Abstract
Loggerhead sea turtle hatchlings (Caretta caretta) use regional magnetic fields as open-ocean navigational markers during trans-oceanic migrations. Little is known, however, about the ontogeny of this behaviour. As a first step towards investigating whether the magnetic environment in which hatchlings develop affects subsequent magnetic orientation behaviour, eggs deposited by nesting female loggerheads were permitted to develop in situ either in the natural ambient magnetic field or in a magnetic field distorted by magnets placed around the nest. In orientation experiments, hatchlings that developed in the normal ambient field oriented approximately south when exposed to a field that exists near the northern coast of Portugal, a direction consistent with their migratory route in the northeastern Atlantic. By contrast, hatchlings that developed in a distorted magnetic field had orientation indistinguishable from random when tested in the same north Portugal field. No differences existed between the two groups in orientation assays involving responses to orbital movements of waves or sea-finding, neither of which involves magnetic field perception. These findings, to our knowledge, demonstrate for the first time that the magnetic environment present during early development can influence the magnetic orientation behaviour of a neonatal migratory animal.
Keywords: magnetic field, magnetic orientation and navigation, magnetoreception, ontogeny, sea turtles, Caretta caretta
1. Introduction
Diverse animals detect the Earth's magnetic field and exploit it as a source of information while migrating, homing or moving through their habitat [1,2]. Animals can derive at least two different types of information from the geomagnetic field. The first is directional or compass information, which enables animals to maintain courses in a particular direction such as north or south [1,3]. In addition, at least a few animals derive positional or map information from the geomagnetic field, which enables them to determine where they are relative to a goal or to change direction when they reach a particular geographical area along a migratory route [4–6].
Loggerhead sea turtles, Caretta caretta, undergo one of the longest and most spectacular marine migrations. Hatchling loggerheads that emerge on the east coast of Florida, USA, enter the sea and immediately embark on a trans-oceanic migration [7]. Turtles initially swim eastward to the Gulf Stream current, where many become entrained in the North Atlantic Subtropical Gyre, the circular current system that flows around the Sargasso Sea [8,9]. Many loggerheads migrate across the Atlantic and back before eventually returning to the North American coast [9,10].
The magnetic sense of loggerhead turtles has been studied extensively for more than two decades. Young loggerheads in the open sea are guided at least partly by a ‘magnetic map’, in which regional magnetic fields function as navigational markers and elicit changes in swimming direction at crucial locations along the migratory pathway [11,12]. Responses to regional magnetic fields appear to be inherited, inasmuch as they are present in turtles that have never before been in the ocean [13,14]. Moreover, strong selective pressure probably acts to ensure that, as the geomagnetic field gradually changes over time, the responses of hatchlings change accordingly [13,15].
Although loggerhead hatchlings clearly emerge from their nests with a fully functional magnetic sense, little is known about the ontogeny of their magnetic navigation behaviour. For example, the responses to regional magnetic fields might be genetically encoded and unaffected by environmental variables such as the ambient magnetic field around the nest. Alternatively, the field that exists during development and/or after hatching might influence subsequent magnetic navigation behaviour. These questions are of interest both with regard to the ontogeny of behaviour and from a conservation perspective, given that a common conservation practice is to surround nests of sea turtles with wire mesh cages that protect eggs from predators but distort the ambient magnetic field [16].
As a first step towards investigating these issues, we altered the magnetic field around loggerhead turtle eggs with magnets and then tested whether turtles raised under these conditions responded to a regional magnetic field in the same way as control hatchlings raised in the normal geomagnetic field. Results indicated that turtles raised in the unnatural field failed to respond normally to the regional field. Moreover, effects of this treatment appeared to be limited to orientation behaviour involving magnetoreception, because incubation in the altered field had no effect on orientation to wave motion or on sea-finding behaviour mediated by visual cues. These findings, to our knowledge, demonstrate for the first time that the magnetic environment present during early development can influence subsequent magnetic orientation behaviour of neonates.
2. Material and methods
(a). Locating turtle nests
The study was conducted within a 5 km stretch of beach in Melbourne Beach, Florida, USA, during the spring and summer of 2005. During May and June, we walked along the beach each morning to locate loggerhead nests that had been deposited by nesting female turtles the night before. Wooden stakes placed in the vegetation near the base of the closest dunes were used to mark the location of each nest. Stakes were typically located about 2–15 m from each clutch.
(b). Experimental design and clutch manipulations
Each nest was randomly assigned to one of three treatment groups. For the first group, we changed the magnetic environment in which the eggs developed by placing around each clutch a square PVC pipe frame with magnets attached. Magnets were used to alter the field instead of electrically powered coil systems because of the lack of electricity on the beach and because coil systems could not be installed around nests without moving the eggs. For the second group, eggs were surrounded by a PVC pipe frame of identical dimensions, but with non-magnetic aluminium bars attached instead of magnets; thus, eggs in this treatment were exposed to the disturbance of having a PVC pipe frame placed around the clutch, but the magnetic environment was not disrupted. The final group consisted of nests that were left undisturbed (no frame was placed around the eggs). These eggs developed under natural conditions in the unaltered geomagnetic field.
PVC pipe frames were squares that measured 55.9 cm on a side, dimensions that are large enough to easily surround a clutch without contacting the egg cavity itself (figure 1). Magnets and aluminium bars were attached to opposite sides of the PVC frame, but positioned so that they faced the inside of the frame. The magnets were rectangular neodymium rare earth magnets (N40; National Imports, Falls Church, VA, USA) that measured 0.64 × 1.27 × 10.16 cm. Aluminium bars had identical dimensions. Magnets were arranged so that the north pole of one magnet and the south pole of the second magnet faced inward towards each other. This configuration created an unnatural magnetic field in which the field intensity varied greatly across the clutch. Measurements with a Model 912 digital gaussmeter (Magnetic Instrumentation, Inc., Indianapolis, IN, USA) indicated that field intensity ranged from approximately 36–310 µT within the space occupied by eggs, with the average field intensity in the centre of the frame being about 80 µT. Magnetic inclination angle also presumably varied greatly, but could not be measured accurately because the gaussmeter probe could not be positioned accurately relative to gravity. The natural field intensity at the study site was 46.7 µT and the inclination angle was 57.8° (measurements of the natural field were made with an Applied Physics Systems tri-axial fluxgate magnetometer model 520A).
