Abstract
Behaviour may contribute to changes in fitness prospects with age, for example through effects of age-dependent social dominance on resource access. Older individuals often have higher dominance rank, which may reflect a longer lifespan of dominants and/or an increase in social dominance with age. In the latter case, increasing dominance could mitigate physiological senescence. We studied the social careers of free-living jackdaws over a 12 year period, and found that: (i) larger males attained higher ranks, (ii) social rank increased with age within individuals, and (iii) high-ranked individuals had shorter lifespan suggesting that maintaining or achieving high rank and associated benefits comes at a cost. Lastly, (iv) social rank declined substantially in the last year an individual was observed in the colony, and through its effect on resource access this may accelerate senescence. We suggest that behaviour affecting the ability to secure resources is integral to the senescence process via resource effects on somatic state, where behaviour may include not only social dominance, but also learning, memory, perception and (sexual) signalling. Studying behavioural effects on senescence via somatic state may be most effective in the wild, where there is competition for resources, which is usually avoided in laboratory conditions.
Keywords: dominance, ageing, senescence, birds, corvids
1. Introduction
Optimality theories of senescence [1] assume that resources are limited, which imposes a trade-off between reproductive investment and somatic maintenance or repair, causing senescence. However, individuals show large variation in their ability to secure environmental resources, and this inherently shapes the scope for allocation between reproductive investment and somatic maintenance or repair [2]. Understanding what determines individual success in securing resources, and how it changes with age, is therefore essential to understand the senescence process.
Disentangling factors that determine access to resources is difficult because they interact with each other. For example, body size, a component of physiological state, is positively correlated with the ability to compete over food [3,4]. Increased access to food, in turn, facilitates increased investment in somatic growth, maintenance or repair, positively affecting state and hence the ability to secure resources. The interaction between state and the ability to secure resources is probably modified by age, because: (i) physiological state first improves with age owing to development, and later in life declines (i.e. senescence) [5]; and (ii) the ability to secure resources, in part, depends on learning or experience that is gained over time [6,7]. Lastly, social dominance, or any other behaviour affecting the ability to secure resources, is an integral part of these relationships. Thus, we see social dominance as a trait that can be invested in to gain resource holding potential [8], which through its effect on resource access could modify the balance between somatic maintenance and repair and other competing demands, shaping senescence.
Social dominance has often been shown to depend on age, with older individuals being more dominant [3,4,6,7,9–14], also in jackdaws [10], the subject of this study. However, these studies are cross-sectional, i.e. between individuals, and this leaves open the question whether the observed patterns are owing to changes within individuals as opposed to other processes. For example, high-ranked individuals may have a longer lifespan resulting in selective disappearance of lower-ranked individuals. This would by itself result in a positive correlation between age and dominance in cross-sectional studies. Alternatively, dominance may depend on queuing for higher rank [14–17]. Queuing may arise when all individuals start at the bottom of the social ladder, and only advance to a higher position when a more dominant individual disappears from the population. If such a queuing effect occurs, then the resulting pattern is that dominance increases with age within individuals, despite the fact that this process may be entirely independent of competition, experience or development. Longitudinal studies are required to disentangle these non-mutually exclusive effects, and the few longitudinal studies that we are aware of showed that indeed dominance increases with age within individuals [11] and that this increase is highly variable between individuals [18,19].
We collected longitudinal social dominance data of individual male jackdaws using observations of contest behaviour over food. Earlier work in our colony has shown that social dominance over food is strongly correlated with the ability to secure nest-boxes [20]. This indicates that social dominance in conflicts over food reflects something other than hunger level, and we assume this to be resource holding potential. We determined dominance in males only, because they are dominant over females and the outcome of conflicts between pairs is determined by the rank of the male [20,21]. We disentangled effects of within- from between-subject age [22] to determine whether dominance increased with age within subjects. This approach also allowed us to test whether social dominance is associated with lifespan, because this is reflected in the mean age at which they were observed.
