Abstract
Classic theories of ageing consider extrinsic mortality (EM) a major factor in shaping longevity and ageing, yet most studies of functional ageing focus on species with low EM. This bias may cause overestimation of the influence of senescent declines in performance over condition-dependent mortality on demographic processes across taxa. To simultaneously investigate the roles of functional senescence (FS) and intrinsic, extrinsic and condition-dependent mortality in a species with a high predation risk in nature, we compared age trajectories of body mass (BM) in wild and captive grey mouse lemurs (Microcebus murinus) using longitudinal data (853 individuals followed through adulthood). We found evidence of non-random mortality in both settings. In captivity, the oldest animals showed senescence in their ability to regain lost BM, whereas no evidence of FS was found in the wild. Overall, captive animals lived longer, but a reversed sex bias in lifespan was observed between wild and captive populations. We suggest that even moderately condition-dependent EM may lead to negligible FS in the wild. While high EM may act to reduce the average lifespan, this evolutionary process may be counteracted by the increased fitness of the long-lived, high-quality individuals.
Keywords: functional senescence, body mass, condition-dependent mortality, life-history evolution, lifespan, sex difference
1. Introduction
Actuarial senescence (AS, increasing risk of mortality with advancing age) is a well-defined demographic process in the vast majority of species [1–4]. The increase in mortality probably results from functional senescence (FS, within-individual deterioration of physical or physiological functioning with advancing age), which, along with terminal disease or investment in reproduction at the expense of maintenance [5–8], can expose individuals to extrinsic hazards in a condition-dependent manner. Therefore, only high-quality individuals may survive to an age where FS takes effect, making ageing difficult to observe in cross-sectional studies of natural populations [9–13].
Classic theories on life-history evolution [14] posit that populations with high extrinsic mortality (EM) rates (random mortality from environmental causes) should have a reduced lifespan and age rapidly, and support for this pattern has been found with experimental and comparative work [3,15–18]. In spite of the supposed significance of extrinsic factors in shaping life histories, ageing research is still largely biased towards captive animals living under standard, benign conditions (e.g. [16,19,20]). In the wild, studies of FS have largely focused on long-lived, large-bodied animals that face relatively low levels of environmental hazard (ungulates [10,21], sea-birds [22], seals [23] and primates [6,24]). Because FS is more detectable when EM is low, this taxonomic bias may lead to an overestimation of the prevalence of FS compared with the influences of selective disappearance in natural populations across species. Hence, the study of wild populations with high EM risk is essential for testing hypotheses on the evolution of lifespan and FS. To assess how declining individual performance versus earlier mortality of low-quality individuals shape demographic processes and hence selection pressures, it is necessary to simultaneously estimate functional declines and selective disappearance in a population. So far, few studies have taken this approach [10,12,13], and thus the relative importance of these processes within populations and across taxa is largely unresolved.
The sexes often differ in their life histories, EM hazard and ageing processes [24–27], and female mammals typically enjoy longer lifespans than males [28]. Sex-specific life-history optimization and potential sexually antagonistic selection have been proposed as evolutionary mechanisms for the maintenance of life-history variation within a species [29]. Therefore, a direct comparison of the sexes is essential for deciphering the evolutionary mechanisms behind senescence and lifespan determination.
In this study, we simultaneously assess the influences of intrinsic ‘background’ mortality, extrinsic hazard and non-random mortality of lower quality individuals in creating the observed patterns of FS and lifespan in a species that experiences high EM under natural conditions [30]. We employ long-term body mass (BM) data from one captive and two wild populations (10–18 years covering 7–16 cohorts) of the grey mouse lemur (Microcebus murinus) to characterize variation in body condition. In mammals, including M. murinus [31], body growth typically ceases around the age of sexual maturity [32] (six to eight months in M. murinus [31,33]). Thereafter, BM fluctuates in response to imbalances in energy acquisition and expenditure, cyclic seasonal changes [21,34,35] or senescent muscle loss [36]. BM broadly reflects resources available for allocation to physiological processes, making it a meaningful indicator of FS. We assess the influence of the environment on the rate of FS in M. murinus by comparing patterns of BM decline under captive and wild conditions. Under the benign, captive conditions, any decline should be mainly owing to intrinsic rather than extrinsic causes. In wild animals, these intrinsic processes interact with the environmental hazards [2,37,38] that probably remove low-quality individuals rapidly from the population. This in combination with the modelling of condition-dependent survival permits an assessment of the relative importance of FS and selective disappearance in the species. While rates of AS have previously been found to be lowered in captivity compared with natural populations [18,39,40], this is, to our knowledge, the first study in which data on captive and wild populations spanning several generations have been juxtaposed for testing fundamental hypotheses of ageing theory with respect to FS.
Given the high EM of M. murinus in the natural environment, we predicted average lifespan to be shorter in the wild than in captivity. If individuals are mainly removed from the population via random processes regardless of their condition, earlier or more dramatic FS might be expected in the wild [14,28]. If, however, extrinsic hazard selectively removes individuals in poor condition, evolutionary processes might instead lead to delayed FS or the survival of only the highest quality individuals (showing little senescent decline) to an old age. Assuming a weaker influence of condition on mortality in the predator and pathogen-protected captive colony, FS should in this case be more pronounced there. Consequently, aged animals in the wild should maintain a relatively high BM compared with captivity.
Condition-dependence of mortality is difficult to measure, particularly under natural conditions where the cause and exact timing of death are often unknown and fine-scale condition data difficult to obtain. Non-random mortality may act at different timescales: lifespan and body condition may be associated throughout life, or condition may decline shortly preceding death. While not perfect proof of condition-dependent EM (e.g. terminal declines in condition may indicate intrinsic causes, such as terminal illness), we expect selective disappearance to be indicated by prolonged survival of individuals in good body condition throughout life and by a terminal decline in BM. These intrinsic declines would probably render the individual more vulnerable to EM. As the same physiological processes presumably drive FS, senescent within-individual declines might nevertheless occur in both the wild and captivity. However, in presence of condition-dependent mortality, these declines should be relatively minor in the wild compared with captivity. In the wild, the coping of aged individuals may be especially compromised in the ecologically more demanding dry season when food and water availability decline considerably [34], which might lead to more severe BM senescence (i.e. age-related declines in BM) in this season.
