Abstract
Consistent individual variation in animal behaviour is nearly ubiquitous and has important ecological and evolutionary implications. Additionally, suites of behavioural traits are often correlated, forming behavioural syndromes in both humans and other species. Such syndromes are often described by testing for variation in traits across commonly described dimensions (e.g. aggression and neophobia), independent of whether this variation is ecologically relevant to the focal species. Here, we use a variety of ecologically relevant behavioural traits to test for a colony-level behavioural syndrome in rock ants (Temnothorax rugatulus). Specifically, we combine field and laboratory assays to measure foraging effort, how colonies respond to different types of resources, activity level, response to threat and aggression level. We find evidence for a colony level syndrome that suggests colonies consistently differ in coping style—some are more risk-prone, whereas others are more risk-averse. Additionally, by collecting data across the North American range of this species, we show that environmental variation may affect how different populations maintain consistent variation in colony behaviour.
Keywords: personality, social insects, behavioural syndromes, risk-tolerance
1. Introduction
In 1932, the psychologist McDougall wrote ‘Personality may to advantage be broadly analysed into five distinguishable but separate factors, namely intellect, character, temperament, disposition, and temper’ [1, p. 15], implying that people consistently vary along five independent dimensions in their behaviour. Such reproducible variation across individuals in behaviour, called ‘personality’, is taken for granted in humans. But inter-individual behavioural variation is also well documented across many non-human animal taxa (e.g. gastropods [2], insects [3,4], fish [5], birds [6,7] and mammals [8,9]). While any stable variation across individuals within a species is considered evidence for ‘personality’ in animals, the number of studies that have tested for independent dimensions of personality has only recently begun to grow [10–12].
In the past decade, researchers have begun to focus both on understanding how behavioural traits are correlated in animals and what is driving variation [10,13]. If behavioural or personality traits are related, they are considered part of a behavioural syndrome. Behavioural syndromes, or correlated suites of behavioural traits within a population, have recently been described in many taxa including fish, birds, laboratory rodents, non-human primates and arthropods [14–18]. Behavioural syndromes are a property of the population [17–19]. For example, one individual may be more aggressive than another individual both when defending a food resource and also when guarding a mate. Such an aggressive phenotype would be referred to as the individual's ‘behavioural type’, and if individuals in a population differ consistently in behavioural type, they are considered to have ‘personalities’. Thus far, a relatively small range of behavioural traits has been examined across contexts: particularly boldness, aggressiveness and activity level [20]; we refer to these as ‘classic’ behavioural syndromes [19]. Considerably, less work has related these traits to the wide variety of other, sometimes species-specific, behaviours that animals may exhibit. This may be due both to a bias towards syndromes that appear relatively ubiquitous, as well as practical consideration for behavioural traits that are most easily observed or quantified. It is also less common for studies to examine how traits in these behavioural syndromes are related to one another, meaning if or how traits usually included in one classic behavioural syndrome are related to the traits in another (despite notable exceptions, e.g. Bourne and Sammons [21] found a correlation between boldness, exploration and aggression in male molly fish: Poecilia parae). This is very relevant, because the selection pressures that led to the evolution of a syndrome and variation in behavioural type are near impossible to understand if it is not known which behaviours are included in the syndrome or how many independent dimensions exist.
We suggest more emphasis should be placed on determining which larger behavioural domains interact. Determining such interactions allows for a better understanding of what drives personality. As Bell describes, behavioural correlations may be the result of internal constraints or of adaptation by natural selection [11]. Internal constraints may be particularly important when there is genetic heritability of behaviour. Even among closely related species, there is the possibility for significant variation in heritability of traits [22–24]. In such species that show a heritable component to personality, the genetic architecture of personality's independent dimensions needs to be taken into account when looking at how selection will affect the evolution of behavioural variation [25]. For example, behavioural traits on the same dimension may be constrained due to pleiotropy, and thus remain linked even in the absence of selection for a syndrome. On the other hand, correlations between behavioural traits may emerge as a result of phenotypic plasticity, for example if organisms develop under particular environmental conditions. Differences in genotype, heritability of traits and natural selection can, therefore, create either similarity or dissimilarity between populations. While personality and behavioural syndrome studies may define these dimensions, we still do not have a strong understanding of the mechanisms underlying behavioural correlations. However, before an investigation of mechanism can take place, we must first look at the overall architecture of which behaviours are correlated and how these correlations compare across populations.
Individual variation in social insects has also begun to be considered in a behavioural syndrome framework. Much of this research has focused on behavioural differences among individual workers, such as those associated with behavioural specialization (e.g. nurses versus foragers) and their physiological correlates. For example, some honeybees preferentially forage for pollen over nectar, and these preferences are associated with different sensory biases, locomotive activity and gene regulation [15,26–29]. Other species show different patterns of individual differences within colonies. In Temnothorax ants, individuals show different levels of activity, with some workers consistently less active than others [30]. Inter-individual differences within colonies may have ecologically relevant consequences; for example, the number of individuals with an aggressive behavioural type determines how well the group can defend its nest from social parasites [31,32] and affects the colony lifespan [33,34].
Colonies can also exhibit behavioural variation. For example, colonies have been shown to vary in their levels of aggression [35–37], hygienic behaviour [38,39] and foraging behaviour [40–43]. Colony behaviours may also be correlated across situations [19,44]. For example, in honeybees, colony responses to food and to disturbance may correlate and vary within populations [44,45]. Because the reproductive unit of social insects is the colony, colony-level variation may evolve in a manner similar to individual-level variation in solitary species and is likely to have ecological consequences. In social insects, differences between colonies have been seen in behaviours such as aggression, exploration, sociability and activity between dispersers and residents, though less is known about the timescale of consistency of these behaviours (reviewed in [46]).