2. Methods
(a). Study population
We studied free-living jackdaws in the colony at the Zoological Laboratory in Haren, The Netherlands, a semi-urban environment (see [23,24] for details). Jackdaws are highly social small corvids with a strong and stable dominance hierarchy [10,20,21,23–25]. Individual birds were identified through their unique combination of colour rings. Estimates of adult age are exact for individual birds first ringed as fledglings or yearlings (distinguishable through brown plumage coloration). Birds of unknown age were assigned a minimum age of 2 years, which is the modal age at recruitment in our population. In total, we used 69 males in this study (14 with exact age known). That we did not always know the exact age has little effect on this study, because our primary interest was (change in) social status within individuals. Biometry (tarsus, wing length, and mass) was measured, and we used the tarsus length as a measure of body size because it does not change with age.
(b). Dominance
Social dominance was determined the month before the breeding season (March and first days of April) in the years 1998–2009, with the exception of 1999 and 2002, see [23] for details. In brief, we staged social interactions using two feeding pits (filled only during observation sessions), 30 m apart, where only one jackdaw could eat at a time. Social dominance was determined by the outcome of displacement, threat or fight interactions between males, which were scored for each male that could be identified (mean ± s.e. = 59.5 ± 7.4 interactions per male per year collected during on average 25.6 observation sessions per year, range 9–35). Relative rank on a scale from 0 to 1 was calculated using David's score [26], where 0 was assigned to the most dominant individual (cf. [23,24]).
(c). Statistical analyses
Because social dominance, as we defined it, is bound between 0 and 1, we analysed data with mixed-effects logistic regression using ML-win v. 2.02, with bird identification as a random effect. With dominance as dependent variable, we first tested the effects of tarsus, age, age-squared and [returned?], i.e. whether individuals returned the subsequent year (1 when they returned and 0 when they did not). The latter variable was included to test for terminal effects, as we previously documented for jackdaw telomeres [27]. To further investigate the shape of the relationship between age and social dominance, and whether this depended on body size, we also tested the two-way interactions. Next, we disentangled queuing from within-individual effects, by testing the effect of colony composition. If queuing determines the increase of dominance with age, then the effect of age will depend on the fraction of new or disappeared individuals. To test this hypothesis, we included the effects of [% dead in hierarchy], [% new in hierarchy] and tested the two-way interactions with age.
(d). Separating within- from between-subjects effects
In a standard linear regression, the estimated effect of age is the combined effect of the within- and between-subject age effects (model 1). Relative rank yij of measurement i from subject j is given by
| 2.1 |
where β0 is the intercept and β1 is the dependency of dominance yij on age xij. The terms u0j and e0ij denote the random intercept and residual variance. To disentangle the within- from the between-subjects age components we transformed the model (1) into a model with average age and delta age (model 2) as previously described [22,28]. Average age 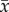 of subject j was obtained by taking the mean of ages i over the years an individual was ranked, resulting in one value for average age per individual
of subject j was obtained by taking the mean of ages i over the years an individual was ranked, resulting in one value for average age per individual 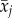 . Delta age is defined as the difference between the age at which individuals are ranked and their average age
. Delta age is defined as the difference between the age at which individuals are ranked and their average age 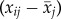 , resulting in multiple values for delta age per individual. Thus, the average age can be used to describe how relative rank is related with age between individuals, whereas delta age can be used to describe the change in dominance with age within individuals. Note that, because our ages are minimum ages in most instances, it is more exact to think of our variable ‘mean age’ as ‘mean number of years since entering the colony at which dominance was measured plus 2’. We retained the term ‘mean age’, however, because this distinction does not affect the interpretation of the results, and because the variation in age at first breeding is limited, the difference is small. The dependency of relative rank on age is then described by
, resulting in multiple values for delta age per individual. Thus, the average age can be used to describe how relative rank is related with age between individuals, whereas delta age can be used to describe the change in dominance with age within individuals. Note that, because our ages are minimum ages in most instances, it is more exact to think of our variable ‘mean age’ as ‘mean number of years since entering the colony at which dominance was measured plus 2’. We retained the term ‘mean age’, however, because this distinction does not affect the interpretation of the results, and because the variation in age at first breeding is limited, the difference is small. The dependency of relative rank on age is then described by
| 2.2 |
where βW is the within individual effect of delta age 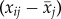 , and βB the between individual effect of average age
, and βB the between individual effect of average age 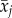 . To test whether slopes βB and
βW differed significantly from each other, which signifies selective (dis)appearance of individuals with high or low dominance, we transformed the model (2) into a model with average age
. To test whether slopes βB and
βW differed significantly from each other, which signifies selective (dis)appearance of individuals with high or low dominance, we transformed the model (2) into a model with average age 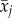 and age xij as follows
and age xij as follows
| 2.3 |
If the coefficient of average age 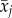 is significantly positive, than individuals with high dominance selectively disappear from the colony, because the slopes of within- versus between-subject age differ significantly [22].