In the highly promiscuous mating system of M. murinus [41–43], female reproductive skew is negligible [44] and female lifetime fitness increases with lifespan. Longevity is slightly male-biased in our captive study population [19,45] but strongly female-biased in the wild [30]. Roaming by males during the mating season [41] coincides with increased male mortality and, along with an age-associated increase in risk taking by males [46], probably drives the overall sex bias in longevity in the wild [30]. Should this also induce male-biased selective disappearance, wild males that survive to old age may be of exceptionally high quality, whereas the condition of long-lived captive males might be relatively low. Faster ageing rates are predicted for the sex that experiences higher adult mortality [28], hence, we expected male BM to decline faster than that of females, especially in the wild.
2. Material and methods
(a). Study species
The grey mouse lemur is a small, sexually monomorphic primate that is emerging as a model species for ageing [19]. For individuals that survive to adulthood (here, to age 12 months or more), the average lifespan in captivity is approximately 5 years [19,45] (maximum in our captive colony was 13.8 years) but only 2–3 years in the wild (lifespan of at least 10 years recorded in our study population). The annual turnover rate in nature is approximately 50% owing to high levels of EM [30] (mainly reptilian, avian and mammalian predation [47]). BM shows cyclic annual fluctuation in response to changes in photoperiod [48] and resource availability [34]. Under natural conditions, individuals in sufficient body condition use torpor to conserve energy during the dry season [49], whereas no spontaneous use of torpor is seen under usual captive conditions [50]. Data from captivity suggest the onset of FS at around age 5 years in males [19,33,45,48].
(b). Body mass in the captive population
The captive colony in Brunoy, France, was set up nearly 40 years ago with M. murinus originating from southwestern Madagascar. All individuals used for this study (258 individuals in 2000–2013) were born in captivity and were not involved in long-term experiments. We included only animals whose entire lifespan was monitored, excluding transfers and animals that were still alive. Food and water were available ad libitum and the animals' BM was measured (precision ± 1 g) at least monthly throughout life, starting at weaning (age four months). Seasonality was induced with a change in photoperiod [45] to promote physiological changes [51]: the short day season (SDS; six months, light 10 out of 24 h) marks the onset of fattening, whereas the long day season (LDS; six months, light 14 out of 24 h) increases activity and initiates reproductive activity. Seasonal BM averages were calculated from six to seven measurements per individual per season (LDS/SDS) for all analyses.
(c). Body mass in the wild population
Grey mouse lemurs were studied in their natural habitat in Kirindy forest in central western Madagascar. The area experiences a distinct dry season in May–October (lean season, reproductive quiescence) and a rainy season in November–April (breeding, fattening), with associated changes in food availability [34]. Long-term data were collected from two subpopulations inhabiting the study areas ‘N5’ (2002–2012, n = 1144/159 (measurements/individuals)) and ‘CS7’ (1993–2011, n = 2324/436). The study sites are situated 3 km apart and the subpopulations do not overlap [52].
Animals were captured with Sherman live traps (for details on capture and handling protocols, see [34,41]) 6–10 times per year with a minimum trapping effort of monthly captures at the end of the rainy season (March–May) and at the end of the dry season (September–November). Only data from the months March–November were used in the analyses to reduce the influence of female pregnancy. Unmarked animals were equipped with a subcutaneous transponder (Trovan EURO ID, Germany) for individual identification. The animals were almost exclusively first captured as juveniles (age less than 12 months), and the age estimates were confirmed by morphometrics. The individuals for which age could not be estimated to the year with reasonable confidence (age at first capture presumably more than 2 years) were excluded from the analyses. Marked individuals were weighed monthly upon subsequent recaptures (precision ± 1 g). For the analyses, we used individuals born in 1993–2008 and captured for the last time at least six months before the last capture session included in the dataset for each subpopulation.
3. Statistics
(a). Modelling approach
Models were created separately for data from the wild and captivity, and all analyses were performed in R v. 3.0.1 [53]. ‘Lifetime’ models were created to describe age trajectories of BM in adulthood (age range 1–11 years). The datasets included 1773 seasonal averages from 258 individuals (females: 1005/156, males: 768/102) in captivity (‘C10’ dataset), and 3468 measurements of 595 individuals (females: 1665/292, males: 1803/303) from the two subpopulations in the wild (‘W10’). The subpopulations were combined for the analyses, but the population identity (‘CS7’ or ‘N5’) was included as a cofactor in all models to account for potential small-scale ecological effects, differences in capture effort and different time windows for the data. We used only adult measurements (age 12 months or above) from animals with minimum lifespan of 1 year or above. All individuals included in the analyses are assumed to have reached adult structural size and sexual maturity. While the lifetime trajectories indicate general patterns of change, we further examined the data for only those individuals that survived to the age of at least 5 years to quantify rates of FS across seasons, settings and sexes. The final ‘old age’ datasets included 316 observations from 70 wild individuals (W5, females: 244/50, males: 72/20) and 339 observations from 105 captive individuals (C5, females: 174/57, males: 165/48).
An a priori set of biologically meaningful candidate models (electronic supplementary material, S3, tables S1–S4) was created for each dataset. Standard Akaike's information criterion values (AIC, for C10 and W10) or AIC corrected for small sample size (AICc, calculated with the MuMIn-package [54], C5 and W5) and differences between models (AICΔ) were calculated for the candidate models. The associated AIC-weights (AICw) were used for model selection. When AICw for the best model was less than 0.9, all models with cumulative AICw of 0.95 or above (‘confidence set’) were used for multimodel inference [55].
The year of measurement and subpopulation identity were included as fixed factors in all W10 and W5 models, and autocorrelation of measurements within an individual was included in all W10 candidate models. All continuous predictor terms were log-transformed, scaled (mean/SD) and centred [56,57], and BM was log-transformed. Further details on the statistical analyses are provided in the electronic supplementary material, S1.
The seasonal patterns between the wild and captive populations do not directly correspond to each other, but for our purposes the factor of importance was the gain and loss of BM. Hence, seasonality based on the expression of maximum (SDS, rainy season) and minimum (LDS, dry season) annual BM provides an estimate of the animals' capacity to respond to environmental cues and is used for across-setting comparison.
