Abstract
Host defences become increasingly costly as parasites breach successive lines of defence. Because selection favours hosts that successfully resist parasitism at the lowest possible cost, escalating coevolutionary arms races are likely to drive host defence portfolios towards ever more expensive strategies. We investigated the interplay between host defence portfolios and social parasite pressure by comparing 17 populations of two Temnothorax ant species. When successful, collective aggression not only prevents parasitation but also spares host colonies the cost of searching for and moving to a new nest site. However, once parasites breach the host's nest defence, host colonies should resort to flight as the more beneficial resistance strategy. We show that under low parasite pressure, host colonies more likely responded to an intruding Protomognathus americanus slavemaker with collective aggression, which prevented the slavemaker from escaping and potentially recruiting nest-mates. However, as parasite pressure increased, ant colonies of both host species became more likely to flee rather than to fight. We conclude that host defence portfolios shift consistently with social parasite pressure, which is in accordance with the degeneration of frontline defences and the evolution of subsequent anti-parasite strategies often invoked in hosts of brood parasites.
Keywords: host–parasite interaction, defence portfolios, brood parasites, social insects, frontline defences
1. Introduction
Why do some organisms express a multitude of defence strategies against exploiters, whereas others fail to employ seemingly adaptive defences? This long-standing question in evolutionary biology led to the development of the strategy blocking hypothesis, which poses that proficiency in one defensive strategy relaxes selection on other traits and thereby inhibits the evolution of further lines of defence [1,2]. Theory suggests that once the first line of defence is breached by exploiters, selection favours victims that mount further defences. This could result in complex defence portfolios, deployed in hierarchical sequence [3,4]. Consequently, in host–parasite systems, the evolution of host defences may not only depend on attributes of parasites, such as parasite pressure and their degree of specialization, but also on the efficacy of other traits in the adaptive portfolios of hosts and parasites [3,5]. Recent modelling approaches conclude that victims have unique evolutionary advantages when coevolution involves multiple traits in hosts and parasites [6]. These findings highlight the need for integrative studies on host defence portfolios to understand the trajectories and outcomes of coevolutionary arms races.
In systems where parasites exploit the brood care behaviour of their host, defences that are expressed prior to parasitation spare the host costly investment in parasitic young [7]. Such frontline defences thus have the greatest potential to minimize the costs inflicted by parasites, while parasite attack becomes increasingly costly as successive lines of defence are breached [7,8]. Because selection favours hosts that successfully defend themselves at the lowest possible cost, the temporal sequence in which defences are employed is thought to reflect the order in which they evolved [5]. For instance, most examples of chick rejection—the most costly defence mode against avian brood parasites—occur in hosts with a long history of exploitation, such as those of the evolutionarily old bronze cuckoo, which breached preceding defences [8]. Thus, with increasing evolutionary age of brood parasite–host associations, anti-parasite strategies appear to shift from frontline defences to those expressed later.
According to this rationale, frontline defences, that circumvent exploitation altogether [7], mark the first phase in the evolutionary arms race between parasites and hosts. Parasites are then under selection to counter these defences. During the next phase, parasites breach the first defence line, potentially causing its decay. Simultaneously, hosts are under selection to mount further defences. If parasites are rare and reciprocal selection pressures are weak, the arms-race may not proceed beyond the first phase. However, at coevolutionary hotspots, further escalation of the arms-race could result in counter adaptations by the parasite, the degeneration of the first line of defence and the evolution of subsequent defensive strategies. Hence, over the geographical range of host species, parasite pressure is predicted to be associated with a hierarchy of defence strategies.
Although the evolutionary principles governing adaptive portfolios are generally applicable to exploiter–victim systems [2], the concept has almost exclusively received attention in avian brood parasites and their hosts [3,5,7]. Nonetheless, hosts of insect social parasites and avian brood parasites show striking similarities in the trajectories and outcomes of their coevolutionary arms races [3]. Like avian hosts, social insects exhibit a range of morphological, behavioural and physiological adaptations to social parasites, which often co-occur in a single host species [9–13]. The apparent depth of these defence portfolios renders social parasitic hosts particularly suitable targets in the study of host defence portfolios [3].
Contrary to avian brood parasites, social parasites target and exploit the brood care behaviour of entire societies [14]. The social lifestyle of the ant, wasp or bee host allows for the evolution of collective defences, which are likely to attenuate asymmetries in individual competitive ability of parasites and hosts. Indeed, organized group defences are one of the most characteristic features of insect societies and greatly contribute to their ecological success [15]. Collective host defences can be represented by fight or flight behaviours [14,16]. Hosts that can evade parasitism through aggressive nest defence avoid the costs associated with giving up their nest site, which is often a limited resource. Hence, aggression, as a first line of defence, is likely to convey the largest fitness benefits [7]. However, once parasites breach the host's nest defence, flight may remain the only beneficial mode of resistance.
