google Scholar]
- 32.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 33.Coffey F, Alabyev B, Manser T. Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles. Immunity. 2009;30:599–609. doi: 10.1016/j.immuni.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitano M, et al. Bcl-6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Casola S, et al. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proc Natl Acad Sci U S A. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calado DP, et al. Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell. 2010;18:580–589. doi: 10.1016/j.ccr.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cattoretti G, et al. Deregulated BCL-6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 38.Strasser A, et al. Enforced BCL-2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwenhuis P, Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984;170:421–435. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- 40.Victora GD, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000;191:1281–1292. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein U, et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 43.Saito M, et al. A signaling pathway mediating downregulation of BCL-6 in germinal center B cells is blocked by BCL-6 gene alterations in B cell lymphoma. Cancer Cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Rickert RC, Rajewsky K, Roes J. Impairment of T cell-dependent B cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 46.Dominguez -Sola D, et al. Role of c-MYC in germinal center cyclic re-entry and maintenance. 2012.
- 47.Duyao MP, Buckler AJ, Sonenshein GE. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci U S A. 1990;87:4727–4731. doi: 10.1073/pnas.87.12.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peukert K, et al. An alternative pathway for gene regulation by Myc. EMBO J. 1997;16:5672–5686. doi: 10.1093/emboj/16.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kepler TB, Perelson AS. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol Today. 1993;14:412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki Y, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.