(b). Lifetime body mass trajectories
To estimate flexible age trajectories of BM and control for the expected nonlinear seasonal BM fluctuation [35], we used additive mixed modelling (GAMM, mgcv-package [57]). For W10, a sex-specific smoother term for ‘day of year’ was created to allow for sex-specific seasonal fluctuations [35]. For W10 and C10, a smoother term for age was used to describe age trajectories of BM. Alternative model terms allowed the age trajectories to be sex or season-specific. Additional terms entered were ‘lifespan’ (based on age at last capture or known date of death), season (wild: dry/rainy; captivity: SDS/LDS), a binary-coded variable ‘last season of life’ to assess terminal change and the interactions between sex and each of these three variables.
(c). Body mass senescence
BM senescence was quantified in further detail using linear mixed models (lme4-package [58]). An identical model set was built for the captive (C5, electronic supplementary material, table S3) and wild (W5, electronic supplementary material, table S4) populations, differing only in the random effects structure (both: individual nested within cohort, W5: year of measurement). Linear effects of age on BM were computed to obtain estimates of absolute change. Additional terms included in candidate models were lifespan, terminal change and interactions of age with sex and season as well as a three-way interaction of sex, season and age. For W5, the data were restricted to the months March–May and September–November.
4. Results
(a). Lifespan in captivity and in the wild
Excluding juvenile mortality, captive males lived on average one season longer than females (males: 5.5 ± 1.7 years; females: 5.0 ± 1.5 years, t330.4 = −3.88, p < 0.001; electronic supplementary material, figure S1a). By contrast, average minimum lifespan of wild females was on average seven months longer than that of males (male: 2.7 ± 1.4 years; females: 3.4 ± 2.0 years, t421.66 = 4.45, p < 0.001; electronic supplementary material, figure S1b). In the wild, the survival difference is probably a conservative estimate because males may be captured closer to their time of death than females owing to their higher recapture probability [30].
(b). Lifetime age trajectories of body mass in captivity (C10)
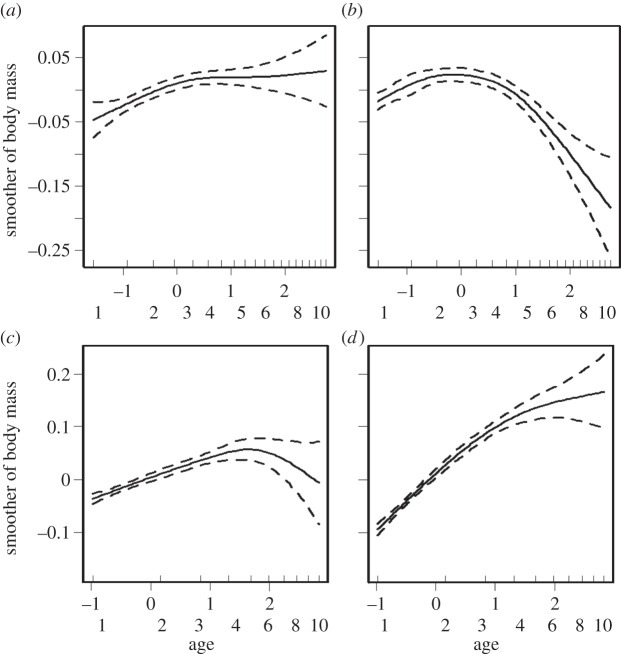
Model selection for C10 indicated a single best model (AICw > 0.99; electronic supplementary material, table S1) that included the terms sex, season and their interaction, a season-specific smoother for age and the terms indicating condition-dependence: lifespan and last season. BM was consistently higher in SDS than in LDS and females had on average higher BM in both seasons, with the sex difference being more pronounced in LDS. In LDS (figure 1a), an asymptote in BM was reached in prime adulthood (approx. age 3 years) with no subsequent declines, but in SDS a decline in BM began at age 4–5 years (figure 1b). Lifespan was positively associated with BM, and terminal decline was indicated by the negative influence of the last season of life on BM (table 1; effect size based on back-transformed means: −5.5 g, i.e. 6% decline). No support was found for sex-specific age trajectories (i.e. sex-specific smoothers of age) or sex differences in terminal decline or condition-dependence of lifespan.
Figure 1.
Age trajectories (smoothers of age based on scaled age (x-axis top row, with the corresponding chronological age in years on x-axis bottom row) of BM in (a) LDS and (b) SDS photoperiods in captivity (C10) and in the (c) dry and (d) rainy season in the wild (W10). The predictions are based on the best model in each setting. The smoother curves and their confidence bands reflect variation around mean BM. Note that scales differ between (a,b) captive and (c,d) wild data.
Table 1.
Parameter estimates for the terms included in the single best model for the captive population lifetime data (C10) and the confidence set of models for the wild population lifetime data (W10). (Temporal within-individual autocorrelation and random effects structure (individual nested within cohort) were applied to all models.)
| term | captive (C10) |
wild (W10)a |
relative importance | ||
|---|---|---|---|---|---|
| β | s.e. | β | s.e. | ||
| sex (ref. female) | −0.134 | 0.017 | −0.075 | 0.008 | 1 |
| season (ref. LDS) | 0.149 | 0.006 | |||
| lifespan | 0.016 | 0.007 | −0.008 | 0.005 | 0.37 |
| last season | −0.059 | 0.010 | −0.018 | 0.007 | 0.75 |
| sex : season | 0.046 | 0.009 | |||
| sex : last season | 0.012 | 0.011 | 0.19 | ||
| s(age) : season (LDS/dry)b | 0.024 | 0.017 | 0.051 | 0.039 | 1 |
| s(age) : season (SDS/rainy)b | −0.032 | 0.027 | 0.296 | 0.039 | 1 |
aSex-specific seasonal smoother, subpopulation and year of measurement included as forced fixed variables.
bEstimated degrees of freedom (edf) for the smoothers: SDS edf = 3.42, LDS edf = 2.15, rainy edf = 2.28 and dry edf = 2.65.
(c). Lifetime age trajectories of body mass in the wild (W10)
In W10, the confidence set of models (cumulative AICw > 0.98) included five models (electronic supplementary material, table S2), each of which contained the terms sex, sex-specific seasonal smoothers and season-specific smoothers of age. The seasonal patterns of BM differed for the sexes (electronic supplementary material, figure S2) but overall, adult females were slightly heavier than males and both sexes weighed more in the rainy season than in the dry season (table 1). BM continued to rise in adulthood until very old age in the rainy season (figure 1d), but in the dry season, BM reached an asymptote or declined slightly after peak values around 4–5 years of age (figure 1c). No support was found for sex-specific age trajectories of BM.