The obligate social parasite Protomognathus americanus is an evolutionarily old parasite that exploits the brood care behaviour of its Temnothorax hosts [17]. Through regular, destructive raids, these slavemakers replenish their slave workforce by capturing the host's brood [18]. Slave raids are preceded by a scouting event during which a single slavemaker worker discovers and inspects a host nest and returns to its colony to recruit nest-mates. The slave raid that follows is often initiated by only one or few slavemakers, which recruit nest-mates before or during the raiding attack. As host workers and the queen are often killed during a raid, few colonies survive a slavemaker attack [19,20]. Hence, frontline defences, directed to fend off slavemaker scouts or raiding parties, are likely to be selected for. Indeed, Temnothorax hosts exhibit both fight and flight behaviour during antagonistic interactions with slavemakers, both of which reduce the costs slavemakers inflict on their host [9–11,13,21].
Here, we investigate how host defence portfolios changed with social parasite pressure across 17 populations of two Temnothorax host species. Collection sites span most of the geographical range of the slavemaker and the two host species and include unparasitized host populations. Specifically, we ask whether fight and flight responses towards the introduction of a slavemaker ant into a host colony change according to parasite pressure in the population from which the host originated. We hypothesize that in populations where the slavemaker is rare or absent, hosts resort to collective aggressive nest defence, as the first line of defence. However, in highly parasitized populations, further escalation of the coevolutionary arms race may have led to the expression of nest evacuation as a further defence strategy down the hierarchy. Hence, we predict that host defence portfolios shift from collective fight to flight behaviours with increasing parasite pressure.
2. Material and methods
(a). Colony collection and maintenance
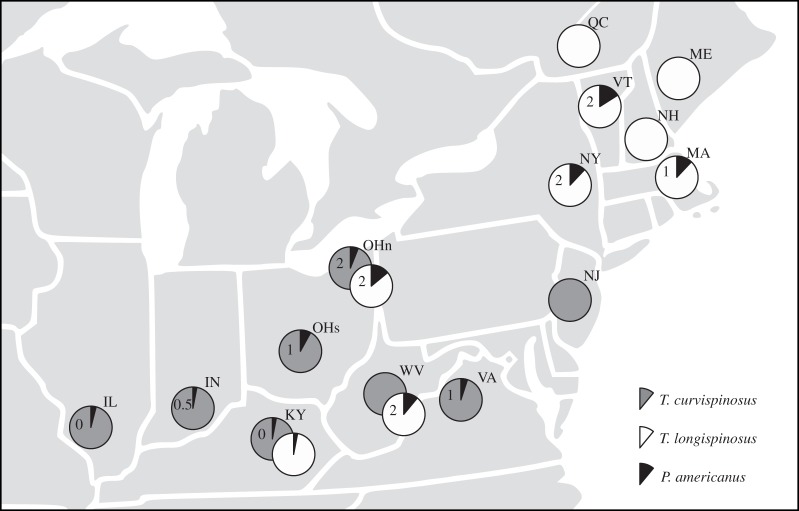
From May to July 2012, we collected 3463 Temnothorax longispinosus, Temnothorax curvispinosus and P. americanus ant colonies from a total of 17 host populations from 14 sites in the United States and Canada (figure 1; electronic supplementary material, table S1). From each population, we sampled approximately 100 colonies or more with the exception of the T. longispinosus population from Kentucky (65 colonies) due to the low local abundance of this species. Ants were collected shortly before the annual raiding season, which takes place between July and September. Hence, even in parasitized populations, colonies did not have slavemaker contact for at least 1 year. All colonies were counted, transferred to artificial glass nest sites (cavity size: 50 × 10 × 3 mm) and kept at a constant 25°C and a 12 L : 12 D cycle. They were housed in plastered nest-boxes (10 × 10 × 3 cm) to prevent desiccation and fed weekly with honey and cricket.
Figure 1.
Distribution of experimental Temnothorax host populations and parasite pressure by the slavemaker ant P. americanus. Pie diagrams and numbers represent parasite prevalence and median slavemaker colony sizes (i.e. the median number of slavemaker workers), respectively, in Illinois (IL), Indiana (IN), Kentucky (KY), Maine (ME), Massachusetts (MA), New Hampshire (NH), New Jersey (NJ), New York (NY), Ohio North (OHn), Ohio South (OHs), Quebec (QC), Vermont (VT), Virginia (VA) and West Virginia (WV). Details on collection sites are provided in the electronic supplementary material.
(b). Parasite pressure estimates
Parasite pressure was estimated by (i) parasite prevalence (i.e. the number of slavemaker colonies relative to the number of host colonies) and (ii) median slavemaker colony size (i.e. the median number of slavemaker workers per slavemaker colony, the queen was not included in this count). Parasite prevalence reflects the likelihood of being attacked by a slavemaker, whereas the median slavemaker colony size is indicative of the potential raiding party size. The latter is important because the decision to fight or flee is taken before host colonies have reliable information on the size of the raiding party, for instance because they face a scout or the first member of the raiding party. For median slavemaker colony size, we assigned a value of zero to populations where the slavemaker was absent (excluding these populations from the analyses yielded qualitatively similar results).
