Abstract
Polyphenisms can be adaptations to environments that are heterogeneous in space and time, but to persist they require conditional-specific advantages. The nematode Pristionchus pacificus is a facultative predator that displays an evolutionarily conserved polyphenism of its mouthparts. During development, P. pacificus irreversibly executes either a eurystomatous (Eu) or stenostomatous (St) mouth-form, which differ in the shape and number of movable teeth. The Eu form, which has an additional tooth, is more complex than the St form and is thus more highly derived relative to species lacking teeth. Here, we investigate a putative fitness trade-off for the alternative feeding-structures of P. pacificus. We show that the complex Eu form confers a greater ability to kill prey. When adults were provided with a prey diet, Eu nematodes exhibited greater fitness than St nematodes by several measures, including longevity, offspring survival and fecundity when followed by bacterial feeding. However, the two mouth-forms had similar fecundity when fed ad libitum on bacteria, a condition that would confer benefit on the more rapidly developing St form. Thus, the two forms show conditional fitness advantages in different environments. This study provides, to our knowledge, the first functional context for dimorphism in a model for the genetics of plasticity.
Keywords: developmental plasticity, feeding behaviour, fitness, polyphenism, Pristionchus pacificus, trade-off
1. Introduction
Developmental plasticity has been proposed to foster adaptation and rapid diversification by providing a ready source of selectable variation [1,2]. Polyphenisms in particular allow jumps to alternative adaptive peaks, including responses to environments that are heterogeneous in space and time, without disruptive selection on a particular morphology. To be both adaptive and evolutionarily stable, alternative forms must each be more favourable under a given set of environmental conditions [3]. Where alternative phenotypes arise from a single genotype, developmental outcomes are often finely tuned to the environment to optimize fitness, such as in response to seasonal changes [4], the presence of predators [5] or the availability of a given food source [6]. However, clear demonstrations of the benefits and costs of polyphenisms under different environmental conditions are still elusive. Separate developmental modules as allowed by a developmental switch [7] could allow diversifying selection on morphs [1,8], but the conditions favouring alternative morphs must be identified to test this principle.
Polyphenisms that allow alternative resource use are of particular interest for the relationship between plasticity and diversity. In contrast to alternative phenotypes that are costly responses to transient antagonistic pressures in the environment, resource polyphenisms enable niche partitioning and its associated character displacement within species [9]. The consequent divergence within species might then be followed by genetic assimilation or accommodation of optimized forms [10,11], possibly leading to morphological diversification [1,2,12,13]. Given the abundance of known resource polyphenisms, such plasticity may be a common diversifying force in nature [1,9,14]. Determining and quantifying fitness effects on alternative phenotypes can test their adaptive value and thus their ability to persist and diverge.
The nematode Pristionchus pacificus, an emerging model for comparative mechanistic studies of developmental traits [15], exhibits a morphological dimorphism that may correlate with variable food resources [16]. The feeding-structure dimorphism of P. pacificus consists of contrasting mouthparts in the adult stage (figure 1a–c). The dimorphism results from an irreversible decision during larval development, as mouthparts are produced during the final moult of the nematode life cycle. Both mouth-forms possess a dorsal tooth that has enabled predation (figure 1d) and, like the dimorphism, is a novelty of the nematode family Diplogastridae [17]. However, one of the forms, the eurystomatous (Eu, ‘wide-mouthed’) form, is equipped with even more complex structures, including an opposing tooth and a heavily serrated plate of denticles [18]. The Eu form is highly derived with respect to the closest outgroups that lack teeth, whereas the alternative, stenostomatous (St, ‘narrow-mouthed’) form is more reminiscent of outgroups in terms of mouth complexity and shape [9]. With such a dimorphism, this model system has been amenable to advances in understanding the genetic regulation of plasticity, including neuroendocrine signalling [19] and a developmental switch from a new gene [7].
Figure 1.
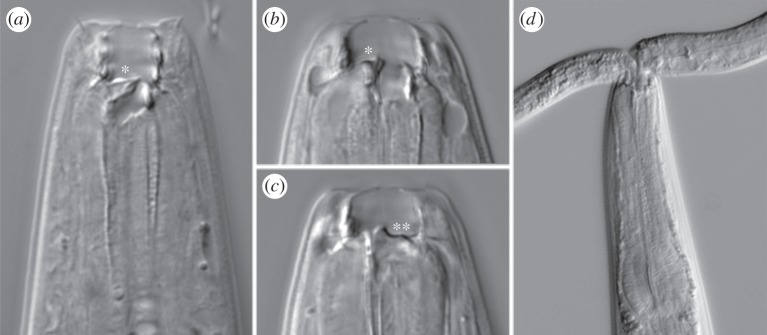
Mouth dimorphism and predation in P. pacificus. Movable teeth and a mouth dimorphism are both novelties of P. pacificus and other Diplogastridae and have enabled predation in this nematode family. (a) St form, with a single, triangular dorsal tooth (asterisk). (b) Eu form, with a claw-like dorsal tooth (asterisk). (c) Right sublateral focal plane of same individual in (b), showing a second, opposing tooth (double asterisks). Compared to the St form, the Eu form is more complex and highly derived with respect to outgroups that lack teeth or a mouth dimorphism. (d) Eu hermaphrodite of P. pacificus attacking a larva of C. elegans. Photograph in (d) by Dan Bumbarger.
The variable environments in which P. pacificus and other diplogastrids proliferate, especially the cadavers of host insects [20,21], may favour a resource polyphenism. These environments are rich and dynamic, being rapidly colonized by various bacteria [22], fungi and other nematodes [23]. Consistent with conditional regulation in response to such an environment, the dimorphism of P. pacificus is influenced by signals that indicate resource availability. Starvation, specifically the deprivation of bacterial food, is one cue that triggers the formation of the Eu form [19]. The presence of conspecifics, which is sensed by small molecules, also induces development of the Eu form [24,25]. By responding to crowding, the dimorphism may result in a frequency-dependent switch to alternative food sources [6]. Specifically, this switch might allow better predation on other nematodes, possibly even cannibalism, which has been anecdotally observed in P. pacificus (V. Serobyan, E. J. Ragsdale 2013, unpublished data).