Support for condition-dependent longevity was also indicated in the top models. Models incorporating an effect of last season on BM were three times more likely than those without. As expected, BM in the last season of life was on average lower than in other seasons (table 1; back-transformed −1.4 g, i.e. 2% decline). Models incorporating the term lifespan also received some support (∑AICw = 0.37), but, contrary to our predictions, the negative association of lifespan with BM suggests that long-lived individuals actually had a slightly lower BM earlier in life than individuals that died at a younger age. Limited support (∑AICw = 0.19) was found for an interaction between sex and last season, with males having higher BM in the last season of life than females (table 1).
(d). Body mass senescence in captivity (C5)
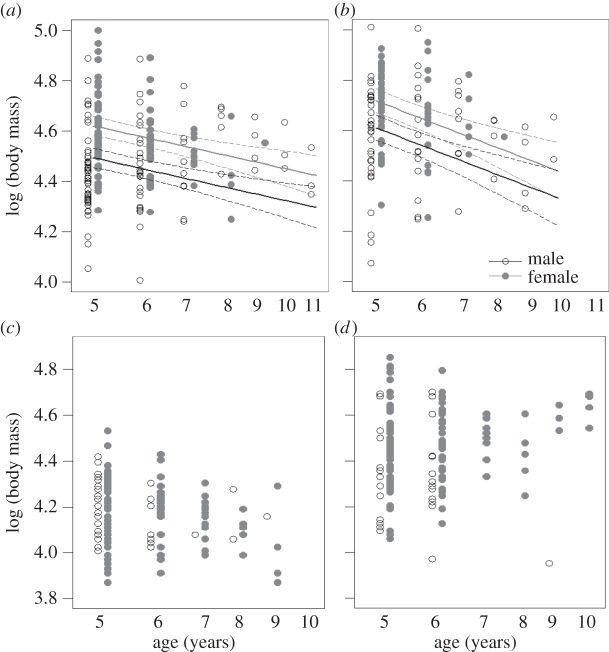
In C5, the confidence set (∑AICw > 0.98; electronic supplementary material, table S3) consisted of three models, each of which contained the terms sex, age, season and lifespan. The interaction terms age × season (∑AICw = 0.43) and lifespan × sex (∑AICw = 0.08) received limited support. Females were heavier in both seasons and BM was higher in both sexes in SDS (table 2; electronic supplementary material, figure S3). A steeper senescent decline was evident in SDS than in LDS (figure 2a,b and table 2). Strong support was found for condition-dependent longevity (∑AICw = 1.0), i.e. a positive relationship between BM and lifespan (table 2), but unlike in C10, terminal decline in BM received no support in C5.
Table 2.
Parameter estimates for the terms included in the confidence set of models for the aged captive animals (C5) and the single best model for the aged wild animals (W5).
| term | captive (C5)a |
relative importance | wild (W5)b |
||
|---|---|---|---|---|---|
| β | s.e. | β | s.e. | ||
| season (ref. LDS/dry) | 0.118 | 0.013 | 1 | 0.316 | 0.019 |
| sex (ref. female) | −0.124 | 0.025 | 1 | 0.011 | 0.038 |
| age | −0.053 | 0.009 | 1 | ||
| lifespan | 0.079 | 0.015 | 1 | ||
| season : age | −0.039 | 0.014 | 0.43 | ||
| sex : lifespan | 0.064 | 0.030 | 0.08 | ||
| season : sex | −0.160 | 0.040 | |||
aRandom effect of individual nested within cohort applied to all models.
bRandom effects of individual nested within cohort and year of measurement applied to all models. Subpopulation entered in all models as a forced fixed factor.
Figure 2.
BM varied as a function of age in the aged animals in captivity (C5), (a) LDS and (b) SDS, but not under natural conditions (W5), (c) dry season and (d) rainy season. Solid line indicates the best-fit based on the top model and dashed lines show the 95% confidence bands.
(e). Body mass senescence in the wild (W5)
In W5, a single model including the terms sex, season and their interaction received strong support (AICw = 0.91; electronic supplementary material, table S4). BM differed between the sexes only in the rainy season, with females being heavier than males (table 2; electronic supplementary material, figure S3). Although a slight senescent decline seemed to be indicated by the lifetime age trajectory in the dry season (W10, figure 1c), an age effect was not supported by the W5 model selection, suggesting an overall plateau with no increase or decrease in BM at old age (figure 2c,d). The seasonal differences in age trajectories seen in W10 were not found in the old animals, which may be partially caused by the very low number of old males present in the rainy season. No evidence was found for an influence of terminal change or lifespan on BM. The results were qualitatively similar when using GAMMs including the sex-specific seasonal trends as specified in W10.
5. Discussion
In this study, we tested fundamental hypotheses of ageing theory by assessing the relative importance of condition-dependent disappearance and FS in shaping populations of a species that naturally experiences heavy predation pressure. By comparing longitudinal data from captive and natural populations, we found evidence of selective disappearance operating in both settings, but the selective processes appear to be more rapid in the wild. FS was evident only in captivity. The disappearance of low BM individuals in captivity indicates intrinsic processes as a likely cause of the functional decline. In the presence of environmental hazard, these processes probably cause such rapid disappearance of individuals whose condition has declined, that FS is undetectable in nature even in this large dataset. This result is in stark contrast with studies of large-bodied herbivores with low EM risk, in which FS has been found after accounting for selective disappearance [10]. We found no support for the prediction that ageing occurs earlier in populations with higher risk of EM. On the contrary, the oldest animals in the wild (unlike in captivity) remained in excellent condition relative to the population average.
(a). Condition-dependent longevity and terminal declines in body mass
An energy imbalance can quickly render an individual susceptible to disease [59] and predation [60] or lower their success in resource competition. The end of life is therefore often characterized by a terminal decline in condition [6–8,27,32]. Our results indicate that M. murinus had a lowered BM in the season preceding death in both the captive and wild populations. As the decline in condition may be sudden but sampling was done only at a seasonal scale, the magnitude and importance of terminal decline especially in the wild is probably an underestimate. Selective mortality is presumably more rapid in the wild than in captivity, because weak individuals may more easily succumb to environmental hazards. Wild males had a slightly higher BM than females in their last season of life, which may indicate that female survival depends more on condition, whereas random external mortality may affect males more owing to their risky behaviours [30,46].