Here, we examine the overall architecture of inter-colony behavioural variation in a social insect, how this variation is structured across different behavioural dimensions and across populations. We use a wide variety of behavioural assays, with a focus on ecologically relevant behaviours. Specifically, we use ants of the species Temnothorax rugatulus and measure foraging distance, foraging effort to familiar and novel resources, activity level and response to threat. We specifically test the following hypotheses: (i) colony behavioural responses are consistent over time; (ii) these behaviours are part of a colony-level behavioural syndrome, i.e. responses in different assays correlate with one another. Lastly, we test (iii) whether there is more than one orthogonal dimension, i.e. syndrome, of colony behaviour. This would imply that a colony's behavioural type with respect to one syndrome is independent of its behavioural type with respect to a second syndrome. Ultimately, we hope to determine whether there are truly independent dimensions of behaviour which will give us clues as to the function and mechanisms of this overall behavioural variation.
2. Material and methods
(a). Model system
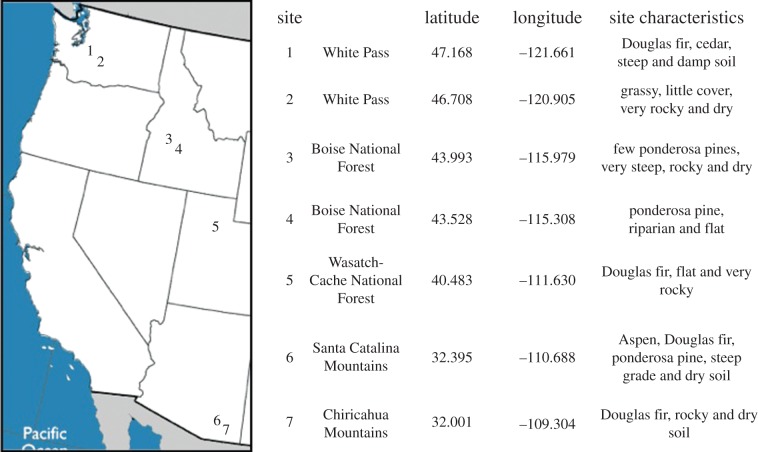
To address these questions, we used the myrmicine ant T. rugatulus. This species ranges from northern Mexico through the inter-mountain range of the western United States and north to southwest Canada. Colonies prefer cool temperatures and are often found in pine and juniper forests. Colonies range in size from usually 50 to 400 ants, with some very large colonies reaching 1300 ants [40]. Species in this genus can be seasonally polydomous (having multiple nests for a single colony), often living in small pre-formed crevices in rocks [47]. The small size of the colony and the locations of their nests allow for easy collection of the entire colony, including queen and brood, without need for excavation. This is important for live collection with minimal loss of workers and brood. Their tolerance for laboratory conditions allows for controlled empirical studies. This species has been successfully used in behavioural studies in many contexts, meaning well-established methods for empirical studies exist (e.g. emigrations [48], group decision-making [49] and starvation resistance [50]). In this study, all ants were collected from seven field sites ranging from northern Washington, USA, to southeastern Arizona, USA, during July 2012 (figure 1).
Figure 1.
A summary of the sites colonies were collected from, including GPS coordinates and qualitative features about each site. (Online version in colour.)
(b). Foraging distance and colony collection
Foraging is one of the most dangerous tasks for a colony to perform. Leaving the nest, foragers are exposed to predators, aggressive conspecifics and potential pathogens. Thus, travelling farther may increase exposure to these risks, and variation in foraging distance may suggest variation in tolerance for these threats. The foraging distance of each colony was established in the field as in Bengston & Dornhaus [40]. A 10 × 10 m grid, separated into one hundred 1 m2 plots, was established in the field with a dish of commercially canned cat food placed in the middle of each 1 m2 plot as bait. When a T. rugatulus forager was seen at any of the baits, the other dishes were removed. That forager was marked with florescent pink powder and followed until it returned to its colony, thus enabling discovery of the nest without disturbing it. The marked forager was collected live with an aspirator before re-entering the nest. The nest was then monitored and the subsequent 20 foragers who emerged from it were similarly marked with powder and followed (20 foragers in total measured for each colony). After the return of the 20th forager, the nest was opened and all workers, brood and queens were collected live.
After collection, the ants were maintained in the laboratory as described by Dornhaus et al. [51]. Each colony was established inside a standard artificial nest which was constructed of a cardboard nest chamber between two glass slides (nest chamber size: 2.75× 2 cm). These nests were kept inside a fluon-coated container (10 × 10 cm). Laboratory temperatures averaged 21°C, but varied between 20 and 23°C. Humidity was not controlled and averaged 40%. Laboratory lighting was on a 12 L : 12 D cycle. Colonies were maintained on a diet of freeze-killed and chopped cockroaches, freeze-killed fruit flies, freeze-killed spring tails, a 10% sucrose solution ad libitum and free access to water.
After colonies were established in the laboratory, each was identified to species. Colonies were also photographed and the number of adult workers, queens and brood items were counted. Colonies that were not T. rugatulus, or did not include brood or a queen in the census, were removed from the study.
(c). Familiar and novel resources
Temnothorax rugatulus ants are thought to primarily forage for small soil arthropods. However, foragers have been seen to exploit other resources such as a larger arthropod corpse or human food waste (SE Bengston 2012, personal observation). Both populations of soil arthropods and these larger food resources are probably very ephemeral and will be exploited by both conspecifics and other species. Therefore, the number of individuals exploiting both familiar and unfamiliar resources (foraging effort) may impact overall colony resource intake and thus reproduction.
Colonies were fed on a weekly basis in a small dish in their container. Over two weeks, 90 min after the standard diet (‘familiar’ food) was placed in the dish, the number of foragers in the dish who were processing food was recorded. To measure how colonies responded to a novel resource, over a different two-week interval, a novel food item was given instead of the standard diet during the normal feeding time. Again, the number of foragers in the dish was recorded 90 min after the food was introduced. After the forager number was measured, the novel food item was removed and the standard diet was returned. The novel food on week one was canned tuna fish. Week two was commercially canned applesauce.