Despite its conditional regulation, the dimorphism is apparently also stochastically regulated. Both mouth-forms are consistently present in populations under all tested experimental conditions. Moreover, the majority of wild P. pacificus populations examined have a mouth-form frequency biased towards the Eu form (i.e. more than 50% of a population) when fed exclusively on bacteria [7], a diet that in nematodes requires neither teeth nor dimorphism. The production of both forms in every generation thus suggests a partial bet-hedging strategy that is based on probabilities for enhanced fitness under variable conditions [26]. The ephemeral environments P. pacificus inhabits are unpredictable, presumably occurring with irregular frequency with respect to the life cycle of the nematode. Therefore, a dimorphism with mixed regulation may be advantageous for exploiting such temporally uncertain and variable niches, although precisely how the two feeding-forms use resources differently was heretofore unknown.
As a first response to this problem, the potential for a fitness trade-off in the dimorphism of P. pacificus was recently demonstrated. Specifically, the St form was found to develop to maturity faster on a bacterial diet, presumably owing to its simpler morphology and accompanying physiological differences [24]. Specifically, St animals proceed through postembryonic development about 6 h faster than Eu animals, resulting in a head start in reproduction for the St form in a population. Given the nematodes' short generation time of 3–4 days, this difference in developmental rate gives the St form of P. pacificus a significant advantage when feeding on bacteria, a benefit that would be particularly exaggerated over the several generations possible on ephemeral resources. However, because individuals of both forms are prolific on a bacterial diet, some fitness advantage for the Eu form would be required to ensure the persistence of a dimorphism. Alternative food sources, including other nematodes, could offer the necessary conditions to favour the Eu form. The ability to feed on other nematodes is a commonly observed phenomenon in Pristionchus and other Diplogastridae (figure 1d), although it was previously unknown whether this ability differs between the two forms. Because of its wide mouth and opposing teeth, the Eu form could be predicted by analogy to be the superior predator; to the best of our knowledge, however, this precept has never been tested.
Fitness tests are especially feasible in P. pacificus owing to the hermaphroditic mode of reproduction in this species. Hermaphrodites (XX morphological females) can also facultatively outcross, as males (X0 animals) are occasionally produced by meiotic X-chromosome non-disjunction [27]. This reproductive strategy makes P. pacificus amenable to direct measurements of fitness of either mated or unmated individuals in response to experimental conditions. Here, by developing experimental assays involving predators and prey, we have tested the functional and fitness advantages of the Eu form of P. pacificus.
2. Material and methods
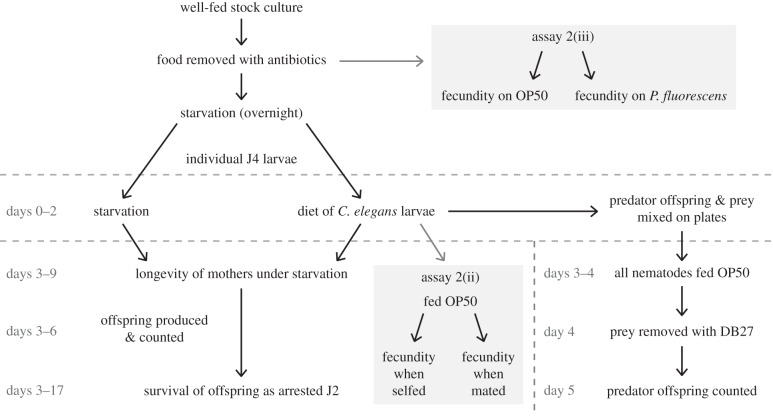
To test the adaptive value of alternative forms, we measured their respective functional and fitness advantages. First, as a test of function of form-specific morphologies, we assayed the ability of each form to kill prey. Second, we measured the fitness of alternative forms that were fed prey as adults (figure 2). Third, we measured the fitness of forms when fed prey and then afterwards allowed to feed on bacteria, which we identified as a much more nutritional food source then prey. Finally, to complement an earlier study on developmental times on a bacterial diet [24], we tested for fitness advantages of the alternative forms in the presence of abundant bacteria.
Figure 2.
An assay for fitness differences between Eu and St mouth-forms fed on prey. Figure corresponds to methods described in §2b of text, with alterations shown for assays in 2(ii) and 2(iii) as described in the electronic supplementary material.
In the following, we describe the methodology of the predatory assay §2a and the fitness differences on prey diet §2b. Other parts of the methodology, i.e. (i) nematode cultivation and phenotypes, (ii) fecundity on a heterogeneous adult diet, (iii) differential fecundity on a bacterial diet, and (iv) statistical analyses are found in the electronic supplementary material.
(a). Predation assay
To test for form-specific differences in the ability to prey on other nematodes, we developed an assay to quantify components of the predatory behaviour of P. pacificus. To prepare P. pacificus for this assay, nematodes were washed from stock-culture plates, rinsed twice in water with antibiotics (50 µg ml−1 ampicillin) to remove residual food, starved overnight on nematode growth medium (NGM) agar plates without cholesterol or peptone and kept at 20°C, and then placed at 12°C the next morning to prevent further starvation before their use in assays that day. Young adults (i.e. carrying 0–2 eggs) were subjected to the assays.
Prey for the predation assay consisted of first-stage (L1) larvae of the nematode Caenorhabditis elegans. This species is a member of a group of nematodes, ‘Rhabditidae’, that P. pacificus often encounters in the wild. Rhabditidae are widespread in ephemeral habitats, which both rhabditid and diplogastrid nematodes can rapidly colonize [28]. Consequently, P. pacificus has been observed to feed on other nematodes of Rhabditidae, including Pelodera spp. (data not shown), which are commonly found on the same beetle hosts of Pristionchus spp. [23]. Furthermore, C. elegans exhibits very similar external morphology to known prey species [29,30], secreted chemical profiles of Rhabditina (including Rhabditidae) are highly conserved [31], and locomotive behaviour, including responses to mechanical stimuli, is stereotypic for nematodes of Rhabditidae. Cultures of C. elegans strain N2 were maintained on NGM agar with OP50 as described above for P. pacificus. To produce larvae for assays, plates of C. elegans were bleached to release eggs and remove all larvae and adults, following standard protocols [32]. After rinsing eggs into water, the density of eggs was estimated by agitating the volume and counting eggs dispensed in two 5 µl drops. Eggs were then dispensed onto NGM plates with no food, cholesterol or peptone and at a standard density of 12 000 eggs per plate. After incubating plates overnight at 20°C, hatched L1 larvae emerged on plates. Prey larvae, although active, were developmentally arrested at this stage, ensuring very little variation in prey age or size within or among replicates and experiments.
