Abstract
Scope
By increasing blood folate concentrations, folic acid supplementation reduces risk for neural tube defect-affected pregnancies, and lowers homocysteine concentrations. We assessed response of red blood cell (RBC) and serum folate to folic acid supplementation, and examined association of response with the genetic polymorphism C677T of the methylenetetrahydrofolate NAD(P)H (MTHFR) gene.
Methods and Results
Randomized, controlled, crossover trial with two folic acid supplement treatment periods and a 30-week washout period. The primary outcome is blood folate (serum and RBC) concentrations. Volunteers (n=142) aged 18-69 were randomized to two of three doses (0, 200, and 400 μg) of folic acid for twelve weeks. Serum folate response depended on treatment period with significant responses to 200 μg seen only in the second treatment periods (4.4 ng/mL or 3.4 ng/mL). Additionally, serum folate increased as folic acid dose increased to 400 μg (p< 0.01) and response was greater after the washout period (8.7 ng/mL), than after a 6-week run-in (2.3 ng/mL). The differential change attributable to a daily supplement of 400 μg compared to 200 μg was 96.8 ng/mL; while the change attributable to 400 μg compared to 0 μg was 121.4. Increases in RBC folate concentrations with 400 μg occurred within MTHFR gene mutation (C677T); and in the African American group.
Conclusions
Serum folate concentration is responsive to modest increases in folic acid intake. Red blood cell folate increases only with higher additional doses of folic acid supplementation, and this is true for each MTHFR C677T genotype.
Keywords: Folic acid, fortification, methylenetetrahydrofolate reductase, supplementation
INTRODUCTION
Low blood folate concentration is associated with low dietary intake of folic acid [1], and with the homozygous state of thermolabile variant (C677T) of the methylenetetrahydrofolate reductase NAD(P)H (MTHFR) gene [2-4]. By increasing blood folate concentrations, folic acid supplementation reduces homocysteine concentrations [5, 6], markedly reduces the frequency of neural tube defect-affected pregnancies [7, 8], hearing loss among the elderly [9], and possibly cognitive decline [10-13]. Contrary to earlier hypotheses, folic acid supplementation does not reduce risk of cancer [14-17], or cardiovascular diseases (CVD) [17-20].
The MTHFR gene has received attention as a factor that might modulate the risk relationship between folic acid intake and CVD [21-23]. The enzyme methylenetetrahydrofolate reductase catalyzes the reduction of 5, 10 –methylenetetrahydrofolate to 5-methyltetrahydrofolate, thus transferring a methyl group to cobalamin, which in turn donates a methyl group for the conversion of homocysteine to methionine. The MTHFR gene has many single nucleotide polymorphisms (SNPs) and the C677T (rs 1801133) is one of these SNPs. The thermolabile (C677T) allele is characterized by a point mutation at position 677 of the MTHFR gene that converts cytosine (C) into a thymine (T) resulting in an amino acid substitution (alanine to valine) in the enzyme. The 677C allele of MTHFR has been shown to have higher enzyme activity than the 677T allele in vitro [24)]. MTHFR C677T has three genotypes: 677CC is normal or wild-type, 677CT is the heterozygote, and 677TT is the mutant. The MTHFR 677TT genotype occurs with a frequency of 5 to 10% in U.S. Caucasian populations and the basic molecular defect can be identified in humans. Recently it has been documented that individuals with 677TT genotype have elevation in plasma homocysteine concentrations that is not correctable with folic acid supplementation [23], in contrast to earlier reports [25, 26].
Prior to folic acid fortification of foods which was initiated in 1998 in the United States (US), folate deficiency was common in some Western populations, especially in subgroups of populations such as older adults, with estimates documented as high 40% [27]. In the Netherlands, Belgium and Germany estimates of folate deficiency have been 30-50% [28].
Although folic acid supplementation is generally considered a simple, cost-effective approach to improving blood folate status, the nature of the relationship between folate intake and serum and red blood cell folate needs to be understood. The response of folate status to folic acid supplementation should be characterized; and the amount of folic acid intake necessary to maintain adequate folate status in healthy individuals should be estimated, as it cannot be assumed that the relationship between folate intake and serum or RBC folate is quantitative and linear.