(d). Activity level
Variation in activity plays an important ecological role in many species. For example, it can be the result of different metabolic investment, energy optimization strategies or response to stimuli [52]. The activity level of the colony, defined as the amount of movement in the colony in the absence of any manipulation, was measured in each colony. To do this, each colony was filmed at a random time between 12.00 and 16.00 for 5 min in standard laboratory conditions. Each video was then analysed using an algorithm which computes the motion of ants between two frames of video using optical flow. Optical flow is computed based on assumptions that objects remain the same colour between frames and move relatively small distances between frames. Motion between two frames is represented as a velocity at each pixel location, the optical flow field. The Horn–Schunck method, which adds a smoothness constraint to the preceding assumptions, further assumes that adjacent pixels usually move together. The optical flow measurements were provided by Hoan Nguyen and Min C. Shin (University of Carolina, Charlotte, NC, USA). Optical flow field was computed for every frame (by comparing to its subsequent frame) and the following measures were calculated: (i) magnitude, i.e. the average magnitude of optical flow for every individual pixels; (ii) percentage of moving pixels, the percentage of pixels that moved at least 2 pixels; (iii) magnitude of movement, the average magnitude of the pixels that moved. All videos were filmed at the same resolution and magnification. We used percentage of moving pixels as a measure of colony activity level, as it best captures the amount of movement within the colony between any two given frames. We assume that all movement is due to ants moving, so the percentage of pixels changed was corrected for differences in colony size by dividing the average change in pixels by the number of ants and thus creating an average movement per ant measure. This is a necessary correction as a very small amount of movement by ants in a large colony may create as much pixel movement as a lot of movement by fewer ants in a smaller colony. This was repeated 14 days later.
(e). Response to threat
In the laboratory, T. rugatulus colonies can and do displace colonies when competing for nest space [53]. Therefore, noting and responding to an intruder can both impact if and how they defend their brood items and the nest. To measure the colony-level response to a threat, we used a live conspecific ‘intruder’ from a colony collected at a population not represented in the tests. The intruder was marked with fluorescent powder to increase visibility (to the observer) and, using forceps, was placed approx. 1 cm into the nest without otherwise disturbing the colony. The colony was filmed for 5 min, and the video was analysed using the same algorithm as in the activity level test. This assay was done immediately after the previous (baseline) activity level measure was recorded and the response to threat was reported as the absolute change between the undisturbed activity level and the activity level after the introduction of the intruder.
(f). Aggression score
A change in activity level in response to threat can be informative about the magnitude but not the directionality of the change in behaviour, meaning an increase in activity may be indicative of either an aggressive (fighting) or a defensive (fleeing) response. This is an important distinction, as during the assay it was noted that fleeing colonies sometimes evacuated all brood and queens from the nest. In the field, these colonies may be easier to evict from nest sites by conspecific colonies. To consider this distinction, the videos of the intruder response were also analysed for the types of behaviours contributing to the change in activity levels. The video was divided into four quadrants to minimize bias towards behaviours more or less proximal to the intruder and five individuals from each quadrant were observed for 5 s every 30 s. These 20 individuals were chosen randomly at each 30 s interval. The behaviours were scored by category, with higher scores indicating more aggressive behaviours (electronic supplementary material, table S1). Individuals who remained inactive or appeared to be wandering without performing a definable task received a 0 score.
All of the assays, except for the measurement of foraging distance, were repeated 14 days after the first trial to measure consistency of these behaviours through time. Foraging distance in the field could not be repeated due to the necessary collection of the colonies at the end of the assay and no reliable way to re-release and track colonies in the field.
3. Results
(a). Inter-colony variation
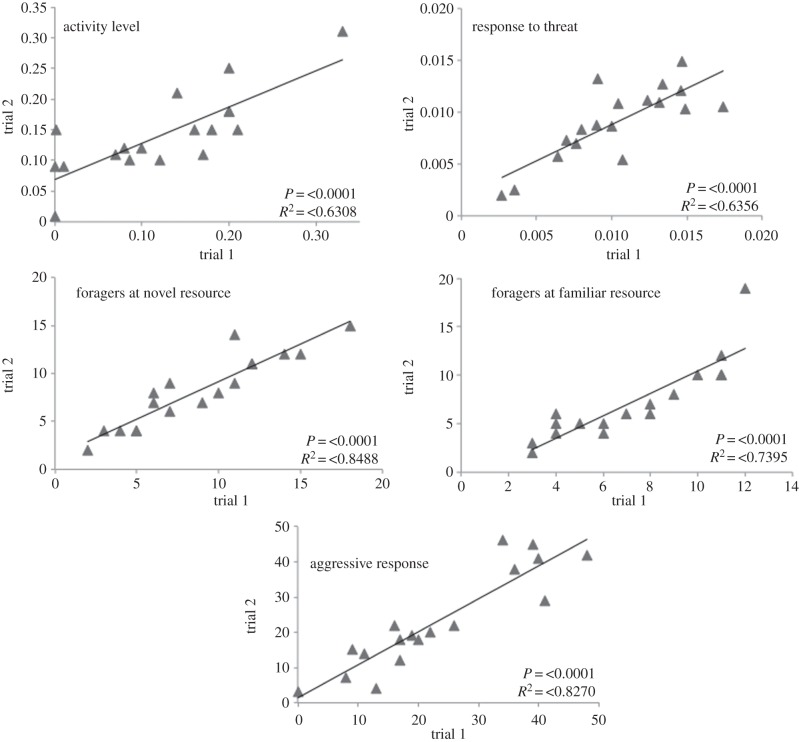
All behaviours (except for foraging distance, which could only be measured once in the field) showed a strong correlation between the first and second trials (figure 2). This suggests that colony behaviour is consistent across at least two weeks. Consistency of traits implies that a colony's behaviour at one time point more closely resembles its own behaviour earlier than that of other colonies, meaning that there is variation in behaviour between the colonies, demonstrating colony ‘personality’. Although the foraging distance assay could not be repeated, there was consistency between foragers within colony, meaning there was a significant difference between colonies (Kruskal–Wallis p < 0.0001). The foraging distances of colonies are summarized in figure 3.
Figure 2.