Coevolutionary dynamics play out over long time-scales in species with long generation times such as Temnothorax ants, in which queens can live for several decades [22]. Whenever possible, we therefore included long-term collection data on parasite pressure from previously studied communities (i.e. Ohio North, West Virginia, Vermont and New York [23–26]). Moreover, we have evidence for consistent parasite occurrence from some other communities that have been sampled sporadically in the past (i.e. T. longispinosus population from Massachusetts and T. curvispinosus populations from West Virginia, Virginia and Ohio South; S. Foitzik 2001–2008, personal observation). Where available, long-term parasite pressure estimates were used in our analyses. Current parasite pressure and its relationship with host defences are reported in the electronic supplementary material, table S1.
(c). Behavioural experiments
From each population, 32.0 ± 3.3 (mean ± s.d.; electronic supplementary material, table S1), average-sized host colonies were selected for standardized fight–flight experiments. Colony sizes did not differ between T. longispinosus and T. curvispinosus colonies (Poisson generalized linear mixed model (GLMM) with colony ID, nested in population ID as random factor: χ2 = 2.88, p = 0.090). Host colony sizes did not differ between 16 out of the 17 host populations (quasi-Poisson GLM: F = 1.37, Δd.f. = 15, p = 0.157). The exception was T. longispinosus colonies from New Hampshire, which were smaller than those from the other 16 populations (all p < 0.005). Excluding New Hampshire from all following analyses yielded qualitatively the same results.
For the experiments, a living P. americanus slavemaker worker was introduced into a host colony and the nest entrance was sealed for 1 h. Upon opening the nest entrance, we recording the number of host workers individually attacking the slavemaker (i.e. biting or stinging) as well as the number of workers involved in collective slavemaker immobilization (i.e. holding). During the latter, multiple host workers immobilize the slavemaker by holding its legs and antennae in their mandibles for prolonged periods of time. Because a single ant is physically not strong enough to hold the larger slavemaker, collective immobilization requires cooperation between host workers. The holding behaviour exhibited by workers immobilizing the slavemaker distinguishes itself from biting (i.e. a form of individual attack) where workers show brief but forceful snapping with the mandibles. Although individual attack may harm the slavemaker, it does not necessarily prevent it from escaping and potentially recruiting nest-mates. In contrast, collective immobilization frequently causes dismemberment and subsequent death of the slavemaker and therefore can prevent the recruitment of a raiding party.
Host colonies were monitored for nest evacuation and slavemaker escape during the 6 h following slavemaker introduction. Slavemaker escape status was assigned based on whether or not the slavemaker was able to leave the colony physically unharmed, as an unharmed scout is likely to return to its colony to recruit nest-mates and initiate a slave raid. Preliminary tests showed that colony evacuation status or slavemaker escape status did not change from 6–24 h. Experiments were conducted at 25°C from August to September 2012, which coincides with the raiding season of P. americanus colonies. To eliminate potential test date and time-of-day effects, we randomly selected an equal number of colonies from each population per test day and randomized test order within test days.
(d). Slavemaker origin
All slavemakers originated from colonies containing slaves of the species they were tested against. As several host populations were unparasitized and could thus not be tested against a sympatric slavemaker, we standardized slavemaker population of origin. Hereto, we only used slavemakers from New York against T. longispinosus colonies and slavemakers from either Ohio South (n = 199) or Illinois (n = 58) against T. curvispinosus colonies. Colony evacuation probability (binomial GLM: Δdeviance = −1.87, Δd.f. = 1, p = 0.172) and the number of immobilizing workers (quasi-Poisson GLM: F1,252 = 1.57, p = 0.211) did not differ between colonies facing a slavemaker from Ohio or Illinois.
Temnothorax longispinosus colonies from New York and T. curvispinosus colonies from Ohio were confronted with a sympatric slavemaker, whereas colonies from the remaining populations faced allopatric slavemakers. To assess whether slavemaker sympatry affected colony responses, we tested each of the 64 experimental T. longispinosus colonies from New York and West Virginia against both a sympatric and an allopatric slavemaker in random order with a 7-day interval. As in previous behavioural studies [10,24], we found no effect of slavemaker sym- or allopatry (GLMMs with colony identity, nested in population identity as random factor: number of aggressive workers: χ2 = 0.01, p = 0.931; evacuation probability: χ2 = 0.05, p = 0.826).
(e). Statistics
We assessed whether collective defences to an intruding slavemaker and the efficiency of such responses were associated with parasite pressure, host species identity and host colony size. Hereto, we analysed the likelihood of collective immobilization (i.e. the holding of a slavemaker by more than one host worker), colony evacuation and slavemaker escape using GLMMs (lmer function implemented in the lme4 package [27]) with binomial error distribution and logit link function. In addition, we tested for differences in individual and collective aggressive defences by analysing the number of workers either attacking or immobilizing the slavemaker. Because there is only a quantitative difference between holding by a single worker and immobilization by multiple workers, we assessed all instances of holding, including those where only a single worker was involved. Holding by a single worker was however rare, as 93% of the 329 holding events involved multiple workers. For these analyses, we used a set of GLMMs [27] with Poisson error distribution and log link function. The effect of parasite pressure was evaluated using separate analyses of parasite prevalence and median slavemaker colony size, as these estimates of parasite pressure were highly correlated (Spearman ρ = 0.76, S = 69.11, p = 0.004). In all analyses, the parasite pressure measure, species identity and their interaction were included as fixed predictors, as was host colony size. Colony identity, nested in population identity was included as random factor to account for pseudo-replication. Only non-evacuating colonies suffer from allowing a slavemaker to escape, recruit and return to raid the colony. Hence, we excluded evacuating host colonies from the analysis of slavemaker escape status.
