Abstract
The role of heat shock proteins (HSPs) in heat tolerance has been demonstrated in cultured cells and animal tissues, but rarely in whole organisms because of methodological difficulties associated with gene manipulation. By comparing HSP70 expression patterns among representative species of reptiles and birds, and by determining the effect of HSP70 overexpression on embryonic development and hatchling traits, we have identified the role of HSP70 in the heat tolerance of amniote embryos. Consistent with their thermal environment, and high incubation temperatures and heat tolerance, the embryos of birds have higher onset and maximum temperatures for induced HSP70 than do reptiles, and turtles have higher onset and maximum temperatures than do lizards. Interestingly, the trade-off between benefits and costs of HSP70 overexpression occurred between life-history stages: when turtle embryos developed at extreme high temperatures, HSP70 overexpression generated benefits by enhancing embryo heat tolerance and hatching success, but subsequently imposed costs by decreasing heat tolerance of surviving hatchlings. Taken together, the correlative and causal links between HSP70 and heat tolerance provide, to our knowledge, the first unequivocal evidence that HSP70 promotes thermal tolerance of embryos in oviparous amniotes.
Keywords: critical thermal maximum, embryonic development, gene overexpression, HSP70, thermal adaptation, thermotolerance
1. Introduction
Thermal tolerance is critical for the survival of most organisms that are often exposed to extreme temperatures, which are common in both terrestrial and aquatic environments [1,2]. Of all physiological and biochemical mechanisms by which an organism can resist thermal extremes [3], heat shock proteins (HSPs) are the most well known and extensively studied system in diverse species from bacteria to plants and animals [1,4–6].
HSPs are involved in transport, folding, assembly and degradation of other proteins; and these tasks are enhanced when organisms are exposed to extreme temperatures as well as to other stressful conditions, such as hypoxia, toxic substances and disease [5,7]. The early literature on HSPs continues to be massive, but few papers address the evolution, ecology and, more specifically, thermal adaptation of HSPs [5,6]. Of the studies that do address the evolutionary and ecological studies of HSPs, most focus on model organisms (e.g. Drosophila) or aquatic animals (especially marine invertebrates): terrestrial vertebrates have received far less attention [4,5,7–10].
HSP70 is the most important and well-studied protein of all HSP families that protect cells, tissues and whole organisms from severe thermal stress. Even so, high levels of HSP70 expression probably impose costs on individuals exposed to thermal stress by influencing their fecundity, growth or survival [5,7–9,11]. Most of these studies infer links between the high expression of HSP70 and thermal adaptation by comparing HSP70 and heat tolerance of animals from different seasons or from different thermal environments. In general, these multi-species comparisons find that HSP70 expression (e.g. the onset (Ton) and maximum (Tmax) temperatures at which HSPs are expressed) correlates positively with the level of stress to which an animal is exposed [5,12,13]. Nonetheless, correlation does not imply causation; organisms and cells could respond to stress in many other ways than involving HSP70. More direct evidence comes from experiments that manipulate HSP70 expression, thereby providing a functional link between HSP70 and heat tolerance.
Transgenic technology, gene knockout and RNA interference have been widely used to study gene function in model organisms [14,15]. However, such technologies are currently challenging, if not impossible, to adopt with higher multicellular animals, which still lack pure strains and cell lines. An alternative way to manipulate gene expression is to use a vector (e.g. plasmid) to transfer a target gene fragment into a target animal. The focal gene is therefore overexpressed, and the recombinant plasmid with functional genes can persist for one to two months without degradation [16,17]. This molecular method thus provides us an effective way to manipulate the hsp70 gene in higher animals such as vertebrates [16].
Embryos are probably more vulnerable to extreme temperature than are post-embryonic individuals, simply because most embryos cannot move to evade extreme temperatures [18]. Given that embryos of oviparous species are constrained within eggs and thus have limited capability to behaviourally thermoregulate to avoid extreme temperatures (but see [19]), embryos might be expected to have well developed capacities to enhance physiological tolerance when exposed to extreme temperatures [18]. Many studies have looked at heat tolerance of embryos or larvae in invertebrates, especially in Drosophila [4,18,20,21]. Rarely, however, has thermal tolerance during embryonic development—or its mechanistic bases—been reported in vertebrate embryos (but see [22,23]).
Extreme high temperatures are probably one of the most common and important stresses experienced by reptile and bird embryos [24,25]. In field nests, for example, reptile and bird embryos may experience fluctuating temperatures and extreme high temperatures that exceed 40°C, at least when reptile eggs are laid in shallow nests or when brooding birds leave the nest to forage [22,26]. Survival of such embryos then depends on their resistance to heat stress, as extreme high temperatures may easily cause embryo damage or even death [22]. Even within the viable range of temperatures, high temperature during embryogenesis can affect the morphology, performance and survival of hatchlings [24]. In addition, cold temperatures may also impose severe stress on reptile embryos and hatchlings, particularly for those species that overwinter as hatchlings in the nest [27,28]. Therefore, embryos provide an excellent model system to study the ecological and evolutionary responses of HSPs to thermal stress. To date, however, such studies are still rare for embryos of egg-laying amniotes.
In this study, we began by comparing HSP70 expression in embryos in response to high temperatures in some representative species from different lineages of reptiles and birds, including a lizard, Takydromus septentrionalis, a turtle, Pelodiscus sinensis, a quail, Coturnix coturnix and a duck, Anas platyrhynchos domestica. Then, we identified the effect of HSP70 overexpression on heat tolerance of embryos by injecting recombinant plasmids with the hsp70 gene into P. sinensis embryos, thus inducing HSP70 overexpression. These experiments evaluate the role of HSP70 in the heat tolerance of reptile and bird embryos in two complementary ways: (i) whether variation in heat tolerance among species is positively and strongly correlated with HSP70 expression, and (ii) whether variation in heat tolerance within species is casually linked with HSP70 expression. In addition, we also measured offspring traits (e.g. thermal tolerance, locomotor performance and early growth) following the manipulations to evaluate potential costs of HSP70 overexpression. Based on studies of HSPs and thermal tolerance of invertebrates [1,4–6], we predicted that: (i) the Ton and Tmax of HSP70 induction would correlate positively with embryonic heat tolerance and also with the temperatures at which these embryos normally develop, and (ii) HSP70 overexpression would enhance the heat tolerance of embryos, but impose costs on embryos as well as hatchlings.
2. Materials and methods
(a). Interspecific comparison of hsp70 expression
(i). Study species
We used two species of reptiles (T. septentrionalis, the northern grass lizard, and P. sinensis, Chinese soft-shelled turtle) and two species of birds (C. coturnix, the quail, and A. platyrhynchos, the Pekin duck) to study the Hsp70 expression pattern in response to heat stress during embryonic development. The northern grass lizard is a small oviparous lacertid reptile (maximum length: 80 mm snout-vent length) found in China [29]. The optimal incubation temperatures for the lizard ranged from 24°C to 27°C [30]. The Chinese soft-shelled turtle is widely distributed in mainland China and southeastern Asia [29]. The optimal incubation temperatures for the turtle ranged from 27°C to 30°C [31,32]. The quail is a common and widespread domestic bird from Phasianidae. The Pekin duck is a large species from Anatidae. The optimal incubation temperatures for these two species are around 37.5°C [25].
