Abstract
Interspecific communication is common in nature, particularly between mutualists. However, whether signals evolved for communication with other species, or are in fact conspecific signals eavesdropped upon by partners, is often unclear. Fork-tailed drongos (Dicrurus adsimilis) associate with mixed-species groups and often produce true alarms at predators, whereupon associating species flee to cover, but also false alarms to steal associating species' food (kleptoparasitism). Despite such deception, associating species respond to drongo non-alarm calls by increasing their foraging and decreasing vigilance. Yet, whether these calls represent interspecific sentinel signals remains unknown. We show that drongos produced a specific sentinel call when foraging with a common associate, the sociable weaver (Philetairus socius), but not when alone. Weavers increased their foraging and decreased vigilance when naturally associating with drongos, and in response to sentinel call playback. Further, drongos sentinel-called more often when weavers were moving, and weavers approached sentinel calls, suggesting a recruitment function. Finally, drongos sentinel-called when weavers fled following false alarms, thereby reducing disruption to weaver foraging time. Results therefore provide evidence of an ‘all clear’ signal that mitigates the cost of inaccurate communication. Our results suggest that drongos enhance exploitation of a foraging mutualist through coevolution of interspecific sentinel signals.
Keywords: eavesdropping, interspecific signal, kleptoparasitism, mutualism, mixed-species group, sentinel call
1. Introduction
Mutualisms, where organisms derive a benefit by providing a ‘service’ that another organism cannot otherwise obtain, are ubiquitous throughout nature [1,2]. Within mutualisms, selection favours the coevolution of behaviours that enhance benefits to both parties, including the production of signals for interspecific communication [3–6]. However, while mutualists often attend to partner species' signals [1,5,7], it is often unclear whether such signals are specifically produced for communication with partners, or whether partners instead eavesdrop on signals produced for conspecific communication, thereby obtaining by-product benefits [7–9]. For interspecific signals to coevolve between mutualists, they must manipulate partner behaviour to the benefit of the signaller [8,9]. Consequently, while eavesdropping on a signal may be a precursor to the evolution of interspecific communication [5], such signals do not represent coevolved adaptations between mutualists [8]. To demonstrate that mutualists produce signals that function in interspecific communication with their partners, we suggest that it is necessary to determine: (i) whether the signal is produced in the appropriate context for interspecific communication, (ii) whether the signal elicits behaviour by the mutualist that benefits the signaller, and (iii) whether such behaviour is specific to the signal and not others in the signalling species' repertoire.
Signals for interspecific communication between mutualists are perhaps most familiar in flowering plants, which possess visual and olfactory signals to attract pollinators [1,7]. In other systems, interspecific signals have additionally been shown to facilitate cooperative foraging [10], or provide predator protection [3,4,11]. For example, treehoppers provision ants with a carbohydrate-rich excretion, but also produce vibrational signals to elicit costly defence towards predators [4]. A similar protection benefit has been suggested for the numerous birds and mammals that form mixed-species foraging mutualisms to reduce predation risk [7,12–14]. By eavesdropping on each other's alarm and sentinel calls, group members can reduce their own investment in antipredator vigilance and thereby increase time available for foraging [14–16]. However, there is currently limited evidence that individuals specifically produce signals for communication with heterospecific group members. Research on racket-tailed drongos (Dicrurus paradiseus), suggests they act as group sentinels, providing predator alarm calls in mixed-species bird flocks [17]. Furthermore, observations and playback experiments suggest that they produce vocal mimicry of other species' non-alarm or alarm calls in contexts that could facilitate species attraction, either to form foraging associations or to mob and potentially deter predators [17–19]. However, such mimicry is also produced by racket-tailed drongos when not associating with heterospecifics, and it remains unclear whether species' responses represent eavesdropping, or whether other species respond to the racket-tailed drongos natural production of vocal mimicry [17–19].
Fork-tailed drongos (Dicrurus adsimilis), henceforth drongos, are common throughout sub-Saharan Africa and appear to play a similar sentinel role in their mixed-species foraging associations [20–22]. Drongos impose costs on associating species by using aggression and false alarm calls to steal food (kleptoparasitism), which provides on average 23% of their mass intake, with a further 10% obtained by capturing prey flushed by associating species [22–24]. Nevertheless, drongos frequently produce true alarms at approaching predators, in response to which associating species flee to cover [22,25], and evidence indicates that drongos alarm at predators that threaten associating species rather than drongos themselves [26]. Furthermore, in response to playback of drongo non-alarm vocalizations, two species that associate with drongos, pied babblers (Turdoides bicolour) and dwarf mongooses (Helogale parvula), reduce their own vigilance, and in the case of the pied babbler, improve their foraging payoffs [20,21]. These results indicate that drongos could produce interspecific sentinel signals to enhance their own foraging [20]. However, it is unknown whether drongos produce a specific vocalization type when with other species, and whether other species' responses are specific to any particular drongo call. Consequently, it remains to be determined whether associating species simply eavesdrop on drongo calls, or whether drongos produce an interspecific sentinel signal.