Figure 1.
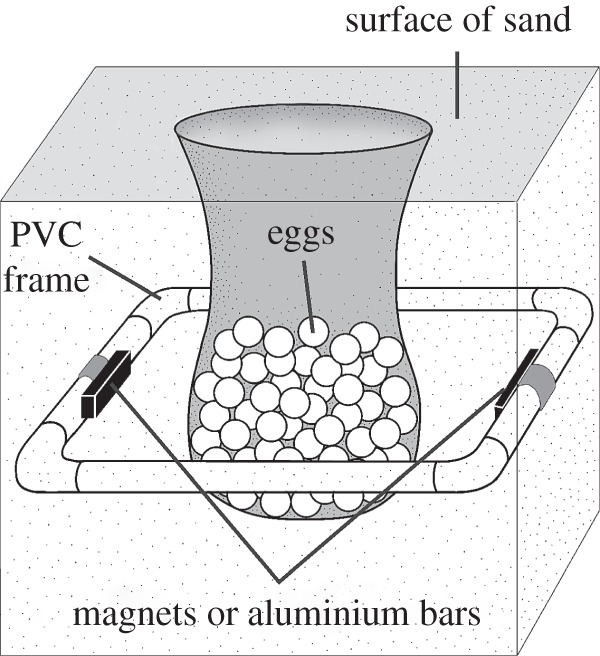
Diagram of a clutch of turtle eggs surrounded by a buried PVC pipe frame. Each frame had either magnets or aluminium bars attached to it. The shaded area surrounding and above the eggs indicates the approximate area typically excavated by a nesting turtle and filled in with sand after egg deposition. See text for details.
Both aluminium bars and magnets were thoroughly wrapped in waterproof plastic bags so that they did not contact the sand. To bury a frame around a nest, a trench was dug around each egg chamber using gardening trowels; care was taken to avoid contacting any eggs during this process. Frames were placed in the trench so that they were 23 cm below the top of the egg chamber, which corresponds to the approximate depth of the centre of the egg chamber in loggerhead nests (Ray Carthy 2005, personal communication). We then filled the trench with the same moist sand that had been dug out. When buried, the sides of the frame that held aluminium bars or magnets were on the east and west sides of the clutch, and aligned so that the long axis of the bars or magnets were parallel with the north–south axis of the local ambient field.
(c). Collection of hatchlings
During morning surveys, we recorded the date that each nest was deposited on the beach. To predict the date when hatchlings would emerge from each nest, we monitored the period of incubation that preceded emergence in other nests deposited on similar dates and locations on the beach. A few hours before an emergence was expected, we gently dug into the nest by hand and removed approximately 20–30 turtles. Hatchlings were placed in a lightproof, Styrofoam cooler and driven by automobile to the laboratory for experiments. During the brief (approx. 5 min) drive, turtles were presumably exposed to unnatural magnetic fields associated with the vehicle, but once at the testing site, the cooler was kept in a location free from magnetic distortions. Turtles remained in complete darkness in the local magnetic field until testing (which typically occurred between 3 and 9 h later). Each animal was used only once for a single experiment and then released later the same night.
(d). Magnetic orientation behaviour
In magnetic orientation experiments, turtles from all three treatment groups were subjected to a magnetic field replicating one found along their migratory route near the coast of Portugal. Procedures are described in detail elsewhere [11,12,17]. Briefly, each hatchling was placed into a nylon-lycra harness that encircled the turtle's carapace without impeding swimming [18]. The turtle was then tethered by a monofilament line to an electronic tracking system in the centre of a water-filled, circular arena that was 91.4 cm in diameter and filled with water to a depth of about 40 cm (figure 2). The tracking system consisted of a graphite tracker arm attached to a digital encoder at the top of a post mounted in the centre of the arena. The tracker arm was free to rotate 360° in the horizontal plane; hatchlings were able to swim in any direction, but were restrained to a circle with a radius of about 35 cm and were unable to contact either the outer edge of the arena or the central post. The digital encoder was wired to a computer in a nearby building. The computer recorded the direction that each turtle swam. The orientation arena was covered with a plywood lid (1.9 cm thick) that could be opened or closed as needed. The lid was painted black. During all experiments, it was closed and covered with four layers of black plastic sheeting that hung down over the sides of the arena to prevent light leaks.
Figure 2.
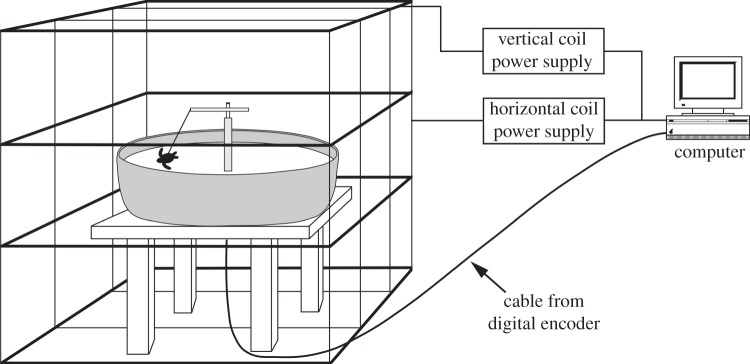
Diagram of the experimental apparatus and data acquisition system used to monitor magnetic orientation. The turtle was harnessed in a nylon-lycra harness and placed in a water-filled arena that was surrounded by two orthogonally arranged coil systems. Computer-controlled power supplies located in a nearby house altered the magnetic field in the arena so that it replicated a magnetic field found at the northeastern boundary of the North Atlantic gyre. The computer also monitored the direction that the turtle swam (adapted from [11]).