(ii). Egg collection and incubation
We obtained 60 fertilized P. sinensis eggs (each from different clutches and with a white patch on the shell surfaces; mean egg mass = 5.41 g) from a turtle farm in Zhejiang, China. Takydromus septentrionalis eggs were collected from field-caught lizards maintained at our laboratory (mean mass = 0.30 g; n = 42 (16 clutches)). The reptilian eggs were randomly allocated to plastic containers (25 × 20 × 10 mm) filled with moist vermiculite of −220 kPa (1 g water per 1 g vermiculite) and maintained in incubators (Ningbo Life Science and Technology Ltd, China) at 28°C for turtle eggs or 24°C for lizard eggs, close to the mean temperature of artificial nests in the field in each species [30,31]. We obtained 42 fertilized quail eggs (each from different clutches, mean mass = 11.16 g), and 42 duck eggs (each from different clutches, mean mass = 95.74 g) from a commercial bird ranch in Beijing, China. The avian eggs were maintained at 37.5°C in an incubator (Grumbach, Compact S84, Germany).
(iii). Tissue collection
To make the data comparable among species, we determined the expression of HSP70 in the liver of late embryos (70% embryonic development throughout their total developmental time) at a series of temperatures from 24°C to the species-specific upper thermal limit for embryonic development. At each temperature treatment, six eggs from each species were exposed for 1 h and then recovered for 1 h at their incubation temperature. The eggs were dissected; the embryo livers were immediately isolated and stored at −80°C for later analysis. Because T. septentrionalis embryos were too tiny to isolate livers, the whole body was frozen and stored for later analysis. The different tissue sampling techniques among species are unlikely to confound our results because hsp70 expression of the whole body is similar to that of the liver in lizards [33].
(iv). Real-time quantitative polymerase chain reaction and Western blot analysis
Fluorescent real-time quantitative polymerase chain reaction (PCR) was performed in a 10 μl total volume, including premix, primers, cDNA template and water to quantify the expression of hsp70 mRNA. Each reaction was performed in triplicate and normalized using β-actin. Western blot analysis followed methods in Huang & Kang [13] with some modifications to determine the expression of the HSP70 protein. A loading control of Coomassie blue staining was used in the Western blot as described by Oshima et al. [34] with some modification. HSP70 expression was quantified as the quotient between HSP70 band intensities and total protein intensities in each lane (see details in the electronic supplementary material, Methods).
(v). The onset (Ton) maximal (Tmax) temperatures for the expression of hsp70 mRNA and HSP70 protein
We designated the first temperature at which the expression level of hsp70 mRNA or HSP70 protein was significantly higher than that of the 24°C control group as the Ton for hsp70 gene synthesis. The temperature at which the expression level was highest was denoted as the Tmax [10,13]. An analysis of variance (ANOVA) was used to determine thermal dependence of the expression level of hsp70 mRNA and HSP70. A Tukey's post hoc multiple comparison test was used to detect differences among temperatures. To understand the relationships between the Ton and Tmax of hsp70 mRNA expression and those of HSP70 protein expression as well as the relationships between the Ton and Tmax of HSP70 expression and the lethal temperature for embryonic development, we used Z-transform tests to combine the probability from independent tests of correlations between these variables [35].
(b). The effect of HSP70 overexpression on embryonic development and hatchling traits
(i). Construction of plasmid pIRES2-EGFP with turtle hsp70 sequence
The total RNA and first strand cDNA of the turtle were prepared using the methods previously described. The hsp70 coding region of P. sinensis was obtained by PCR amplification using cDNA as the template. The primers used were PsHsp70 F1 (5′-CATATGATATCGCCACCATGTCCGGCAAAGCGCCT-3′, underlined sequence, EcoRV site) and PsHsp70 R1 (5′- CTCGAGATATCGTCGACTTCTTCAATGGTTGGT-3′, underlined sequence, EcoRV site), which were designed on the basis of the reported P. sinensis hsp70 mRNA sequence (JF694990.1). The fragment was then ligated to PMD 19-T Simple Vector (TaKaRa) using T4 DNA ligase (TaKaRa), following the manufacturer's protocol. The 1.9 kb turtle hsp70 gene fragment was excised from the T vector using EcoRV and inserted into pIRES2-EGFP at SmaI site to construct pIRESPsHsp70.
(ii). Study species and experimental treatment
We collected P. sinensis eggs from a turtle farm in Zhejiang, China to study the effect of HSP70 overexpression on embryonic development and hatchling traits. To avoid pseudo-replication, one egg was collected from each clutch to use in the following experiments. The collected eggs were incubated in plastic containers (25 × 20 × 10 mm) filled with moist vermiculite of −220 kPa, and randomly assigned to two treatments: 34°C sham control and 34°C treatment of HSP70 overexpression. The eggs from HSP70 overexpression treatments were injected with 15 µg of plasmid pIRES2-EGFP (Clontech, Mountain View, CA) cloned with turtle hsp70 sequences at the edge of the chalky white spot (indicative of fertilization) using 1 ml syringes, whereas those from sham controls were only injected with 15 μg of plasmid pIRES2-EGFP. In addition, a 34°C phosphate buffered saline (PBS) control was used in the experiments of detecting plasmids and HSP70 expression; eggs from this group were injected with 15 µg PBS. The injection was done on an aseptic laboratory table, and the puncture was sealed with beeswax subsequently.
(iii). Detection of plasmids and HSP70 expression
The embryos (n = 5 for each treatment) at two weeks after injection and the liver of hatchlings (n = 5 for each treatment) were used for DNA extraction using the EasyPure Genomic DNA Kit (Transgen, Beijing, China) as well as total RNA extraction and cDNA synthesis as previously described. PCR, fluorescence immunohistochemistry and Western blot were used to detect the expression of exogenous HSP70. The embryos and hatchling livers were washed with PBS and then fixed in 4% paraformaldehyde for 24 h. Fixed samples were dehydrated in 30% sucrose for 48 h, embedded in specimen holders, frozen in a cryostat machine and sectioned using a microtome. The sections (14 µm thick) were observed under a fluorescence microscope (Nikon Eclipse 90i, Japan). ANOVA and a Tukey's post hoc multiple comparison test were used to compare among-treatment differences in the expression level of HSP70.