is significantly positive, than individuals with high dominance selectively disappear from the colony, because the slopes of within- versus between-subject age differ significantly [22].
Variation in apparent survival rate can be due to variation in recapture probability as well as variation in survival, and both probabilities can be estimated separately using capture–mark–recapture models [29]. However, such models were not a suitable tool in this study; first, because such models cannot accommodate continuously distributed trait changes with age such as we found for social dominance. Second, capture probability within the colony was very high (we oversaw the colony from our offices) whereas being very low outside the colony, and hence there is little scope for capture probability estimates to affect our survival estimates or to estimate dispersal.
3. Results
(a). Size, age and dominance
Birds with a longer tarsus were more dominant (model A; table 1 and figure 1). Average age was not related to dominance (model A; table 1), indicating that there was no cross-sectional relationship between age and social dominance in our population. Dominance did however increase with age within individuals (model A; table 1). None of the quadratic terms or other two-way interactions significantly improved the model (table 1; model A), indicating a linear relationship between age and dominance within individuals. Within individual slopes may be biased by individual outliers [30], but a random slope for the delta age effect explained a negligible part of the variance, indicating little individual variation in the relationship between dominance and delta age.
Table 1.
Model A. Social dominance in relation to age and body size. (Note that a negative slope signifies increasing dominance. The effect of delta age βW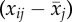 , average age βB(
, average age βB(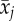 ) (equation 2.2), tarsus size and the variable [returned?], i.e. whether individuals return to the colony in the subsequent year. n = 149 bird years, of 69 individuals. Deviance denotes the −2 log-likelihood value of the model fit.)
) (equation 2.2), tarsus size and the variable [returned?], i.e. whether individuals return to the colony in the subsequent year. n = 149 bird years, of 69 individuals. Deviance denotes the −2 log-likelihood value of the model fit.)
| model A | deviance | fixed effect | slope | s.e. | p-value |
|---|---|---|---|---|---|
| null | 83.94 | intercept (β0) | |||
| final | 57.12 | intercept (β0) | 11.135 | 4.225 | <0.001 |
| tarsus | −0.229 | 0.092 | 0.013 | ||
delta age (βW
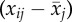 ) ) |
−0.221 | 0.069 | 0.001 | ||
average age (βB
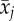 ) ) |
0.052 | 0.048 | 0.276 | ||
| returned? | −0.581 | 0.281 | 0.038 | ||
| rejected terms | |||||
| (average age)2 | 0.009 | 0.016 | 0.577 | ||
| (delta age)2 | 0.001 | 0.028 | 0.998 | ||
| average age × returned? | 0.098 | 0.110 | 0.377 | ||
| tarsus × returned? | −0.037 | 0.213 | 0.860 | ||
| average age × tarsus | −0.023 | 0.037 | 0.524 | ||
Figure 1.
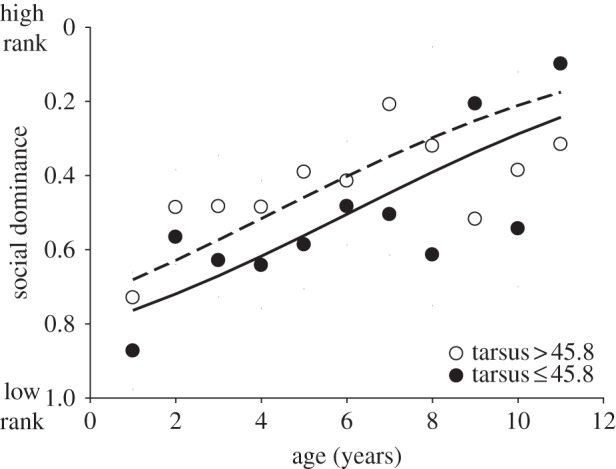
Social dominance in relation to age and size (tarsus length). Statistical analysis was carried out using raw data, but for graphical purposes, individuals were grouped by tarsus size (tarsus ≤ 45.8; or >45.8) and age. Lines show the predicted values of the logistic regression in table 1 for the average tarsus length of each group (44.8 and 46.8). Low rank indicates high dominance.