For each assay, a single P. pacificus individual was gently transferred to a prey plate and monitored with a Zeiss Discovery V.12 stereomicroscope. The assay was started after the predator was allowed to recover from handling, namely upon detection of predator locomotion. Recovery time did not differ between mouth-forms (Kruskal–Wallis ANOVA, p > 0.2; data not shown). Predators were placed near the centre of the plate, throughout which prey were distributed by their own dispersal behaviour. In the assay, the behaviour of P. pacificus was scored for ‘encounters’, ‘attacks’ and ‘kills’. An encounter was recorded when the anterior tip (lips) of the predator made perpendicular contact with a C. elegans larva; an attack was recorded if, during an encounter, anterior body movement of the predator ceased and pharynx pumping initiated, which included deployment of the dorsal tooth; a kill was recorded if the body wall of the prey was ruptured, as evident from the release of body pressure or contents from prey. In the case of a kill, the time period from the start of the fatal attack to the rupture of prey cuticle was measured. The assay was performed for 10 min, unless a prey item was successfully killed, at which time the assay was ended. Predatory ability for each strain and mouth-form combination was assessed by the proportion of tested individuals able to successfully kill a prey item. Three strains of P. pacificus were assayed for predatory ability: (i) PS312, the laboratory reference strain (‘California’); (ii) JU482 (Japan), which was previously noted to be a relatively successful predatory strain; and (iii) RS5205 (South Africa), which has a ratio of mouth-forms close to 1 : 1 in well-fed culture [7]. For each strain, 30 individuals of each mouth-form were assayed.
(b). Fitness differences on a prey diet
To measure fitness differences between forms when offered a diet of prey, we developed an assay to allow potential predators to feed on only prey at early adulthood (figure 2). In this experiment, prey plates were prepared as for the predation assay, except that prey was administered at a density of 8000 C. elegans larvae per plate, and thus all potential predators were able to feed ad libitum. Strain RS5205 of P. pacificus was selected as the predatory strain, owing to the likelihood of selecting either mouth-form before adult morphology could be determined.
To start the experiment, 140 J4 (pre-adult) stage larvae of P. pacificus were transferred to individual prey plates and maintained at 20°C. Predators were kept on this feeding regimen for 2 days (days 0–1 of assay), during which the predators began expressing one of the two mouth-forms and would be able to acquire nutrition differentially as enabled by their feeding morphologies. As a control, 140 J4 P. pacificus larvae were transferred to identically prepared NGM double-agar (3.4%) plates with no prey and no bacteria. After this 2-day period (day 2), P. pacificus mothers from both types of plates were checked for their mouth phenotype and transferred to individual wells of a 96-well plate, with each well containing 250 µl S-medium with antibiotics.
The first fitness measure taken in this experiment was the fecundity of Eu and St P. pacificus hermaphrodites following a prey diet. All larvae and embryos with dividing cells were counted as offspring; any non-dividing embryos were considered dead eggs and not included in counts. After removal from prey and control (starvation) plates, hermaphrodites were transferred daily to new individual wells with S-medium, and screened for offspring until 2 days after removal from plates (day 4), by which time all hermaphrodites in wells stopped laying eggs. In this manner, offspring produced on each day could be counted separately. To count offspring produced on prey plates during feeding (days 0–1, pooled as day 1), predators needed to be separated from prey, as the similar body shapes of the two species rendered distinction of P. pacificus larvae among thousands of prey larvae impossible. To accomplish this, we first transferred all individuals on prey plates to NGM plates with OP50, letting both species recover from starvation for 2 days (days 3–4). Following this recovery period, we recruited a pathogenic strain of bacteria, Bacillus thuringiensis strain DB27, that after 24 h of exposure is completely lethal to C. elegans but not at all to P. pacificus [33,34]. One day after administering DB27 to plates in a quantity of 60 µl in lysogenic broth per plate (day 5), P. pacificus offspring could be readily distinguished and counted. Offspring produced on control plates, which contained no prey, were simply screened 1 day after removing P. pacificus mothers from plates.
Second, we assayed the longevity of Eu and St P. pacificus hermaphrodites following a prey diet. To do this, we continued the daily transfer of hermaphrodites, already assayed for fecundity, to new wells containing S-medium (with antibiotics) until 7 days after removal from plates (day 9). At that time, the only three survivors were additionally checked for their ability to produce offspring if fed and mated, although they had stopped producing self-offspring at least 4 days earlier. These individuals were placed along with one adult male onto NGM agar plates with OP50 and let to feed and mate indefinitely.
Third, to obtain a measure of quality for the offspring of prey-fed mothers, we assayed the survival of offspring as developmentally arrested larvae. In this assay, a sample of 96 J2 hatchlings produced the first day after removal from prey plates were taken for each test group. Larvae were placed in individual wells containing 250 µl S-medium (with antibiotics) and monitored at intervals of 12 h until dead. Death was scored if larvae were no longer motile, even after mild agitation of wells.
3. Results
(a). Eurystomatous animals have a functional advantage in predation
In spite of their morphological differences, the differential function of the two forms of P. pacificus, or of other dimorphic diplogastrid nematodes, was previously unknown. In this study, three strains of P. pacificus were assayed for their ability to kill other nematodes provided as prey (figure 3a). In each strain, the Eu form was able to kill significantly more often than the St form (PS312, χ2 = 8.40, p < 0.005; JU482, χ2 = 6.83, p < 0.005; RS5205, χ2 = 4.35, p < 0.05). At the level of individuals, therefore, the Eu form was functionally a more successful predator. To test whether predatory ability could be owing to differences in hunting behaviour, the rates of encounters per minute and encounters per attack were compared between forms of strain JU482, the most successful predators of the strains tested. No differences were detected between mouth-forms with either successful or unsuccessful predatory behaviour (Kruskal–Wallis, p > 0.1; electronic supplementary material, figure S1). Nevertheless, in successful acts of predation, the time required to kill (figure 3b) was found to be significantly greater for the St form (KW-H1,26 = 5.80, p < 0.05), such that the Eu form could kill in half the time needed for the St form. Taken together, our assay showed the Eu form to be a superior predator. This ability was not attributed to locating prey, but to the efficiency of turning an attack into a kill.