The orientation arena was surrounded by two magnetic coil systems arranged orthogonally (figure 2). Each was a Merritt 4 coil system [19]. The first coil (228.6 cm on a side) was aligned along the north–south axis of the ambient magnetic field and was used to control the field's horizontal component. The second (251.5 cm on a side) controlled the vertical field component. Each coil was powered by a computer-controlled power supply. Custom software enabled us to control the electrical current through each coil, and thus to reproduce the magnetic field that exists at any location in the Atlantic Ocean. To avoid field distortions from buildings or electrical wiring, the coil and arena were placed outdoors in an area free of magnetic distortions.
During experiments (see below), hatchlings were exposed to a magnetic field that replicated one found at the northeast boundary of the North Atlantic Subtropical Gyre (44.5° N, 20° W). The field had an inclination angle of 60.1° and a total intensity of 49.1 µT (measured with a tri-axial fluxgate magnetometer: Applied Physics Systems model 520). The field parameters were based on estimates provided by the International Geomagnetic Reference Field model, version 2000, for July, 2005, the month when the experiments began.
Experiments were conducted between 20.30 and 02.00, the time when most loggerheads emerge from their nests and enter the sea [20]. Prior to each trial, the magnetic coils were turned off so that turtles began each trial in the local magnetic field. A light-emitting diode (LED; peak λ = 520 nm) located on the eastern side of the arena was illuminated. A hatchling was then placed in the harness and allowed to swim in the tank. Healthy hatchlings swim vigorously towards light after emerging from their nests; thus, the hatchling's swimming response verified that the animal was behaviourally competent [11,17]. Those few turtles that failed to swim towards the LED were replaced with other individuals, prior to the start of the trial.
Each hatchling was permitted to swim towards the light in the local magnetic field for 10 min. After this time, the LED was turned off and the coils were simultaneously turned on to replicate the magnetic field described above. Turtles were given 3 min to acclimatize to the new field and to swimming in darkness. The computer then recorded the hatchling's heading every 10 s for 5 min. The turtle's mean heading was calculated by averaging all data points collected during this 5 min trial. No more than four turtles from any one nest were tested. Each hatchling was tested a single time and released later that night.
For each of the three treatment groups, Rayleigh tests [21] were used to determine whether turtles were significantly oriented as a group. Distributions from the three groups were compared using a Mardia–Watson–Wheeler test [21].
(e). Wave orientation tests
In principle, the altered magnetic field during incubation might have produced behavioural deficits unrelated to magnetoreception. We therefore used a second behavioural assay to determine whether turtles in both groups could respond appropriately to non-magnetic cues. Previous work has demonstrated that hatchlings swim directly into waves when they leave the beach, a response that guides turtles away from shore and towards the open ocean [22–25]. Additionally, turtles determine wave direction by monitoring the circular patterns of movements that occur as waves propagate [26,27]. A turtle swimming steadily into oncoming waves will experience forces pushing it in a particular sequence of directions: upward, backward, downward and forward in a circle [23,26]. Similarly, if waves approach the turtle from its right, then the turtle will be moved to its left, down, to its right and then up again in a circle. In the latter case, turtles respond by turning to the right until oriented into the waves [26].
This ability was demonstrated in the laboratory using a ‘wave simulator’, a machine that reproduces the circular patterns of movement that occur as waves propagate (figure 3) [26]. Turtles can be aligned on the simulator so that they experience the motion associated with swimming in different directions relative to wave movement. Surprisingly, turtles act out their swimming behaviour when suspended on the simulator in air; moreover, when not aligned with the simulated waves, they attempt to turn until they are [26,28].
Figure 3.
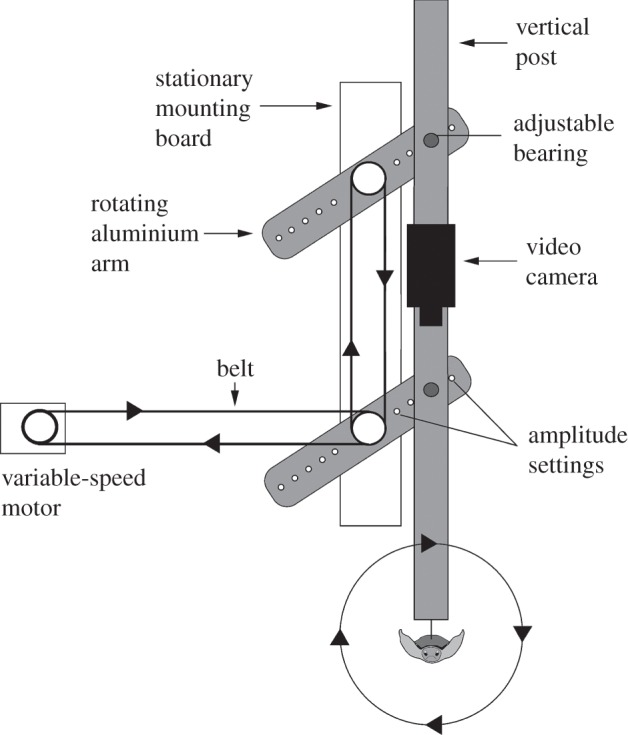
Diagram of the wave simulator used to test the responses of turtles to orbital movements associated with small ocean waves. A motor turned a belt, which rotated a wheel, in turn driving a second belt attached to two identical rotating aluminium arms. A vertical post attached to the arms moved a hatchling (suspended in a cloth harness at the bottom of the post) through a series of orbital movements. An infrared video camera mounted on the post was used to monitor the hatchling's turning behaviour when it was subjected to motion simulating waves approaching from the turtle's right (as shown) or left (adapted from [26]).
In this study, hatchlings were tested on a wave simulator (figure 3) with circular movements that replicated those produced by typical waves along the east coast of Florida during summer (radius = 0.156 m; period = 5 s) [26]. Prior to a trial, each hatchling was allowed to swim in water for at least 10 min to ensure that it was in the behavioural state in which it would readily respond to waves [26]. Next, each hatchling was placed in a nylon-lycra harness, which was attached to the simulator. The turtle was then subjected to circular movements simulating waves approaching from either its left or right side. The direction of orbital movement was reversed after each trial by reversing the direction of the motor; thus, half of the turtles were exposed to simulated waves from the right and half to simulated waves from the left. All trials were videotaped for later analysis.