Analyses including parasite prevalence and slavemaker colony size were based on all 17 and a subset of 16 host populations, respectively, because colony sizes of slavemakers with T. longispinosus slaves from Kentucky were not recorded. For all analyses, we used a backwards-stepwise procedure for model selection (α = 0.05). Model selection tables are provided in the electronic supplementary material. All analyses were performed in R v. 3.0.0 [28].
3. Results
(a). Defence portfolios and parasite pressure
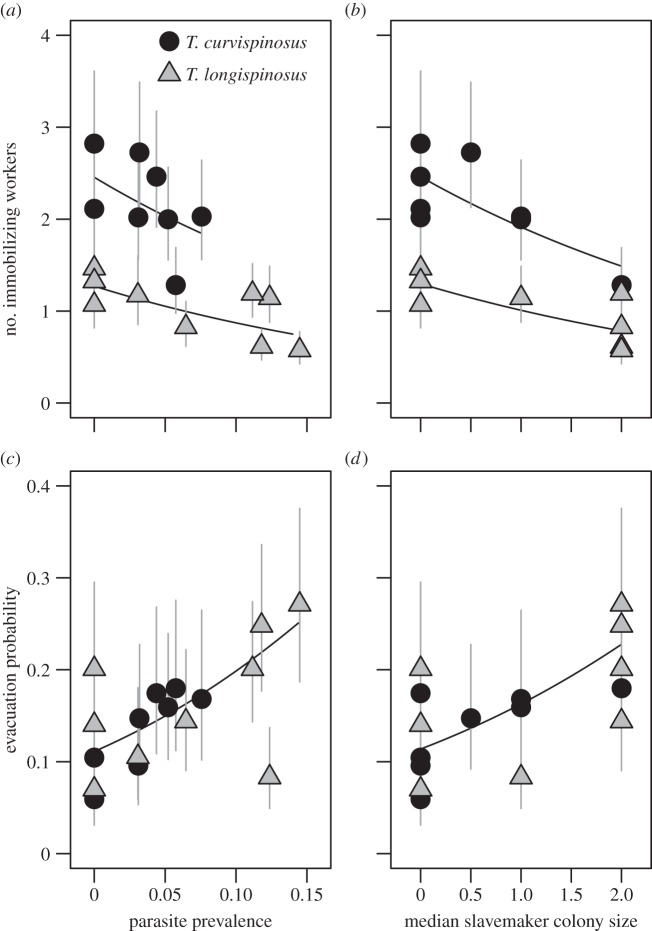
Host defence portfolios shifted from collective fight to flight with parasite pressure (figure 2). Both the likelihood of collective slavemaker immobilization (estimate ± s.e. = −4.36 ± 2.05, z = −2.13, p = 0.033) and the number of immobilizing host workers (figure 2a, p = 0.004) decreased with parasite prevalence. In contrast, the evacuation probability of host populations increased with parasite prevalence (figure 2c; p = 0.004). Thus, host populations that are under severe parasite pressure are more likely to flee rather than fight when they encounter a slavemaker in their nest, which is supported by a strong negative relationship between colony evacuation probability and the number of immobilizing workers (estimate ± s.e. = −0.499 ± 0.074, z = −6.78, p < 0.0001).
Figure 2.
Collective host defences in relation to social parasite pressure. Parasite pressure is represented by the parasite prevalence (a,c) and the median slavemaker colony size (b,d). Symbols represent the estimate ± s.e. per population, standardized for average host colony size (i.e. population estimates + colony size estimate × average host colony size). Regression lines are derived from the following GLMM estimates and back-transformed to the original data scale. (a) Estimate ± s.e. = −3.77 ± 1.31, z = −2.89, p = 0.004; (b) −0.25 ± 0.07, z = −3.49, p < 0.001; (c) 6.84 ± 2.40, z = 2.85, p = 0.004 and (d) 0.42 ± 0.14, z = 3.02, p = 0.003.
Contrary to collective fight and flight responses, individual aggressive defences (i.e. the number of attacking workers) were unrelated to parasite prevalence (χ2 = 0.97, Δd.f. = 1, p = 0.324). Parasite prevalence was not associated with the likelihood that the slavemaker escaped (χ2 = 0.08, Δd.f. = 1, p = 0.780).
(b). Species differences in defence portfolios
Changes in host defence strategies with parasite prevalence were consistent across the two hosts, indicated by the absence of interaction effects with species identity (all p > 0.05; see the electronic supplementary material for model selection results).