A summary of the two trials of each behavioural assay, trial 1 on the X-axis and trial 2 on the Y-axis. Activity (average % of pixels moved/0.3 s/worker), response to threat (change in average activity after intruder introduction), foragers at novel and familiar resources (no. of workers) and aggressive response (average aggression score). The correlation coefficient and R2 value are reported for the correlation between trial one and trial two, performed 21 days apart. A correlation between the two trials shows repeatability and thus inter-colony variation in behaviour.
Figure 3.

Variation in foraging distance between colonies in the field. Twenty foragers were measured for each colony; colonies varied significantly in their foraging distance (Kruskal–Wallis p < 0.0001). Box plots show the 25th and 75th percentiles; centre line: median; whiskers are 1.5 times interquartile range of the data; asterisks show data points beyond 1.5 times the interquartile range.
(b). Behavioural syndrome
Using Pearson's product-moment correlation test, we found positive pairwise correlations between ‘response to threat’ and ‘foraging at a familiar resource’, ‘response to threat’ and ‘foraging at a novel resource’ and foraging at both familiar and novel resources. We found negative correlations between foraging distance and ‘foraging at a novel resource’, foraging distance and ‘foraging at a familiar resource’, foraging distance and ‘response to threat’ and finally between ‘response to threat’ and ‘aggression’ (all p < 0.05, figure 4). Activity level did not show a significant correlation with any other behaviour. All p-values for pairwise correlations were corrected using the False Discovery Rate method [44].
Figure 4.
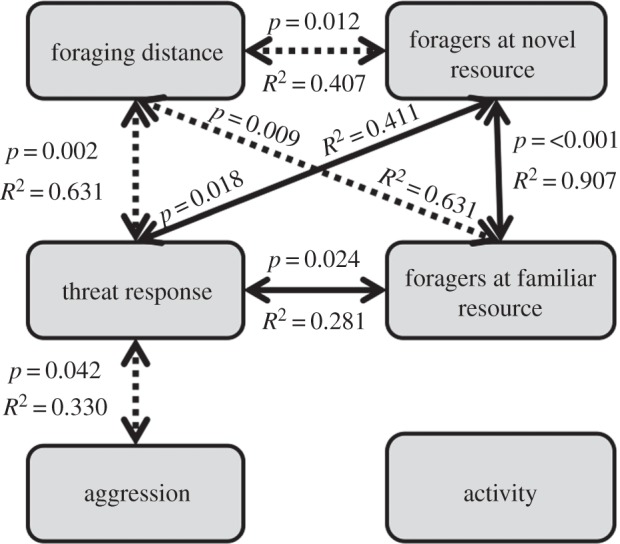
A summary of the pairwise correlations between the behavioural traits. Significant correlations are shown with arrows; dashed arrows indicate a negative correlation and solid lines indicate a positive correlation. P-values shown are false discovery rates corrected for multiple comparisons (see text). Activity did not significantly correlate with any other behaviour.
These relationships between traits seen in the pairwise correlations were confirmed by a principal component analysis (PCA). Data were transformed (so that it was on the same scale and no one variable was overly contributing) by dividing each score for every assay by the overall mean value for that assay. The first two principal components were used to define the two behavioural dimensions, as per the Kaiser–Guttman stopping rule where only components with an eigenvalue greater than the mean are accepted [54,55]. The first behavioural dimension (PC1) was primarily explained by ‘activity’, whereas the second dimension (PC2) was explained by a suite of correlated traits (behavioural syndrome; figure 5a). Because ‘activity’ was not correlated to the behavioural syndrome, we performed an additional PCA with the activity assay removed. In this analysis, PC1 was the only significant dimension (Kaiser–Guttman stopping rule). The percentage of variation explained by the components of each PCA is summarized in figure 5b. These results suggest that there are two independent behavioural dimensions: a behavioural syndrome consisting of several correlated behavioural traits, and a second driven by ‘activity’.
Figure 5.
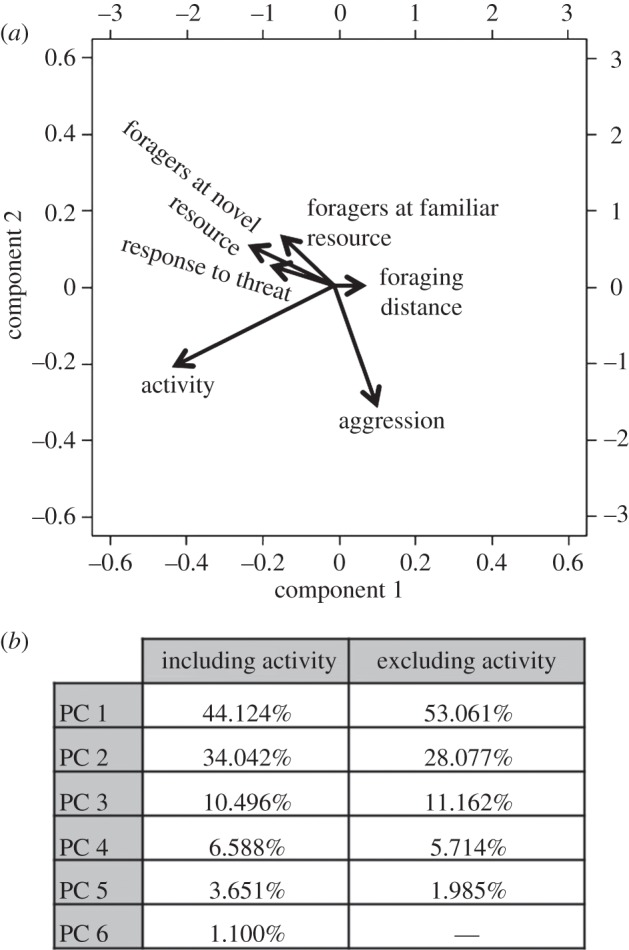
(a) PCA of all behaviours measured. Activity is almost perpendicular to other behaviours, which are all strongly correlated. (b) Per cent of variation explained by each axis (PC) in a PCA. When activity was included in the analysis, the first two components explained the majority of the variation. This is consistent with the two behavioural axes found; the behavioural syndrome and activity. When activity was removed from the analysis in a second PCA, the first component, which reflects the behavioural syndrome we focus on, explained over half of the variation.
