Abstract
Membrane trafficking involves the collection of cargo into nascent transport vesicles that bud off from a donor compartment, translocate along cytoskeletal tracks, and then dock and fuse with their target membranes. Docking and fusion involve initial interaction at a distance (tethering), followed by a closer interaction that leads to pairing of vesicle SNARE proteins (v-SNAREs) with target membrane SNAREs (t-SNAREs), thereby catalyzing vesicle fusion. When tethering cannot take place, transport vesicles accumulate in the cytoplasm. Tethering is generally carried out by two broad classes of molecules: extended, coiled-coil proteins such as the so-called Golgin proteins, or multi-subunit complexes such as the Exocyst, COG or Dsl complexes. This review will focus on the most recent advances in terms of our understanding of the mechanism by which tethers carry out their roles, and new structural insights into tethering complex transactions.
Keywords: Membrane traffic, vesicle tethering, Rab GTPase, Golgi complex, endosomes
Molecular tethering – linking a vesicle to its target
Several excellent reviews have recently appeared and include compilations of identified tethering factors and their confirmed binding partners (Short et al., 2005; Sztul and Lupashin, 2006; Cai et al., 2007a; Munson and Novick, 2006; Hughson and Reinisch, 2010). We will restrict our discussion here to new developments in this area.
Transport vesicle tethering factors function by binding a component of the transport vesicle and a component of the target membrane surface, to facilitate their productive interaction. Most tethers seem to share the ability to bind to Rab GTPases, SNARE proteins and vesicle coat complexes (Cai et al., 2007a; Short et al., 2005). In some cases, Rab GTPases contribute to tethering protein localization, but they are likely to also regulate tether interactions with other components. SNARE protein complexes may be assembled by interaction with tethers, and tethers may collect SNAREs to function at a given cellular location. Moreover, coat complex interactions help tethers bind to their vesicle substrates. Yet to be determined, of course, is the hierarchy of these interactions: how are they orchestrated to permit selective recruitment of tethers at a single target membrane, and which of these interactions are the most critical for the tethering process? The importance of determining the relative contributions of multiple interactions is emphasized by the recent observation that some tethers can bind numerous and distinct Rab GTPases all across their lengths (Sinka et al., 2008; Hayes et al., 2009). And to make matters all the more interesting, some tethers (such as Rabaptin 5 and the TRAPP and HOPS complexes) also bind to (or encode) guanine nucleotide exchange factors that re-activate specific Rab GTPases locally (Horiuchi et al., 1997; Wang et al., 2000; Wurmser et al., 2000).
The first challenge for a tether is to somehow distinguish transport vesicles from their donor membrane compartment. This is not always trivial, as the donor membraneis the source for these components. Antonny and coworkers (Drin et al., 2008) have provided an exciting new paradigm for how one class of tethers may make this distinction.
GMAP-210 (Golgi microtubule associated protein of 210 kDa) is a cis-Golgi localized protein that is important for the maintenance of Golgi structure; its overexpression disrupts anterograde transport through the Golgi and retrograde transport to that compartment (Rios et al., 1994; Pernet-Gallay et al., 2002). GMAP210 is comprised of an N-terminal ALPS motif (“amphipathic lipid-packing sensor”) and a C-terminal GRAB domain (GRIP-related Arf1 binding). The ALPS motif forms an amphipathic helix on the surface of small liposomes, and interacts preferentially with curved membrane surfaces (r < 50nm) (Drin et al., 2007). This N-terminal domain would be predicted to interact preferentially with vesicles or with the highly curved rims of the Golgi complex. GMAP-210’s C-terminal, GRAB domain was shown to bind to the Golgi-localized Arf1 GTPase in the presence of liposomes (Drin et al., 2008). Thus, the C-terminus could localize GMAP-210 to the Golgi surface via Arf1 binding. Consistent with this model, a mini- GMAP-210 construct containing the N-terminus, C-terminus and one third of the internal coiled-coil domain was able to cluster small, highly curved liposomes onto the surface of larger liposomes containing the Arf1 GTPase (Drin et al., 2008).