The aims of this investigation are to compare changes in red blood cell folate and serum folate status in response to modest change in folic acid intake, to estimate the amount of time required following folic acid supplementation for serum and RBC folate concentrations to return to those observed at a baseline visit, and to examine the association of response with the MTHFR C677T genotype.
MATERIALS AND METHODS
Study Design
The study was designed to be a randomized, double-blind, controlled, crossover study of different doses of folic acid supplementation. Randomization to study and first dose assignment was within one of four strata, defined by MTHFR C677T genotype and African American race. A separate stratum was created for African Americans because of known differences in frequency of MTHFR C677T genotypes than other race-ethnic groups. In order to keep the strata of similar size, we screened a large number of volunteers and used a different sampling fraction for each stratum for conditional invitation to join the randomized study. Therefore, there was an initial screening visit, which was followed by a 6-week run-in period, followed by a 54-week main study period.
As soon as there were at least six members of a group, individuals were scheduled for a visit to the UW’s Clinical Research Center. At this visit, eligibility was checked, the study was explained again, and placebo pills were dispensed for the 6-week run-in period. The purpose of this initial run-in period was to assess compliance with study procedures and to provide a washout for previous vitamin use. Adequate compliance was defined as missing no more than one study pill per week during run-in. At the end of the run-in period, those with adequate compliance were invited to continue with the 54-week long study. These 54 weeks included a baseline visit, a 12-week intervention period (first period), a 30-week washout period (i.e., cessation of intervention folic acid supplementation) and another 12-week intervention period (after washout). Two doses - 200 μg and 400 μg - of folic acid were evaluated in the two parallel randomized studies.
Those invited to continue were asked to maintain their usual diets and lifestyle, to report any illnesses over the study period, and to abstain from additional folic acid supplements and foods fortified with 100% of the recommended dietary allowance of folic acid during the entire study. Foods fortified with 100% of the recommended dietary allowance included breakfast cereals and cereal bars.
The folic acid doses used in the study were selected to correspond with folic acid supplementation strategies that were hypothesized to reduce total homocysteine concentrations. The 200 μg dose is similar to the folic acid intake accrued from two servings of folic acid fortified cereal or grain product in the US, while the 400 μg dose is similar to the amount of folic acid obtained in a regular dietary supplement. The trial was conducted in Seattle, Washington at the University of Washington (UW) from 1998-2001 - i.e. after folic acid fortification began in the U.S. The study protocol was approved by the UW Human Subjects Review Board, and volunteers signed informed consent forms.
Study Participants
All interested volunteers were interviewed by phone to determine eligibility for the study. Participants were eligible if they were between the ages of 18 and 69 years, willing to take only the folic acid supplements provided by the study for 54 weeks, not pregnant or planning a pregnancy, free from chronic disease or diseases known to interfere with folate metabolism, and not taking medications that may decrease folic acid absorption or utilization such as methotrexate, bactrim, and phenobarbital. All volunteers who met study eligibility criteria were screened for the thermolabile variant C677T genotype of MTHFR and for plasma homocysteine concentrations. Homocysteine concentrations were analyzed by the Clinical Nutrition Research Unit laboratory at the University of Washington, using a modification of the method employing monobromobimane derivatization and high-performance liquid chromatography. Briefly, 30 μl of plasma was mixed with internal standard (cysteamine) and then reduced with NaBH4. The resulting mixture was derivatized with monobromobimane in nethylmorpholine buffer; the reaction was terminated with glacial acetic acid. The derivatized thiol compounds are stable for at least one weak after derivatization. After standing on ice, protein precipitate was removed, and 20 μl of the sample was injected on to a 150 × 4.6 mm Hypersil ODS column equilibrated with an ammonium formate buffer, pH 3.2. The chromatogram is developed by an acetonitrile gradient, and derivatized thiols are detected by fluorescence. Collection and analysis of data were computerized, using the EZChrom chromatography data system. The laboratory runs high and low control samples for each batch of assays as part of their in-house quality control procedures. For homocysteine, the between run coefficients of variation were 8.6% and 7.3% for high and low control samples respectively; the intra-assay CVs were 6.4% and 3.7% respectively (n=20).