(iv). Thermal tolerance of embryos
Thermal tolerance of embryos (roughly at stage 20, based on the normal series of embryonic development in the turtle [36]; n = 11 for sham control and n = 13 for HSP70 overexpression) was measured two weeks after the embryos had been injected with the plasmids. We candled the egg to localize the embryo, inserted the thermocouple (TCTTT140: Temperature Controls Pty Ltd., Sefton, Australia) near the embryo and then sealed the puncture using paraffin wax to prevent moisture loss. This methodology was used in other reptile eggs and did not affect embryo survival [37]. Eggs were heated from 34°C in a closed 10 × 10 × 6 cm plastic container containing damp vermiculite (−200 kPa) inside an incubator at an increasing rate of 1.2°C min−1. Internal temperatures of eggs were monitored every minute using the thermocouples connected to a data-taker (DT-80: Datataker, Scoresby, Australia). When the temperature reached 41°C, the egg was placed inside an infrared heart-rate detector (Buddy Digital Egg Monitor: Avian Biotech, Cornwall, UK; see [37] to monitor heart rate). Immediately after this recording, the egg was returned to the plastic container, and the heart rate of embryos was monitored at 0.5°C increments thereafter. The entire procedure was repeated until we were unable to detect a heartbeat. The highest temperature at which we could record a heart rate was used as the upper thermal limit of embryonic tolerance (i.e. upper lethal temperature) [23]. Student's t-test was used to detect the effect of HSP70 expression on the upper lethal temperature of embryos.
(v). Hatching success and hatchling traits
Hatching success. A total of 174 eggs (n = 79 for sham control and n = 95 for HSP70 overexpression) were incubated at the two treatments. Once the first egg piped in each treatment, the jars in that treatment were monitored once a day for newly emerging hatchlings. Hatching success was expressed as the percentage of eggs that hatched live turtles at the end of experiment. A two-by-two frequency table was used to detect the effect of HSP70 expression on the hatching success of embryos.
Body size and locomotor performance. After emergence, the hatchlings were weighed to the nearest 1 mg after the yolk had been entirely absorbed. Prior to the locomotion test, the hatchlings were placed in an incubator at 29°C for 1 h. We then chased each hatchling along a 1.2 m racetrack filled with 40 mm of deep water at a constant temperature of 29°C, and recorded its swimming performance using a Panasonic video camera. Videotapes were later examined for sprint speed in the fastest 30 mm interval. One-way ANCOVAs with egg mass as the covariate or one-way ANOVAs were used to determine the influence of HSP70 expression on hatchling body mass or the swimming speed of hatchlings.
Critical thermal maximum (CTmax). The hatchlings (n = 21 for sham control and n = 25 for HSP70 overexpression) were heated from 28°C at the rate of 0.25°C per minute in an incubator. The body temperature (cloacal temperature) associated with a transient loss of righting response (the testing animals did not respond to intense stimulation and could not return to the upright position when turned over) at high thermal limits was used as endpoints for critical thermal maximum [38]. Student's t-test was used to detect the effect of HSP70 expression on the CTmax of hatchlings.
Post-hatching growth. The remain hatchlings (n = 15 for sham control and n = 43 for HSP70 overexpression) were individually kept in cages placed in a temperature-controlled room at 28 ± 1°C, with a 12 L : 12 D cycle, and fed with commercial food (approx. 10% water, 60% proteins, 5% lipids, 5% carbohydrates and 20% minerals) ad libitum. The turtles were monitored for early growth for two months. One-way ANOVAs were used to determine the influence of HSP70 expression on the specific growth rate of hatchlings.
3. Results
(a). Interspecific comparison of HSP70 expression
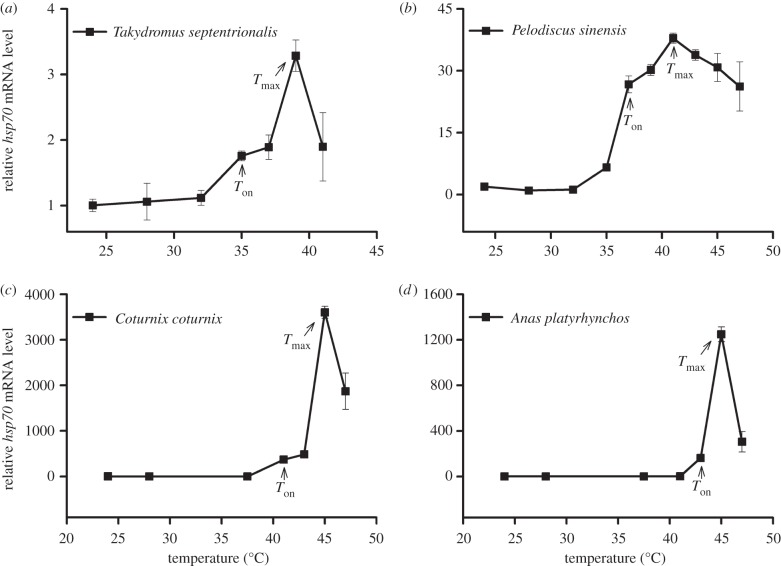
In the embryos of reptiles and birds investigated, hsp70 gene expression was significantly induced under heat stress, but decreased at overly high temperatures (figure 1: T. septentrionalis, F6,59 = 22.8, p < 0.0001; P. sinensis, F9,118 = 157.5, p < 0.0001; C. coturnix, F6,88 = 466.5, p < 0.0001; A. platyrhynchos, F6,88 = 268.3, p < 0.0001). Tukey's test indicated that Ton and Tmax of hsp70 mRNA expression were 35°C and 39°C in T. septentrionalis, 37°C and 41°C in P. sinensis, 41°C and 45°C in C. coturnix, and 43°C and 45°C in A. platyrhynchos, respectively. The Ton and Tmax of reptile embryos were lower than those of bird embryos (figure 1). Within reptiles, the Ton and Tmax for T. septentrionalis embryos were lower than those for P. sinensis embryos. In addition, the hsp70 gene under heat stress increased many times more in bird (3603 times normal levels in C. coturnix) than in reptile (38 times in P. sinensis) embryos (figure 1).
Figure 1.
Expression profiles of hsp70 mRNA in the embryos of (a,b) two reptilian species and (c,d) two avian species exposed to different temperatures. For all species, temperatures ranged from 24°C to lethal temperature. The first temperature at which the expression level was significantly higher than control (24°C) was described as onset temperature (Ton) for hsp70 expression. The temperature at which the expression level of hsp70 was highest was described as maximal expression temperature (Tmax). For each species, relative hsp70 mRNA levels were expressed as the factorial increase above the baseline level of the control. Data are shown as means ± s.e. (n = 6 for each temperature treatment). Differences among mean values were determined using Tukey's post hoc test.