Nonetheless, the two host species resorted to different aggressive defence strategies (figure 3a,b), independent of parasite pressure. The collective defence of immobilizing the slavemaker with multiple host workers more often occurred in T. curvispinosus than in T. longispinosus colonies (est. ± s.e. = 0.810 ± 0.202, z = 4.01, p < 0.0001) and more workers were involved in slavemaker immobilization in T. curvispinosus (figure 3a; p < 0.0001). In contrast, T. longispinosus colonies primarily showed individual attack (figure 3b; p < 0.0001). Slavemaker escape probability decreased with the number of immobilizing host workers (est. ± s.e. = −0.46 ± 0.05, z = −9.64, p < 0.0001) but was unrelated to the number of attacking host workers (z = 0.01, p = 0.990). Hence, slavemakers were more likely to escape from T. longispinosus colonies (figure 3c; p < 0.0001). Host species did not differ in evacuation probability (χ2 = 0.05, Δd.f. = 1, p = 0.816).
Figure 3.
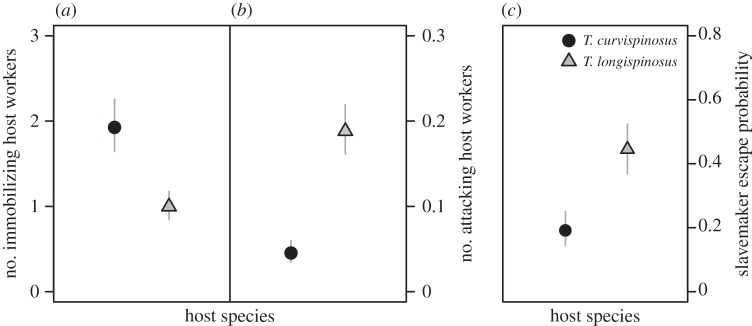
Differences in aggressive defence strategies and efficiency between host species. Symbols represent the GLMM estimates ± s.e. (a) z = −5.45, p < 0.0001; (b) z = 4.54, p < 0.0001; (c) z = 5.30, p < 0.0001.
(c). Defence strategies and colony size
Large host colonies evacuated less often (estimate ± s.e. = −0.074 ± 0.013, z = −5.60, p < 0.0001) and were more likely to show collective immobilization (0.052 ± 0.008, z = 6.21, p < 0.0001), in which more workers were involved (0.038 ± 0.004, z = 9.06, p < 0.0001). Moreover, large host colonies were less likely to let the slavemaker escape (−0.052 ± 0.009, z = −5.35, p < 0.0001).
In contrast, host colonies that co-occurred with large slavemaker colonies evacuated more often (figure 2d; p = 0.003) and were less likely to show collective immobilization (−0.324 ± 0.116, z = −2.80, p = 0.005), and fewer workers immobilized the slavemaker (figure 2b, p < 0.001). The number of workers showing individual attack was unrelated to colony sizes of the host (χ2 = 1.02, Δd.f. = 1, p = 0.312) or the slavemaker (z = 0.62, p = 0.538).
4. Discussion
We have demonstrated that host defence portfolios shift from collective fight to flight with social parasite pressure over large geographical ranges. Host populations in which the slavemaker is rare or absent more frequently show collective aggression, whereas highly parasitized populations are more likely to respond to an intruding slavemaker by evacuating their nest site. These changes in collective defence strategies were consistent across the two host species, despite clear interspecific differences in the geographical distribution of populations that occurred in sympatry with the slavemaker (figure 1). This finding renders it unlikely that environmental conditions govern the evolution of defence strategies in the hosts. Rather, convergence in the association between parasite pressure and defence strategies across species suggests universal patterns in host–parasite coevolution. We further found distinct differences in defence portfolios between the two hosts, pointing to a lower efficiency of averting parasitic exploitation by the preferred host T. longispinosus.
The strategy blocking hypothesis poses that as long as early lines of defence are effective, there is limited selection for subsequent costly defences due to diminishing returns. In accordance, we found that host populations that responded to an intruding slavemaker by collective immobilization were less likely to abandon their nest site. Indeed, flight may involve substantial costs as nest sites are known to be limiting for T. longispinosus [29]. Colonies that can successfully evade parasitation through collective slavemaker immobilization should thus not abandon their nest site, which is indeed what we found. Likewise, avian host species that are highly aggressive towards adult brood parasites are less likely to reject parasitic eggs, for instance through nest desertion [30,31] (but see [32,33]).
Interestingly, our study shows that collective aggression towards the parasite decreased with parasite prevalence. This contrasts with studies on other host–parasite systems which show that hosts facing high parasite pressure exhibit more aggressive defences [34–38]. Previous studies on Temnothorax ants further indicate that parasitized populations are not less aggressive per se. On the contrary, aggression during raiding attacks was more pronounced in host populations that were exposed to high parasite pressure [10]. In addition, colony aggression towards conspecific workers strongly increased with parasite prevalence [39]. Our finding that Temnothorax hosts do not always employ the aggressive potential revealed in different contexts would support that collective aggression is disadvantageous as a frontline defence against the social parasite under severe parasite pressure. Moreover, the fact that Temnothorax ants from parasitized populations can be highly aggressive in different situations [10,39] renders it unlikely that low aggression has led to high parasite pressure rather than the other way around. Nonetheless, we cannot rule out that variation in parasite pressure between host populations is the result of differences in host defence strategies and not its cause.