Although drongo sentinel signals may be reliable and cooperative, they will ultimately be produced to manipulate other species' behaviour to the advantage of drongos, potentially facilitating drongo behaviour that is costly to partners. Consequently, a sentinel signal may be predicted to have three further functions: (i) to attract other species, thereby facilitating associations, (ii) to sound the ‘all clear’ after false alarms, mitigating the costs of frequent disruption to foraging [16,27], and (iii) to increase the likelihood other species respond to a drongos' false alarm calls. This final function may reflect the fact that associating species decrease their own vigilance in response to reliable sentinel calls and might therefore become more dependent on drongo alarm vocalizations, even when occasionally unreliable.
To determine whether drongos produce an interspecific sentinel signal, we tested our three criteria outlined above. We considered interactions between drongos and the species with which they most commonly associate in the Kalahari Desert, the sociable weaver (Philetairus socius) [23]. We first asked whether drongos specifically make a sentinel call when associating with weavers, and in contexts that might facilitate weaver attraction, or their post-alarm resumption of foraging. To investigate what functional benefits sentinel calls provide, we asked whether weavers increase their foraging behaviour and decrease their vigilance when naturally associating with drongos. We then undertook a sentinel call playback experiment to confirm that such benefits are in part attributable to sentinel calls, and to exclude the possibility that drongos only associate with weavers when payoffs are high. To investigate further functions for sentinel calls, we undertook playback experiments testing whether: (i) drongo sentinel calls attract weavers, (ii) whether weavers resume foraging more quickly after false alarms when sentinel calls are made, and (iii) whether weavers more frequently flee in response to drongo false alarms if sentinel calls were made prior to an alarm. We included control playbacks in all experiments to confirm that behavioural responses were specific to sentinel vocalizations.
2. Material and methods
(a). Study site and study species
Our study was undertaken in an area of xeric savannah in the southern Kalahari (26°58′ S, 21°50′ E). From April to May 2013, we collected observational and experimental data on 12 wild sociable weaver colonies tolerant of observers at distances of more than 10 m, and observed interactions with 96 wild drongos that were individually recognizable by colour rings on their legs and habituated to observers at less than 5 m. Sociable weavers are colonially breeding birds, creating massive communal nests containing 20–500 individuals [28]. Adults forage in flocks throughout the day and seldom feed further than 1.5 km from their nests [29]. To obtain data on foraging behaviour, flocks were followed for 3–4 h each morning from when they left their nest to begin foraging after dawn, until observations were complete or the flock was lost from view. To locate a second colony later in the morning, the nest was visited and departing sociable weavers were followed to the main foraging flock. Drongos frequently associated with foraging sociable weaver flocks, defined as watching a sociable weaver flock at a distance of less than 20 m. Drongos also foraged alone, defined as foraging when more than 20 m from another foraging species and not watching other species. Both when associating with other species and foraging alone, drongos foraged as loose parties, typically comprising a territorial pair and their offspring from that year.
(b). Do drongos produce sentinel calls when associating with sociable weavers?
To determine whether drongos produce a sentinel call for communication with foraging mutualists and in what contexts these calls are produced, we first identified whether drongos made any specific call type when associating with weavers, but not when foraging alone. We obtained recordings of drongo vocalizations from 19 one-hour focal sound recordings made on 19 drongos, selected from a dataset of 292 focal observations (mean focal length (±s.e.) 55 ± 1 min) collected from March to July 2008 (see [25] for further details). The focal selected for each drongo was that where the individual foraged alone and associated with weavers during the same focal; where a drongo foraged in both contexts in more than one focal, we selected the focal with the smallest difference between the time spent in each context. Sound recordings were displayed in spectrograms (see the electronic supplementary material for sound recording and spectrogram details) and we visually identified the drongo call types produced, recording the behavioural context in which they were made. Drongos produced numerous species-specific call types, but only one was produced when drongos were associating with sociable weavers and not engaged in alarm behaviour or interactions with other drongos. This call type was considered a possible sentinel call (figure 1) and was consequently used for subsequent analyses and experiments. Drongos typically produced these sentinel calls in bouts with on average 2.15 ± 0.04 calls per bout per drongo (min–max = 1–12; n = 1008 call bouts by 19 drongos); bouts were separated by a minimum of 1 s.
Figure 1.
Spectrograms of: (a) drongo sentinel, (b) drongo territorial and (c) white-browed sparrow-weaver (WBSW) calls used in playbacks; three exemplar sets are shown.