Participants were assigned to one of four groups: 677CC, 677CT, 677TT genotype (if race was other than African American), or African American. Sampling fractions were applied to the available volunteers to enroll 60 individuals classified as 677CC, 60 as 677CT, 40 as 677TT and 40 African Americans for a recruitment goal of 200 participants.
Randomization and Supplement Doses
Stratified random allocation was used to assign participants to dose trial and within trial to treatment order. Strata were defined by MTHFR C677T genotype, African American and low or high homocysteine concentrations (<10 μmol/L or ≥10 μmol/L). Three identical pills were formulated by Tishcon Corporation containing 0, 200 and 400 μg folic acid respectively. One of the dose trials compared 0 μg (placebo) with 200 μg folic acid. The other dose trial compared 200 μg with 400 μg folic acid. Each participant was randomly assigned to one of the two trials; and to one dose order such that they received two treatments with a washout period in between.
At the baseline clinic visit, a fasting blood sample was drawn to provide baseline concentrations of serum and RBC folate. A 45-day supply of folic acid pills was given to volunteers, and a follow-up appointment was scheduled for 6 weeks later. Three weeks after the baseline study visit, the volunteer was called by the study coordinator to make sure that study pills were being taken, and to answer study-related questions. Compliance with study pills was determined by counting the contents of the bottles brought back to the clinic at the end of the 6-week period.
Additional clinic visits for fasting blood draws occurred at weeks 6, 12, 18, 24, 30, 36, 42, 48 and 54. Each treatment period lasted 12 weeks, and was characterized by measures at the start, the middle and the end of the period. The study protocol for each participant visit was therefore 12 weeks of treatment one, 30 weeks of washout, and 12 weeks of treatment two.
Statistical Methods
Statistical analyses were conducted using Statistical Analysis System software (version 9.1; SAS Institute, Inc.). Descriptive statistics (means and percent) were used to describe baseline participant characteristics. Paired t-test analyses were used to compare the mean effect of the three doses within and between dose trials. Analysis of variance compared mean changes in folate status during treatment within each dose trial by treatment group and treatment order. Where significant differences were found by treatment order, estimates from the two periods could not be combined. Where no significant differences were found, estimates of the same dose effect were combined to increase power. In analyses within MTHFR C677T genotype, African Americans were excluded, and results for African Americans are reported separately since the 677TT genotype occurred in less than 10% of this group. P values <0.05 were considered significant.
RESULTS
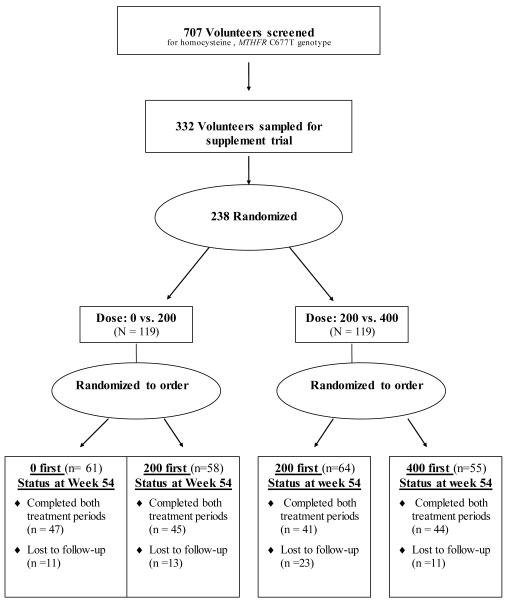
These analyses include 142 male and female volunteers. Of the 707 participants screened, 238 were randomized (Figure 1). Volunteers ranged in age from 18-69 years. Overall, the mean age of participants was 42 years, 46.7% were male, 74.7% were white, and the mean body mass index (BMI) was 27.4 kg/m2. As shown in Table 1, there were no significant differences among the four groups with respect to age, gender or baseline concentrations of serum and RBC folate. Group B had a slightly higher percentage of 677TT genotype and Group C had a slightly lower percentage of African American participants.