To test whether the within- and between-subject effects of age differed significantly from each other, we replaced delta age in model A (equation (2.2)) with age (equation (2.3); model B and the electronic supplementary material, table S1). Average age was significant in this model, in the presence of age (model B and the electronic supplementary material, table S1), which shows that the coefficients of average age and delta age in model A differ significantly from each other [22,28]. In other words, birds with low average age, implying they were in the population for a short time, had higher dominance for their age than birds with high average age (figure 2). Hence, dominant birds disappeared at a younger age from the colony when compared with subordinates (figure 2). This result differs from our conclusion on this relationship in an earlier paper in which survival appeared to be independent of dominance [24]. However, there was already a trend towards more dominant birds to have lower survival in that dataset, and the dataset has increased substantially since that time, increasing statistical power to detect survival effects of dominance.
Figure 2.
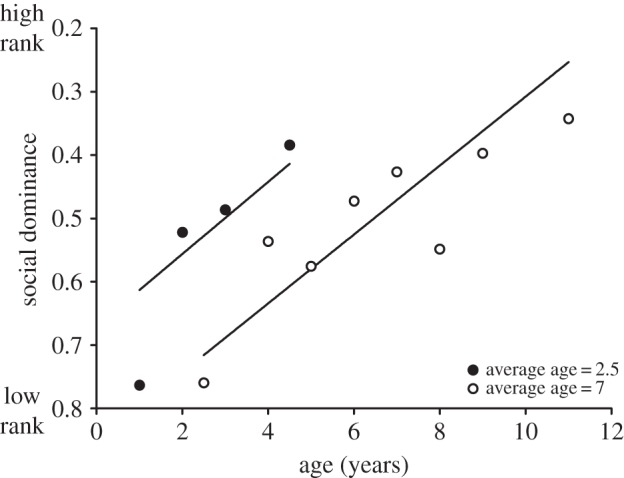
Social dominance in relation to age and lifespan. Statistical analysis was carried out using raw data, but for graphical purposes, individuals were grouped by median average age and age. Lines show the predicted values of the logistic regression (equation (2.3)) for the mean values of average age (2.6 and 6.8). Low rank indicates high dominance.
We previously showed that telomere-shortening rate is elevated in the year before disappearance, indicating a terminal decline [27]. We therefore tested whether social dominance also shows a terminal decline, by including the variable [returned?] in model A. This test showed that birds decreased in rank when they did not return to the colony the subsequent year (table 1), contrasting strongly with the increase in rank over the preceding years (figure 3).
Figure 3.
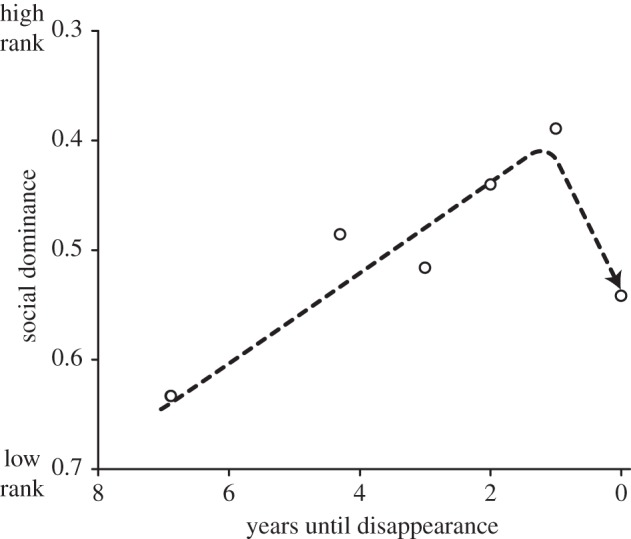
Terminal decline in social dominance. To show the terminal decline, social dominance is plotted in relation to years prior to disappearance (death), but note that the terminal effect was tested in a model based on age rather than age until death (table 1) and hence the line was drawn by eye rather than being a model fit. Low rank (0) indicates high dominance.