To confirm that drongos produced sentinel calls when associating with weavers, but not when foraging alone, we counted the number of sentinel call bouts focal drongos produced when foraging alone or associating with sociable weavers per focal, divided by the total foraging time spent in these foraging contexts, respectively. The presence of other drongos was unlikely to differentially affect sentinel call production in these contexts, because focal drongos were within 20 m of another drongo for on average 65 ± 5% of the time when foraging alone and 73% ± 7% of the time when associating with weavers (Wilcoxon test: v = 71, p = 0.3525, n = 19 drongos). To determine whether drongos might produce sentinel calls to attract or retain sociable weavers, we counted the number of calls produced per sentinel call bout when a weaver flock was foraging, or when moving (more than 50% of the flock in flight between locations on the ground, in the absence of an alarm). Finally, to determine whether drongos might produce sentinel calls to reduce foraging disruption following alarm calls, we counted the number of sentinel calls made within the 20 s prior to when a false alarm had begun (pre-alarm), and within the 20 s after the alarm ended, noting whether the weaver flock had fled to cover (more than 50% of a weaver flock flew to cover in bushes or trees) in response to the alarm (post-alarm flee) or not (post-alarm ignore). False alarm calls were defined as calls produced when a drongo was associating with sociable weavers and watching an individual handling food; no predator was ever observed during a false alarm [25]. Drongos produced true alarms during focal observations (13 true alarms by seven drongos), but weavers responded by ceasing foraging and leaving the area on four occasions resulting in a dataset of 10 true alarms by five drongos, which was too small for inclusion in analyses.
(c). Do sociable weavers gain foraging benefits when naturally associating with drongos?
To determine whether sociable weavers gain foraging and vigilance benefits when naturally associating with drongos, we undertook focal observations on individual sociable weavers located on the ground in foraging flocks. Focals were recorded using a Sony HDR-XR160 Camcorder (42× extended zoom) at distances varying from 5 to 30 m. Whenever a focal individual was lost or moved to a perch, a focal was ended and a new focal on a different individual was begun. At the beginning of each focal, we noted: (i) the focal weaver's location, and (ii) whether a drongo was present. It was not possible to identify individual sociable weavers, but birds continuously joined and left the flock, with flocks always exceeding 10 birds and typically 50, thus reducing the chance that a focal individual was sampled more than once. To balance the potential effects of weaver location within a flock on foraging behaviour, the observer alternated between focals on weavers located at the centre (at least one weaver between the focal and the flock edge) and periphery (no weaver between the focal and the flock edge) of the foraging flock. This additionally reduced the chance of sampling the same bird twice when the bird was lost from view. Focal video footage was analysed using VLC media player (v. 2.0.8) recording: (i) vigilance rate, defined as the number of times a bird raised its head throughout a focal (above 0° parallel to the ground), divided by focal duration (seconds), and (ii) foraging time, defined as the proportion of total focal time (seconds) that the bird spent digging in the substrate with its bill. A total of 175 focals were undertaken at 11 colonies (mean per colony = 16 focals, min–max = 8–34); each colony had a mean 1168 ± 120 s of focal footage (min–max = 513–1875; n = 11) collected during a minimum of two morning observation periods. The mean duration of focals was 75.8 ± 3.5 s (min–max = 10–233; n = 175) and the ratio of central to peripheral focals was 58 : 42.
(d). Playback experiments: what benefits are obtained by drongos and sociable weavers from drongo sentinel calls?
To determine whether weavers modified their behaviour in response to drongo sentinel calls, we undertook three playback experiments at 12 sociable weaver colonies. In all the experiments, we played three call types to control for the possibility that weaver responses were not specific to drongo sentinel calls, but rather to drongo calls more generally or even to any bird call, including those of species that do not typically associate with weavers. The three call types were: (i) drongo sentinel calls (sentinel), (ii) drongo territorial calls (territorial), and (iii) white-browed sparrow-weaver (Plocepasser mahali) (WBSW) calls (figure 1). Drongo territorial calls were identified in spectrograms and from their behavioural context; they are produced by drongos at approximately 20 s intervals from a high perch (>5 m), and drongos on neighbouring territories typically respond by matching these calls [25]. Twelve exemplar sets of three calls (sentinel, territorial and WBSW) were composed using the program Cool Edit Pro (v. 2.0) for playback in experimental treatments. The same set of 12 exemplars was used in each of the three experiments, but the same exemplar was never played at the same colony twice and playback treatment order was different for each experiment at the same colony. To create exemplars, 12 sentinel and territorial calls were obtained from focal recordings on 12 different drongos. WBSW were rarely observed with sociable weavers during the study period and 12 WBSW calls were obtained from recordings of singing individuals at 12 nest trees (see the electronic supplementary material for further details of call exemplars).
(i). Do sociable weavers improve their foraging in response to drongo sentinel calls?
To determine whether weavers decrease their vigilance and increase foraging time in response to drongo sentinel calls, we played three call treatments (sentinel, territorial and WBSW) at 12 weaver colonies and simultaneously undertook focal observations. For all call treatments, a single call of the treatment type was played at 11 s intervals (11 s of silence inserted between calls), which corresponded to the natural frequency with which drongos produce sentinel vocalizations when associating with weavers (11.06 ± 1.01 s; n = 19 drongos). The 11 s interval was used for all treatments in all playbacks to standardize call rate (see the electronic supplementary material for further details of exemplars for this and subsequent playback experiments). The three call treatments were played for a period of 10 min when the weaver colony was foraging, with a minimum 10 min break between treatments during a single observation session. On occasions when the weaver flock ceased foraging, we paused playback and resumed once the flock resumed foraging (more than 50% flock members). Calls were played from a speaker carried by the observer who maintained a distance of 5–30 m from the edge of the flock. Focal recordings were undertaken throughout call treatment playback; methods for recording focals and analysing videos were the same as previously outlined for focal observations. The presence of a drongo was similarly recorded to control for any effect this had, because associating drongos invariably produced sentinel calls. Each colony had a mean 1035 ± 46 s (min–max = 773–1301; n = 12) total focal time collected during experimental playbacks. The mean duration of focals was 70.2 ± 3.5 s (min–max = 12–241; n = 177) and the ratio of central to peripheral focals was 53 : 47.