To perform further analysis, we needed a quantitative way to describe the behavioural type (the expression of the behavioural syndrome) of each colony. To do this, we used PC1 from the PCA that excluded activity as a phenotype score for the foraging and defensive syndrome as it included all of the multivariate components of the behaviours within the syndrome. PC1 is calculated for individual colonies using the loadings of each trait as weights on the traits. A high score indicated that a colony showed an increased foraging effort and response to threat and a decreased level of aggression and shorter foraging distances. A low score indicated lower foraging effort, but with longer foraging distances and higher levels of aggression (figure 6).
Figure 6.
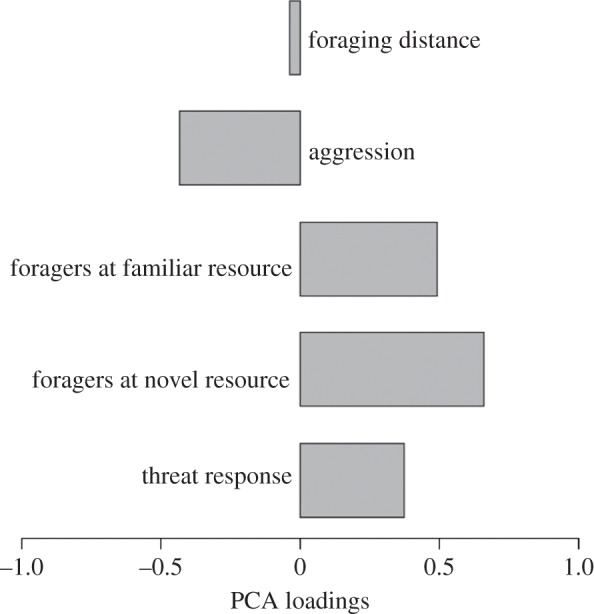
The loadings of variables for the first principal component in a PCA of all behavioural traits excluding activity. We used this principal component as a ‘phenotype score’ to test for effects of colony composition and collection site. Low scores indicate a higher level of aggression and increased foraging distances; high scores indicate increased foraging effort and response to threat.
(c). Explaining inter-colony variation
To determine what may be driving inter-colony variation, two separate generalized linear models (GLMs) were used to look at how activity and phenotype score are affected by latitude. Latitude may reflect environmental characteristics on a larger scale, such as overall weather patterns or day length, or it may reflect microclimatic variation between sites, such as soil moisture or forest type. Latitudes differed significantly in the phenotype scores of colonies found at them (Kruskal–Wallis p = 0.009, d.f. = 4; figure 7). Latitude had a significant negative effect on colony phenotype score (GLM Gaussian; latitude: p = 0.0262, d.f. = 14), meaning colonies with a low score (lower foraging effort, longer foraging distances and higher levels of aggression) were found at more northern latitudes. Additional colony attributes including number of queens, colony size and number of brood showed no significant effect on phenotype score (all p > 0.05, d.f. = 14).
Figure 7.
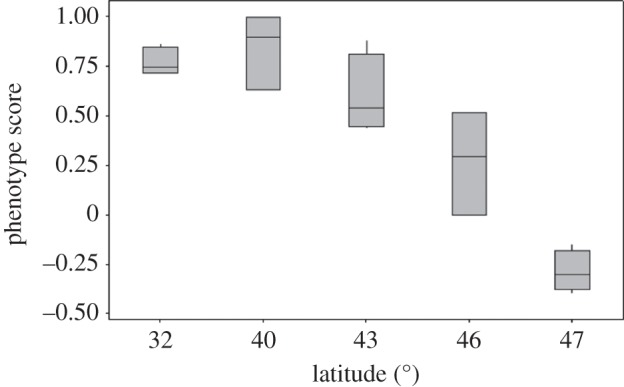
Colonies were collected across the North American range of the species. Latitude significantly affected phenotype score (Kruskal–Wallis p = 0.009, d.f. = 4) and correlated negatively with it (see text).
Activity was not affected by latitude (GLM Gaussian; latitude: p = 0.2087, d.f. = 14). Neither was activity affected by the number of queens in a colony, colony size or number of brood (all p > 0.05, d.f. = 14); however, as the brood to worker ratio increased, there was a marginally significant positive effect on activity (number of brood : number of workers p = 0.048).
4. Discussion
In this study, we have shown that colonies of the ant T. rugatulus show consistent behavioural types, i.e. ‘personalities’: colonies differ repeatably in their behaviour. We also show a colony-level behavioural syndrome: colonies with high foraging effort at resources (both known and novel) also show increased activity in response to threat, lower levels of aggressive behaviour and shorter foraging distances in the field. The behavioural type of colonies with regard to this syndrome correlates with the latitude of the site where they were collected. Colonies also differ in another dimension, activity level, and this trait is independent of the syndrome, and also independent of latitude. This means that there are at least two independent behavioural dimensions in the species studied here.
In general, colony-level behavioural differences and correlations of behaviours have been described in a variety of social insects [19,30]. Our study measures behaviours that are known to be relevant to the ecology of this species [40] and includes more traits, and thus more potential behavioural dimensions, than are included in many behavioural syndrome studies. However, the behavioural syndrome demonstrated here includes behaviours often considered part of a bold/shy syndrome, an exploratory syndrome and an aggressive syndrome, suggesting that in this ant species these are not independent dimensions, as often described in other species [56,57], but instead are interrelated. Activity, on the other hand, is not related to this behavioural syndrome. This deviates from some other studies, which find a relationship between it and other classic behavioural syndrome traits such as aggression (e.g. crickets (Gryllus integer) [58] and crayfish (Pacifastacus leniusculus) [59]).