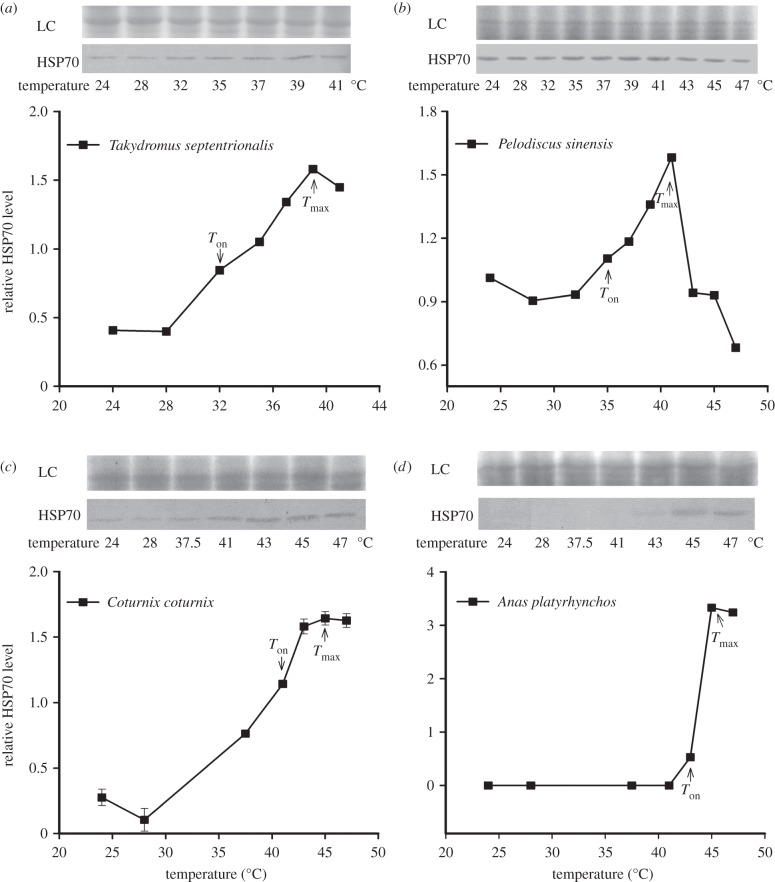
The heat-induced expression of hsp70 was also seen in protein levels (figure 2: T. septentrionalis, F6,14 = 1801.4, p < 0.0001; P. sinensis, F9,20 = 36635, p < 0.0001; C. coturnix, F6,14 = 432.1, p < 0.0001; A. platyrhynchos, F6,14 = 50061, p < 0.0001). Tukey's test indicated that Ton and Tmax of HSP70 expression were 32°C and 39°C in T. septentrionalis, 35°C and 41°C in P. sinensis, 41°C and 45°C in C. coturnix, and 43°C and 45°C in A. platyrhynchos, respectively (figure 2). Thus, patterns of Ton and Tmax of HSP70 protein expression were very consistent with those of hsp70 mRNA expression (r = 0.999, n = 4, p < 0.00001). In addition, when the eggs were exposed to extreme high temperatures for 1 h, T. septentrionalis embryos died at 41°C, which was lower than that (47°C) of P. sinensis and two bird-species embryos. This among-species difference in the lethal temperature corresponded to the difference in the Ton and Tmax of HSP70 expression (r = 0.963, n = 4, p < 0.05).
Figure 2.
Western blot analysis of HSP70 protein in the embryos of (a,b) two reptilian species and (c,d) of two avian species. Treatments are the same as in figure 1. Each lane of a 10% SDS–polyacrylamide gel was loaded with equal amounts of protein (123 µg for Takydromus septentrionalis, 50 µg for Pelodiscus sinensis, 221 µg for Coturnix coturnix, 528 µg for Anas platyrhynchos). HSP70 expression was quantified as the quotient between HSP70 band intensities and total protein intensities (the loading control of Coomassie blue staining, LC) in each lane. The first temperature at which the expression level was significantly higher than the control (24°C) was described as onset temperature (Ton) for HSP70 expression. The temperature at which the expression level of HSP70 was highest was described as maximal expression temperature (Tmax). Data are shown as means ± s.e. (n = 3 for each temperature treatment). Differences among mean values were determined using Tukey's post hoc test.
(b). Effect of HSP70 overexpression on embryonic development and hatchling traits
(i). Expression of PsHSP70 in turtle tissues
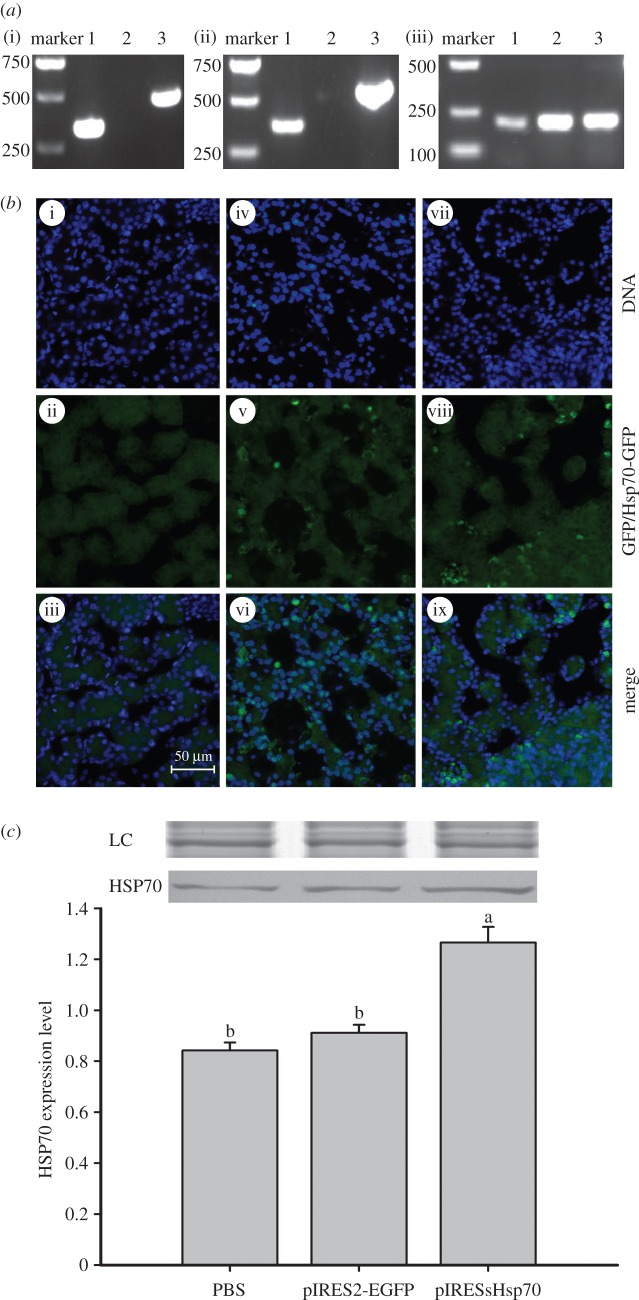
When transported into turtle tissues, the plasmid was stable as shown by comparing pIRESPsHsp70 and pIRES2-EGFP in turtle embryos (figure 3a(i)). mRNA transcripts of pIRESPsHsp70-derived PsHsp70 were detected in embryos injected with pIRESPsHsp70, but not in those with pIRES2-EGFP (figure 3a(ii)). Consistent with the distribution and transcription results, the fluorescence observation showed that both pIRESPsHsp70 and pIRES2-EGFP could be detected in embryos with green fluorescence (figure 3b). The protein level of HSP70 was significantly higher in embryos injected with pIRESPsHsp70 than embryos injected with pIRES2-EGFP or PBS (F2,6 = 81.48, p < 0.0001; figure 3c). Similar results of HSP70 expression were also seen in livers of the hatchlings (electronic supplementary material, figure S1).