(b). Are birds that disappeared dead?
The pattern that dominant males disappear at younger ages (figure 2) may reflect their shorter lifespan, but alternatively dominant males dispersed more often, which would yield the same pattern. However, a jackdaw colony consists of resident birds that each year return to the colony to breed, and intruders that may return or disperse to breed elsewhere [20]. We therefore recalculated model B with only residential males, that bred in the colony more than one year, where dispersal is likely to be negligible [20,29]. This subset yielded results that were indistinguishable from the result in the complete dataset (average age (βB − βW) = 0.193 ± 0.089 versus 0.178 ± 0.085; residents versus total). We therefore consider it safe to conclude that more dominant birds achieved lower lifespan, rather than showing higher dispersal rate.
(c). Does dominance increase via queuing?
When queuing effects caused the increase in dominance with age, we expect that individuals increase in rank more when there are many new individuals in the colony. We examined this hypothesis by testing the variables [% newrank] (percentage new birds in the hierarchy), [% deadrank] (percentage of disappeared birds from the hierarchy) and the two-way interactions with delta age. None of these variables significantly decreased the deviance (electronic supplementary material, table S2), indicating that dominance did not increase with age owing to queuing effects.
4. Discussion
Dominance affects access to resources, shaping life histories and when dominance is age-dependent it could modulate how fitness prospects change with age. We therefore studied social dominance in relation to age in a colony of free-living jackdaws, also taking body size and colony composition into account.
Larger males were more dominant (figure 1), which is consistent with previous findings in other species [3,4,24]. Adult structural size in birds is generally fixed early in life, and in our study population, the tarsus length of adults is strongly correlated with their tarsus length at fledging (r = 0.96, n = 23 birds, p < 0.0001). Thus, adult size, and hence a part of the variation in social dominance, is determined early in life by a combination of genetic and environmental effects related to growth.
Dominance rank increased with age within individuals (figure 2), except that individuals lost dominance in the last year before disappearing from the colony (figure 3). We further conclude that the increase in dominance with age was not owing to a ‘queuing effect’, because the increase in rank with age did not depend on the proportion of new birds in the colony. Males that already attained high dominance early in life disappeared from the colony at younger ages (figure 2) suggesting that dominance is costly by reducing lifespan.
Natural selection will reward investment in dominance only if the benefits of dominance outweigh the costs of reduced lifespan. Given that extra-pair fertilizations are practically absent in jackdaws [31,32], only reproductive success with the partner could outweigh the negative effect of a shortened lifespan. However, we previously showed that dominant jackdaws in this colony consistently achieved lower reproductive success than subdominants, and hence it was concluded that more dominant birds had lower fitness [24]. Our finding in this study that more dominant birds have shorter lifespans indicates that the negative fitness effect of dominance in our colony is even stronger than we assumed on the basis of data on reproductive success. We previously discussed possible mechanistic explanations of this finding, and suggested that dominants in this study colony may have had very high testosterone titres, because they were involved in substantially more agonistic interaction than subdominants, with negative effects on care for offspring and partner [24]. We believe this to be a specific feature of our colony, related to the close proximity of the nest-boxes (see [24] for a full discussion). Given that experimentally increased testosterone level has been shown to reduce survival in another bird species [33], high testosterone may also be involved in the dominance effect on lifespan. In addition to testosterone, stress hormones (cortisol, corticosterone) may also be involved, because this often varies with rank, and literature reviews suggest that dominants have higher stress hormone levels in particular when being dominant involves more aggression [34–37].