BM throughout life was positively associated with lifespan in captivity, indicating that selective disappearance of low-quality individuals operated there also at a longer timescale. Unexpectedly, however, lifespan was negatively associated with BM in the wild, implying that heavier individuals may disappear from the population at an earlier age. The causes for this effect are unknown but it is possible that heavier or larger individuals incur higher mortality owing to higher predation pressure, parasite loads or, potentially, investment in reproduction. The lowest quality individuals in the wild might die as juveniles, thus being excluded from our sample, whereas their lifespan in captivity may be prolonged. However, the negative effect of BM on survival was quite weak, and heterogeneity in capture probabilities may contribute to the unexpected result, if individuals in a worse condition enter traps more frequently. Therefore, while the negative trend was highly unexpected, BM may have little influence on lifespan at a long timescale and rather operate mainly via rapid terminal declines in the wild. This view is supported by the fact that the oldest surviving individuals in the wild were in an excellent condition. Random and condition-dependent mortality presumably both occur in the natural setting and their influences are difficult to disentangle. However, even moderate levels of condition-dependent mortality in the wild would lead to prolonged survival of high-quality individuals.
In the oldest animals, terminal changes were negligible in both the captive and wild setting and longevity was only condition-dependent in captivity, suggesting that the final declines in BM observed in the lifetime data are not directly associated with FS or terminal investment. The declines more likely follow from rapid processes such as illness, which may predispose the individual to a higher risk of EM, e.g. predation [61]. The absence of condition-dependent longevity in the aged wild animals, while a negative connection was found in the lifetime data, may indicate that any selective mortality of heavier or larger individuals occurs earlier in life.
The BM of an individual is largely dependent on body size, and condition-dependent longevity might partially reflect body size variation. However, as our sample contained only mature individuals, which are considered to have reached adult size, we are confident that BM is a good approximation of resources available for physiological maintenance in this species. In support of this, additional analyses (data not shown) of BM corrected for structural size (scaled mass index [49,62] using opportunistic measurements of adults in the wild) revealed no evidence for FS in body condition. Moreover, we found no support for individual variation (random slopes) in ageing rates (electronic supplementary material, S1).
(b). Patterns of functional senescence differ in the presence and absence of natural hazard
BM fluctuations over the adult lifespan were consistently, strongly dependent on sex, season and age. Senescent declines in BM in captivity began at 4–5 years of age, coinciding with the onset of decline in physical functioning [19] found in other studies (balance performance [33] and muscle strength (A. Hämäläinen, M. Dammhahn, F. Aujard, C. Kraus 2014, unpublished data)), therefore probably reflecting muscle deterioration. It is plausible that wild animals which succumb to a similar degree of physiological senescence are more likely to succumb to EM. By contrast, we found no evidence of further changes in BM after the fifth year of life in the wild, even though the lifetime trajectories appeared to suggest a declining pattern (figure 1c).
Seasonality modulated the age trajectories of BM across settings. In aged captive animals, the amplitude of seasonal BM fluctuation was diminished, which is attributable to a declined ability to regain BM. This pattern has been described earlier for captive aged males [45,48] and was confirmed in this study for both sexes. By contrast, wild, aged animals' BM remained at a level similar to prime aged adults in the dry season (figure 1; electronic supplementary material, S4), and they regained BM in the rainy season even more efficiently than younger animals, supporting the conclusion that the surviving old animals in the wild are of high quality. The observed plateau in BM in the dry season might reflect senescent muscle loss or longer periods of anorexia owing to intense torpor use by old individuals [49].
Despite the weak evidence for selective mortality at long timescales in the wild, these differences between the captive and wild populations suggest that condition-dependent EM [63–65] operates in the natural population. In both settings, terminal processes eliminates individuals whose condition deteriorates, but this process may be intensified in the hazardous environment, as implied by the relatively high threshold condition apparently required for wild individuals to survive to old age. Unlike in species with low EM rates, this interaction probably eliminates individuals from the natural population before they show FS. The interplay of FS, selective disappearance of individuals in an inferior condition and EM rate is probably a universal process [63–65], but its detection under natural conditions is challenging. While captive research is vital for understanding mechanisms of senescence, information gained from captivity may be of limited relevance for wild animals in species whose life histories are evolutionarily shaped by high EM. Despite high EM rates and AS, condition-dependent mortality may even lead to negligible FS under natural conditions [63,66].
(c). Sex-specific patterns of seasonal body mass change
Our data confirm and further detail sex-specific patterns of seasonal BM fluctuation in the natural population [35] that probably reflect sex differences in seasonal energy requirements owing to reproduction and torpor use [67]. Sex differences in ageing rate are thought to stem from selection optimizing reproductive capacity for each sex [28,29], leading males to show more rapid functional declines than females [24,26–28]. However, our models indicated no support for sex differences in ageing in either captive or wild animals. It is possible that sex biases in mortality may lead to sex differences in FS, but these differences are masked by the strong seasonal effects and rapid terminal changes. Based on a visual inspection of the sex-specific age trajectories of BM (figure 2; electronic supplementary material, figure S4), the trends in senescent declines concur with the direction of each population's sex bias in mortality.
(d). Lifespan determination in captivity and nature
In support of previously formulated [63,64] and tested [65] hypotheses, our results suggest that lifespan is determined at population-level by an interaction of intrinsic mortality rates with environmental influences. Condition-dependent EM can cause selection to favour improved somatic maintenance [60,63,65,68] that increases predator-avoidance success and lifespan and permits the manifestation of physiological ageing only in the absence of extrinsic hazard. As expected, the estimated lifespans of wild and captive M. murinus differ substantially: captive males live on average twice as long and females 50% longer than their wild counterparts. Such variation reflects highly plastic life histories that may have helped the species to adapt to the gradient of ambient temperatures found within its natural geographical distribution, and contribute to the proposed pace-of-life variation found across this range [69]. Our results concur with previous studies [39,40] that have found longer lifespan and lower AS rates in captivity than in nature.
The particularly strong plasticity in M. murinus males results in a contrasting sex bias in lifespan across settings. This intriguing phenomenon may reflect stronger selection for robust males [28,29], resulting from their higher EM, as suggested previously [70]. Alternatively, adjustment to captivity may evoke differential responses in males and females owing to physiological differences associated with reproduction, somatic maintenance [38,71], hormone levels and susceptibility to disease [72,73] or torpor use [74].