Because hatchling turtles use their rear flippers as rudders when turning, we monitored turning behaviour on the wave simulator by observing extension of the rear flippers, as in previous experiments [26,28]. On the simulator, hatchlings attempt to turn left by extending their left rear flipper and attempt to turn right by extending their right rear flipper. During analysis of the videotaped trial, each hatchling was given a 1 min adjustment period after the simulator was turned on; the turtle's rear flipper extension was then analysed for the next 3 min.
For both groups of turtles (i.e. those that developed in the altered field and those that developed in the natural field), the time spent turning either left or right in response to each of the two simulated wave conditions was compared using a Wilcoxon signed-rank test. The time spent turning either left or right in response to these same wave conditions was then compared between treatment groups using Mann–Whitney U-tests.
(f). Sea-finding behaviour
As an additional check for non-specific effects of the magnetic treatment, we also investigated whether development in an altered magnetic field influenced the ability of hatchling turtles to find the sea after emerging from their nests. Sea-finding in hatchlings is a robust behaviour in which the newly emerged turtles quickly crawl down the beach and enter the ocean. Visual cues, including light reflecting off the sea, guide turtles to the water [29].
All sea-finding trials were conducted between 21.00 and 00.30, a period during which most loggerhead hatchlings emerge from their nests naturally [20]. Hatchlings were drawn from four nests with magnets around them and four control nests surrounded by aluminium bars; four to five hatchlings from each nest were tested. Hatchlings awaiting testing were kept in lightproof, Styrofoam coolers during the trials. A small amount of moist sand was added to the cooler before the trials to stimulate turtle activity.
To test sea-finding behaviour, a single hatchling was placed at the centre of a 15 m diameter circle inscribed in the sand on the natal beach. Each turtle was tested only once and was released facing north, west, south or east; release directions were used in sequence so that equal numbers of hatchlings were released facing each direction. After release, observers retreated from the turtle as far as possible without losing sight of it. As the turtle began to crawl, its progress was monitored visually and the bearing at which the turtle crossed the circle's circumference was recorded with a digital compass (Autohelm personal compass, Nautech Limited, UK). The range of bearings that led towards the ocean from the circle's centre was approximately 40°–80°.
Rayleigh tests were used to determine whether turtles from each group were significantly oriented [21]. The distributions from the two groups were compared using a Watson U2-test [21].
3. Results
(a). Magnetic orientation behaviour
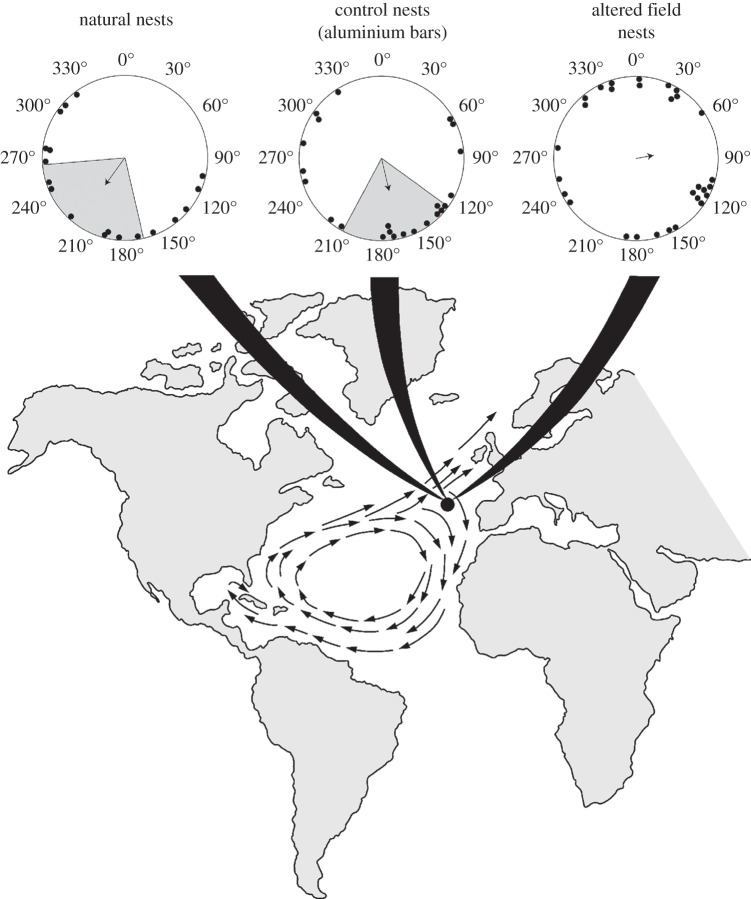
As in previous experiments [11,12], hatchlings that developed in the natural ambient magnetic field responded to a field replicating one in the northeastern gyre by swimming approximately south (figure 4). This direction is consistent with the migratory path of the turtles in this geographical area [9,10]. Turtles that developed in natural nests were significantly oriented (Rayleigh test, r = 0.42, n = 18, p < 0.05) with a mean angle of 216°. Similarly, turtles from nests surrounded by non-magnetic aluminium bars were significantly oriented (Rayleigh test, r = 0.39 n = 23, p < 0.05) with a mean angle of 167°. By contrast, turtles that developed in altered magnetic fields had orientation that was statistically indistinguishable from random (Rayleigh test, r = 0.17; n = 29 p = 0.42). The Mardia–Watson–Wheeler test [21] indicated that significant differences existed among the three groups (W = 11.58, p < 0.05). Pairwise comparisons indicated that the orientation of turtles raised in the altered field differed from the orientation of turtles from natural nests (W = 8.51, p = 0.014). The orientation of the altered field group also differed significantly from the orientation of turtles from nests surrounded by aluminium bars (W = 6.59, p = 0.037). No difference existed between the orientation of turtles from natural nests and those from the aluminium bar group (W = 1.86, p = 0.395).