The behavioural syndrome found here suggests that colonies might show different levels of risk-tolerance: some colonies deploy more foragers to exploit closer resources and increase their defensive activity in the response to threat, but avoid travelling farther distances or aggressively engaging conspecific invaders. This might be considered a risk-averse behavioural type. Other colonies travel farther and respond more aggressively when confronted with a conspecific, but appear to invest less (in terms of number of ants reacting) in each given incident or food source. This might be considered a more risk-prone behavioural type, i.e. colonies may tolerate more danger in favour of potentially reaping higher rewards. Risk-averse or risk-prone behaviours are often linked to different life-history strategies [16,60]. For example, in the continuum of r- versus K-type life-history strategies, r-individuals are more risk-prone, whereas K-individuals are more risk-averse. This is probably due to environmental stability; r-type individuals may be best suited to unpredictable conditions in which they need to be bolder in order to exploit a new resource and allocate more energy towards reproduction early, for example. Conversely, K-type individuals are expected to be found in more stable conditions, where less risk-taking is advantageous and long-term self-maintenance is beneficial [61–63]. Thus, the behavioural type of an individual should always be matched to the life-history strategy of the individual, while variation in life-history strategy may be dependent upon external, environmental conditions such as mortality risk, competition and environmental predictability. In our study, the collection sites probably differed in the length of the reproductive season experienced by ants: sites 1 and 2 often see snow into late May, while those in sites 3, 4 and 5 usually see snowmelt in April (figure 1). Sites 6 and 7 may not maintain a consistent snowpack overwinter (as based on data from the past 5 years from the National Weather Service). Therefore, colonies at higher latitudes may take greater risks to acquire the necessary resources fast enough to produce reproductive alate queens and males. However, further research is necessary to determine what mechanisms, either adaptively or as a constraint, are shaping behavioural-type variation across a latitudinal gradient.
In research on intra-species behavioural variation (‘personalities’) and clusters of correlated behavioural traits (‘behavioural syndromes’), often neither the evolutionary forces maintaining the variation nor those maintaining the correlation are known [16,57]. In our study, including several different behavioural measures relevant to the ecology of our study species helped discover a broad behavioural syndrome that was not restricted to the ‘classic’ traits [19]. The correlated traits all seem linked to risk-taking behaviour and thus may have consequences for colony life history, though more research is needed, such as information on growth rates, and reproductive investment. Intra-species variability in life-history strategies, and the ecological and evolutionary forces maintaining both diversity of such strategies as well as their consistency across contexts, are well studied [63–65]. Therefore, if behavioural syndromes are often linked to such life-history strategies, and perhaps emerge as a result of variation in life-history strategy, that would answer many of the outstanding questions about them [16,60]. In our study system, it seems indeed likely that the differing colony behavioural types reflect high-risk, fast growth versus low-risk, slow growth life-history strategies.
Besides testing multiple ecologically relevant behaviours, a second goal of our study was to broadly measure which behaviours were linked and which were independent. We found that general activity level (movement inside the nest) differed strongly and consistently between colonies, but was not correlated with any other behaviours or the overall colony behavioural type in the risk-tolerance syndrome. Activity level may of course be linked to other behavioural or physiological traits not measured in this study, such as metabolic rate. It is important to note that ‘activity level’ has been used to describe several different behavioural measures, such as the distance travelled to forage, the number of foragers outside at a given time or, as in this study, the in-nest movement of ants. However, we show here that neither foraging distance nor number of active foragers can be used to predict the level of in-nest activity; these should therefore be considered unrelated traits. Foraging distance is probably a better reflection of overall foraging effort, recruiting effort, diet preference, resource distribution or search patterns; in-nest movement may reflect physiological state, task specialization or other traits. It is thus important to consider how traits may play different roles in a species' ecology, as opposed to only focusing on a generalized terminology. Considering the ecology of a species can guide us towards otherwise missed components.
To conclude, we have sampled enough behaviours to show that T. rugatulus colonies have at least two independent behavioural dimensions, one of which makes up a behavioural syndrome and the other being dominated by activity level. The behavioural syndrome that colonies show may reflect differences among colonies in risk-tolerance, and thus in life-history strategy. This syndrome is likely to be driven by different environmental conditions, possibly the length of the reproductive season, though further research is necessary. The existence of a behavioural syndrome at the colony level in ants opens up a new line of investigation for those interested in the evolution of linked behavioural traits: it represents an independent origin of such processes at a different level of biological organization. If ant colonies evolve ‘personalities’ and behavioural syndromes in connection with their life-history evolution just as solitary animals do, this would be strong evidence for the universality of this process.
Supplementary Material
Acknowledgements
We would like to thank Min C. Shin and Hoan Nguyen for the collaboration with the optical flow algorithm, the Dornhaus laboratory members, Stephen Pratt, Jennifer Jandt and an anonymous reviewer for helpful feedback and Chantal Binder and Kevin Harrington for their assistance in the field and laboratory.
Data accessibility
Dryad; doi:10.5061/dryad.p2qt8.
Funding statement
We also thank the NSF for funding (grants no. IOS-1045239 and IOS-0841756).