(ii). Do drongo sentinel calls attract sociable weavers?
To determine whether weavers approach drongo sentinel calls when departing their nest, we played three call treatments (sentinel, territorial and WBSW), on three separate days at 12 weaver nests. Call treatments consisted of the treatment call repeated at 11 s intervals for 5 min. For this experiment, the treatment was played on repeat until the experiment was finished. Before dawn, the speaker was placed 80 m from the nest on a 1 m stand to simulate a drongo in sentinel position and the same location was used for each treatment; the observer sat 50 m from the speaker. The call treatment was started when the weavers began to vocalize from within the nest and continued until 5 min after the first weaver had left the nest to forage. Video footage was analysed afterwards, recording whether or not any weavers approached and landed on the ground within 20 m of the speaker.
(iii). Do drongo sentinel calls reduce foraging disruption resulting from false alarm calls and increase weaver response to false alarms?
To determine whether drongo sentinel calls decrease the time it takes weavers to resume foraging following a drongo false alarm and increase the likelihood weavers respond to false alarms, we played three call treatments (sentinel, territorial and WBSW) to 12 weaver colonies, with playback of a drongo false alarm call preceding and following each treatment. The three treatments were played during a single observation session to the foraging weaver flock, with a minimum 10 min break between treatments. The pre-treatment alarm call was composed to maximize the likelihood that foraging weavers fled to cover and consisted of a drongo alarm combining the three most frequently produced drongo alarm call types (‘weep’, ‘pee-pee kerrr’ and ‘skyeek’; see the electronic supplementary material, figure S1) [25]. This was followed by 5 min of call treatment playback, beginning 3 s after the alarm call and reproduced at 11 s intervals. Finally, a post-treatment ‘skyeek’ false alarm call was played 11 s after the final treatment call. During playbacks, video recordings were made and we subsequently analysed: (i) the time weavers took to resume foraging after the pre-treatment alarm (more than 50% of colony return to the ground), and (ii) the colonies response to the post-treatment alarm (did more than 50% flee to cover, or not). Weavers fled to cover in response to the pre-treatment alarm on 33 of 36 playbacks, resulting in three missing data points.
(e). Statistical analyses
Analyses were conducted using R (v. 3.0.1). Linear mixed models (LMMs) and generalized linear mixed models (GLMMs) were undertaken where analyses included both fixed and random terms (see the electronic supplementary material for further model details). Post-hoc Tukey tests were undertaken to check for significant differences between factor levels.
For analyses of sentinel call production when weavers were moving, we used a Poisson GLMM with the number of calls produced per sentinel call bout as the response variable and weaver behaviour (foraging, moving) as the explanatory term; focal drongo identity was included as a random term. For analyses of sentinel call production following drongo false alarms, we used a zero-inflated negative binomial GLMM, with the number of sentinel calls made in the 20 s period pre- or post-false alarm call as the response variable distinguishing between occasions when weavers had fled to cover or not (pre-alarm, post-flee, post-ignore); false alarm call nested within focal drongo identity were included as random terms.
For analysis of foraging time and vigilance rates during natural focal observations, we used a GLMM with a binomial response variable of foraging time over focal length, and an LMM with vigilance per second (log10 transformed) as the response variable. Explanatory terms for both models included weaver location (centre, periphery) and presence of a drongo (present, absent). Weaver colony was included as a random term and in all subsequent analyses. For playback experiment (i), two models were undertaken similar to those for natural focal observations; focal order within a playback treatment was included as an explanatory term, as were call treatment (sentinel, territorial and WBSW) and call order (first, second and third), which were also included in subsequent analyses. For playback experiment (ii), we undertook a GLMM with a binomial response variable of whether weavers approached or not (1/0). For playback experiment (iii), we undertook two models: the first was an LMM with a response variable of time taken to resume foraging after a pre-treatment alarm (square-root transformed); the second was a GLMM with a binomial response variable of whether a colony fled or not (1/0) to a post-treatment alarm.
3. Results
(a). Do drongos produce sentinel calls when associating with sociable weavers?
Drongos produced only one call type, considered a sentinel call, when associating with weavers and not engaged in other behaviour. This sentinel call was produced more frequently by all 19 focal drongos when associating with weavers (2.20 ± 0.28 per min), than when foraging alone, when it was rarely produced at all (0.09 ± 0.28 per min) (figure 2a). Drongos also made more sentinel calls per bout when a weaver flock was moving (2.84 ± 0.20) compared with when a flock was foraging (1.96 ± 0.10), (figure 2b and the electronic supplementary material, table S1). Furthermore, when weavers had fled to cover in response to drongo false alarm calls, drongos made more sentinel calls (6.67 ± 1.56) compared with the period preceding the alarm (1.34 ± 0.26), but did not do so when weavers had not fled (1.86 ± 0.30), (figure 2c and the electronic supplementary material, table S2).