Figure 3.
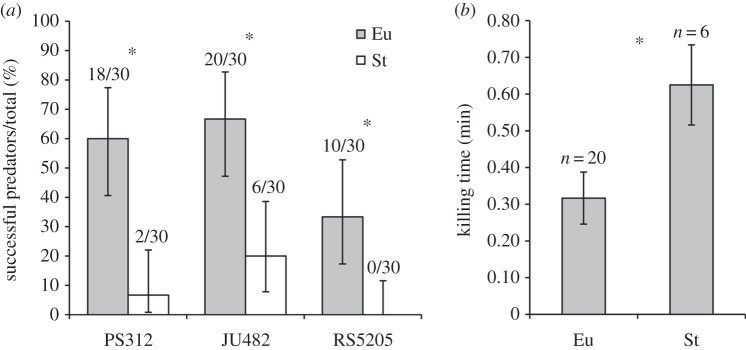
Predatory (killing) ability in Eu versus St hermaphrodites of P. pacificus. (a) The percentage of assayed individuals able to kill a prey larva within 10 min, as tested for three strains of P. pacificus. In all assayed strains, the Eu form was the more successful predator (*p < 0.05, χ2-test). Absolute numbers of successful predators are given above bars. Whiskers represent a 95% binomial confidence interval. (b) The time required for hermaphrodites P. pacificus strain JU482 to kill their prey. Individuals tested were those successful in the predation assay. Time was measured from the onset of the final attack until rupture of the prey body cuticle. Whiskers represent the standard error. *p < 0.05, Student's t-test.
(b). Differential fecundity on a prey diet
To test whether the functional advantage of the Eu form translated to a greater adaptive value, we tested for fitness differences between forms following a diet enriched in live prey (table 1). When potential predators were fed ad libitum on C. elegans larvae, there were no differences in the total brood size produced after feeding by the Eu and St forms or test (prey-fed) and control (starved) groups (KW-H3,145 = 7.96, p = 0.07), which had a median brood size of 12. However, differences were detected in the daily brood size in both test and control groups: the St form produced more offspring the first day after feeding (KW-H3,145 = 53.9, p < 0.0005), whereas the Eu form produced more on the second day (KW-H3,145 = 70.7, p < 0.0005) and on the third and fourth days (KW-H3,145 = 35.2, p < 0.0005). This result confirms the faster developmental time to maturity for the St form [24].
Table 1.
Brood sizes of P. pacificus (RS5205) hermaphrodites that were starved or prey-fed as adults. (On day 0, virgin J4 (pre-adult) hermaphrodites were introduced to the experimental feeding regime (figure 2). On day 2, hermaphrodites were removed from experimental feeding regimen to wells for starvation. Offspring were counted throughout reproductive lifetime of hermaphrodites. Values are given in the form: median (lower quartile-upper quartile).)
| day 1 | day 2 | days 3–4 | total | |
|---|---|---|---|---|
| Eu starved | 1 (1–3) | 10 (7–12) | 0 (0–1) | 12 (10–16) |
| St starved | 6 (3–9) | 1 (0–3) | 1 (0–4) | 11 (8–13) |
| Eu prey-fed | 0 (0–2) | 7 (5–9) | 4 (1–6) | 13 (10–16) |
| St prey-fed | 8 (2–11) | 3 (1–5) | 0 | 10 (6–15) |
Although the Eu and St forms showed no fecundity differences when starved after a prey diet, we tested in a separate experiment whether prey-fed hermaphrodites could produce more offspring when reintroduced to bacterial food (figure 4a). In contrast to the starved hermaphrodites, which showed no difference between forms (KW-H1,21 = 0.6, p = 0.44), the fecundity of Eu hermaphrodites was significantly higher than that of St hermaphrodites when reintroduced to bacteria (KW-H1,32 = 10.4, p < 0.005). Fecundity experiments thus suggest that an adult diet of prey is challenging to both mouth-forms, but that when reintroduced to a nutritionally rich food source, namely bacteria, the Eu form recovered better than the St form.
Figure 4.