The localization of GMAP-210 would be further refined if Arf1-GTP was excluded from curved membranes (and vesicles) that should not recruit this tether’s C-terminus. Interestingly, the ArfGAP1 enzyme also contains ALPS motifs that favor its colocalization with the GMAP-210 N-terminal ALPS motif on highly curved membranes (Mesmin et al., 2007). The presence ArfGAP1 would disfavor interaction of GMAP-210’s Arf-binding GRAB domain, thereby orienting the interaction of this tether: the N-terminus on vesicles, and the C-terminus on a flatter membrane surface containing Arf1. The precise role of GMAP-210 in organizing the structure of the Golgi awaits additional experimental analysis.
The challenge of recognizing transport vesicles for tethering purposes may also be facilitated by tethers binding directly to transport vesicle coat proteins (reviewed in Cai et al., 2007a). The TRAPP-I tethering complex participates in the delivery of proteins from the endoplasmic reticulum (ER) to the Golgi complex (Sacher et al., 1998). Its Bet3p constituent was recently shown to bind directly to the Sec23p coat subunit that is important for cargo recruitment into nascent transport vesicles (Cai et al., 2007b). Thus, in the case of ER-to-Golgi transport, tethering interactions may be initiated even before the vesicle has budded off from the donor ER membrane. This type of molecular link ensures accurate delivery of abundant transport vesicles that bud from the ER. Similarly, the retrograde transport tethering complexes, COG and Dsl, both interact with COP-I coat components (Zolov and Lupashin, 2005; Andag and Schmitt, 2003); as mentioned earlier, GMAP-210 interacts with ArfGAP1 which may also be a COP-I vesicle coat component (Yang et al., 2002; Lee et al., 2005).
Symmetric Tethering Fully Reconstituted
So far, we have considered the asymmetric tethering of transport vesicles to their respective target membranes. Tethering also includes symmetric tethering of equivalent compartments, such as early endosome-early endosome tethering that precedes fusion of these compartments. Homotypic tethering of early endosomes was first shown in 1999 by Zerial and coworkers (Christoforidis et al., 1999) using rhodamine-transferrin-containing endosomes incubated with either cytosolic proteins or purified EEA1 protein (early endosome antigen 1). EEA1 is recruited onto the surface of early endosomes by Rab5 GTPase and phosphatidylinositol 3-phosphate (PI3P; Simonsen et al., 1998). This dimeric, coiled-coil protein facilitates homotypic interaction between two early endosome compartments. In a recent reconstitution of the same process using entirely purified components, two tethering proteins seemed to work together: EEA1 and Rabenosyn5-HVps45 (Ohya et al., 2009). Both EEA1 and Rabenosyn 5 interact with PI3P via FYVE domains and bind to Rab5 for endosome localization; Rabenosyn 5 also binds the HVps45 protein that can interact with Syntaxins 4, 6 and 13 (Nielsen et al., 2000). EEA1 also interacts with SNARE proteins (Simonsen et al., 1999). Importantly, EEA1 and Rabenosyn 5 were both needed for full fusion in the reconstituted system, suggesting that their activities are non-redundant (Ohya et al., 2009). The basis for this distinction in terms of the precise roles of each of these tethering proteins in early endosome fusion will be important to ascertain. In addition, how the proteins engage each other physically during coalescence of donor and acceptor endosomes will be interesting to determine.
In their studies of the homotypic fusion of vacuolar compartments, Wickner and colleagues reported an obligate requirement for the multi-subunit HOPS tethering complex in their fully reconstituted vacuolar fusion system (Stroupe et al., 2009). Like the early endosomal tethering proteins, HOPS complex subunits interact with a Rab GTPase: in this case, the Rab7 homolog, Ypt7p, as well as phosphoinositides on vacuolar membrane surfaces (Stroupe et al., 2006). HOPS also interacts with the SNAREs Vam3p and Vam7p. Moreover, as mentioned earlier, one of its subunits carries out nucleotide exchange on the Ypt7 GTPase, driving Ypt7 activation (Wurmser et al., 2000). In the fully reconstituted vacuolar fusion study, Stroupe et al. (2009) argued that Rab:tether interactions did not seem to be sufficient to detect stable tethering of proteoliposomes; SNARE proteins were also required. Most recently, Hickey and Wickner (2010) can now detect HOPS tethering of liposomes prior to SNARE engagement (2010), consistent with the conclusions of a study of early endosomal fusion in which SNAREs were less important for tethering (Geumann et al., 2008). Since early endosome fusion requires multiple tethers (EEA1 and Rabenosyn 5), perhaps double tether participation decreases the requirement for SNAREs in stabilizing tethered complexes. In any event, much remains to be learned in terms of how, when and why tethers interact with lipids, Rab GTPases and SNARE proteins.