Figure 2.
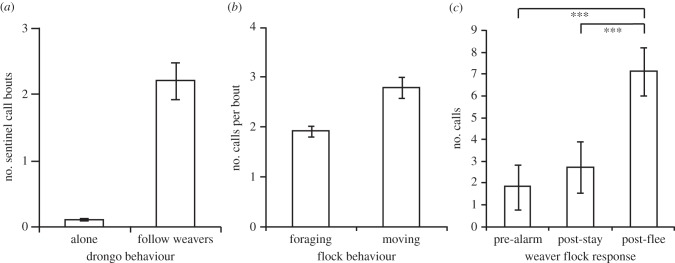
Drongo sentinel call production. (a) Drongos made more sentinel call bouts when with weavers then when foraging alone (Wilcoxen test: v = 0, p ≤ 0.001; n = 19) and (b) made more sentinel calls during bouts when weavers were moving rather than foraging (GLMM: Z = 6.47, p ≤ 0.001; n = 1008 bouts by 19 drongos; electronic supplementary material, table S1). (c) Drongos made more sentinel calls following false alarms when weavers fled to cover (post-flee), than during the period preceding the alarm (pre-alarm), but did not do so when weavers ignored the alarm (post-stay), (GLMM: Z = −5.11, p ≤ 0.001; n = 131 alarms by 17 drongos). All figures show predicted means ± 1 s.e. where applicable; for Tukey contrasts the following is the guide: *p ≤ 0.05, **p ≤ 0.001, ***p ≤ 0.001.
(b). Do sociable weavers gain foraging benefits when naturally associating with drongos and by attending to drongo sentinel calls?
When associating with drongos under natural conditions, weavers increased their foraging time and reduced their vigilance rates (figure 3a,b and the electronic supplementary material, table S3). Similarly, weavers foraged for longer in response to experimental playback of drongo sentinel calls, than to control territorial or WBSW calls, so long as a drongo was absent from a foraging flock (figure 3c and the electronic supplementary material, table S4). When a drongo was present, foraging time did not differ because weavers increased the time they spent foraging regardless of call playback (figure 3c and the electronic supplementary material, table S4). Weavers also decreased their vigilance in response to sentinel calls compared with territorial and WBSW calls (figure 3d and the electronic supplementary material, table S4); but in contrast to expectations based on the results for foraging time, the presence of a drongo did not significantly decrease vigilance during control playbacks (LMM: F = 0.07, p = 0.789, n = 177 focals during 36 playbacks; electronic supplementary material, table S4).
Figure 3.
Sociable weaver foraging and vigilance when naturally associating with drongos, and in response to call playbacks. When drongos were naturally associating with a weaver flock, weavers (a) foraged for longer and (b) were less frequently vigilant, than when drongos were absent (GLMM foraging: Z = 0.045, p = 0.045; LMM vigilance: F = 23.82, p ≤ 0.001; n = 175 focals at 11 colonies; electronic supplementary material, table S3). Similarly, (c) weavers foraged for longer in response to playback of drongo sentinel calls than to territorial or WBSW calls, but only when drongos were absent during playbacks (GLMM foraging: Z = 10.35, p ≤ 0.001; n = 177 focals during 36 playbacks at 12 colonies; electronic supplementary material, table S4). (d) Weavers were also less frequently vigilant when sentinel calls were played than territorial or WBSW calls (LMM: F = 42.20, p ≤ 0.001; electronic supplementary material, table S4).
(c). Do drongo sentinel calls: (i) attract sociable weavers, (ii) reduce weaver foraging disruption resulting from false alarm calls, and (iii) increase weaver response to false alarms?
Sociable weavers were significantly more likely to approach playback of drongo sentinel calls than control territorial and WBSW calls (figure 4a and the electronic supplementary material, table S5). Furthermore, weavers that had fled to cover in response to playback of drongo false alarm calls resumed foraging more quickly when sentinel calls were played, compared with playback of WBSW calls (figure 4b and the electronic supplementary material, table S6); a similar but non-significant pattern was observed between sentinel and territorial call playbacks. However, playback of sentinel calls did not increase the likelihood that weavers fled to cover in response to a drongo false alarm call, compared with territorial or WBSW calls (GLMM: χ2 = 1.28, p = 0.410, n = 33; electronic supplementary material, table S6).
Figure 4.

Sociable weaver movement and alarm behaviour in response to call playbacks. (a) When leaving their communal nest to forage, weaver flocks were more likely to approach playback of drongo sentinel calls than territorial or WBSW calls (GLMM: Z = −3.40, p ≤ 0.001; n = 36 playbacks at 12 nests; electronic supplementary material, table S5). (b) When weaver flocks fled to cover following playback of drongo false alarm calls, flocks resumed foraging more quickly to playback of sentinel calls than WBSW calls (LMM: F = 5.17, p = 0.004; n = 33 playbacks at 12 colonies; electronic supplementary material, table S6), but despite a similar trend, they did not do so significantly (n.s.) more quickly than in response to territorial calls (Tukey contrast: p = 0.166).