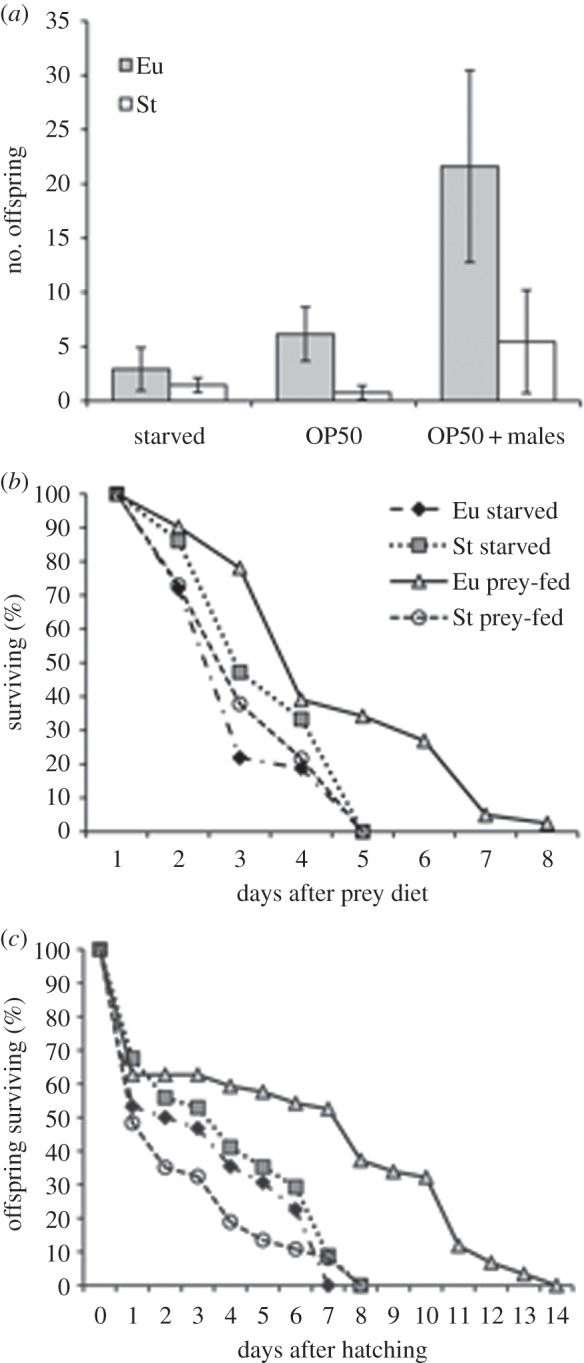
Fitness measures of Eu and St hermaphrodites of P. pacificus RS5205 after feeding on prey. (a) Fecundity (brood size) of hermaphrodites after a prey diet and when also fed bacteria (OP50) or fed bacteria and mated (OP50 + males). Whiskers represent the standard error. *p < 0.05, Kruskal–Wallis. (b) Longevity of starved and prey-fed hermaphrodites. Individuals were introduced as J4 (pre-adult) larvae to starvation or prey plates on day 0. On day 8, the last surviving hermaphrodites were removed from assay to test their fertility, for which two out of three were positive. (c) Survival of arrested J2 (first post-eclosion stage) offspring produced by starved or prey-fed hermaphrodites.
When prey-fed hermaphrodites were introduced to both bacterial food and males, Eu hermaphrodites also produced more offspring than St hermaphrodites (KW-H1,31 = 8.8, p < 0.005), indicating that the nutritional challenge of a prey diet affected self-sperm production in both forms but was less detrimental to egg production in the Eu form than in the St form. Furthermore, hermaphrodites of the Eu form produced more offspring when mated versus not mated (KW-H1,33 = 5.8, p < 0.05), although the St form showed no such increase when mated (KW-H1,30 = 0.8, p = 0.36). Therefore, the nutritional challenge to the more resilient Eu form can be explained by the effect of a poor diet on self-sperm production.
Finally, when fed only bacteria, the two mouth-forms showed no fecundity differences on either OP50 (T58 = 0.998, p = 0.32; electronic supplementary material, figure S2) or a naturally associated strain of P. fluorescens (T56 = −0.784, p = 0.44; electronic supplementary material, figure S2), indicating that both forms were equally efficient in converting energy acquired from bacteria to reproductive output when fed ad libitum. This finding yields two major conclusions. First, it shows that bacteria are a far better food resource than prey for either mouth-form (figure 4a and table 1). This confirms that the Eu form is adaptive as a stress-resistant form in conditions of low bacteria but high prey availability. Second, the ability of the St form to produce as many offspring as the Eu form, when combined with a shorter generation time for the St form [24], could translate to higher fitness for that form in a bacteria-rich environment.
(c). Survival differences of prey-fed mothers and their offspring
In another measure of fitness, we assayed the longevity of hermaphrodites fed on prey (figure 4b). Prey-fed Eu hermaphrodites survived significantly longer than prey-fed St, starved Eu, and starved St hermaphrodites (log-rank, χ23 = 38.9, p < 0.0005), further indicating the Eu form to be more resilient to the absence of bacterial food but presence of nematode prey. By contrast, the latter three groups did not differ from each other (log-rank, χ22 = 3.66, p = 0.16). Because in this assay, longer lived hermaphrodites outlived their capacity to produce self-offspring, at the end of the assay we tested whether the surviving hermaphrodites were still fertile by providing them with bacterial food and a mate. Seven days following their removal from plates, including 4 days without laying eggs, two of the three tested survivors produced hatching offspring, one of them even producing over 40 offspring (data not shown).
Finally, a measure of the quality of offspring produced by mothers fed on prey was taken by measuring the survival of hatched offspring that were themselves never fed (figure 4c). Larvae remained arrested in the first post-eclosion (J2) stage, presumably persisting on energy reserves provisioned by the mother. In this assay, the offspring of prey-fed Eu hermaphrodites survived significantly longer than those of prey-fed St, starved Eu and starved St hermaphrodites (log-rank, χ23 = 44.0, p < 0.0005). By contrast, the latter three groups did not differ from each other (log-rank, χ22 = 3.66, p = 0.27). The survival of arrested young larvae thus revealed Eu mothers to be more fit than St mothers after a diet of prey.
4. Discussion
In spite of mechanistic advances towards understanding the regulation and evolution of a developmental dimorphism in P. pacificus [7,19,25], the ecological context for morphological differences in this model organism was heretofore untested. Furthermore, the precise costs and benefits of resource polyphenisms under different environments have been generally difficult to determine in nature. Here, we have used an experimental approach in a genetically and environmentally controlled setting to determine the condition-dependent advantages of the more complex of the two feeding-forms. This study thus provides, to our knowledge, the first functional and evolutionary context for plasticity in P. pacificus, and it establishes a laboratory model for interpreting costs and benefits of a polyphenism as it responds to cues in the wild.
(a). A superior predatory form in Pristionchus pacificus
Following our development of a predation assay, we have shown with empirical evidence that the Eu form confers an advantage over the St form in this mode of feeding. Given a set length of time and abundant nematode larvae, more Eu than St individuals were able to kill their prey. In cases where the St form was able to kill a prey item, the predator required more time to complete the task, indicating either a difference in pharyngeal behaviour, such as rapidity of tooth movement or morphological limitations. A functional explanation for the superior killing ability of the Eu form would be sufficient based on morphological differences alone. First, the hooked subventral tooth of the Eu form may enable that form to engage the body wall of its prey, whereas the dorsal tooth is deployed opposite to slice the substrate, as described for the diplogastrid Tylopharynx foetida when fed on fungal spores [35]. By contrast, the St form lacks this tooth and must therefore rely on suction to keep its prey engaged. Second, the wider mouth of the Eu form may simply allow a substrate with a given elasticity or curvature to enter deeper into the mouth cavity. Because differences between the two forms in their killing ability are now established, testing differences at a neurological level may also be ultimately possible, given the reconstructed wiring for the pharyngeal nervous system in P. pacificus as a reference [36].