Tether regulation of SNARE Complex assembly
The first indication that tethers may catalyze SNARE complex assembly was obtained by Shorter et al. (2002) in their work studying the Golgi tether p115. The presence of p115 resulted in an increased rate of complex formation between two cognate SNAREs involved in COPI vesicle fusion. Recent work suggests that other tethering complexes will share this capacity. The multisubunit HOPS complex interacts with individual SNAREs and assembled SNARE complexes (Starai et al., 2008). This has now also been shown for the GARP complex involved in endosome to Golgi transport (Perez-Victoria and Bonifacino, 2009). But the most detailed picture of SNARE-tether interactions comes from Hughson and colleagues in their studies of the Dsl complex (Ren et al., 2009).
The Dsl complex is a three-subunit tether required for Golgi to ER retrograde transport (Reilly et al., 2001; Andag et al., 2001). The complex, comprised of Dsl1, Sec39 (Dsl3) and Tip20 proteins, is localized to the ER at least in part by direct interactions with two ER SNAREs: Sec20 and Use1 (Kraynack et al., 2005; Tripathi et al., 2009). Dsl is thought to mediate retrograde vesicle tethering by binding to components of the COP-I coat (see below).
Different Dsl subunits interact with different SNAREs via their N--terminal regulatory domains: Sec39 binds Use1 while Tip20 binds Sec20. By binding to individual SNARE components, this arrangement would facilitate the disassembly of non-functional SNARE protein clusters and also have the capacity to drive the formation of active SNARE complexes, by bringing together Sec20, Use1 and eventually, Ufe1 proteins. Indeed, the Dsl complex enhanced, at least somewhat, the rate of SNARE complex formation detected using a fluorescence polarization assay (Ren et al., 2009). In addition, the Dsl tether subunits interact with N-terminal regulatory domains on the SNAREs, rather than the SNARE motif itself. Thus, SNARE complex formation would not necessarily be impeded by the presence of the Dsl tether.
In addition to catalyzing SNARE complex formation, tethering factors could also regulate SNARE pairing by reducing the rate of complex assembly until some set of criteria important for trafficking are met. Munson and colleagues first identified this type of SNARE regulation in their work on the Exocyst component Sec6 (Sivaram et al., 2005). The Exocyst is a multi-subunit tethering complex required for delivery of post-Golgi vesicles to the plasma membrane (Terbush et al., 1996). Sec6 binds to Sec9, a t-SNARE at the plasma membrane. Sec9 assembles into a t-SNARE complex with Sso1, and the inclusion of Sec6 in an in vitro assembly reaction lowered the rate of assembly approximately 3.5 fold (Sivaram et al., 2005). It is possible that the Exocyst, a large complex with many binding partners, is able to integrate a number of signals to help determine the proper set of conditions that must be met before SNARE pairing is allowed to proceed.
Dsl: A structural view of the tethering process
Structural studies have provided invaluable information regarding how interaction of the Dsl complex with COP-I coat proteins may contribute to the tethering process. The Dsl1 subunit interacts with multiple COP-I vesicle coat components (Andag and Schmitt, 2003). Zink et al. (2009) found that an unstructured loop in Dsl1 binds to the same short region in alpha-COP that is used to stabilize COP-I coat structures by binding to epsilon-COP. This implies that interaction with Dsl would favor coat destabilization. The authors favor a model in which the Dsl complex inhibits repolymerization of COP-I coat constituents at the fusion target site. It seems nevertheless highly likely that Dsl tethering includes interaction with at least partially disassembled coats on incoming transport vesicles.