Figure 3.
Detection of the plasmids and the expression of PsHSP70 in the embryos of Pelodiscus sinensis. (a) hsp70 gene expression. Embryos injected with pIRESPsHsp70 (lane 1) or pIRES2-EGFP (lanes 2 and 3) were used. (i) Extracted DNA was used for PCR with primers specific for pIRESPsHsp70 (lanes 1 and 2) or specific for pIRES2-EGFP (lane 3); (ii) extracted RNA was used for PCR with the primers described in (a); (iii) extracted RNA was used for PCR with primers specific to β-actin (internal control). (b) Distribution and expression of plasmids. Embryos injected with either PBS (i–iii), pIRES2-EGFP (iv–vi) or pIRESPsHsp70 (vii–ix) were fixed and observed under fluorescence microscope for DAPI nuclear staining (i, iv, vii) with an exposure time of 20 ms or green fluorescence with an exposure time of 200 ms (ii, v, viii). (c) HSP70 expression in embryos injected with PBS, pIRES2-EGFP or pIRESPsHsp70. The loading amounts of total protein were all 300 µg. HSP70 expression was quantified as the quotient between HSP70 band intensities and total protein intensities (the loading control of Coomassie blue staining, LC) in each lane. Bands of LC were shown above the Western blots. Data are shown as means ± s.e. (n = 3 for each treatment). Means with different letters above the error bars are statistically different (Tukey's post hoc test).
(ii). Thermal tolerance and hatching success of turtle embryos
The upper lethal temperature of embryos was greater in the treatment of HSP70 overexpression than in the sham control when turtle eggs were incubated at 34°C (t21 = 2.86, p < 0.01; figure 4a). Correspondingly, hatching success of eggs was considerable higher in the treatment of HSP70 overexpression (78%) than the sham control (52%; χ2 = 13.01, p < 0.001; electronic supplementary material, figure S2).
Figure 4.
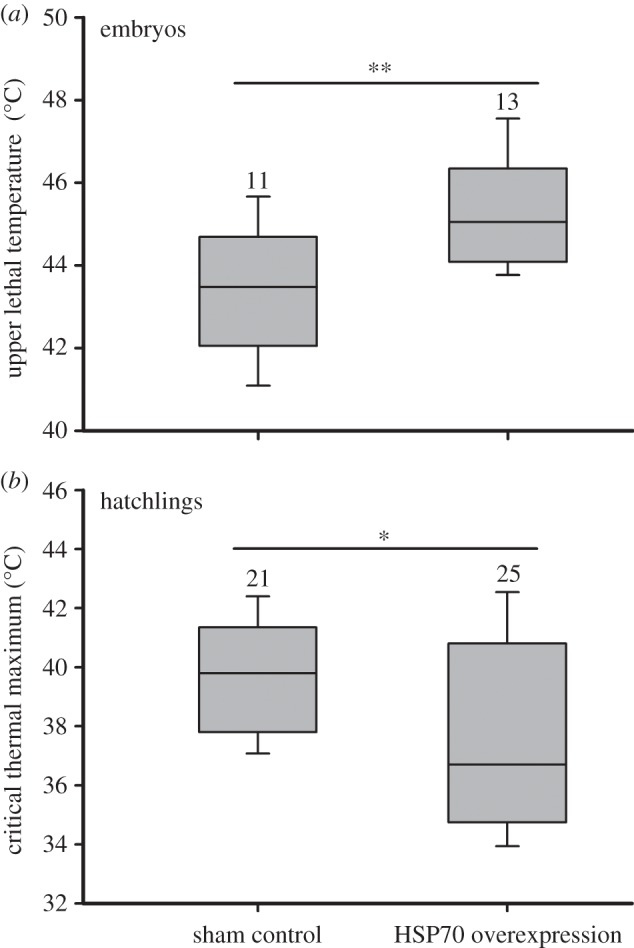
The upper lethal temperature of (a) embryos and (b) the critical thermal maximum of hatchlings from different HSP70 expression treatments in Pelodiscus sinensis. The eggs from HSP70 overexpression treatments were injected with pIRESPsHsp70, whereas those from the shame control with pIRES2-EGFP. For embryos, heart rate was monitored, and the highest temperature with a detectable heart rate was used as the critical thermal maximum. For hatchlings, body temperature associated with a transient loss of righting response at high temperature was used for the critical thermal maximum. Statistical significance between groups was calculated by Student's t test (*p < 0.05; **p < 0.01). Data are shown as means ± s.e. Numbers above the error bars are sample size.
(iii). Hatchling traits
When eggs were incubated at 34°C, HSP70 overexpression hatchlings had lower CTmax than did sham controls (t44 = 2.34, p = 0.02; figure 4b). However, HSP70 overexpression did not affect body mass (F1,55 = 1.62, p = 0.21), swimming speed (F1,56 = 3.01, p = 0.09) and specific growth rate (F1,56 = 0.45, p = 0.51) of hatchlings (electronic supplementary material, figure S3).
4. Discussion
HSPs play important roles in helping defend diverse organisms against thermal stress, and might be a general mechanism of evolutionary adaptation to extreme temperatures, along with behavioural, physiological or biochemical adaptations [6]. By comparing HSP70 expression among species and also by overexpressing HSP70 to identify its function, we provide, to our knowledge, the first unequivocal evidence that HSP70 affects the heat tolerance of oviparous amniote embryos. The Ton and Tmax of HSP70 induction were higher in the two bird than the two reptile embryos. Interestingly, HSP70 overexpression in the turtle (P. sinensis) enhanced heat tolerance and hatching success of embryos at extreme high temperatures, but later decreased the heat tolerance of surviving hatchlings, probably owing to the carryover effect of overexpression during embryonic stage. In the following, we discuss these results in turn as well as the role of HSP70 in the heat tolerance of amniote embryos.