Figure 4.
Orientation of hatchlings exposed to a magnetic field that exists near the northeastern boundary of the North Atlantic subtropical gyre. The location where the field exists is indicated by the black dot on the map. ‘Natural nests’ indicate headings from turtles that incubated in undisturbed nests; ‘control nests (aluminium bars)’ depict headings from turtles that incubated in nests surrounded by non-magnetic aluminium bars. ‘Altered field nests’ depict headings from turtles that incubated in nests surrounded by magnets. In the circular orientation diagrams, each dot represents the mean heading of a single hatchling. The arrow indicates the mean direction of turtles in the group. The length of the arrow is proportional to the magnitude of the mean vector r, with the radius of the circle corresponding to r = 1. The shaded sectors inside the circular diagrams represent the 95% CIs for the two groups that were significantly oriented. Turtles that developed in natural nests were significantly oriented (Rayleigh test, r = 0.42, n = 18, p < 0.05) with a mean angle of 216°. Turtles from nests surrounded by aluminium bars were also significantly oriented (r = 0.39, n = 23, p < 0.05) with a mean angle of 167°. By contrast, turtles that developed in fields altered by magnets had orientation that was statistically indistinguishable from random (r = 0.17, n = 29, p = 0.42). Data are plotted relative to magnetic north, which is indicated by 0°.
(b). Orientation to wave orbital movements
In tests involving orientation responses to wave orbital movements, no differences were detected in the behaviour of hatchlings that developed in nests surrounded by magnets and those that developed in nests surrounded by aluminium bars (table 1). When simulated waves approached the turtles’ left side, both groups of hatchlings spent significantly more time turning left into the perceived oncoming wave than turning right (Wilcoxon signed-rank test; natural magnetic field group: n = 25, W = 323.0, p < 0.01; altered magnetic field group: n = 25, W = 325.0, p < 0.01). The responses of the two groups were statistically indistinguishable (Mann–Whitney U; time turning left: U = 306.0, p = 0.91; time turning right: U = 297.0, p = 0.77).
Table 1.
Time (mean ± s.d.) turtles spent turning in the different wave conditions on the wave simulator.
| treatment groups | waves approach hatchling's left side |
waves approach hatchling's right side |
||||
|---|---|---|---|---|---|---|
| time spent turning left (s) | time spent turning right (s) | within-individual comparisonsa | time spent turning left (s) | time spent turning right (s) | within-individual comparisonsa | |
| local magnetic field | 142.9 ± 37.8 | 4.8 ± 11.0 |
W = 323.0 p < 0.01 |
3.4 ± 6.9 | 145.5 ± 38.0 |
W = –325.0 p < 0.01 |
| altered magnetic field | 151.3 ± 21.9 | 2.9 ± 5.4 |
W = 325.0 p < 0.01 |
8.0 ± 12.7 | 146.1 ± 26.7 |
W = –325 p < 0.01 |
| between-group comparisonsb |
U = 306.0 p = 0.91 |
U = 297.0 p = 0.77 |
U = 233.5 p = 0.12 |
U = 288.0 p = 0.64 |
||
aComparison of the time that turtles in each wave simulator condition spent turning right or left using Wilcoxon signed-rank tests.
bComparison of the time that turtles in each treatment group spent turning right and left in each wave simulator condition using Mann–Whitney U-tests.
Similarly, when simulated waves approached from the turtles’ right side, both groups spent significantly more time turning right than left (Wilcoxon signed-rank test; natural magnetic field group: n = 25, W = −325.0, p < 0.01; altered magnetic field group: n = 25, W = −325, p < 0.01). Responses of the two groups were statistically indistinguishable (Mann–Whitney U; time turning left: U = 233.5, p = 0.12; time turning right: U = 288.0, p = 0.64).
(c). Orientation towards the sea
Hatchlings from the two groups had nearly identical sea-finding behaviour. Hatchlings that incubated in nests surrounded by aluminium bars oriented significantly as a group towards the east-northeast (Rayleigh test; mean angle = 56°, r = 0.97, n = 31, p < 0.001). Hatchlings that incubated in nests surrounded by magnets were also oriented significantly as a group in the same east-northeastward direction (Rayleigh test; mean angle = 55°, r = 0.97, n = 25 p < 0.001). The orientation of both groups was directed towards the ocean and the two distributions were statistically indistinguishable (Watson U2 = 0.056, p > 0.50).
4. Discussion
Our results provide evidence that the magnetic environment in which hatchling loggerhead turtles incubate affects subsequent magnetic navigation behaviour. When exposed to a magnetic field that resembles one at the northeastern boundary of the circular migratory route, turtles that developed in a natural magnetic field oriented southward as in previous experiments [11,12]. By contrast, hatchlings that developed in an altered magnetic environment had orientation that was statistically indistinguishable from random (figure 4).
Turtles that developed under distorted and normal magnetic environments did not differ in their responses to two non-magnetic orientation cues that are critical to hatchlings [26,29]: (i) orbital movements associated with ocean waves (table 1), and (ii) visual cues that underlie sea-finding behaviour. These findings imply that the effects of developing in a distorted magnetic environment are specific to behaviour involving magnetoreception, rather than reflecting more general developmental, neural or behavioural deficits.
The ability of hatchling loggerheads to use regional magnetic fields as navigational markers appears to be an adaptive mechanism that helps turtles advance along their migratory pathway and avoid straying into water that is lethally cold [11,12,17]. The failure of hatchlings that developed in an altered field to orient south, when tested in a regional field that normally elicits southward swimming, implies that the magnetic environment in which turtles develop somehow influences this navigational system. To the best of our knowledge, this is the first demonstration that the ambient magnetic field present during early development influences subsequent magnetic navigation behaviour of neonate migratory animals.