Remaining Mysteries
Why are most coiled-coil tethers so long? It has been suggested that many of these tethering factors could extend from organelle surfaces to capture incoming vesicles, but experimental evidence for this possibility is not yet in hand. Experiments investigating the interactions of single vesicles or organelles with purified tethering proteins will provide important information to further inform current models. Do tethering factors undergo structural transitions that facilitate progression from more distant tethering to closer docking states? Electron microscopy showed that the Dsl complex may exist in either an extended or bent conformation (Ren et al., 2009), and it will be of interest to determine whether different binding interactions with components of either the target membrane or the transport vesicle favor one of these conformations over the other. The fundamental question of how tethers recognize vesicles and prime them for productive interaction with target membranes will surely continue to engage us for many years to come.
Figure 1.
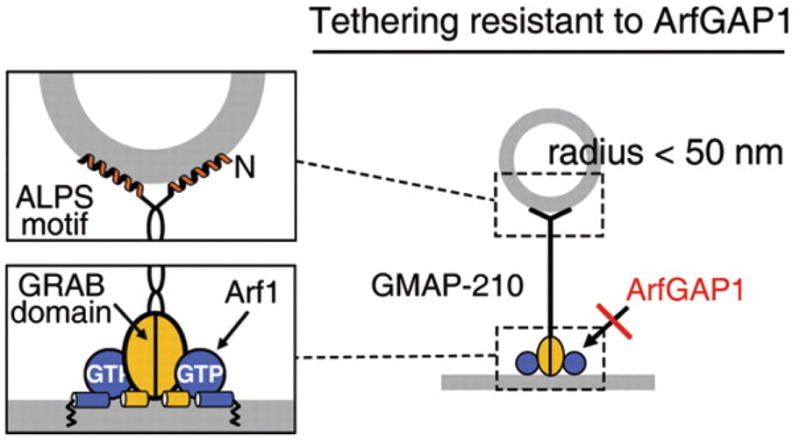
Tethering orientation of GMAP-210. The N-terminus has an ALPS motif that binds preferentially to curved membrane surfaces; the C-terminus has a GRAB domain that binds Arf1-GTP. The presence of ArfGAP1 on curved membranes keeps drives GMAP-210’s C terminus onto flattened membranes. Reprinted from Drin et al. (2008) with permission from AAAS.
Figure 2.
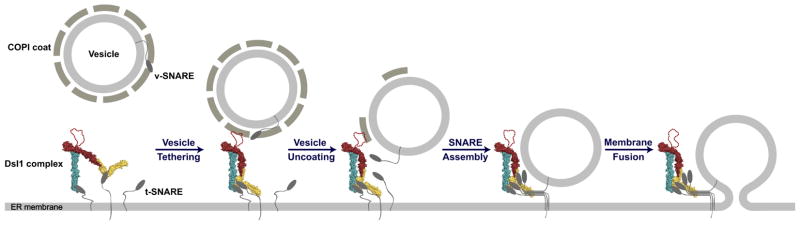
The Dsl complex is located at the ER membrane. Here it organizes SNAREs and an unstructured loop recognizes coat components to lasso the incoming vesicle to the target membrane. Reprinted from Ren et al. (2009) with permission from Elsevier.
Acknowledgments
This work was funded by research grants from the US National Institutes of Health (DK37332 and GM79322).