Figure 1.
Flow diagram of progress through study phases
Table 1.
Baseline characteristics of study population
| Treatment Group* | ||||
|---|---|---|---|---|
| A n=42 |
B n=34 |
C n=34 |
D n=32 |
|
| Mean Age (y) | 40.9 | 39.1 | 43.1 | 44.4 |
| Gender (% male) | 45.2 | 47.1 | 44.1 | 40.6 |
|
Mean Baseline Serum Folate
(ng/mL) |
13.3 | 13.8 | 14.3 | 13.9 |
|
Mean Baseline RBC Folate
(ng/mL) |
515 | 562 | 520 | 517 |
| % 677TT Genotype | 14.3 | 23.5 | 17.6 | 9.4 |
| % African-American | 16.7 | 20.5 | 5.9 | 18.8 |
A=0 and 200 μg/day, B=200 and 0 μg/day, C=200 and 400 μg/day, D=400 and 200 μg/day
Participant compliance was determined to be good on the basis of pill counts. Most (85.3%) volunteers were high compliers and on average forgot to take no more than one pill per week during the entire study period. Poor compliance was defined as missing three or more pills per week. There were no participants with poor compliance based on pill counts. The CV for serum folate was about 0.25 and for RBC folate, about 0.35.
Serum folate varied by period endpoints and treatment group in the first treatment period, but variations between the groups had disappeared by the end of washout. As shown in Table 2, serum folate response to folic acid depended on treatment period with significant responses to 200 μg seen only in the second treatment periods (4.4 ng/mL or 3.4 ng/mL). Supplementation with 400 μg resulted in an increase (p<0.01) in serum folate in each treatment period but the magnitude of response was greater after the 30-week washout (8.7 mg/mL) than in the first treatment period (2.3 ng/mL).
Table 2.
Serum folate (ng/mL) response to supplementation (mean (s.e.))
| Start | Middle | End | Response estimate at 12 weeks (95%CI) |
|
|---|---|---|---|---|
| DOSE 0 μg | 0 | 0 | ||
| Treatment Group A First period (n=42) |
13.3 (0.37) | 13.0 (0.45) | 13.0 (0.35) | −0.1 (−1.7, 1.5) |
| Treatment Group B (After washout) (n=34) |
11.6 (0.53) | 11.5 (0.73) | 11.8 (0.64) | 0.4 (−1.4, 2.2) |
| Dose 200μg | 200 | 200 | ||
| Treatment Group B (First period) (n=34) |
13.8 (0.46) | 14.9 (0.46) | 14.9 (0.38) | 1.0 (−0.8, 2.8) |
| Treatment Group C (First period) (n=34) |
14.3 (0.38) | 15.0 (0.33) | 14.5 (0.34) | 0.2 (−2.0, 2.4) |
| Treatment group A (After washout) (n=42) |
11.8 (0.42) | 15.5 (0.76) | 16.2 (1.34) | 4.4 (2.8, 6.0)b |
| Treatment Group D (After washout) (n=32) |
11.0 (0.49) | 13.7 (0.71) | 14.2 (0.72) | 3.4 (1.2, 5.6)b |
| Dose 400μg | 400 | 400 | ||
| Treatment Group D (First period) (n=32) |
13.9 (0.49) | 15.8 (0.87) | 16.1 (0.72) | 2.3 (0.1, 4.5)a |
| Treatment Group C (After washout) (n=34) |
11.3 (0.47) | 17.6 (2.04) | 20.0 (1.69) | 8.7 (6.7, 10.7)b |
Significant at p<0.05,
Significant at p<0.01
There was an increase in RBC folate concentrations after supplementation with active doses of folic acid for twelve weeks (Table 3). The RBC folate response did not depend on treatment period and response was seen in both periods. Table 4 shows the effect of different doses of folic acid, with groups combined, on RBC folate. When the effect estimates are combined, the differential change attributable to a daily supplement of 400 μg compared to 200 μg is 96.8 ng/mL (p<0.01) and the differential change attributable to a dose of 400 μg compared to placebo is 121.4 ng/mL. As shown in Table 5, the increases in RBC folate concentrations seen with 400 μg folic acid are found within each MTHFR C677T genotype, with individuals with 677TT genotype having mean (95%CI) response [246.6 ng/mL (139.0, 354.1)] , apparently larger than those with 677CC [(72.2 (3.4, 141.0)] or 677CT [(87.6 (24.3, 150.9)]. For African Americans, a significant response in RBC folate concentration was seen with supplementation with 400 μg only (Table 5). The results for African Americans are not divided by genotype, and 15 of them were 677CC, 7 were 677CT and 0 were 677TT in our dataset.