In principle, the field in which turtles develop might influence subsequent orientation and navigation in several different ways. One possibility is that incubating in an altered field prevented turtles from obtaining directional or ‘compass’ information from the geomagnetic field [30,31], but still allowed them to obtain positional information from it [11,12]. If so, then young turtles might have been able to recognize the regional magnetic field used in the experiment by correctly detecting the magnetic inclination and intensity, but they might have been unable to establish or maintain the appropriate directional heading because the magnetic compass had been impaired.
A second possibility is that incubating in an unnatural field prevented turtles from obtaining positional or ‘map’ information from the Earth's magnetic field, but without compromising the magnetic compass. In this scenario, young turtles were unable to perceive or recognize the regional field to which they were exposed, even though they were capable of maintaining a consistent compass heading in any direction. It is possible, of course, that incubating in the altered field affected both the compass and ‘map’ of the turtle.
A related but slightly different possibility is that the responses of turtles to regional magnetic fields are ‘set’ or ‘calibrated’ relative to the field in which the turtles develop. In other words, recognition of specific regional fields might be encoded in young turtles not in terms of specific values of inclination and intensity [13,17], but instead based on how the fields at distant sites vary relative to the field at the home beach. Viewed in this way, the failure of turtles raised in a field altered by magnets to respond correctly to a regional field along the migratory route might result not because of a deficit in magnetoreception per se, but instead because the unnatural field ensured that the parameters at the distant site did not differ from those at the natal site by the normal amounts. Although this idea has some appeal, a potential problem is that, as the Earth's field gradually changes, the difference between the field at the home beach and the field at many locations along the migratory path does not remain constant and instead varies unpredictably (N. F. Putman & K. J. Lohmann 2011, unpublished data). Nevertheless, the possibility that responses to regional fields are linked in some way to the field at the home beach is worth considering.
An additional way that the ontogeny of the magnetic sense might influence sea turtle navigation is through interactions between the magnetic compass and other compass systems. Studies in migratory birds have provided evidence that nestlings learn relationships between their magnetic and celestial compasses, and that these compasses interact during their first migration [32,33]. Sea turtles, like birds, eventually acquire and use celestial compasses [34,35], but hatchling turtles, unlike birds, develop underground and cannot perceive celestial cues until they leave their nest [36]. In this study, turtles were tested in darkness and thus had no access to, or experience with, celestial cues. Nevertheless, interactions between the turtle magnetic compass and other environmental cues have been demonstrated; for example, hatchlings can initiate headings on the basis of visual cues or waves, and then transfer the heading to the magnetic compass [37]. Similar interactions between magnetic and celestial compasses are possible as turtles mature.
Although our experiments involved hatchling turtles, the findings have interesting implications for the navigational strategies of turtles at other life-history stages. Adult female sea turtles return to the vicinity of their natal beach to nest [38,39], though how they navigate back from distant sites after being away for many years is not known [40]. One hypothesis is that turtles imprint on the magnetic field of their natal region and then use this information to return [41,42]. For this to be true, a turtle's early experience must necessarily influence its behaviour as an adult. Although our results do not directly support or refute this hypothesis, the finding that the magnetic environment experienced by turtles during development affects subsequent navigation is broadly consistent with the idea of early experience affecting the behaviour of older turtles. Additional studies will be needed to determine whether gravid females do indeed use geomagnetic cues to guide themselves to their natal beaches to nest, as initial studies suggest [43,44].
A limitation of this study is that it does not reveal how long the effects of a distorted magnetic environment during incubation persist. In our experiment, hatchlings were tested a single time on the night that they would have emerged naturally. Although the observed effects might be long lasting or permanent, it is also conceivable that the effects are transient and disappear as the turtles mature and gain migratory experience. Additional studies are needed to resolve this issue.
Finally, our findings have implications for sea turtle conservation. Protective cages constructed of galvanized wire are commonly placed around sea turtles nests to protect them from predators [45,46]. At least some of these cages significantly alter the magnetic field around the incubating clutch [16]. Our results suggest the possibility that hatchlings developing under such cages might have compromised navigational abilities. Similarly, other anthropogenic structures, such as beachfront condominiums and sea walls, typically contain steel beams, iron rebar and other metallic materials that distort the local magnetic field. In some locations, sea turtles preferentially nest in front of these structures [47,48]. Thus, our findings raise the question of whether hatchlings that develop in such areas have impaired navigational abilities, and whether anthropogenic magnetic fields represent a previously overlooked threat to jeopardized populations of sea turtles.
Acknowledgements
We thank J. Wang, R. Katz, C. Lohmann, L. Ehrhart and D. Bagley for assistance with experiments and logistics, B. Eastwood for the data acquisition software, and the Hubbs-Sea World Research Institute for providing laboratory space.
All procedures described herein were approved by the University of North Carolina, Chapel Hill's Institutional Animal Care and Use Committee, as well as the appropriate state and federal government agencies.
Data accessibility
Data reported in this manuscript are deposited in a Dryad Digital Repository (doi:10.5061/dryad.4gv6t)
Funding statement
The research was authorized under Florida FWC special permit TP-065 and funded by NSF grants IOS-0718991 and IOS-1022005 to K.J.L.