Despite its ubiquity, aggressive host defences are not universal and, in some circumstances, non-adaptive [7,40]. In avian hosts, the lack of aggression has often been attributed to small host body size which prohibits successful nest defence. Indeed, enemy attack can involve considerable costs and fights only escalate when the strength asymmetry between opponents is small [41]. Aggressive defences may thus be selected against if the chance of winning antagonistic encounters is limited. In our study, large host colonies were more likely to respond with collective immobilization than with nest evacuation when confronted with a slavemaker. We also found the highest collective aggression in colonies originating from populations where slavemaker colonies were typically small. The collective defence of immobilizing a slavemaker is probably disadvantageous when facing a large raiding party, as valuable time and workforce is lost on the retention of one out of multiple opponents, which cannot be used for brood or queen rescue. Thus, the inability to win a fight may render evacuation the only feasible option. Analogously, physical constraints to remove a potential threat to avian hosts of brood parasites may leave desertion and re-nesting as the only beneficial mode of defence [42–45].
Although the shift from fight to flight was consistent across host species, the two host species also showed distinct defence strategies towards an intruding slavemaker. Temnothorax longispinosus mainly responded by individual attack on the slavemaker, whereas T. curvispinosus more often showed collective defence by pinning the slavemaker down. Only the latter strategy reduced the likelihood that the slavemaker escaped and subsequent raiding risk. Brandt & Foitzik [24] demonstrated higher aggression in T. curvispinosus towards slavemakers, a higher fraction of slavemakers killed and more brood saved during raiding attacks. Such interspecific differences in host defence strategies have also been reported in hosts of brood parasites [30] and social parasitic hosts [46] and may reflect host preference by the parasite [46]. Although we cannot rule out that differences in defence strategies and efficiencies between our two host species resulted from the use of different slavemaker populations, it could provide a mechanistic explanation for P. americanus’ preference for its primary host, T. longispinosus [20,24].
In theory, evolutionary divergence in defence portfolios could arise in the absence of intrinsic differences between host species, provided they are at different stages in the coevolutionary arms-race with their parasite [2]. In practice, however, host species invariably differ in ecology, life-history and morphology, which may impose differential constraints on the evolution of specific host defences [47]. Such differences may greatly restrict formal tests of the predictions of the strategy blocking hypothesis using interspecific comparisons. As an alternative, we assessed the interplay between parasite pressure and host defence portfolios between multiple populations of the same host species. Such comparisons have proved highly valuable in the study of single-trait pair coevolutionary arms races [48,49], especially when they cover the entire geographical range of host species, including populations where the parasite is absent [50]. Nonetheless, intraspecific variation in host defence portfolios has rarely been studied [36], let alone across geographically distant populations.
In conclusion, we demonstrate that host defence portfolios shift consistently along a social parasite pressure gradient. Collective aggression, as a first line of defence against the slavemaker, is less frequently employed by host populations that are under severe parasite pressure. Instead, these populations resort to an alternative collective defence strategy in the form of nest evacuation. Degeneration in the first line of defence and the evolution of subsequent anti-parasite strategies has been invoked in a number of hosts of both brood and social parasites [3]. However, this study is the first to demonstrate consistent shifts in host defence portfolios along a social parasite pressure gradient.
Supplementary Material
Acknowledgements
We thank Joel Meunier and two anonymous reviewers for their comments on the manuscript.
Ant collection permits were obtained from parks/preserves or we asked private land owners for permission to collect ant colonies. Import and export licences are not required for the transport of our study species. We followed the guidelines of the Study of Animal Behaviour and the legal and institutional rules.
Funding statement
This study was funded by the Deutsche Forschungsgemeinschaft (Fo 298/9-1 and Fo 298/11-2) and the E.N. Huyck preserve, NY, USA.