References
- Andag U, Neumann T, Schmitt HD. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J Biol Chem. 2001;276:39150–60. doi: 10.1074/jbc.M105833200. [DOI] [PubMed] [Google Scholar]
- Andag U, Schmitt HD. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J Biol Chem. 2003;278:51722–34. doi: 10.1074/jbc.M308740200. [DOI] [PubMed] [Google Scholar]
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007a;12:671–82. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. Coats, Tethers, Rabs and SNAREs work together to mediate the intracellular destination of a transport vesicle. Nature. 2007b;445:941–4. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–5. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol. 2007;14:138–46. doi: 10.1038/nsmb1194. [DOI] [PubMed] [Google Scholar]
- Drin G, Morello V, Casella JF, Gounon P, Antonny B. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 2008;320:670–3. doi: 10.1126/science.1155821. [DOI] [PubMed] [Google Scholar]
- Geumann U, Barysch SV, Hoopmann P, Jahn R, Rizzoli SO. SNARE function is not involved in early endosome docking. Mol Biol Cell. 2008;19:5327–37. doi: 10.1091/mbc.E08-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20:209–17. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Wickner W. HOPS initiates vacuole docking by tethering membranes prior to trans-SNARE complex assembly. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-01-0044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–59. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hughson FM, Reinisch KM. Structure and mechanism in membrane trafficking. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.03.011. [Online] Available at: http://dx.doi.org/10.1016/j.ceb.2010.03.011. [DOI] [PMC free article] [PubMed]
- Kraynack BA, Chan A, Rosenthal E, Essid M, Umansky B, Waters MG, Schmitt HD. Dsl1p, Tip20p, and the novel Dsl3(Sec39) protein are required for the stability of the Q/t-SNARE complex at the endoplasmic reticulum in yeast. Mol Biol Cell. 2005;16:3963–77. doi: 10.1091/mbc.E05-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168:281–90. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesmin B, Drin G, Levi S, Rawet M, Cassel D, Bigay J, Antonny B. Two lipid-packing sensor motifs contribute to the sensitivity of ArfGAP1 to membrane curvature. Biochemistry. 2007;46:1779–90. doi: 10.1021/bi062288w. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–81. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–12. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya T, Miaczynska M, Coskun U, Lommer B, Runge A, Drechsel D, Kalaidzidis Y, Zerial M. Reconstitution of Rab- and SNARE-dependent membrane fusion by synthetic endosomes. Nature. 2009;459:1091–7. doi: 10.1038/nature08107. [DOI] [PubMed] [Google Scholar]
- Perez-Victoria FJ, Bonifacino JS. Dual roles of the mammalian GARP complex in tethering and SNARE complex assembly at the trans-Golgi network. Mol Cell Biol. 2009;29:5251–63. doi: 10.1128/MCB.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet-Gallay K, Antony C, Johannes L, Bornens M, Goud B, Rios RM. The overexpression of GMAP-210 blocks anterograde and retrograde transport between the ER and the Golgi apparatus. Traffic. 2002;3:822–32. doi: 10.1034/j.1600-0854.2002.31107.x. [DOI] [PubMed] [Google Scholar]
- Reilly BA, Kraynack BA, VanRheenen SM, Waters MG. Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit. Mol Biol Cell. 2001;12:3783–96. doi: 10.1091/mbc.12.12.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Yip CK, Tripathi A, Huie D, Jeffrey PD, Walz T, Hughson FM. A structure-based mechanism for vesicle capture by the multisubunit tethering complex Dsl1. Cell. 2009;139:1119–29. doi: 10.1016/j.cell.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios RM, Tassin AM, Celati C, Antony C, Boissier MC, Homberg JC, Bornens M. A peripheral protein associated with the cis-Golgi network redistributes in the intermediate compartment upon brefeldin A treatment. J Cell Biol. 1994;125:997–1013. doi: 10.1083/jcb.125.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates JR, 3rd, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744:383–95. doi: 10.1016/j.bbamcr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Chrisoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–8. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Gaullier JM, D’Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–60. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183:607–15. doi: 10.1083/jcb.200808018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaram MV, Saporita JA, Furgason ML, Boettcher AJ, Munson M. Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry. 2005;44:6302–11. doi: 10.1021/bi048008z. [DOI] [PubMed] [Google Scholar]
- Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–8. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–89. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Hickey CM, Mima J, Burfeind AS, Wickner W. Minimal membrane docking requirements revealed by reconstitution of Rab GTPase-dependent membrane fusion from purified components. Proc Natl Acad Sci. 2009;106:17626–33. doi: 10.1073/pnas.0903801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E, Lupahin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol. 2006;290:C11–26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–94. [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Ren Y, Jeffrey PD, Hughson FM. Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex. Nat Struct Mol Biol. 2009;16:114–23. doi: 10.1038/nsmb.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Sacher M, Ferro-Novick S. TRAPP stimulates guanine nucleotide exchange on Ypt1p. J Cell Biol. 2000;151:289–96. doi: 10.1083/jcb.151.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–62. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink S, Wenzel D, Wurm CA, Schmitt HD. A link between ER tethering and COP-I vesicle uncoating. Dev Cell. 2009;17:403–16. doi: 10.1016/j.devcel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Zolov SN, Lupashin VV. Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J Cell Biol. 2005;168:747–59. doi: 10.1083/jcb.200412003. [DOI] [PMC free article] [PubMed] [Google Scholar]