(b). Condition-dependent fitness advantages
In the presence of prey nematodes, the Eu form of P. pacificus showed higher fitness than the St form by several measures: (i) fecundity when offered bacteria as food after feeding on prey; (ii) longevity; and (iii) survival of starved, developmentally arrested offspring. By contrast, survival assays showed no advantage for the St form over starved individuals of either form, suggesting a poor ability to convert prey as a food resource to reproductive output. Therefore, under conditions restricted in bacterial resources but replete with prey, the Eu form was shown to confer the higher adaptive value to P. pacificus.
The Eu form was more prolific when fed a heterogeneous diet as adults, although neither morph showed an advantage when fed on prey and subsequently starved. Additionally, lifetime brood sizes of virgin, prey-fed hermaphrodites were small relative to those fed exclusively on bacteria, indicating prey to be an inferior food resource to bacteria for both mouth-forms. In our assay, an adult diet of only prey elicited a starvation-like response, recalling the ‘adult reproductive diapause’ of C. elegans, whereby hermaphrodites starved from the fourth larval stage onwards experience a similar reduction of fecundity [37]. In C. elegans, this reduction of brood size reflects starvation-induced shrinkage of the germline, presumably to divert nutrition to the few developing eggs produced [38]. In the wild, however, it is more likely that P. pacificus encounters both bacterial and animal food sources [22,23], albeit in variable abundances. Consequently, assays of reproductive recovery when fed with prey followed by bacteria indicate a fitness benefit for the Eu form on a heterogeneous food resource. Specifically, the superior ability of Eu individuals to recover from a nutritionally poor diet of only prey suggests the Eu form to be more stress-resistant than the St form. We speculate, therefore, that in environments where bacteria are present but limited, the higher fecundity of the Eu form when supplemented with prey confers an adaptive advantage for that form.
In contrast to the small brood sizes of virgin hermaphrodites fed on prey, Eu hermaphrodites that were subsequently fed bacteria and mated were more fecund. This result suggests that in starved or prey-fed hermaphrodites, the number of available self-sperm is reduced, possibly as a sacrifice for provisioning the few eggs that can be fertilized and laid [38]. Restricted food conditions may therefore favour the Eu form, at least under some conditions as tested here, because it can exhibit greater fitness on a mixed diet. The latter consequence would provide an advantage of the Eu form for exploiting co-occurring microbivorous species, which compete for the same diminishing bacterial food source, as potential prey.
The survival curves for both prey-fed mothers and their offspring show the advantage of Eu individuals and their offspring to persist under prey-enriched conditions, presumably owing to greater energy reserves. The longer survival of arrested larvae, if similar in function to the ageing-suspending, stress-resistant L1 arrested larvae of C. elegans [39,40], would, in principle, also impart a greater probability of reaching maturity and reproducing. Enhanced survival of young P. pacificus larvae would allow their more successful dispersal to new bacteria-rich habitats, such as with the death of their insect hosts.
(c). A fitness trade-off supports maintenance of alternative forms
The conditional advantages of the Eu form, demonstrated here on a regimen of predatory feeding, complement the recent finding that the St form reaches the age of maturity faster on a bacterial diet [24]. Consistent with that finding, hermaphrodites that mature from the pre-adult stage on either a diet of prey or under starvation conditions, the St was reproductively active sooner than the Eu form. The absence of a fecundity advantage for either form when fed ad libitum on bacteria supports an advantage for the St form under conditions rich with that food source. The presence of environmentally dependent fitness optima for the two forms suggests a fitness trade-off, satisfying the predicted conditions for the persistence of a discontinuous dimorphism in evolution [3]. The equilibrium of forms might expectedly change in populations as an adaptation to a particular host or microhabitat [41], and a diversity of ratios has indeed been observed in P. pacificus [7]. However, the persistence of conditions benefitting both forms would favour a dimorphism, provided that the cost of maintaining plasticity is not itself prohibitive [42].
This study complements discussions on the adaptive significance of alternative phenotypes as the result of developmental switches. Previous analyses of polyphenisms have predicted fitness advantages by inferring costs and benefits of alternative forms [43–46]. In examples analogous to the predatory feeding dimorphism of P. pacificus, the density-dependent induction of predatory or cannibalistic morphs in ciliates [47,48], rotifers [49], Ambystoma salamanders [50–52] and spadefoot toads [6] have shown in particular how plasticity allows a rapid adaptive response that includes novel feeding strategies. However, the measurements of direct fitness that are required to test the adaptive value of alternative forms, specifically in the absence of heritable and environmental variation, are difficult to achieve in many systems. By testing the potential trade-off of a resource polyphenism in a hermaphroditic laboratory model, we have distinguished such adaptive values. The inferred trade-off thus suggests evolutionary advantages for specific genetic regulators of dimorphism as have been identified in P. pacificus. Furthermore, support for a persistent dimorphism suggests its macroevolutionary potential to buffer further adaptive variability [1,53]. In such a case, the adaptive significance of the mouth dimorphism would be of central importance for buffering the diversity of feeding morphology seen in Pristionchus and other dimorphic Diplogastridae [18,54,55].
Supplementary Material
Acknowledgements
We are thankful to Andreas Weller, who established an early protocol for the predation assay that we developed for this study. We also thank Robbie Rae and Vladislav Susoy for their advice on the project, and we thank Cameron Weadick for his insightful suggestions on the manuscript. V.S. and E.J.R. designed the research; V.S. performed the research; E.J.R., V.S. and R.J.S. wrote the manuscript.
Funding statement
We gratefully acknowledge the Alexander von Humboldt Foundation for the support of a fellowship awarded to E.J.R.