References
- 1.McDougall W. 1932. Of the words character and personality. J. Pers. 1, 3–16. ( 10.1111/j.1467-6494.1932.tb02209.x) [DOI] [Google Scholar]
- 2.Burrows MT, Hughes RN. 1991. Optimal foraging decisions by dogwhelks, Nucella lapillus: influences of mortality risk and rate-constrained digestion. Funct. Ecol. 5, 461–475. ( 10.2307/2389628) [DOI] [Google Scholar]
- 3.Müller U. 1996. Inhibition of nitric oxide synthase impairs a distinct form of long-term memory in the honeybee, Apis mellifera. Neuron 16, 541–549. ( 10.1016/S0896-6273(00)80073-2) [DOI] [PubMed] [Google Scholar]
- 4.Papaj DR, Rausher MD. 1983. Individual variation in host location by phytophagous insects. In Herbivorous insects: host-seeking behavior and mechanisms (ed. Ahmad S.), pp. 77–124. New York, NY: Academic Press. [Google Scholar]
- 5.Kohda M. 1994. Individual specialized foraging repertoires in the piscivorous cichlid fish, Lepidiolamprologus profundicola. Anim. Behav. 48, 1123–1131. ( 10.1006/anbe.1994.1345) [DOI] [Google Scholar]
- 6.Sutherland WJ, Ens BJ. 1987. The criteria determining the selection of mussels Mytilus edulis by oystercatchers Haematopus ostralegus. Behaviour 103, 187–202. ( 10.1163/156853987X00341) [DOI] [Google Scholar]
- 7.Golet GH, Kuletz KJ, Roby DD, Irons DB. 2000. Adult prey choice affects chick growth and reproductive success in pigeon guillemots. Auk 117, 82–91. ( 10.1642/0004-8038(2000)117[0082:APCACG]2.0.CO;2) [DOI] [Google Scholar]
- 8.Hoelzel AR, Dorsey EM, Stern SJ. 1989. The foraging specializations of individual minke whales. Anim. Behav. 38, 786–794. ( 10.1016/S0003-3472(89)80111-3) [DOI] [Google Scholar]
- 9.Mattson DJ, Reinhart DP. 1995. Influences of cutthroat trout (Oncorhynchus clarki) on behaviour and reproduction of Yellowstone grizzly bears (Ursus arctos), 1975–1989. Can. J. Zool. 73, 2072–2079. ( 10.1139/z95-244) [DOI] [Google Scholar]
- 10.King JE, Figueredo AJ. 1997. The five-factor model plus dominance in chimpanzee personality. J. Res. Pers. 31, 257–271. ( 10.1006/jrpe.1997.2179) [DOI] [Google Scholar]
- 11.Bell AM. 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 18, 464–473. ( 10.1111/j.1420-9101.2004.00817.x) [DOI] [PubMed] [Google Scholar]
- 12.Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 76, 1128–1138. ( 10.1111/j.1365-2656.2007.01284.x) [DOI] [PubMed] [Google Scholar]
- 13.Endler JA. 1995. Multiple-trait coevolution and environmental gradients in guppies. Trends Ecol. Evol. 10, 22–29. ( 10.1016/S0169-5347(00)88956-9) [DOI] [PubMed] [Google Scholar]
- 14.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page RE, Fondrk MK, Rüeppell O. 2012. Complex pleiotropy characterizes the pollen hoarding syndrome in honey bees (Apis mellifera L.). Behav. Ecol. Sociobiol. 66, 1459–1466. ( 10.1007/s00265-012-1400-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 17.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 18.Sih A, Bell A, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 19.Jandt JM, Bengston SE, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, Sih A. 2013. Behavioural syndromes and social insects: personality at multiple levels. Biol. Rev. 89, 48–67. ( 10.1111/brv.12042) [DOI] [PubMed] [Google Scholar]
- 20.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 21.Bourne GR, Sammons AJ. 2008. Boldness, aggression and exploration: evidence for a behavioural syndrome in male pentamorphic livebearing fish, Poecilia parae. AACL Bioflux 1, 39–49. [Google Scholar]
- 22.McCrae RR, Costa PT. 1997. Personality trait structure as a human universal. Am. Psychol. 52, 509–516. ( 10.1037/0003-066X.52.5.509) [DOI] [PubMed] [Google Scholar]
- 23.Bouchard TJ, Jr, Loehlin JC. 2001. Genes, evolution, and personality. Behav. Genet. 31, 243–273. ( 10.1023/A:1012294324713) [DOI] [PubMed] [Google Scholar]
- 24.Weiss A, King JE, Figueredo AJ. 2000. The heritability of personality factors in chimpanzees (Pan troglodytes). Behav. Genet. 30, 213–221. ( 10.1023/A:1001966224914) [DOI] [PubMed] [Google Scholar]
- 25.Gosling SD, John OP. 1999. Personality dimensions in nonhuman animals a cross-species review. Curr. Dir. Psychol. Sci. 8, 69–75. ( 10.1111/1467-8721.00017) [DOI] [Google Scholar]
- 26.Page RE, Waddington KD, Hunt GJ, Fondrk KM. 1995. Genetic determinants of honey bee foraging behaviour. Anim. Behav. 50, 1617–1625. ( 10.1016/0003-3472(95)80015-8) [DOI] [Google Scholar]
- 27.Linksvayer TA. 2006. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution 60, 2552–2561. ( 10.1111/j.0014-3820.2006.tb01889.x) [DOI] [PubMed] [Google Scholar]
- 28.Rüeppell O, Pankiw T, Nielsen DI, Fondrk MK, Beye M, Page RE. 2004. The genetic architecture of the behavioral ontogeny of foraging in honeybee workers. Genetics 167, 1767–1779. ( 10.1534/genetics.103.021949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rüeppell SC, Pankiw T, Fondrk M, Beye M, Hunt G, Page R. 2005. The genetic architecture of sucrose responsiveness in the honey bee (Apis mellifera L.). Genetics 172, 243–251. ( 10.1534/genetics.105.046490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charbonneau D, Dornhaus A. Submitted Workers ‘specialized’ on inactivity: behavioural consistency of inactive workers and their role in task allocation. [Google Scholar]
- 31.Pamminger T, Modlmeier AP, Suette S, Pennings PS, Foitzik S. 2012. Raiders from the sky: slavemaker founding queens select for aggressive host colonies. Biol. Lett. 8, 748–750. ( 10.1098/rsbl.2012.0499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf I, Modlmeier AP, Fries S, Tirard C, Fotzik S. 2012. Characterizing the collective personality of ant societies: aggressive colonies do not abandon their home. PLoS ONE 7, e33314 ( 10.1371/journal.pone.0033314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruitt JN. 2012. Behavioral traits of colony founders affect the life history of their colony. Ecol. Lett. 15, 1026–1032. ( 10.1111/j.1461-0248.2012.01825.x) [DOI] [PubMed] [Google Scholar]