Table 3.
Red blood cell folate (ng/mL )response to supplementation (s.e.)
| week 0 | week 12 | |
|---|---|---|
| DOSE 0 μg | 0 | |
| Treatment Group A First period (n=42) | 516 (29) | 505 (27) |
| Treatment Group B (After washout) (n=34) | 586 (33) | 571 (34) |
| Dose 200μg | 200 | |
| Treatment Group B (First period) (n=34) | 562 (34) | 584 (29) |
| Treatment Group C (First period) (n=34) | 520 (40) | 546 (42) |
| Treatment group A (After washout) (n=42) | 533 (26) | 566 (27) |
| Treatment Group D (After washout) (n=32) | 583 (41) | 552 (31) |
| Dose 400μg | 400 | |
| Treatment Group D (First period) (n=32) | 517 (23) | 603 (33) |
| Treatment Group C (After washout) (n=34) | 528 (26) | 654 (44) |
Table 4.
Effect on RBC folate of different doses of folic acid (combining groups)
| Effect | Difference Estimate* | CI | p-value |
|---|---|---|---|
| 200 vs. 0 | 24.6 | −4.6, 78.3 | 0.18 |
| 400 vs. 200 | 96.8a | 67.5, 157.4 | <.001 |
| 400 vs. 0 | 121.4 a | 88.2, 210.5 | <.001 |
Difference of the mean difference of both doses.
Significant at p<.01
Table 5.
Dose effect estimates (mean (95% CI) on RBC folate by MTHFR C677T genotype and race
| 0* | 200* | 400* | |
|---|---|---|---|
|
677CC
(n = 58) |
7.3 (−33.9, 48.4) | 19.9 (−17.5, 57.3) | 72.2 (3.4, 141.0)a |
|
677CT
(n = 61) |
−46.0 (−83.0, −9.1)a | 10.9 (−23.7, 45.6) | 87.6 (24.3, 150.9)b |
|
677TT
(n = 23) |
11.6 (−38.8, 62.0) | −8.5 (−60.8, 43.8) | 246.6 (139.0, 354.1)b |
| African Americans | −3.6 (−45.4, 38.2) | 11.5 (−5.8, 58.9) | 105.6 (55.4, 155.8)b |
Significant at p<.05
Significant at p<.01
Randomization groups and time points are combined for each dose level.
DISCUSSION
In this folic acid intervention study, using a crossover design, we observed that blood folate status can be improved with modest doses of folic acid supplements. Significant responses in serum folate concentrations were seen after supplementation with 200 μg and 400 μg. RBC folate concentrations increased after taking 400 μg, but were not responsive to 200 μg. Increases in RBC folate concentrations seen with 400 μg depended on MTHFR C677T genotype with 677TTs having a larger response than 677CCs or 677CTs.
Our findings of an increase in serum and RBC folate with increasing folic acid intake are consistent with results from observational studies as well as randomized clinical trials. Serum folate has been shown to be highly influenced by recent intake, peaking two to three hours after a load in most people [29, 30]. Red cell folate, however, reflects an integrated measure of intake over about 120 days, because this is the life of a red blood cell and the folate content is set before the erythrocyte (red cell) leaves the bone marrow [29, 31]. Furthermore, data from the Framingham Offspring Study cohort showed increased mean serum folate concentrations in middle-aged and older adults consuming cereal-grain products fortified with folic acid [1]. Likewise, comparisons between 1988–1994 National Health and Nutrition Examination Survey (NHANES) III data and 1999 NHANES –collected after folic acid fortification of the food supply—showed increased serum and RBC folate concentrations among women of childbearing age [32]. These early NHANES findings were corroborated by later NHANES reports [33]. Additionally, trials conducted in several parts of the world consistently documented increases in serum folate concentrations after intervention with folic acid fortified foods [34-37] or supplements [34, 38-42].