The Ton and Tmax of heat shock response, which are tightly correlated with organismal thermotolerance, are largely determined by heat shock gene expression and differ significantly among species inhabiting thermally distinct environments (e.g. tropical versus arctic zones, terrestrial versus aquatic) [5,39–43]. Bird eggs are normally brooded by parents, but are sometimes directly exposed to solar radiation (hence extreme high temperature) when brooding parents leave for foraging [22]. The eggs of bird species in our study have relatively high optimal incubation temperatures (ca 38°C [25]) and high lethal temperatures (up to 47°C for acute exposure), whereas the turtle and lizard eggs are typically laid in underground nests and have much lower optimal temperatures (27–30°C for the turtle, and 24–27°C for the lizards) and lower lethal temperatures (up to 35°C or 33°C for chronic exposure, and 47°C or 41°C for acute exposure in the turtle or lizard) [30,31]. Similarly, Ton and Tmax of HSP70 expression were higher in the embryos of birds than of reptiles, and expression in embryos of the turtle was higher than in the lizard (figure 1). Although we investigated only four species in this study, the divergence between bird and reptile embryos in the Ton and Tmax of HSP70 expression is probably widespread, given most bird eggs have higher incubation temperatures (around 38°C) than do reptile eggs (mostly less than 32°C) [24,25,44]. In addition to mean temperatures, the fluctuation of nest temperatures significantly differ between bird and reptile eggs, with relative constant temperatures in bird nests, but much more fluctuation in reptile nests [24,25]. It would be of great interest to understand how HSPs function in response to temperature fluctuations in future studies.
Our results are generally consistent with patterns that have been widely reported in post-embryonic ectothermic animals from a diversity of lineages from arthropods to reptiles. The Ton and Tmax of HSP induction, which might be a result of separate evolutionary histories among species [12], are higher for both aquatic and terrestrial animals from warm regions than for those from cold regions [4,5,7,13,33,39]. Despite the overwhelming evidence of positive correlations between HSP70 expression and heat tolerance in diverse taxa, several reasons dictate that caution is still required to definitively link interspecific comparisons of inducible HSP70 expression to thermal adaptation. First, this positive correlation is not universal: for example, HSP70 expression and thermotolerance are not correlated in embryos of American horseshoe crab (Limulus polyphemus L.) [45], or in early embryos of fruitfly pests (Ceratitis capitata) [21]. In addition, some highly adapted species that inhabit extreme environments (such as Antarctica, deserts and intertidal zone) adopt alternative strategies to resist thermal stress: for example, some maintain a high constitutive level of HSP70 rather than enhancing the expression of inducible HSP70 [39,41,45–47]. Accordingly, the big difference in the expression of the hsp70 gene between bird and reptile embryos may not necessary reflect the difference in heat tolerance between the two lineages (figure 1). We do not understand why bird embryos show extremely high expression of the hsp70 gene compared with reptile embryos, but perhaps this could reflect strong responses to acute temperature change in bird embryos that normally develop at relatively constant temperatures, or high expression of the hsp70 gene is essential for HSP70 synthesis in birds. Second, the conclusions derived from interspecific comparisons may suffer confounding effects of some other factors, such as phylogenetic history of species [48] and phenotypic plasticity of HSP70 expression [11,12].
Although interspecific and interpopulation comparisons suggest that HSP70 expression is related to heat tolerance, direct evidence of a functional link between them requires experimentally manipulating HSP70 expression, as has been well established in cultured cells and animal tissues [49,50], but not in whole organisms (but see [20,51]). This is probably owing to the methodological difficulty of gene manipulations in non-model organisms (especially higher animals) for which genetic background information are still insufficient. In this study, we circumvented methodological obstacles of HSP overexpression in non-model organisms using a plasmid to transfer extra exogenous hsp70 genes into turtle embryos. Plasmids with the hsp70 gene were transported successfully into turtle embryos, and the hsp70 gene was expressed both at transcriptional and at translational levels (figure 3). This enabled us to overexpress HSP70 in the embryos and thereby unambiguously demonstrate that high expression of HSP70 enhanced the heat tolerance of non-model amniotes under acute heat exposure. Moreover, this manipulation also increased heat tolerance of embryos incubated at 34°C (figure 4a), which is near the upper temperature permitting embryonic development in this species [31]. Previous studies in Drosophila demonstrated that hsp70 copy number directly influences heat-induced expression of HSP70 and tolerance of high temperatures; flies with more gene copies showed greater HSP70 expression and had higher heat tolerance [20,51]. Other experiments on Drosophila also provide support for a functional link between HSP expression and heat tolerance. For example, laboratory artificial selection experiments showed that heat shock response may be regulated by genetic factors that shift during evolution in different temperature regimes [52]. By contrast, one study shows that Hsp70 expression is correlated with the heat tolerance of larvae, but not adult Drosophila [53].
HSP70 expression obviously generates benefits (e.g. increasing heat tolerance), but may also impose costs (e.g. decreasing fecundity, developmental and survival rates), because initiation of heat shock responses usually reduces synthesis of other proteins, and imposes greater energy costs and potentially toxic effects owing to high concentrations of HSPs [5,7,54,55]. In our study, artificial overexpression of HSP70 in turtle embryos generated benefits by increasing heat tolerance embryos, but also imposed costs: heat tolerance of hatchlings with overexpressed HSP70 was actually reduced for turtle embryos that had been incubated at a high temperature (figure 4b). This suggests that trade-offs between benefits and costs of HSP70 overexpression may exist not only within a life-history stage [5,55], but also between life-history stages.
Embryos of oviparous reptiles and birds are excellent models for studying the ecological adaptation of organisms to thermal stress, because temperature is one of the most important environmental factors that can significantly affect embryonic development and hatchling fitness and thus may act as a critical selection force in amniote embryos [2,24,56]. Our study not only explicitly demonstrates HSP70's role in the heat tolerance of amniote embryos, but also provides suggestions of future studies that can evaluate the ecological role of HSP expression in heat tolerance of amniote embryos. For one thing, embryos probably activate other families of HSPs (not just HSP70) in response to thermal stress [7,10]. Studies of these other families will be necessary to understand the expression patterns of HSPs among species and populations living in distinct thermal environments (e.g. seasonal variation and latitudinal cline), and thus to provide a broader picture of the response of HSPs to thermal and other stresses in the embryos of reptiles and birds. In addition, the composition and expression of the regulatory components underlying the HSP system perhaps vary among species as well as among populations from distinct thermal environments. These regulatory mechanisms include the structural and/or regulatory regions of HSPs, mutations in heat shock transcription factors or other pathways involved in HSP expression [10,33]. Third, other candidate genes and proteins than HSPs might be involved in thermal adaptation [57]. Novel molecular technologies such as transcriptomics and proteomics provide efficient tools to identify these kind of genes and proteins, and would therefore greatly deepen our knowledge on the molecular basis of embryonic response to thermal variation [6,58].
Supplementary Material
Acknowledgements
We thank L. Kang, Y.-H. Sun, H.-L. Lu, M.-X. Wu and T.-T. Wang for their help in the laboratory. We are grateful to R. Huey and three anonymous reviewers for their suggestions on the manuscript.
Research was performed under approvals from the Animal Ethics Committee at the Institute of Zoology, Chinese Academy of Sciences (IOZ14001).