References
- 1.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.West-Eberhard MJ. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549. ( 10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran NA. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989. ( 10.1086/285369) [DOI] [Google Scholar]
- 4.Brakefield PM, Reitsma N. 1991. Phenotypic plasticity, seasonal climate and the population of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 16, 291–303. ( 10.1111/j.1365-2311.1991.tb00220.x) [DOI] [Google Scholar]
- 5.Stibor H. 1992. Predator induced life-history shifts in a freshwater cladoceran. Oecologia 92, 162–165. ( 10.1007/BF00317358) [DOI] [PubMed] [Google Scholar]
- 6.Pfennig DW. 1990. The adaptive significance of an envrionmentally-cued developmental switch in an anuran tadpole. Oecologia 85, 101–107. ( 10.1007/BF00317349) [DOI] [PubMed] [Google Scholar]
- 7.Ragsdale EJ, Müller MR, Rödelsperger C, Sommer RJ. 2013. A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell 155, 922–933. ( 10.1016/j.cell.2013.09.054) [DOI] [PubMed] [Google Scholar]
- 8.West-Eberhard MJ. 1989. Phenotypic plasticity and the origins of diversity. Annu. Rev. Ecol. Syst. 20, 249–278. ( 10.1146/annurev.es.20.110189.001341) [DOI] [Google Scholar]
- 9.Smith TB, Skúlason S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133. ( 10.1146/annurev.ecolsys.27.1.111) [DOI] [Google Scholar]
- 10.Waddington CH. 1953. Genetic assimilation of an acquired character. Evolution 7, 118–126. ( 10.2307/2405747) [DOI] [Google Scholar]
- 11.Suzuki Y, Nijhout HF. 2006. Evolution of a polyphenism by genetic accommodation. Science 311, 650–652. ( 10.1126/science.1118888) [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Mestre I, Buchholz DR. 2006. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl Acad. Sci. USA 103, 19 021–19 026. ( 10.1073/pnas.0603562103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledon-Rettig CC, Pfennig DW, Nascone-Yoder N. 2008. Ancestral variation and the potential for genetic accommodation in larval amphibians: implications for the evolution of novel feeding strategies. Evol. Dev. 10, 316–325. ( 10.1111/j.1525-142X.2008.00240.x) [DOI] [PubMed] [Google Scholar]
- 14.Pfennig DW, McGee M. 2010. Resource polyphenism increases species richness: a test of the hypothesis. Phil. Trans. R. Soc. B 365, 577–591. ( 10.1098/rstb.2009.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommer RJ, McGaughran A. 2013. The nematode Pristionchus pacificus as a model system for integrative studies in evolutionary biology. Mol. Ecol. 22, 2380–2393. ( 10.1111/mec.12286) [DOI] [PubMed] [Google Scholar]
- 16.Kiontke K, Fitch DHA. 2010. Phenotypic plasticity: different teeth for different feasts. Curr. Biol. 20, R710–R712. ( 10.1016/j.cub.2010.07.009) [DOI] [PubMed] [Google Scholar]
- 17.Sudhaus W, Fürst von Lieven A. 2003. A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda). J. Nematode Morphol. Syst. 6, 43–90. [Google Scholar]
- 18.Fürst von Lieven A, Sudhaus W. 2000. Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. J. Zool. Syst. Evol. Res. 38, 37–63. ( 10.1046/j.1439-0469.2000.381125.x) [DOI] [Google Scholar]
- 19.Bento G, Ogawa A, Sommer RJ. 2010. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 466, 494–497. ( 10.1038/nature09164) [DOI] [PubMed] [Google Scholar]
- 20.Herrmann M, Mayer WE, Sommer RJ. 2006. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology 109, 96–108. ( 10.1016/j.zool.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 21.Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, Sommer RJ. 2007. The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the Oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zool. Sci. 24, 883–889. ( 10.2108/zsj.24.883) [DOI] [PubMed] [Google Scholar]
- 22.Rae R, Riebesell M, Dinckelacker I, Wang Q, Herrmann M, Weller AM, Dieterich C, Sommer RJ. 2008. Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J. Exp. Biol. 211, 1927–1936. ( 10.1242/jeb.014944) [DOI] [PubMed] [Google Scholar]
- 23.Weller AM, Mayer WE, Rae R, Sommer RJ. 2010. Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogeneous distribution. J. Parasitol. 96, 525–531. ( 10.1645/GE-2319.1) [DOI] [PubMed] [Google Scholar]
- 24.Serobyan V, Ragsdale EJ, Müller MR, Sommer RJ. 2013. Feeding plasticity in the nematode Pristionchus pacificus is influenced by sex and social context and is linked to developmental speed. Evol. Dev. 15, 161–170. ( 10.1111/ede.12030) [DOI] [PubMed] [Google Scholar]
- 25.Bose N, Ogawa A, von Reuss SH, Yim JJ, Ragsdale EJ, Sommer RJ, Schroeder FC. 2012. Complex small-molecule architectures regulate phenotypic plasticity in a nematode. Angew. Chem. 51, 12 438–12 443. ( 10.1002/anie.201206797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard Smith J. 1982. Evolution and the theory of games. New York, NY: Cambridge University Press. [Google Scholar]
- 27.Sommer RJ, Carta LK, Kim SY, Sternberg PW. 1996. Morphological, genetic and molecular description of Pristionchus pacificus n. sp. (Nematoda: Neodiplogastridae). Fund. Appl. Nematol. 19, 511–521. [Google Scholar]
- 28.Bongers T. 1999. The Maturity Index, the evolution of nematode life-history traits, adaptive radiation and cp-scaling. Plant Soil 212, 13–22. ( 10.1023/A:1004571900425) [DOI] [Google Scholar]
- 29.Scholze VS, Sudhaus W. 2011. A pictorial key to current genus groups of ‘Rhabditidae’. J. Nematode Morphol. Syst. 14, 105–112. [Google Scholar]