Theories of ageing posit that molecular damage builds up at old age because selection is inefficient against deleterious effects that arise after individuals have passed on their genes [14]. However, if lifetime fitness is sufficiently enhanced by living longer, selection may favour somatic maintenance and counteract the accumulation of damage [2,29,65]. Microcebus murinus have a long potential lifespan relative to their body size and life-history characteristics [75,76], yet a distinctly shorter average lifespan in nature. While high EM may be associated with evolutionary processes that shorten lifespan, these processes might be partially counteracted in M. murinus by their lifelong reproduction [45,51] and ability to efficiently fatten and use torpor in late adulthood. Overall, our results support the view that condition-dependent EM may shape FS and, eventually, lifespan. Further research on these simultaneous processes in other systems is required to identify the specific constraints on lifespan evolution, with emphasis on the influences that individual condition, environmental plasticity and the flexible nature of senescence may have on the observed patterns of senescence and lifespan.
6. Conclusion
Using long-term BM data from grey mouse lemurs, we demonstrate selective disappearance in both captive and wild populations. BM senescence is apparently absent in the wild, although clearly present in captivity. We identify condition-dependent mortality as a potentially significant demographic process that can influence ageing processes but has been largely overlooked in ageing studies thus far. Its underestimation can lead to biased conclusions on the prevalence of FS and the evolution of lifespan. FS is, indeed, sometimes undetectable in the wild since in species with high EM risk, the oldest individuals tend to be of high quality. Ageing may also be masked by environmental variables, respond to subtle changes in conditions or be altogether absent. An increased understanding of this variability will bring us closer to resolving open questions on life-history determinants across taxa.
Supplementary Material
Acknowledgements
We thank Rodin Rasoloarison and Léonard Razafimanantsoa for logistic support, and Tiana Andrianjanahary, the late Jean-Claude de Beroboka, Bruno Tsiverimana and l'Equipe Kirindy and research technicians in Brunoy for indispensable assistance in long-term data collection. Henning Lahmann helped with the Kirindy database. Comments by Adeelia Goffe, Alexei Maklakov and two anonymous reviewers improved earlier versions of this paper. We acknowledge the authorization and support of this study by Prof. D. Rakotondravony (Département de Biologie Animale, Universite d'Antananarivo), Commission Tripartite and CAFF of Direction des Eaux et Fôrets and CNFEREF Morondava.
Research in Madagascar was approved by the Ministère de l'Environment et des Eaux et Fôrets, MINEEF and complies with the applicable national laws of Madagascar. Research at the Brunoy breeding colony is authorized by the agreement DDPP, Essonne, France no. E91-114-1.
Data accessibility
The data used in this paper is available via the Dryad repository, doi:10.5061/dryad.t01gt.
Funding statement
Funding was provided by DFG (CK: KR3834/1-1, PK: Ka 1082/10-1&2), Margot-Marsh Biodiversity Foundation (M.D.), Christian-Vogel-Fonds (M.D.) and German Primate Center, Göttingen.
References
- 1.Gaillard JM. 1994. Senescence in natural populations of mammals: a reanalysis. Evolution 48, 509–516. ( 10.2307/2410110) [DOI] [PubMed] [Google Scholar]
- 2.Promislow DEL. 1991. Senescence in natural populations of mammals: a comparative study. Evolution 45, 1869–1887. ( 10.2307/2409837) [DOI] [PubMed] [Google Scholar]
- 3.Ricklefs RE. 1998. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Nat. 152, 24–44. ( 10.1086/286147) [DOI] [PubMed] [Google Scholar]
- 4.Jones OR, et al. 2014. Diversity of ageing across the tree of life. Nature 505, 169–173. ( 10.1038/nature12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH. 1984. Reproductive effort and terminal investment in iteroparous animals. Am. Nat. 123, 212–229. ( 10.1086/284198) [DOI] [Google Scholar]
- 6.Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D. 2010. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav. Ecol. 21, 972–978. ( 10.1093/beheco/arq098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weladji RB, Holand Ø, Gaillard J-M, Yoccoz NG, Mysterud A, Nieminen M, Stenseth NC. 2010. Age-specific changes in different components of reproductive output in female reindeer: terminal allocation or senescence? Oecologia 162, 261–271. ( 10.1007/s00442-009-1443-5) [DOI] [PubMed] [Google Scholar]
- 8.Isaac JL, Johnson CN. 2005. Terminal reproductive effort in a marsupial. Biol. Lett. 1, 271–275. ( 10.1098/rsbl.2005.0326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM. 2008. Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 22, 393–406. ( 10.1111/j.1365-2435.2008.01408.x) [DOI] [Google Scholar]
- 10.Nussey DH, Coulson T, Delorme D, Clutton-Brock TH, Pemberton JM, Festa-Bianchet M, Gaillard J-M. 2011. Patterns of body mass senescence and selective disappearance differ among three species of free-living ungulates. Ecology 92, 1936–1947. ( 10.1890/11-0308.1) [DOI] [PubMed] [Google Scholar]
- 11.van de Pol M, Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within-and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 12.Bouwhuis S, Sheldon BC, Verhulst S, Charmantier A. 2009. Great tits growing old: selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc. R. Soc. B 276, 2769–2777. ( 10.1098/rspb.2009.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayward AD, Wilson AJ, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2013. Reproductive senescence in female Soay sheep: variation across traits and contributions of individual ageing and selective disappearance. Funct. Ecol. 27, 184–195. ( 10.1111/1365-2435.12029) [DOI] [Google Scholar]