4. Discussion
Our results suggest that fork-tailed drongos produce interspecific sentinel calls that provide several benefits both to drongos and their foraging mutualist, the sociable weaver. Weavers naturally associating with drongos benefit by increasing their foraging effort and decreasing vigilance behaviour. Although drongos might only follow weavers when foraging returns are high, playbacks of drongo sentinel calls confirmed that changes in weaver foraging and vigilance are in part mediated by sentinel calls, which drongos specifically produced when associating with weavers. Furthermore, drongos made sentinel calls most often when weavers were moving, and weavers approached sentinel call playback, suggesting these calls facilitate the formation of associations. Finally, sentinel calls appear to reduce disruption resulting from drongo false alarms, because they were produced most often when weavers had fled following a false alarm, and weavers resumed foraging more quickly to sentinel call playback. However, sentinel calls did not appear to benefit drongos by increasing weaver response to the drongos' false alarm calls. Overall, drongo sentinel calls benefit weavers by increasing time available for foraging, and benefit drongos by increasing opportunities for kleptoparasitism or capture of flushed prey [20]. Our results therefore provide strong evidence that a cooperative, yet manipulative, interspecific signal has coevolved for communication between mixed-species foraging mutualists.
Previous research has shown that mixed-species group members eavesdrop on each other's alarm calls [14], and could in theory produce interspecific signals for communication with associating species [18–20]. However, we here tested the conditions necessary to distinguish between eavesdropping and interspecific signalling. We showed that drongo sentinel calls: (i) were produced when associating with other species, but rarely if at all in other contexts, (ii) elicited a specific behavioural response from other species that benefited the signaller, and (iii) that other calls in the signaller's repertoire or that of non-associating species did not elicit similar behaviour. Future investigation of interspecific signalling should consider these three criteria to confirm that signals are specifically produced for communication with other species.
The drongo sentinel signal appears to serve multiple functions within mutualistic associations. In common with sentinel signals produced by other species, it increases foraging returns for group members [20,30,31]. Further, it attracts foraging mutualists, potentially facilitating the formation and maintenance of associations, as suggested for signals made by other mutualists [4,10]. Finally, drongo sentinel signals may sound the ‘all clear’ following alarm disruption, mitigating the costs of alarm responses. Such ‘all clear’ signals could either take the form of structurally distinct signals only produced after alarms when predators are absent, or as is the case for the drongo sentinel call, they may be signals produced in all non-alarm contexts where variation in the rate of call production effectively signals that a predator is no longer present [32]. Research has previously shown that species use the resumption of non-alarm calling by conspecifics or heterospecifics as a cue to resume foraging following predator alarms, but such behaviour is considered eavesdropping rather than signalling [16,33]. Further, some sentinel-calling species increase call rates following alarms when a threat is no longer present suggesting a possible all clear function [32]. However, how group members respond is unclear and such signals may instead indicate greater predation threat increasing vigilance and decreasing foraging [34]. By contrast, we show that drongos increased sentinel call frequency after alarms, but where no predator had been present, and weavers responded by resuming foraging. Consequently, our results represent strong evidence that species produce ‘all clear’ signals. Such behaviour could provide significant benefits to group-living species, whose foraging is frequently disrupted by alarms, particularly inaccurate alarms (including those made in kleptoparasitism or in response to non-threatening stimuli), which may account for up to 75% of alarm signals [16,35].
Our results illustrate how communication between mutualists can increase benefits obtained from the relationship. Such behaviour can play a key role in stabilizing association payoffs, thereby preventing fluctuation between parasitic and mutualistic relationships [1,36]. This is because mutualism payoffs are typically dynamic and transition to parasitic relationships, or vice-versa, may occur where mutualist partners incur costs as well as benefits from associations [1,5,36]. Whether drongo sentinel calls originally facilitated transition from parasitic to mutualistic relations between drongos and weavers is unknown [20]. Nevertheless, these species currently appear to be engaged in a cooperative mutualism towards which the drongo's sentinel signal contributes, because weavers increase their foraging when associating with drongos and actively approach sentinel vocalizations. However, it remains unclear how the drongo's interspecific sentinel signal, and interspecific signals more generally, coevolve between signallers and receivers [14]. Further research is necessary to investigate the contribution of ‘innate’ mechanisms versus individual and social learning, to the production of interspecific signals and partner species' responses to these signals.
The benefits weavers derive from attending to the drongo's cooperative sentinel calls may ironically result in tolerance of a higher frequency of costly kleptoparasitism. However, we might speculate that as in other cooperative systems, weaver flocks could employ sanctions [1,37] to reduce kleptoparasitic cheating, either by ignoring sentinel and alarm signals when the costs of kleptoparasitism exceed the benefits of drongo vigilance, or by switching to another drongo's territory [37]. Partner switching may not however, be very effective in group-living species such as sociable weavers, where individuals within groups probably vary in the costs and benefits of switching depending on their individual state. Consequently, group members suffering net costs from associating with drongos may be unable to effect coordinated group movement when other individuals continue to gain net benefits [38]. Future research should consider whether individuals increase partner cooperation by imposing sanctions to reduce cheating, and by monitoring how often alternative partners cheat.