Terminal declines have been reported in several traits [5,38–40], but the underlying mechanism determining whether physiology declines gradually or through a collapse prior to death/disappearance remains poorly understood. We previously showed that the rate of telomere attrition is strongly elevated in the year before disappearance from the colony [27], and speculated that this could reflect a more general physiological collapse heralding death. If such a terminal decline indeed characterizes jackdaw senescence, we expected that this would also be apparent on the behavioural level. In agreement with our telomere results, we found that birds which were in their last year substantially lost dominance status, which contrasted with the steady increase observed in the years up to this point (figure 3). This finding is in line with a study in lemurs showing that the oldest individuals could not maintain high social status [12], but we are not aware of other studies demonstrating such effects. Terminal declines may reflect a general physiological collapse prior to death and apparently both social dominance and telomere dynamics could potentially function as a biomarker of such effects.
Phenotypic state, dominance and resource acquisition can be viewed as three interacting factors, which may all be independently affected by age. This interaction can be positive, with animals climbing up the social ladder, which through knock-on effects on resource acquisition may enhance social dominance via positive resource effects on somatic state (figure 4a). We found that dominance increased with age within individuals, and we interpret this to be at least in part the result of a positive reinforcement loop across these three factors (figure 4a). An increase in phenotypic state will support higher dominance, which in turn increases the availability of resources for somatic maintenance or repair. An upward spiral over time can thus be envisioned (figure 4a), counteracting negative age effects on physiological state (i.e. physiological senescence). Such processes may explain why early in life reproductive success generally increases with age. However, at some point in time, physiological state may deteriorate sufficiently owing to senescence, to cause a decline in social dominance, in particular in species such as jackdaws where the more dominant individuals participate in more agonistic interactions [24], suggesting that dominance becomes too costly to maintain. When such a threshold is crossed, the reinforcing loop may reverse, leading to a collapse through negative reinforcement, with reduced dominance leading to lower resource acquisition, leading to lower phenotypic state and so on (figure 4b). Thus, phenotypic state and social dominance should perhaps not be seen as stable factors, but instead as dynamically interacting traits, and we see the characterization of these dynamics as the way ahead.
Figure 4.
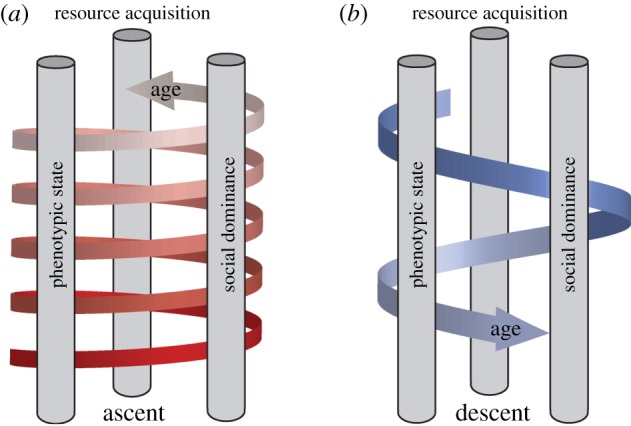
Reinforcement effects of behaviour up and down the social ladder. (a) Positive and (b) negative reinforcement leading to increasing and decreasing social dominance with age at different life stages. Each pillar (phenotypic state, dominance and the ability to secure resources) will positively affect the next pillar, causing an upward spiral with age (a). Physiological state deteriorates with age owing to senescence, ultimately turning the direction of the effects (b). (Online version in colour.)
Behaviour is not generally considered as part of the senescence process, probably, because most senescence research is carried out in model organisms in the laboratory. Behaviour is of limited importance in captivity, because social competition for resources is usually minimized, and predators are absent. By contrast, we propose that under natural conditions different aspects of behaviour may delay or accelerate the senescence process. Senescence may be delayed owing to increasing dominance, knowledge and experience, whereas senescence may be accelerated when an initial decline in performance is amplified owing to downstream effects on, for example, the ability to secure resources. Cognitive abilities may be as important in this context as competitive abilities; indeed, in figure 4, behaviour (social dominance) can be replaced with cognitive abilities (learning, memory), and perhaps also with other aspects of behaviour such as sexual signalling. Thus, we suggest that behaviour is an integral part of the ageing syndrome and studying social life histories and other aspects of behaviour may therefore be important to understand the senescence process.