- 14.Monaghan P, Charmantier A, Nussey D, Ricklefs R. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. ( 10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 15.Austad SN. 1993. Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana). J. Zool. 229, 695–708. ( 10.1111/j.1469-7998.1993.tb02665.x) [DOI] [Google Scholar]
- 16.Stearns S, Ackermann M, Doebeli M, Kaiser M. 2000. Experimental evolution of aging, growth, and reproduction in fruitflies. Proc. Natl Acad. Sci. USA 97, 3309–3313. ( 10.1073/pnas.97.7.3309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reznick DN, Bryant MJ, Roff D, Ghalambor CK, Ghalambor DE. 2004. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 431, 1095–1099. ( 10.1038/nature02936) [DOI] [PubMed] [Google Scholar]
- 18.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. 2002. The aging baboon: comparative demography in a non-human primate. Proc. Natl Acad. Sci. USA 99, 9591–9595. ( 10.1073/pnas.142675599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Languille S, et al. 2012. The grey mouse lemur: a non-human primate model for ageing studies. Ageing Res. Rev. 11, 150–162. ( 10.1016/j.arr.2011.07.001) [DOI] [PubMed] [Google Scholar]
- 20.Takeda T, et al. 1981. A new murine model of accelerated senescence. Mech. Ageing Dev. 17, 183–194. ( 10.1016/0047-6374(81)90084-1) [DOI] [PubMed] [Google Scholar]
- 21.Toïgo C, Gaillard J-M, Van Laere G, Hewison M, Morellet N. 2006. How does environmental variation influence body mass, body size, and body condition? Roe deer as a case study. Ecography 29, 301–308. ( 10.1111/j.2006.0906-7590.04394.x) [DOI] [Google Scholar]
- 22.Pardo D, Barbraud C, Authier M, Weimerskirch H. 2013. Evidence for an age-dependent influence of environmental variations on a long-lived seabird's life-history traits. Ecology 94, 208–220. ( 10.1890/12-0215.1) [DOI] [PubMed] [Google Scholar]
- 23.Hindle AG, Horning M, Mellish J-AE, Lawler JM. 2009. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii). J. Exp. Biol. 212, 790–796. ( 10.1242/jeb.025387) [DOI] [PubMed] [Google Scholar]
- 24.Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC. 2010. Life history context of reproductive aging in a wild primate model. Ann. NY Acad. Sci. 1204, 127–138. ( 10.1111/j.1749-6632.2010.05531.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgenson JT, Festa-Bianchet M, Gaillard J-M, Wishart WD. 1997. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78, 1019–1032. ( 10.1890/0012-9658(1997)078[1019:EOASDA]2.0.CO;2) [DOI] [Google Scholar]
- 26.Greiner S, Nagy M, Mayer F, Knörnschild M, Hofer H, Voigt CC. 2014. Sex-biased senescence in a polygynous bat species. Ethology 120, 197–205. ( 10.1111/eth.12193) [DOI] [Google Scholar]
- 27.Tafani M, Cohas A, Bonenfant C, Gaillard J-M, Lardy S, Allainé D. 2013. Sex-specific senescence in body mass of a monogamous and monomorphic mammal: the case of Alpine marmots. Oecologia 172, 427–436. ( 10.1007/s00442-012-2499-1) [DOI] [PubMed] [Google Scholar]
- 28.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. 2008. Sexual selection, sexual conflict and the evolution of ageing and life span. Funct. Ecol. 22, 443–453. ( 10.1111/j.1365-2435.2008.01417.x) [DOI] [Google Scholar]
- 29.Maklakov AA, Lummaa V. 2013. Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays 35, 717–724. ( 10.1002/bies.201300021) [DOI] [PubMed] [Google Scholar]
- 30.Kraus C, Eberle M, Kappeler PM. 2008. The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate. Proc. R. Soc. B 275, 1635–1644. ( 10.1098/rspb.2008.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanet J, Croci S, Aujard F, Perret M, Cubo J, Margerie Ed. 2004. Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus. J. Zool. 263, 31–39. ( 10.1017/S0952836904004844) [DOI] [Google Scholar]
- 32.Karkach A. 2006. Trajectories and models of individual growth. Demogr. Res. 15, 347–400. ( 10.4054/DemRes.2006.15.12) [DOI] [Google Scholar]
- 33.Némoz-Bertholet F, Aujard F. 2003. Physical activity and balance performance as a function of age in a prosimian primate (Microcebus murinus). Exp. Gerontol. 38, 407–414. ( 10.1016/S0531-5565(02)00244-9) [DOI] [PubMed] [Google Scholar]
- 34.Dammhahn M, Kappeler P. 2008. Comparative feeding ecology of sympatric Microcebus berthae and M. murinus. Int. J. Primatol. 29, 1567–1589. ( 10.1007/s10764-008-9312-3) [DOI] [Google Scholar]
- 35.Schmid J, Kappeler PM. 1998. Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus). Behav. Ecol. Sociobiol. 43, 125–132. ( 10.1007/s002650050474) [DOI] [Google Scholar]
- 36.Baumgartner RN. 2000. Body composition in healthy aging. Ann. NY Acad. Sci. 904, 437–448. ( 10.1111/j.1749-6632.2000.tb06498.x) [DOI] [PubMed] [Google Scholar]
- 37.Hayflick L. 2000. The future of ageing. Nature 408, 267–269. ( 10.1038/35041709) [DOI] [PubMed] [Google Scholar]
- 38.Kirkwood TB, Austad SN. 2000. Why do we age? Nature 408, 233–238. ( 10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 39.Lemaître J-F, Gaillard J-M, Bingaman Lackey L, Clauss M, Müller DW. 2013. Comparing free-ranging and captive populations reveals intra-specific variation in aging rates in large herbivores. Exp. Gerontol. 48, 162–167. ( 10.1016/j.exger.2012.12.004) [DOI] [PubMed] [Google Scholar]
- 40.Magalhães JPd, Costa J, Church GM. 2007. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. A Biol. Sci. Med. Sci. 62, 149–160. ( 10.1093/gerona/62.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberle M, Kappeler P. 2004. Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav. Ecol. Sociobiol. 57, 77–90. ( 10.1007/s00265-004-0826-1) [DOI] [Google Scholar]
- 42.Eberle M, Perret M, Kappeler P. 2007. Sperm competition and optimal timing of matings in Microcebus murinus. Int. J. Primatol. 28, 1267–1278. ( 10.1007/s10764-007-9220-y) [DOI] [Google Scholar]
- 43.Huchard E, Canale CI, Le Gros C, Perret M, Henry P-Y, Kappeler PM. 2012. Convenience polyandry or convenience polygyny? Costly sex under female control in a promiscuous primate. Proc. R. Soc. B 279, 1371–1379. ( 10.1098/rspb.2011.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eberle M, Kappeler PM. 2004. Selected polyandry: female choice and inter-sexual conflict in a small nocturnal solitary primate (Microcebus murinus). Behav. Ecol. Sociobiol. 57, 91–100. ( 10.1007/s00265-004-0823-4) [DOI] [Google Scholar]