In summary, our results illustrate how coevolution between mutualists can result in cooperative behaviour, such as the drongo sentinel call, that can increase benefits in mixed-species foraging associations [6,36]. Nevertheless, drongos produce sentinel calls to manipulate their mutualist, enhancing host foraging behaviour to facilitate kleptoparasitic exploitation. This is illustrated by the drongos' tactical production of sentinel calls to sound the ‘all clear’ after false alarms. As such, sentinel calls can be considered both a cooperative signal and an adaptation to kleptoparasitic behaviour. This apparent paradox highlights the fact that interactions between organisms, including mutualists, are characterized by both conflict and cooperation.
Supplementary Material
Acknowledgements
Access to the study site was kindly provided by the Kalahari Research Trust, T. H. Clutton-Brock and M. Manser. We also thank Peter Ryan, Claire Spottiswoode, Rafael Mares and Martha Nelson-Flower for supervision, help and advice. Two anonymous reviewers provided valuable comments that improved this manuscript.
Northern Cape Conservation provided research permission (FAUNA 270/2008) and the University of Cape Town provided ethical permission (2010/V20/TF).
Data accessibility
Data for all analyses presented are available in the DRYAD Repository (doi:10.5061/dryad.m3263).
Funding statement
Funding was provided by the DST/NRF Centre of Excellence at the Percy FitzPatrick Institute of African Ornithology, the University Research Council at the University of Cape Town and Drs C. and R. Baigrie.
References
- 1.Leigh J. 2010. The evolution of mutualism. J. Evol. Biol. 23, 2507–2528. ( 10.1111/j.1420-9101.2010.02114.x) [DOI] [PubMed] [Google Scholar]
- 2.Herre E, Knowlton N, Mueller U, Rehner S. 1999. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 14, 49–53. ( 10.1016/S0169-5347(98)01529-8) [DOI] [PubMed] [Google Scholar]
- 3.Axén AH, Leimar O, Hoffman V. 1996. Signalling in a mutualistic interaction. Anim. Behav. 52, 321–333. ( 10.1006/anbe.1996.0178) [DOI] [Google Scholar]
- 4.Morales MA, Barone JL, Henry CS. 2008. Acoustic alarm signalling facilitates predator protection of treehoppers by mutualist ant bodyguards. Proc. R. Soc. B 275, 1935–1941. ( 10.1098/rspb.2008.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostan KM. 2002. The evolution of mutualistic interspecific communication: assessment and management across species. J. Comp. Psychol. 116, 206 ( 10.1037/0735-7036.116.2.206) [DOI] [PubMed] [Google Scholar]
- 6.Connor RC. 1995. The benefits of mutualism: a conceptual framework. Biol. Rev. 70, 427–457. ( 10.1111/j.1469-185X.1995.tb01196.x) [DOI] [Google Scholar]
- 7.Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD. 2010. Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 25, 354–361. ( 10.1016/j.tree.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 8.Diggle SP, Gardner A, West SA, Griffin AS. 2007. Evolutionary theory of bacterial quorum sensing: when is a signal not a signal? Phil. Trans. R. Soc. B 362, 1241–1249. ( 10.1098/rstb.2007.2049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard Smith J, Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Vail AL, Manica A, Bshary R. 2013. Referential gestures in fish collaborative hunting. Nat. Commun. 4, 1765 ( 10.1038/ncomms2781) [DOI] [PubMed] [Google Scholar]
- 11.Travassos MA, Pierce NE. 2000. Acoustics, context and function of vibrational signalling in a lycaenid butterfly–ant mutualism. Anim. Behav. 60, 13–26. ( 10.1006/anbe.1999.1364) [DOI] [PubMed] [Google Scholar]
- 12.Ridley AR, Wiley EM, Thompson AM. 2014. The ecological benefits of interceptive eavesdropping. Funct. Ecol. 28, 197–205. ( 10.1111/1365-2435.12153) [DOI] [Google Scholar]
- 13.Magrath RD, Bennett TH. 2012. A micro-geography of fear: learning to eavesdrop on alarm calls of neighbouring heterospecifics. Proc. R. Soc. B 279, 902–909. ( 10.1098/rspb.2011.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magrath RD, Haff TM, Fallow PM, Radford AN. In press. Eavesdropping on heterospecific alarm calls: from mechanisms to consequences. Biol. Rev. ( 10.1111/brv.12122) [DOI] [PubMed] [Google Scholar]
- 15.Rasa OAE. 1983. Dwarf mongoose and hornbill mutualism in the Taru Desert, Kenya. Behav. Ecol. Sociobiol. 12, 181–190. ( 10.1007/BF00290770) [DOI] [Google Scholar]
- 16.Sullivan KA. 1984. Information exploitation by downy woodpeckers in mixed-species flocks. Behaviour 91, 294–311. ( 10.1163/156853984X00128) [DOI] [Google Scholar]