Supplementary Material
Supplementary Material
Acknowledgements
Comments of two anonymous referees improved the paper.
The study was carried out under licence from the Ethical Committee for animal experiments of the University of Groningen.
Funding statement
H.M.S was supported by an NWO Vici grant to S.V.
References
- 1.Partridge L, Barton NH. 1993. Optimality, mutation and the evolution of aging. Nature 362, 305–311. ( 10.1038/362305a0) [DOI] [PubMed] [Google Scholar]
- 2.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 3.Berdoy M, Smith P, MacDonald DW. 1995. Stability of social status in wild rats: age and the role of settled dominance. Behaviour 132, 193–212. ( 10.1163/156853995X00694) [DOI] [Google Scholar]
- 4.Favre M, Martin JGA, Festa-Bianchet M. 2008. Determinants and life-history consequences of social dominance in bighorn ewes. Anim. Behav. 76, 1373–1380. ( 10.1016/j.anbehav.2008.07.003) [DOI] [Google Scholar]
- 5.Nussey DH, Coulson T, Delorme D, Clutton-Brock TH, Pemberton JM, Festa-Bianchet M, Gaillard J-M. 2011. Patterns of body mass senescence and selective disappearance differ among three species of free-living ungulates. Ecology 92, 1936–1947. ( 10.1890/11-0308.1) [DOI] [PubMed] [Google Scholar]
- 6.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 7.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker GAG. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 9.Arcese P, Smith JNM. 1985. Phenotypic correlates and ecological consequences of dominance in song sparrows. J. Anim. Ecol. 54, 817–830. ( 10.2307/4380) [DOI] [Google Scholar]
- 10.Henderson IG, Hart PJB. 1995. Dominance, food acquisition and reproductive success in a monogamous passerine: the jackdaw Corvus monedula. J. Avian Biol. 26, 217–224. ( 10.2307/3677322) [DOI] [Google Scholar]
- 11.Weiss BM, Kotrschal K, Foerster K. 2011. A longitudinal study of dominance and aggression in greylag geese (Anser anser). Behav. Ecol. 22, 616–624. ( 10.1093/beheco/arr020) [DOI] [Google Scholar]
- 12.Aujard F, Perret M. 1998. Age-related effects on reproductive function and sexual competition in the male prosimian primate, Microcebus murinus. Physiol. Behav. 64, 513–519. ( 10.1016/S0031-9384(98)00087-0) [DOI] [PubMed] [Google Scholar]
- 13.Thouless CR, Guinness FE. 1986. Conflict between red deer hinds: the winner always wins. Anim. Behav. 34, 1166–1171. ( 10.1016/S0003-3472(86)80176-2) [DOI] [Google Scholar]
- 14.Bridge C, Field J. 2007. Queuing for dominance: gerontocracy and queue-jumping in the hover wasp Liostenogaster flavolineata. Behav. Ecol. Sociobiol. 61, 1253–1259. ( 10.1007/s00265-007-0355-9) [DOI] [Google Scholar]
- 15.Ens BJ, Weissing FJ, Drent RH. 1995. The despotic distribution and deferred maturity: two sides of the same coin. Am. Nat. 146, 625–650. ( 10.1086/285818) [DOI] [Google Scholar]
- 16.Wiley RH, Rabenold KN. 1984. The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution 38, 609–621. ( 10.2307/2408710) [DOI] [PubMed] [Google Scholar]
- 17.East ML, Hofer H. 2001. Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 12, 558–568. ( 10.1093/beheco/12.5.558) [DOI] [Google Scholar]
- 18.DuVal EH. 2012. Variation in annual and lifetime reproductive success of lance-tailed manakins: alpha experience mitigates effects of senescence on siring success. Proc. R. Soc. B 279, 1551–1559. ( 10.1098/rspb.2011.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert KA, Mennill DJ, Ramsay SM, Otter KA, Boag PT, Ratcliffe LM. 2007. Variation in social rank acquisition influences lifetime reproductive success in black-capped chickadees. Biol. J. Linn. Soc. 90, 85–95. ( 10.1111/j.1095-8312.2007.00713.x) [DOI] [Google Scholar]