References
- 1.Planqué R, Britton NF, Franks NR, Peletier MA. 2002. The adaptiveness of defence strategies against cuckoo parasitism. Bull. Math. Biol. 64, 1045–1068. ( 10.1006/bulm.2002.0311) [DOI] [PubMed] [Google Scholar]
- 2.Britton NF, Planqué R, Franks NR. 2007. Evolution of defence portfolios in exploiter-victim systems. Bull. Math. Biol. 69, 957–988. ( 10.1007/s11538-006-9178-5) [DOI] [PubMed] [Google Scholar]
- 3.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Phil. Soc. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 4.Svennungsen TO, Holen ØH. 2010. Avian brood parasitism: information use and variation in egg-rejection behavior. Evolution 64, 1459–1469. ( 10.1111/j.1558-5646.2009.00919.x) [DOI] [PubMed] [Google Scholar]
- 5.Langmore NE, Kilner RM. 2010. The coevolutionary arms race between Horsfield's Bronze-Cuckoos and Superb Fairy-wrens. Emu 110, 32–38. ( 10.1071/MU09032) [DOI] [Google Scholar]
- 6.Gilman RT, Nuismer SL, Jhwueng D-C. 2012. Coevolution in multidimensional trait space favours escape from parasites and pathogens. Nature 483, 328–330. ( 10.1038/nature10853) [DOI] [PubMed] [Google Scholar]
- 7.Feeney WE, Welbergen JA, Langmore NE. 2012. The frontline of avian brood parasite–host coevolution. Anim. Behav. 84, 3–12. ( 10.1016/j.anbehav.2012.04.011) [DOI] [Google Scholar]
- 8.Spottiswoode CN, Kilner RM, Davies NB. 2012. Brood parasitism. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M.), pp. 226–239. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Alloway TM. 1990. Slave-species ant colonies recognize slavemakers as enemies. Anim. Behav. 39, 1218–1220. ( 10.1016/S0003-3472(05)80797-3) [DOI] [Google Scholar]
- 10.Foitzik S, DeHeer CJ, Hunjan DN, Herbers JM. 2001. Coevolution in host–parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. Lond. B 268, 1139–1146. ( 10.1098/rspb.2001.1627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt M, Heinze J, Schmitt T, Foitzik S. 2005. A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J. Evol. Biol. 18, 576–586. ( 10.1111/j.1420-9101.2004.00867.x) [DOI] [PubMed] [Google Scholar]
- 12.Achenbach A, Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. ( 10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 13.Pamminger T, Scharf I, Pennings PS, Foitzik S. 2011. Increased host aggression as an induced defense against slave-making ants. Behav. Ecol. 22, 255–260. ( 10.1093/beheco/arq191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hölldobler B, Wilson EO. 1990. The ants. Berlin, Germany: Springer. [Google Scholar]
- 15.Hermann HR. 1984. Defensive mechanisms in social insects. New York, NY: Praeger. [Google Scholar]
- 16.Wilson EO, Regnier FE. 1971. The evolution of the alarm-defense system in the formicine ants. Am. Nat. 105, 279–289. ( 10.1086/282724) [DOI] [Google Scholar]
- 17.Beibl J, Stuart RJ, Heinze J, Foitzik S. 2005. Six origins of slavery in formicoxenine ants. Insectes Soc. 52, 291–297. ( 10.1007/s00040-005-0808-y) [DOI] [Google Scholar]
- 18.Alloway TM. 1979. Raiding behaviour of two species of slave-making ants Harpagoxenus americanus (Emery) and Leptothorax duloticus Wesson (Hymenoptera: Formicidae). Anim. Behav. 27, 202–210. ( 10.1016/0003-3472(79)90140-4) [DOI] [Google Scholar]
- 19.Foitzik S, Herbers JM. 2001. Colony structure of a slavemaking ant. II. Frequency of slave raids and impact on the host population. Evolution 55, 316–323. ( 10.1111/j.0014-3820.2001.tb01296.x) [DOI] [PubMed] [Google Scholar]
- 20.Blatrix R, Herbers JM. 2003. Coevolution between slave-making ants and their hosts: host specificity and geographical variation. Mol. Ecol. 12, 2809–2816. ( 10.1046/j.1365-294X.2003.01947.x) [DOI] [PubMed] [Google Scholar]
- 21.Wesson LG. 1939. Contributions to the natural history of Harpagoxenus americanus Emery (Hymenoptera : Formicidae). Trans. Am. Entomol. Soc. 65, 97–122. [Google Scholar]
- 22.Keller L. 1998. Queen lifespan and colony characteristics in ants and termites. Insectes Soc. 45, 235–246. ( 10.1007/s000400050084) [DOI] [Google Scholar]
- 23.Herbers JM, Foitzik S. 2002. The ecology of slavemaking ants and their hosts in north temperate forests. Ecology 83, 148–163. ( 10.1890/0012-9658(2002)083[0148:TEOSAA]2.0.CO;2) [DOI] [Google Scholar]
- 24.Brandt M, Foitzik S. 2004. Community context and specialization influence coevolution between a slavemaking ant and its hosts. Ecology 85, 2997–3009. ( 10.1890/03-0778) [DOI] [Google Scholar]
- 25.Foitzik S, Backus VL, Trindl A, Herbers JM. 2004. Ecology of Leptothorax ants: impact of food nest sites and social parasites. Behav. Ecol. Sociobiol. 55, 484–493. ( 10.1007/s00265-003-0718-9) [DOI] [Google Scholar]