References
- 1.Wiltschko W, Wiltschko R. 2005. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 191, 675–693. ( 10.1007/s00359-005-0627-7) [DOI] [PubMed] [Google Scholar]
- 2.Johnsen S, Lohmann KJ. 2005. The physics and neurobiology of magnetoreception. Nat. Rev. Neurosci. 6, 703–712. ( 10.1038/nrn1745) [DOI] [PubMed] [Google Scholar]
- 3.Akesson S, Hedenstrom A. 2007. How migrants get there: migratory performance and orientation. Bioscience 57, 123–133. ( 10.1641/B570207) [DOI] [Google Scholar]
- 4.Boles LC, Lohmann KJ. 2003. True navigation and magnetic maps in spiny lobsters. Nature 421, 60–63. ( 10.1038/nature01226) [DOI] [PubMed] [Google Scholar]
- 5.Lohmann KJ, Lohmann CMF, Ehrhart LM, Bagley DA, Swing T. 2004. Geomagnetic map used in sea turtle navigation. Nature 428, 909–910. ( 10.1038/428909a) [DOI] [PubMed] [Google Scholar]
- 6.Putman NF, Scanlan MM, Billman EJ, O'Neil JP, Couture RB, Quinn TP, Lohmann KJ, Noakes DLG. 2014. An inherited magnetic map guides ocean navigation in juvenile Pacific salmon. Curr. Biol. 24, 446–450. ( 10.1016/j.cub.2014.01.017) [DOI] [PubMed] [Google Scholar]
- 7.Carr A. 1987. New perspectives on the pelagic stage of sea turtle development. Conserv. Biol. 1, 103–121. ( 10.1111/j.1523-1739.1987.tb00020.x) [DOI] [Google Scholar]
- 8.Mansfield KL, Wyneken J, Porter WP, Luo J. 2014. First satellite tracks of neonate sea turtles redefine the ‘lost years’ oceanic niche. Proc. R. Soc. B 281, 20133039 ( 10.1098/rspb.2013.3039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putman NF, Verley P, Shay TJ, Lohmann KJ. 2012. Simulating transoceanic migrations of young loggerhead sea turtles: merging magnetic navigation behavior with an ocean circulation model. J. Exp. Biol. 215, 1863–1870. ( 10.1242/jeb.067587) [DOI] [PubMed] [Google Scholar]
- 10.Bolten AB, Bjorndal KA, Martins HR, Dellinger T, Biscoito MJ, Encalada SE, Bowen BW. 1998. Transatlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analysis. Ecol. Appl. 8, 1–7. ( 10.1890/1051-0761(1998)008[0001:TDMOLS]2.0.CO;2) [DOI] [Google Scholar]
- 11.Fuxjager MJ, Eastwood BS, Lohmann KJ. 2011. Orientation of hatchling loggerhead sea turtles to regional magnetic fields along a transoceanic migratory pathway. J. Exp. Biol. 214, 2504–2508. ( 10.1242/jeb.055921) [DOI] [PubMed] [Google Scholar]
- 12.Lohmann KJ, Cain SD, Dodge SA, Lohmann CMF. 2001. Regional magnetic fields as navigational markers for sea turtles. Science 294, 364–366. ( 10.1126/science.1064557) [DOI] [PubMed] [Google Scholar]
- 13.Lohmann KJ, Putman NF, Lohmann CMF. 2012. The magnetic map of hatchling loggerhead sea turtles. Curr. Opin. Neurobiol. 22, 336–342. ( 10.1016/j.conb.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 14.Collett TS, Collett M. 2011. Animal navigation: following signposts in the sea. Curr. Biol. 21, R843–R846. ( 10.1016/j.cub.2011.09.002) [DOI] [PubMed] [Google Scholar]
- 15.Lohmann KJ, Lohmann CMF. 2003. Orientation mechanisms of hatchling loggerheads. In Loggerhead sea turtles (eds Bolton AB, Witherington BE.), pp. 44–62. Washington, DC: Smithsonian Books. [Google Scholar]
- 16.Irwin WP, Horner A, Lohmann KJ. 2004. Magnetic field distortions produced by protective cages around sea turtle nests: unintended consequences for orientation and navigation? Biol. Conserv. 118, 117–120. ( 10.1016/j.biocon.2003.07.014) [DOI] [Google Scholar]
- 17.Putman NF, Endres CS, Lohmann CMF, Lohmann KJ. 2011. Longitude perception and bicoordinate magnetic maps in sea turtles. Curr. Biol. 21, 463–466. ( 10.1016/j.cub.2011.01.057) [DOI] [PubMed] [Google Scholar]
- 18.Salmon M, Wyneken J. 1987. Orientation and swimming behavior of hatchling loggerhead turtles (Caretta caretta L) during their offshore migration. J. Exp. Mar. Biol. Ecol. 109, 137–153. ( 10.1016/0022-0981(87)90012-8) [DOI] [Google Scholar]
- 19.Merritt R, Purcell C, Stroink G. 1983. Uniform magnetic-field produced by 3-square, 4-square, and 5-square coils. Rev. Sci. Instrum. 54, 879–882. ( 10.1063/1.1137480) [DOI] [Google Scholar]
- 20.Witherington BE, Bjorndal KA, McCabe CM. 1990. Temporal pattern of nocturnal emergence of loggerhead turtle hatchlings from natural nests. Copeia 4, 1165–1168. ( 10.2307/1446507) [DOI] [Google Scholar]
- 21.Batschelet E. 1981. Circular statistics in biology. New York, NY: Academic Press Inc. [Google Scholar]
- 22.Lohmann KJ. 1992. How sea turtles navigate. Sci. Am. 266, 100–106. ( 10.1038/scientificamerican0192-100) [DOI] [Google Scholar]
- 23.Lohmann KJ, Lohmann CMF. 1992. Orientation to oceanic waves by green turtle hatchlings. J. Exp. Biol. 171, 1–13. [Google Scholar]
- 24.Lohmann KJ, Salmon M, Wyneken J. 1990. Functional autonomy of land and sea orientation systems in sea turtle hatchlings. Biol. Bull. 179, 214–218. ( 10.2307/1541772) [DOI] [PubMed] [Google Scholar]
- 25.Salmon M, Lohmann KJ. 1989. Orientation cues used by hatchling loggerhead sea turtles (Caretta caretta L) during their offshore migration. Ethology 83, 215–228. ( 10.1111/j.1439-0310.1989.tb00530.x) [DOI] [Google Scholar]