- 45.Perret M. 1997. Change in photoperiodic cycle affects life span in a Prosimian primate (Microcebus murinus). J. Biol. Rhythms 12, 136–145. ( 10.1177/074873049701200205) [DOI] [PubMed] [Google Scholar]
- 46.Dammhahn M. 2012. Are personality differences in a small iteroparous mammal maintained by a life-history trade-off? Proc. R. Soc. B 279, 2645–2651. ( 10.1098/rspb.2012.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman SM, O'Connor S, Langrand O. 1993. A review of predation on lemurs: implications for the evolution of social behavior in small, nocturnal primates. In Lemur social systems and their ecological basis (eds Kappeler PM, Ganzhorn JU.), pp. 51–66. New York, NY: Springer. [Google Scholar]
- 48.Perret M, Aujard F. 2001. Regulation by photoperiod of seasonal changes in body mass and reproductive function in gray mouse lemurs (Microcebus murinus): differential responses by sex. Int. J. Primatol. 22, 5–24. ( 10.1023/A:1026457813626) [DOI] [Google Scholar]
- 49.Vuarin P, Dammhahn M, Henry P-Y. 2013. Individual flexibility in energy saving: body size and condition constrain torpor use. Funct. Ecol. 27, 793–799. ( 10.1111/1365-2435.12069) [DOI] [Google Scholar]
- 50.Canale CI, Perret M, Théry M, Henry P-Y. 2011. Physiological flexibility and acclimation to food shortage in a heterothermic primate. J. Exp. Biol. 214, 551–560. ( 10.1242/jeb.046987) [DOI] [PubMed] [Google Scholar]
- 51.Perret M. 1992. Environmental and social determinants of sexual function in the male lesser mouse lemur (Microcebus murinus). Folia Primatol. 59, 1–25. ( 10.1159/000156637) [DOI] [PubMed] [Google Scholar]
- 52.Fredsted T, Pertoldi C, Schierup MH, Kappeler PM. 2005. Microsatellite analyses reveal fine-scale genetic structure in grey mouse lemurs (Microcebus murinus). Mol. Ecol. 14, 2363–2372. ( 10.1111/j.1365-294X.2005.02596.x) [DOI] [PubMed] [Google Scholar]
- 53.R Development Core Team. 2013. R: a language and environment for statistical computing. (3.0.1 ed.). Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 54.Barton K. 2013. MuMIn: multi-model inference. R package v. 1.9.5. See http://CRAN.R-project.org/package=MuMln. [Google Scholar]
- 55.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 56.Grueber C, Nakagawa S, Laws R, Jamieson I. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711. ( 10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 57.Wood S. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: CRC Press. [Google Scholar]
- 58.Bates D, Maechler M, Bolker B. 2013. lme4: linear mixed-effects models using S4 classes. R package v. 0.999999-2. See http://CRAN.R-project.org/package=lme4. [Google Scholar]
- 59.Coop RL, Kyriazakis I. 1999. Nutrition-parasite interaction. Vet. Parasitol. 84, 187–204. ( 10.1016/S0304-4017(99)00070-9) [DOI] [PubMed] [Google Scholar]
- 60.Murray DL. 2002. Differential body condition and vulnerability to predation in snowshoe hares. J. Anim. Ecol. 71, 614–625. ( 10.1046/j.1365-2656.2002.00632.x) [DOI] [Google Scholar]
- 61.Dowling DK. 2012. Aging: evolution of life span revisited. Curr. Biol. 22, R947–R949. ( 10.1016/j.cub.2012.09.029) [DOI] [PubMed] [Google Scholar]
- 62.Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118, 1883–1891. ( 10.1111/j.1600-0706.2009.17643.x) [DOI] [Google Scholar]
- 63.Williams PD, Day T. 2003. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution 57, 1478–1488. ( 10.1111/j.0014-3820.2003.tb00356.x) [DOI] [PubMed] [Google Scholar]
- 64.Williams PD, Day T, Fletcher Q, Rowe L. 2006. The shaping of senescence in the wild. Trends Ecol. Evol. 21, 458–463. ( 10.1016/j.tree.2006.05.008) [DOI] [PubMed] [Google Scholar]
- 65.Chen H-Y, Maklakov AA. 2012. Longer life span evolves under high rates of condition-dependent mortality. Curr. Biol. 22, 2140–2143. ( 10.1016/j.cub.2012.09.021) [DOI] [PubMed] [Google Scholar]
- 66.McNamara JM, Houston AI. 1996. State-dependent life histories. Nature 380, 215–221. ( 10.1038/380215a0) [DOI] [PubMed] [Google Scholar]
- 67.Schmid J. 1999. Sex-specific differences in activity patterns and fattening in the gray mouse lemur (Microcebus murinus) in Madagascar. J. Mammal. 80, 749–757. ( 10.2307/1383244) [DOI] [Google Scholar]
- 68.Bronikowski AM, Promislow DEL. 2005. Testing evolutionary theories of aging in wild populations. Trends Ecol. Evol. 20, 271–273. ( 10.1016/j.tree.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 69.Lahann P, Schmid J, Ganzhorn JU. 2006. Geographic variation in populations of Microcebus murinus in Madagascar: resource seasonality or Bergmann's rule? Int. J. Primatol. 27, 983–999. ( 10.1007/s10764-006-9055-y) [DOI] [Google Scholar]
- 70.Maklakov AA, et al. 2009. Sex differences in nutrient-dependent reproductive ageing. Aging Cell 8, 324–330. ( 10.1111/j.1474-9726.2009.00479.x) [DOI] [PubMed] [Google Scholar]
- 71.Kirkwood TB. 2002. Evolution of ageing. Mech. Ageing Dev. 123, 737–745. ( 10.1016/S0047-6374(01)00419-5) [DOI] [PubMed] [Google Scholar]
- 72.Klein SL. 2000. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behav. Process 51, 149–166. ( 10.1016/S0376-6357(00)00125-X) [DOI] [PubMed] [Google Scholar]
- 73.Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. 2007. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. B 274, 819–825. ( 10.1098/rspb.2006.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turbill C, Bieber C, Ruf T. 2011. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B 278, 3355–3363. ( 10.1098/rspb.2011.0190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey PH, Clutton-Brock TH. 1985. Life history variation in primates. Evolution 39, 559–581. ( 10.2307/2408653) [DOI] [PubMed] [Google Scholar]
- 76.Ross C. 1998. Primate life histories. Evol. Anthropol. 6, 54–63. () [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this paper is available via the Dryad repository, doi:10.5061/dryad.t01gt.