- 17.Goodale E, Kotagama SW. 2008. Response to conspecific and heterospecific alarm calls in mixed-species bird flocks of a Sri Lankan rainforest. Behav. Ecol. 19, 887–894. ( 10.1093/beheco/arn045) [DOI] [Google Scholar]
- 18.Goodale E, Kotagama SW. 2006. Vocal mimicry by a passerine bird attracts other species involved in mixed-species flocks. Anim. Behav. 72, 471–477. ( 10.1016/j.anbehav.2006.02.004) [DOI] [Google Scholar]
- 19.Goodale E, Ratnayake CP, Kotagama SW. 2014. Vocal mimicry of alarm-associated sounds by a drongo elicits flee and mobbing responses from other species that participate in mixed-species bird flocks. Ethology 120, 266–274. ( 10.1016/j.anbehav.2006.02.004) [DOI] [Google Scholar]
- 20.Radford AN, Bell MBV, Hollén LI, Ridley AR. 2011. Singing for your supper: sentinal calling by kleptoparasitites can mitigate the cost to victims. Evolution 65, 900–906. ( 10.1111/j.1558-5646.2010.01180.x) [DOI] [PubMed] [Google Scholar]
- 21.Sharpe LL, Joustra AS, Cherry MI. 2010. The presence of an avian co-forager reduces vigilance in a cooperative mammal. Biol. Lett. 6, 475–477. ( 10.1098/rsbl.2009.1016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridley AR, Raihani NJ. 2007. Facultative response to a kleptoparasite by the cooperatively breeding pied babbler. Behav. Ecol. 18, 324–330. ( 10.1093/beheco/arl092) [DOI] [Google Scholar]
- 23.Flower TP, Child M, Ridley AR. 2013. The ecological economics of kleptoparasitism: pay-offs from self-foraging versus kleptoparasitism. J. Anim. Ecol. 82, 245–255. ( 10.1111/j.1365-2656.2012.02026.x) [DOI] [PubMed] [Google Scholar]
- 24.Flower TP, Gribble M. 2012. Kleptoparasitism by attacks versus false alarms in the fork-tailed drongo. Anim. Behav. 83, 403–410. ( 10.1016/j.anbehav.2011.11.009) [DOI] [Google Scholar]
- 25.Flower T. 2011. Fork-tailed drongos use deceptive mimicked alarm calls to steal food. Proc. R. Soc. B 278, 1548–1555. ( 10.1098/rspb.2010.1932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridley AR, Child MF, Bell MBV. 2007. Interspecific audience effects on the alarm-calling behaviour of a kleptoparasitic bird. Biol. Lett. 3, 589–591. ( 10.1098/rsbl.2007.0325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse DH. 1982. Behavioral mechanisms in ecology. Cambridge, MA: Harvard University Press. [Google Scholar]
- 28.Maclean G. 1973. The sociable weaver, part 1: description, distribution, dispersion and populations. Ostrich 44, 176–190. ( 10.1080/00306525.1973.9639158) [DOI] [Google Scholar]
- 29.Maclean G. 1973. The sociable weaver, part 5: food, feeding and general behaviour. Ostrich 44, 254–261. ( 10.1080/00306525.1973.9639162) [DOI] [Google Scholar]
- 30.Manser MB. 1999. Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc. R. Soc. Lond. B 266, 1013–1019. ( 10.1098/rspb.1999.0737) [DOI] [Google Scholar]
- 31.Hollén LI, Bell MB, Radford AN. 2008. Cooperative sentinel calling? Foragers gain increased biomass intake. Curr. Biol. 18, 576–579. ( 10.1016/j.cub.2008.02.078) [DOI] [PubMed] [Google Scholar]
- 32.Kern JM, Radford AN. 2013. Call of duty? Variation in use of the watchman's song by sentinel dwarf mongooses, Helogale parvula. Anim. Behav. 85, 967–975. ( 10.1016/j.anbehav.2013.02.020) [DOI] [Google Scholar]
- 33.Wingelmaier K, Winkler H, Nemeth E. 2007. Reed bunting (Emberiza schoeniclus) males sing an ‘all-clear’ signal to their incubating females. Behaviour 144, 195–206. ( 10.1163/156853907779947319) [DOI] [Google Scholar]
- 34.Bell MBV, Radford AN, Rose R, Wade HM, Ridley AR. 2009. The value of constant surveillance in a risky environment. Proc. R. Soc. B 276, 2997–3005. ( 10.1098/rspb.2009.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cresswell W, Hilton GM, Ruxton GD. 2000. Evidence for a rule governing the avoidance of superfluous escape flights. Proc. R. Soc. Lond. B 267, 733–737. ( 10.1098/rspb.2000.1064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy B, Kirchner J. 2000. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54, 51–63. ( 10.1111/j.0014-3820.2000.tb00007.x) [DOI] [PubMed] [Google Scholar]
- 37.Raihani NJ, Thornton A, Bshary R. 2012. Punishment and cooperation in nature. Trends Ecol. Evol. 27, 288–295. ( 10.1016/j.tree.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 38.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2011.12.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for all analyses presented are available in the DRYAD Repository (doi:10.5061/dryad.m3263).