Funding statement
This work was supported by grants from the ‘Hundred Talents Programme’ of the Chinese Academy of Sciences, National Natural Science Foundation of China (31200310) and Zhejiang Provincial Natural Science Foundation (Z13C030006).
References
- 1.Johnston IA, Bennett AF. 1996. Animals and temperature: phenotypic and evolutionary adaptation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Hochachka PW, Somero GN. 2002. Biochemical adaptation. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Hoffmann AA, Sorensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 5.Feder ME, Hofmann GE. 1999. Heat-shock proteins,molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 6.Sørensen JG. 2010. Application of heat shock protein expression for detecting natural adaptation and exposure to stress in natural populations. Curr. Zool. 56, 703–713. [Google Scholar]
- 7.Sørensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. ( 10.1046/j.1461-0248.2003.00528.x) [DOI] [Google Scholar]
- 8.Shatilina ZM, Riss HW, Protopopova MV, Trippe M, Meyer EI, Pavlichenko VV, Bedulina DS, Axenov-Gribanov DV, Timofeyev MA. 2011. The role of the heat shock proteins (HSP70 and sHSP) in the thermotolerance of freshwater amphipods from contrasting habitats. J. Therm. Biol. 36, 142–149. ( 10.1016/j.jtherbio.2010.12.008) [DOI] [Google Scholar]
- 9.Karl I, Sorensen JG, Loeschcke V, Fischer K. 2009. HSP70 expression in the copper butterfly Lycaena tityrus across altitudes and temperatures. J. Evol. Biol. 22, 172–178. ( 10.1111/j.1420-9101.2008.01630.x) [DOI] [PubMed] [Google Scholar]
- 10.Tomanek L. 2002. The heat-shock response: its variation, regulation and ecological importance in intertidal gastropods (genus Tegula). Integr. Comp. Biol. 42, 797–807. ( 10.1093/icb/42.4.797) [DOI] [PubMed] [Google Scholar]
- 11.Hamdoun AM, Cheney DP, Cherr GN. 2003. Phenotypic plasticity of HSP70 and HSP70 gene expression in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol. Bull. 205, 160–169. ( 10.2307/1543236) [DOI] [PubMed] [Google Scholar]
- 12.Tomanek L, Somero GN. 1999. Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J. Exp. Biol. 202, 2925–2936. [DOI] [PubMed] [Google Scholar]
- 13.Huang L-H, Kang L. 2007. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol. Biol. 16, 491–500. ( 10.1111/j.1365-2583.2007.00744.x) [DOI] [PubMed] [Google Scholar]
- 14.Sui GC, Soohoo C, Affar E, Gay F, Shi YJ, Forrester WC, Shi Y. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl Acad. Sci. USA 99, 5515–5520. ( 10.1073/pnas.082117599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakso M, Sauer B, Mosinger B, Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. 1992. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl Acad. Sci. USA 89, 6232–6236. ( 10.1073/pnas.89.14.6232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philippe S, Sarkis C, Barkats M, Mammeri H, Ladroue C, Petit C, Mallet J, Serguera C. 2006. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl Acad. Sci. USA 103, 17 684–17 689. ( 10.1073/pnas.0606197103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. 2003. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 301, 338–343. ( 10.1016/S0006-291X(02)03026-7) [DOI] [PubMed] [Google Scholar]
- 18.Feder ME, Krebs RA. 1998. Natural and genetic engineering of the heat-shock protein Hsp70 in Drosophila melanogaster: consequences for thermotolerance. Am. Zool. 38, 503–517. ( 10.1093/icb/38.3.503) [DOI] [Google Scholar]
- 19.Zhao B, Li T, Shine R, Du W-G. 2013. Turtle embryos move to optimal thermal environments within the egg. Biol. Lett. 9, 20130337.. ( 10.1098/rsbl.2013.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welte MA, Tetrault JM, Dellavalle RP, Lindquist SL. 1993. A new method for manipulating transgenes: engineering heat tolerance in a complex, multicellular organism. Curr. Biol. 3, 842–853. ( 10.1016/0960-9822(93)90218-D) [DOI] [PubMed] [Google Scholar]
- 21.Kalosaka K, Soumaka E, Politis N, Mintzas AC. 2009. Thermotolerance and HSP70 expression in the Mediterranean fruit fly Ceratitis capitata. J. Insect Physiol. 55, 568–573. ( 10.1016/j.jinsphys.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 22.Webb DR. 1987. Thermal tolerance of avian embryos: a review. The Condor 89, 874–898. ( 10.2307/1368537) [DOI] [Google Scholar]
- 23.Angilletta MJ, Zelic MH, Adrian GJ, Hurliman AM, Smith CD. 2013. Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Conserv. Physiol. 1, cot018 ( 10.1093/conphys/cot018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeming DC. 2004. Reptilian incubation: environment, evolution and behaviour. Nottingham, UK: Nottingham University Press. [Google Scholar]
- 25.Deeming DC. 2002. Avian incubation: behaviour, environment and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Shine R, Elphick MJ, Barrott EG. 2003. Sunny side up: lethally high, not low, nest temperatures may prevent oviparous reptiles from reproducing at high elevations. Biol. J. Linn. Soc. 78, 325–334. ( 10.1046/j.1095-8312.2003.00140.x) [DOI] [Google Scholar]
- 27.Packard GC, Lang JW, Lohmiller LD, Packard MJ. 1997. Cold tolerance in hatchling painted turtles (Chrysemys picta): supercooling or tolerance for freezing? Physiol. Zool. 70, 670–678. [DOI] [PubMed] [Google Scholar]
- 28.Costanzo JP, Dinkelacker SA, Iverson JB, Lee RE. 2004. Physiological ecology of overwintering in the hatchling painted turtle: multiple-scale variation in response to environmental stress. Physiol. Biochem. Zool. 77, 74–99. ( 10.1086/378141) [DOI] [PubMed] [Google Scholar]
- 29.Zhao E, Adler K. 1993. Herpetology of China. Oxford, UK: SSAR. [Google Scholar]
- 30.Du WG, Ji X. 2006. Effects of constant and fluctuating temperatures on egg survival and hatchling traits in the northern grass lizard (Takydromus septentrionalis, Lacertidae). J. Exp. Zool. A 305, 47–54. ( 10.1002/jez.a.243) [DOI] [PubMed] [Google Scholar]
- 31.Du WG, Ji X. 2003. The effects of incubation thermal environments on size, locomotor performance and early growth of hatchling soft-shelled turtles, Pelodiscus sinensis. J. Therm. Biol. 28, 279–286. ( 10.1016/S0306-4565(03)00003-2) [DOI] [Google Scholar]