- 26.Lohmann KJ, Swartz AW, Lohmann CMF. 1995. Perception of ocean wave direction by sea turtles. J. Exp. Biol. 198, 1079–1085. [DOI] [PubMed] [Google Scholar]
- 27.Lohmann KJ, Lohmann CMF, Endres CS. 2008. The sensory ecology of ocean navigation. J. Exp. Biol. 211, 1719–1728. ( 10.1242/jeb.015792) [DOI] [PubMed] [Google Scholar]
- 28.Manning EL, Cate HS, Lohmann KJ. 1997. Discrimination of ocean wave features by hatchling loggerhead sea turtles, Caretta caretta. Mar. Biol. 127, 539–544. ( 10.1007/s002270050043) [DOI] [Google Scholar]
- 29.Salmon M, Wyneken J, Fritz E, Lucas M. 1992. Seafinding by hatchling sea turtles: role of brightness, silhouette, and beach slope as orientation cues. Behaviour 122, 56–77. ( 10.1163/156853992X00309) [DOI] [Google Scholar]
- 30.Lohmann KJ, Lohmann CMF. 1994. Acquisition of magnetic directional preference in hatchling loggerhead sea turtles. J. Exp. Biol. 190, 1–8. [DOI] [PubMed] [Google Scholar]
- 31.Lohmann KJ. 1991. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J. Exp. Biol. 155, 37–49. [DOI] [PubMed] [Google Scholar]
- 32.Weindler P, Wiltschko R, Wiltschko W. 1996. Magnetic information affects the stellar orientation of young bird migrants. Nature 383, 158–160. ( 10.1038/383158a0) [DOI] [Google Scholar]
- 33.Alerstam T, Hogstedt G. 1983. The role of the geomagnetic field in the development of birds compass sense. Nature 306, 463–465. ( 10.1038/306463a0) [DOI] [Google Scholar]
- 34.Avens L, Lohmann KJ. 2003. Use of multiple orientation cues by juvenile loggerhead sea turtles Caretta caretta. J. Exp. Biol. 206, 4317–4325. ( 10.1242/jeb.00657) [DOI] [PubMed] [Google Scholar]
- 35.Mott CR, Salmon M. 2011. Sun compass orientation by juvenile green sea turtles (Chelonia mydas). Chelonian Conserv. Biol. 10, 73–81. ( 10.2744/CCB-0888.1) [DOI] [Google Scholar]
- 36.Ackerman RA. 1996. The nest environment and the embyronic development of sea turtles. In The biology of sea turtles, vol. 1 (eds Lutz PL, Musick JA.), pp. 83–106. Boca Raton, FL: CRC Press. [Google Scholar]
- 37.Goff M, Salmon M, Lohmann KJ. 1998. Hatchling sea turtles use surface waves to establish a magnetic compass direction. Anim. Behav. 55, 69–77. ( 10.1006/anbe.1997.0577) [DOI] [PubMed] [Google Scholar]
- 38.Bowen B, Avise JC, Richardson JI, Meylan AB, Margaritoulis D, Hopkins-Murphy SR. 1993. Population structure of loggerhead turtles (Caretta caretta) in the northwestern Atlantic Ocean and Mediterranean Sea. Conserv. Biol. 7, 834–844. ( 10.1046/j.1523-1739.1993.740834.x) [DOI] [Google Scholar]
- 39.Encalada SE, Bjorndal KA, Bolten AB, Zurita JC, Schroeder B, Possardt E, Sears CJ, Bowen BW. 1998. Population structure of loggerhead turtle (Caretta caretta) nesting colonies in the Atlantic and Mediterranean as inferred from mitochondrial DNA control region sequences. Mar. Biol. 130, 567–575. ( 10.1007/s002270050278) [DOI] [Google Scholar]
- 40.Lohmann KJ, Lohmann CMF, Brothers JR, Putman NF. 2013. Natal homing and imprinting in sea turtles. In The biology of sea turtles (eds Wyneken J, Lohmann KJ, Musick JA.), pp. 59–77. Boca Raton, FL: CRC Press. [Google Scholar]
- 41.Lohmann KJ, Hester JT, Lohmann CMF. 1999. Long-distance navigation in sea turtles. Ethol. Ecol. Evol. 11, 1–23. ( 10.1080/08927014.1999.9522838) [DOI] [Google Scholar]
- 42.Lohmann KJ, Putman NF, Lohmann CMF. 2008. Geomagnetic imprinting: a unifying hypothesis of long-distance natal homing in salmon and sea turtles. Proc. Natl Acad. Sci. USA 105, 19 096–19 101. ( 10.1073/pnas.0801859105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohmann KJ, Luschi P, Hays GC. 2008. Goal navigation and island-finding in sea turtles. J. Exp. Mar. Biol. Ecol. 356, 83–95. ( 10.1016/j.jembe.2007.12.017) [DOI] [Google Scholar]
- 44.Luschi P, Benhamou S, Girard C, Ciccione S, Roos D, Sudre J, Benvenuti S. 2007. Marine turtles use geomagnetic cues during open-sea homing. Curr. Biol. 17, 126–133. ( 10.1016/j.cub.2006.11.062) [DOI] [PubMed] [Google Scholar]
- 45.Ratnaswamy MJ, Warren RJ, Kramer MT, Adam MD. 1997. Comparisons of lethal and nonlethal techniques to reduce raccoon depredation of sea turtle nests. J. Wildl. Manage 61, 368–376. ( 10.2307/3802593) [DOI] [Google Scholar]
- 46.Yerli S, Canbolat AF, Brown LJ, Macdonald DW. 1997. Mesh grids protect loggerhead turtle, Caretta caretta, nests from red fox Vulpes vulpes predation. Biol. Conserv. 82, 109–111. ( 10.1016/S0006-3207(97)00003-7) [DOI] [Google Scholar]
- 47.Mrosovsky N, Lavin C, Godfrey MH. 1995. Thermal effects of condominiums on a turtle beach in Florida. Biol. Conserv. 74, 151–156. ( 10.1016/0006-3207(95)00022-V) [DOI] [Google Scholar]
- 48.Salmon M, Reiners R, Lavin C, Wyneken J. 1995. Behavior of loggerhead sea turtles on an urban beach. I. Correlates of nest placement. J. Herpetol. 29, 560–567. ( 10.2307/1564739) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data reported in this manuscript are deposited in a Dryad Digital Repository (doi:10.5061/dryad.4gv6t)