- 34.Pruitt JN. 2013. A real-time eco-evolutionary dead-end strategy is mediated by the traits of lineage progenitors and interactions with colony invaders. Ecol. Lett. 16, 879–886. ( 10.1111/ele.12123) [DOI] [PubMed] [Google Scholar]
- 35.Crosland MWJ. 1990. Variation in ant aggression and kin discrimination ability within and between colonies. J. Insect Behav. 3, 359–379. ( 10.1007/BF01052114) [DOI] [Google Scholar]
- 36.Suarez AV, Holway DA, Liang DS, Tsutsui ND, Case TJ. 2002. Spatiotemporal patterns of intraspecific aggression in the invasive Argentine ant. Anim. Behav. 64, 697–708. ( 10.1006/anbe.2002.4011) [DOI] [Google Scholar]
- 37.Pearce AN, Huang ZY, Breed MD. 2001. Juvenile hormone and aggression in honey bees. J. Insect Physiol. 47, 1243–1247. ( 10.1016/S0022-1910(01)00109-3) [DOI] [PubMed] [Google Scholar]
- 38.Paleolog J. 2009. Behavioural characteristics of honey bee (Apis mellifera) colonies containing mix of workers of divergent behavioural traits. Anim. Sci. Papers Rep. 27, 237–248. [Google Scholar]
- 39.Wray MK, Mattila HR, Seeley TD. 2011. Collective personalities in honeybee colonies are linked to colony fitness. Anim. Behav. 81, 559–568. ( 10.1016/j.anbehav.2010.11.027) [DOI] [Google Scholar]
- 40.Bengston SE, Dornhaus A. 2013. Colony size does not predict foraging distance in the ant Temnothorax rugatulus: a puzzle for standard scaling models. Insectes Sociaux 60, 93–96. ( 10.1007/s00040-012-0272-4) [DOI] [Google Scholar]
- 41.Pinter-Wollman N. 2012. Personality in social insects: how does worker personality determine colony personality? Curr. Zool. 58, 580–588. [Google Scholar]
- 42.Ings TC, Raine NE, Chittka L. 2009. A population comparison of the strength and persistence of innate colour preference and learning speed in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 63, 1207–1218. ( 10.1007/s00265-009-0731-8) [DOI] [Google Scholar]
- 43.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman BB, Thain H, Coughlin J, Hughes WOH. 2011. Behavioural syndromes at multiple scales in Myrmica ants. Anim. Behav. 82, 391–397. ( 10.1016/j.anbehav.2011.05.019) [DOI] [Google Scholar]
- 45.Giray T, Guzman-Novoa E, Aron CW, Zelinskey B, Fahrback SE, Robinson GE. 2000. Genetic variation in worker temporal polyethism and colony defensiveness in the honey bee, Apis mellifera. Behav. Ecol. 11, 44–55. ( 10.1093/beheco/11.1.44) [DOI] [Google Scholar]
- 46.Cote J, Clobert J, Brodin T, Fogarty S, Sih A. 2010. Personality dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065–4076. ( 10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partridge LW, Partridge KA, Franks NR. 1997. Field survey of a monogynous leptothoracine ant (Hymenoptera, Formicidae) evidence of seasonal polydomy? Insectes Sociaux 44, 75–83. ( 10.1007/s000400050031) [DOI] [Google Scholar]
- 48.Cao TT, Dornhaus A. 2012. Ants use pheromone markings in emigrations to move closer to food-rich areas. Insectes Sociaux 59, 87–92. ( 10.1007/s00040-011-0192-8) [DOI] [Google Scholar]
- 49.Sasaki T, Pratt SC. 2011. Emergence of group rationality from irrational individuals. Behav. Ecol. 22, 276–281. ( 10.1093/beheco/arq198) [DOI] [Google Scholar]
- 50.Rüeppell O, Kirkman RW. 2005. Extraordinary starvation resistance in Temnothorax rugatulus (Hymenoptera, Formicidae) colonies: demography and adaptive behavior. Insectes Sociaux 52, 282–290. ( 10.1007/s00040-005-0804-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dornhaus A, Holley J-A, Franks N. 2009. Larger colonies do not have more specialized workers in the ant Temnothorax albipennis. Behav. Ecol. 20, 922–929. ( 10.1093/beheco/arp070) [DOI] [Google Scholar]
- 52.Careau V, Thomas D, Humphries MM, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 53.Franks NR, Dornhaus A, Hitchcock G, Guillem R, Hooper J, Webb C. 2007. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 73, 525–534. ( 10.1016/j.anbehav.2006.05.020) [DOI] [Google Scholar]
- 54.Guttman L. 1954. Some necessary conditions for common-factor analysis. Psychometrika 19, 149–161. ( 10.1007/BF02289162) [DOI] [Google Scholar]
- 55.Jackson DA. 1993. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74, 2204–2214. ( 10.2307/1939574) [DOI] [Google Scholar]
- 56.Wilson AD, Godin JGJ. 2009. Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav. Ecol. 20, 231–237. ( 10.1093/beheco/arp018) [DOI] [Google Scholar]
- 57.Sih A, Bell AM. 2008. Insights for behavioral ecology from behavioral syndromes. Adv. Study Behav. 38, 227–281. ( 10.1016/S0065-3454(08)00005-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kortet R, Hedrick ANN. 2007. A behavioural syndrome in the field cricket Gryllus integer: intersexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 91, 475–482. ( 10.1111/j.1095-8312.2007.00812.x) [DOI] [Google Scholar]
- 59.Pinto LM, Sih A, Bauer ML. 2008. Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117, 1629–1636. ( 10.1111/j.1600-0706.2008.16578.x) [DOI] [Google Scholar]
- 60.Stamps JA. 2007. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363. ( 10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 61.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 62.Pianka ER. 1970. On r and K selection. Am. Nat. 104, 592–597. ( 10.1086/282697) [DOI] [Google Scholar]
- 63.Krebs JR, Davies NB. 1984. Behavioural ecology: an evolutionary approach. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 64.Stearns SC. 1992. The evolution of life histories, vol. 249 Oxford, UK: Oxford University Press. [Google Scholar]
- 65.Figueredo AJ, Vásquez G, Brumbach BH, Sefcek JA, Kirsner BR, Jacobs WJ. 2005. The K-factor: individual differences in life history strategy. Pers. Individual Differ. 39, 1349–1360. ( 10.1016/j.paid.2005.06.009) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dryad; doi:10.5061/dryad.p2qt8.