- 20.Röell A. 1978. Social behaviour of the jackdaw, Corvus monedula, in relation to its niche. Behaviour 64, 1–124. ( 10.1163/156853978X00459) [DOI] [Google Scholar]
- 21.Wechsler B. 1988. Dominance relationships in jackdaws (Corvus monedula). Behaviour 106, 252–264. ( 10.1163/156853988X00278) [DOI] [Google Scholar]
- 22.van de Pol M, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758. ( 10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 23.Salomons HM, Dijkstra C, Verhulst S. 2008. Strong but variable associations between social dominance and clutch sex ratio in a colonial corvid. Behav. Ecol. 19, 417–424. ( 10.1093/beheco/arm149) [DOI] [Google Scholar]
- 24.Verhulst S, Salomons HM. 2004. Why fight? Socially dominant jackdaws, Corvus monedula, have low fitness. Anim. Behav. 68, 777–783. ( 10.1016/j.anbehav.2003.12.020) [DOI] [Google Scholar]
- 25.Tamm S. 1977. Social dominance in captive jackdaws (Corvus monedula). Behav. Proc. 2, 293–299. ( 10.1016/0376-6357(77)90032-8) [DOI] [PubMed] [Google Scholar]
- 26.Gammell MP, de Vries H, Jennings DJ, Carlin CM, Hayden TJ. 2003. David‘s score: a more appropriate dominance ranking method than Clutton-Brock et al.'s index. Anim. Behav. 66, 601–605. ( 10.1006/anbe.2003.2226) [DOI] [Google Scholar]
- 27.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snijders TAB, Bosker RJ. 2011. Multilevel analysis: an introduction to basic and advanced multilevel modeling. London, UK: Sage. [Google Scholar]
- 29.Boonekamp JJ, Salomons M, Bouwhuis S, Dijkstra C, Verhulst S. 2014. Reproductive effort accelerates actuarial senescence in wild birds: an experimental study. Ecol. Lett. 17, 599–605. ( 10.1111/ele.12263) [DOI] [PubMed] [Google Scholar]
- 30.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. ( 10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson IG, Hart PJB, Burke T. 2000. Strict monogamy in a semi-colonial passerine: the jackdaw Corvus monedula. J. Avian Biol. 31, 177–182. ( 10.1034/j.1600-048X.2000.310209.x) [DOI] [Google Scholar]
- 32.Liebers D, Peter HU. 1998. Intraspecific interactions in jackdaws Corvus monedula: a field study combined with parentage analysis. Ardea 86, 221–235. [Google Scholar]
- 33.Dufty AM. 1989. Testosterone and survival: a cost of aggressiveness? Horm. Behav. 23, 185–193. ( 10.1016/0018-506X(89)90059-7) [DOI] [PubMed] [Google Scholar]
- 34.Abbott DH, et al. 2002. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67–82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 35.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 36.Kotrschal K, Hirschenhauser K, Möstl E. 1997. The relationship between social stress and dominance is seasonal in greylag geese. Anim. Behav. 55, 6 ( 10.1006/anbe.1997.0597) [DOI] [PubMed] [Google Scholar]
- 37.Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. 2011. Life at the top: rank and stress in wild male baboons. Science 333, 357–360. ( 10.1126/science.1207120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulson T, Fairweather JA. 2001. Reduced reproductive performance prior to death in the black-legged kittiwake: senescence or terminal illness? J. Avian Biol. 32, 146–152. ( 10.1034/j.1600-048X.2001.320207.x) [DOI] [Google Scholar]
- 39.Rattiste K. 2004. Reproductive success in presenescent common gulls (Larus canus): the importance of the last year of life. Proc. R. Soc. Lond. B 271, 2059–2064. ( 10.1098/rspb.2004.2832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed TE, Kruuk LEB, Wanless S, Frederiksen M, Cunningham EJA, Harris MP. 2008. Reproductive senescence in a long-lived seabird: rates of decline in late life performance are associated with varying costs of early reproduction. Am. Nat. 171, E89–E101. ( 10.1086/524957) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