- 26.Foitzik S, Achenbach A, Brandt M. 2009. Locally adapted social parasite affects density social structure and life history of its ant hosts. Ecology 90, 1195–1206. ( 10.1890/08-0520.1) [DOI] [PubMed] [Google Scholar]
- 27.Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. R package version 0.999999–0 (http://cran.r-project.org/package=lme4)
- 28.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Found. Stat. Comput; (http://www.r-project.org/) [Google Scholar]
- 29.Herbers JM. 1986. Nest site limitation and facultative polygyny in the ant Leptothorax longispinosus. Behav. Ecol. Sociobiol. 19, 115–122. ( 10.1007/BF00299946) [DOI] [Google Scholar]
- 30.Robertson RJ, Norman RF. 1976. Behavioral defences to brood parasitism by potential hosts of the Brown-headed Cowbird. Condor 78 166–173. ( 10.2307/1366851) [DOI] [Google Scholar]
- 31.Neudorf DL, Sealy SG. 1992. Reactions of four passerine species to threats of predation and Cowbird parasitism: enemy recognition or generalized responses? Behaviour 123 84–105. ( 10.1163/156853992X00138) [DOI] [Google Scholar]
- 32.Moksnes A, Røskaft E, Braa AT, Korsnes L, Lampe HM, Pedersen HC. 1991. Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour 116 64–89. ( 10.1163/156853990X00365) [DOI] [Google Scholar]
- 33.Røskaft E, Moksnes A, Stokke BG, Bicík V, Moskát C. 2002. Aggression to dummy cuckoos by potential European cuckoo hosts. Behaviour 139 613–628. ( 10.1163/15685390260136735) [DOI] [Google Scholar]
- 34.Briskie JV, Sealy SG, Hobson KA. 1992. Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 46 334–340. ( 10.2307/2409854) [DOI] [PubMed] [Google Scholar]
- 35.Hale K, Briskie JV. 2007. Response of introduced European birds in New Zealand to experimental brood parasitism. J. Avian Biol. 38, 198–204. ( 10.1111/j.2007.0908-8857.03734.x) [DOI] [Google Scholar]
- 36.Lindholm AK, Thomas RJ. 2000. Differences between populations of reed warblers in defences against brood parasitism. Behaviour 137, 25–42. ( 10.1163/156853900501854) [DOI] [Google Scholar]
- 37.Welbergen JA, Davies NB. 2009. Strategic variation in mobbing as a front line of defense against brood parasitism. Curr. Biol. 19, 235–240. ( 10.1016/j.cub.2008.12.041) [DOI] [PubMed] [Google Scholar]
- 38.Thorogood R, Davies NB. 2013. Reed warbler hosts fine-tune their defenses to track three decades of cuckoo decline. Evolution 67, 3545–3555. ( 10.1111/evo.12213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleeberg I, Jongepier E, Foitzik S. In preparation What shapes collective behavior? Colony aggression depends on context and parasite pressure. [Google Scholar]
- 40.Zamora-Munoz C, Ruano F, Errard C, Lenoir A, Hefetz A, Tinaut A. 2003. Coevolution in the slave-parasite system Proformica longiseta–Rossomyrmex minuchae (Hymenoptera: Formicidae). Sociobiology 42, 299–317. [Google Scholar]
- 41.Savolainen R, Vepsäläinen K. 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51, 135–155. ( 10.2307/3565636) [DOI] [Google Scholar]
- 42.Davies NB, Bourke AFG, Brooke MdeL. 1989. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278. ( 10.1016/0169-5347(89)90202-4) [DOI] [PubMed] [Google Scholar]
- 43.Moksnes A, Røskaft E, Braa AT. 1991. Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk 108, 348–354. [Google Scholar]
- 44.Hosoi S, Rothstein S. 2000. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim. Behav. 59, 823–840. ( 10.1006/anbe.1999.1370) [DOI] [PubMed] [Google Scholar]
- 45.Peer BD, Sealy SG. 2004. Correlates of egg rejection in hosts of the brown-headed cowbird. Condor 106, 580–599. ( 10.1650/7412) [DOI] [Google Scholar]
- 46.Mori A, Ettorre RD, Moli EL. 1995. Host nest usurpation and colony foundation in the European amazon ant Polyergus rufescens Latr. (Hymenoptera: Formicidae). Insectes Soc. 286, 279–286. [Google Scholar]
- 47.Servedio MR, Hauber ME. 2006. To eject or to abandon? Life history traits of hosts and parasites interact to influence the fitness payoffs of alternative anti-parasite strategies. J. Evol. Biol. 19, 1585–1594. ( 10.1111/j.1420-9101.2006.01124.x) [DOI] [PubMed] [Google Scholar]
- 48.Brodie ED, Ridenhour BJ, Brodie ED. 2002. The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56, 2067–2082. ( 10.1111/j.0014-3820.2002.tb00132.x) [DOI] [PubMed] [Google Scholar]
- 49.Thompson JN, Cunningham BM. 2002. Geographic structure and dynamics of coevolutionary selection. Nature 417, 735–738. ( 10.1038/nature00810) [DOI] [PubMed] [Google Scholar]
- 50.Gomulkiewicz R, Drown DM, Dybdahl MF, Godsoe W, Nuismer SL, Pepin KM, Ridenhour BJ, Smith CI, Yoder JB. 2007. Dos and don'ts of testing the geographic mosaic theory of coevolution. Heredity (Edinb.) 98, 249–258. ( 10.1038/sj.hdy.6800949) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.