- 32.Ji X, Chen F, Du WG, Chen HL. 2003. Incubation temperature affects hatchling growth but not sexual phenotype in the Chinese soft-shelled turtle, Pelodiscus sinensis (Trionychidae). J. Zool. 261, 409–416. ( 10.1017/S0952836903004266) [DOI] [Google Scholar]
- 33.Zatsepina O, Ulmasov KA, Beresten S, Molodtsov V, Rybtsov S, Evgen'ev M. 2000. Thermotolerant desert lizards characteristically differ in terms of heat-shock system regulation. J. Exp. Biol. 203, 1017–1025. [DOI] [PubMed] [Google Scholar]
- 34.Oshima G, Wennerberg J, Yamatodani T, Kjellen E, Mineta H, Johnsson A, Ekblad L. 2012. Autocrine epidermal growth factor receptor ligand production and cetuximab response in head and neck squamous cell carcinoma cell lines. J. Cancer Res. Clin. Oncol. 138, 491–499. ( 10.1007/s00432-011-1127-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitlock MC. 2005. Combining probability from independent tests: the weighted Z-method is superior to Fisher's approach. J. Evol. Biol. 18, 1368–1373. ( 10.1111/j.1420-9101.2005.00917.x) [DOI] [PubMed] [Google Scholar]
- 36.Tokita M, Kuratani S. 2001. Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae). Zool. Sci. 18, 705–715. ( 10.2108/zsj.18.705) [DOI] [Google Scholar]
- 37.Du WG, Tu MC, Radder RS, Shine R. 2013. Can reptile embryos influence their own rates of heating and cooling? PLoS ONE 8, e67095 ( 10.1371/journal.pone.0067095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MX, Hu LJ, Dang W, Lu HL, Du WG. 2013. Effect of thermal acclimation on thermal preference, resistance and locomotor performance of hatchling soft-shelled turtle. Curr. Zool. 59, 718. [Google Scholar]
- 39.Gehring WJ, Wehner R. 1995. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc. Natl Acad. Sci. USA 92, 2994–2998. ( 10.1073/pnas.92.7.2994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbuz D, Evgenev MB, Feder ME, Zatsepina OG. 2003. Evolution of thermotolerance and the heat-shock response: evidence from inter/intraspecific comparison and interspecific hybridization in the virilis species group of Drosophila. I. Thermal phenotype. J. Exp. Biol. 206, 2399–2408. ( 10.1242/jeb.00429) [DOI] [PubMed] [Google Scholar]
- 41.Ulmasov KA, Shammakov S, Karaev K, Evgen'ev MB. 1992. Heat shock proteins and thermoresistance in lizards. Proc. Natl Acad. Sci. USA 89, 1666–1670. ( 10.1073/pnas.89.5.1666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fangue NA, Hofmeister M, Schulte PM. 2006. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 209, 2859–2872. ( 10.1242/jeb.02260) [DOI] [PubMed] [Google Scholar]
- 43.Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK. 2002. Heat shock protein genes and their functional significance in fish. Gene 295, 173–183. ( 10.1016/s0378-1119(02)00687-x) [DOI] [PubMed] [Google Scholar]
- 44.Deeming DC, Ferguson MWJ. 1991. Egg incubation: its effect on embryonic development in birds and reptiles. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Botton ML, Pogorzelska M, Smoral L, Shehata A, Hamilton MG. 2006. Thermal biology of horseshoe crab embryos and larvae: a role for heat shock proteins. J. Exp. Mar. Biol. Ecol. 336, 65–73. ( 10.1016/j.jembe.2006.04.014) [DOI] [Google Scholar]
- 46.Narum SR, Campbell NR, Meyer KA, Miller MR, Hardy RW. 2013. Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Mol. Ecol. 22, 3090–3097. ( 10.1111/mec.12240). [DOI] [PubMed] [Google Scholar]
- 47.Rinehart JP, Hayward SAL, Elnitsky MA, Sandro LH, Lee RE, Denlinger DL. 2006. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc. Natl Acad. Sci. USA 103, 14 223–14 227. ( 10.1073/pnas.0606840103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garland T, Huey RB, Bennett AF. 1991. Phylogeny and coadaptation of thermal physiology in lizards: a reanalysis. Evolution 45, 1969–1975. ( 10.2307/2409846) [DOI] [PubMed] [Google Scholar]
- 49.Kiang JG, Tsokos GC. 1998. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol. Ther. 80, 183–201. ( 10.1016/S0163-7258(98)00028-X) [DOI] [PubMed] [Google Scholar]
- 50.Ohtsuboa T, Kanob E, Uedac K, Matsumotob H, Saitoa T, Hayashib S, Hatashitab M, Jinb Z-H, Saitoa H. 2000. Enhancement of heat-induced heat shock protein (hsp) 72 accumulation by doxorubicin (Dox) in vitro. Cancer Lett. 159, 49–55. ( 10.1016/S0304-3835(00)00528-0) [DOI] [PubMed] [Google Scholar]
- 51.Feder ME, Cartano NV, Milos L, Krebs RA, Lindquist SL. 1996. Effect of engineering Hsp70 copy number on Hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J. Exp. Biol. 199, 1837–1844. [DOI] [PubMed] [Google Scholar]
- 52.Lerman DN, Feder ME. 2001. Laboratory selection at different temperatures modifies heat-shock transcription factor (HSF) activation in Drosophila melanogaster. J. Exp. Biol. 204, 315–323. [DOI] [PubMed] [Google Scholar]
- 53.Jensen LT, Cockerell FE, Kristensen TN, Rako L, Loeschcke V, McKechnie SW, Hoffmann AA. 2010. Adult heat tolerance variation in Drosophila melanogaster is not related to Hsp70 expression. J. Exp. Zool. A, Ecol. Genet. Physiol. 313, 35–44. ( 10.1002/jez.573) [DOI] [PubMed] [Google Scholar]
- 54.Parsell DA, Lindquist S. 1994. Heat shock proteins and stress tolerance. In The biology of heat shock proteins and molecular chaperones (eds Morimoto RI, Tissieres A, Georgopoulos C.), pp. 457–494. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 55.Silbermann R, Tatar M. 2000. Reproductive costs of heat shock protein in transgenic Drosophila melanogaster. Evolution 54, 2038–2045. ( 10.1111/j.0014-3820.2000.tb01247.x) [DOI] [PubMed] [Google Scholar]
- 56.DuRant SE, Hopkins WA, Hepp GR, Walters JR. 2013. Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol. Rev. 88, 499–509. ( 10.1111/brv.12015) [DOI] [PubMed] [Google Scholar]
- 57.Haney PJ, Badger JH, Buldak GL, Reich CI, Woese CR, Olsen GJ. 1999. Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc. Natl Acad. Sci. USA 96, 3578–3583. ( 10.1073/pnas.96.7.3578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fields PA, Cox KM, Karch KR. 2012. Latitudinal variation in protein expression after heat stress in the salt marsh mussel Geukensia demissa. Integr. Comp. Biol. 52, 636–647. ( 10.1093/icb/ics086) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.