- 30.Sudhaus W. 2011. Phylogenetic systemisation and catalogue of paraphyletic ‘Rhabditidae’ (Secernentea, Nematoda). J. Nematode Morphol. Syst. 14, 113–178. [Google Scholar]
- 31.Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW. 2012. Ascaroside signaling is widely conserved among nematodes. Curr. Biol. 22, 772–780. ( 10.1016/j.cub.2012.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sulston J, Hodgkin J. 1988. Methods. In The nematode Caenorhabditis elegans (ed. Wood WB.), pp. 587–606. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 33.Rae R, Iatsenko I, Witte H, Sommer RJ. 2010. A subset of naturally isolated Bacillus strains show extreme virulence to the free-living nematodes Caenorhabditis elegans and Pristionchus pacificus. Environ. Microbiol. 12, 3007–3021. ( 10.1111/j.1462-2920.2010.02278.x) [DOI] [PubMed] [Google Scholar]
- 34.Iatsenko I, Boichenko I, Sommer RJ. 2013. Bacillus thuringiensis DB27 produces two novel toxins, Cry21Fa1 and Cry21Ha1, which act synergistically against nematodes. Appl. Environ. Micrbiol. 80, 3266–3275. ( 10.1128/AEM.00464-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fürst von Lieven A. 2002. Functional morphology, origin and phylogenetic implications of the feeding mechanisms of Tylopharynx foetida (Nematoda: Diplogastrina). Russ. J. Nematol. 10, 11–23. [Google Scholar]
- 36.Bumbarger D, Riebesell M, Rödelsperger C, Sommer RJ. 2013. System-wide rewiring underlies behavioral difference in predatory and bacterial-feeding nematodes. Cell 152, 109–119. ( 10.1016/j.cell.2012.12.013) [DOI] [PubMed] [Google Scholar]
- 37.Angelo G, Van Gilst MR. 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326, 954–958. ( 10.1126/science.1178343) [DOI] [PubMed] [Google Scholar]
- 38.Seidel HS, Kimble J. 2011. The oogenic germline starvation response in C. elegans. PLoS ONE 6, e28074 ( 10.1371/journal.pone.0028074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson TE, Mitchell DH, Kline S, Kemal R, Foy J. 1984. Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 28, 23–40. ( 10.1016/0047-6374(84)90150-7) [DOI] [PubMed] [Google Scholar]
- 40.Baugh LR, Sternberg PW. 2006. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 16, 780–785. ( 10.1016/j.cub.2006.03.021) [DOI] [PubMed] [Google Scholar]
- 41.Pfennig DW. 1992. Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionary stable strategy. Evolution 46, 1408–1420. ( 10.2307/2409946) [DOI] [PubMed] [Google Scholar]
- 42.DeWitt TJ, Sih A, Sloan Wilson D. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 43.Gilbert JJ. 1980. Further observations on developmental polymorphism and its evolution in the rotifer Brachionus calyciflorus. Freshw. Biol. 10, 281–294. ( 10.1111/j.1365-2427.1980.tb01202.x) [DOI] [Google Scholar]
- 44.Denno RF, Olmstead KL, McCloud ES. 1989. Reproductive cost of flight capability: a comparison of life history traits in wing dimorphic planthoppers. Ecol. Entomol. 14, 31–44. ( 10.1111/j.1365-2311.1989.tb00751.x) [DOI] [Google Scholar]
- 45.McCollum SA, Van Buskirk J. 1996. Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50, 583–593. ( 10.2307/2410833) [DOI] [PubMed] [Google Scholar]
- 46.Miner BG. 2005. Evolution of feeding structure plasticity in marine invertebrate larvae: a possible trade-off between arm length and stomach size. J. Exp. Mar. Biol. Ecol. 315, 117–125. ( 10.1016/j.jembe.2004.09.011) [DOI] [Google Scholar]
- 47.Ryals PE, Smith-Somerville HE, Buhse HE., Jr 2002. Phenotype switching in polymorphic Tetrahymena: a single-cell Jekyll and Hyde. Int. Rev. Cytol. 212, 209–238. ( 10.1016/S0074-7696(01)12006-1) [DOI] [PubMed] [Google Scholar]
- 48.Kopp M, Tollrian R. 2003. Trophic size polyphenism in Lembadion bullinum: costs and benefits of an inducible offense. Ecology 84, 641–651. ( 10.1890/0012-9658(2003)084[0641:TSPILB]2.0.CO;2) [DOI] [Google Scholar]
- 49.Gilbert JJ. 1973. Induction and ecological significance of gigantism in the rotifer Asplanchia sieboldi. Science 181, 63–66. ( 10.1126/science.181.4094.63) [DOI] [PubMed] [Google Scholar]
- 50.Collins JP, Cheek JE. 1983. Effect of food and density on development of typical and cannibalistic salamander larvae in Ambystoma tigrinum nebulosum. Am. Zool. 23, 77–84. [Google Scholar]
- 51.Nyman S, Wilkinson RF, Hutcherson JE. 1993. Cannibalism and size relations in a cohort of larval ringed salamanders (Ambystoma annulatum). J. Herpetol. 27, 78–84. ( 10.2307/1564909) [DOI] [Google Scholar]
- 52.Walls SC, Beatty JJ, Tissot BN, Hokit DG, Blaustein AR. 1993. Morphological variation and cannibalism in a larval salamander (Ambystoma macrodactylum columbianum). Can. J. Zool. 71, 1543–1551. ( 10.1139/z93-218) [DOI] [Google Scholar]
- 53.Moczek AP. 2007. Developmental capacitance, genetic accommodation, and adaptive evolution. Evol. Dev. 9, 299–305. ( 10.1111/j.1525-142X.2007.00162.x) [DOI] [PubMed] [Google Scholar]
- 54.Kanzaki N, Ragsdale EJ, Herrmann M, Sommer RJ. 2012. Two new species of Pristionchus (Rhabditida: Diplogastridae): P. fissidentatus n. sp. from Nepal and La Réunion Island and P. elegans n. sp. from Japan. J. Nematol. 44, 80–91. [PMC free article] [PubMed] [Google Scholar]
- 55.Ragsdale EJ, Kanzaki N, Röseler W, Herrmann M, Sommer RJ. 2013. Three new species of Pristionchus (Nematoda: Diplogastridae) show morphological divergence through evolutionary intermediates of a novel feeding polymorphism. Zool. J. Linnean Soc. 168, 671–698. ( 10.1111/zoj.12041) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.