Our finding that serum folate response depends on treatment period might be because folate concentrations at week 42 of our study reflect a more accurate non-supplemented measure than folate concentrations at week 0. It is possible that the six week run-in period may not have been long enough to washout any prior folic acid supplementation, such that serum folate response in the first treatment period may have been mis-estimated. The use of folic acid containing vitamins among study participants varied at week 0, but was apparently washed out by week 42 (30 weeks after the cessation of supplements). It has been documented that the magnitude of change in blood folate concentrations relative to baseline will be influenced by the initial status of the subjects [37]. In our study, the comparability of baseline folate status of individuals in each folate treatment group reduces the potential influence of difference in folate body stores on folate response. Our finding that RBC folate concentrations increased after taking 400 μg, but were not responsive to 200 μg suggests that intake of up to 200μg may not appreciably change the longer term measure of folate status reflected in RBC folate.
Our findings regarding the association of folic acid response with MTHFR C677T genotype are generally consistent with other published reports [23, 43-45]. A large cross-sectional analysis of data from a Dutch cohort revealed that at any folic acid intake level, individuals with 677TT genotype had lower plasma folate concentrations than those with 677CT or 677CC [44]. Further, our findings are similar to results from a folic acid exclusion study that tested the effect of a folic acid exclusion diet versus a folate-rich diet versus a folic acid supplement showed that plasma folate was higher after folate supplementation [43], and there was no difference in response between genotypes [43]. In another trial that evaluated the effect of daily supplementation for two months of 0.5 mg folic acid on serum and red cell folate concentrations, individuals with 677TT genotype initially had the lowest absolute increase in serum folate concentration [45], however, after four months of supplementation, additional increases were seen in serum and red cell folate and there were no significant differences among the three genotype groups. We found that increases in RBC folate concentrations after 400 μg/day of supplements depend on MTHFR C677T genotype with 677TTs having a larger estimated response than 677CCs or 677CTs. However, because the differences were not statistically significant, we use caution in interpreting this finding.
There are limitations and strengths of the study worth noting. We may not have been able to fully characterize the response to folic acid supplementation for the MTHFR C677T genotypes because of a limitation of the folate assay. The assay we used for quantification of blood folate concentrations is unable to distinguish between different folate vitamers such as methyl tetrahydrofolate, formyl tetrahydrofolate, and others. Folate vitamer distribution in the blood may different among the MTHFR C677T genotypes and the vitamers are not equally detected by the assay. Also, our results have limited generalizability, and should be applied to populations with folic acid fortification and the resultant higher concentrations of blood folate. Strengths of this study include it’s randomized, controlled, crossover design; and relatively long intervention and washout periods.
This study protocol involved the assessment of blood folate status over a 54-week period in response to folic acid supplementation with 0, 200, or 400 μg/day. An important aspect of the supplement doses used is that they are comparable to two common public health strategies to improve folic acid consumption. The 400 μg/day dose represents the use of vitamin supplements containing folic acid, and the 200 μg/day dose represents the consumption of foods fortified with folic acid. Our results suggest that daily supplementation with 400 μg resulted in blood folate concentrations that were almost twice as high as folate concentrations after daily supplementation with 200 μg. The results of this study suggest that vitamin supplements that contain 400 μg of folic acid provide a greater blood folate response than the intake of 200 μg/day folic acid from folic acid-fortified foods. However, additional food fortification with low doses of folic acid, or behavioral change to increase the servings of folate-fortified foods may be good options to achieve moderate increases in blood folate status.
The results of this study suggest that moderate doses of folic acid can provide change in folate status. This is important given the observed relationships of low folate status with neural tube defect affected pregnancies and elevated homocysteine concentrations. Persons at risk for these conditions can expect an increase in serum folate concentrations after supplementation with 200 μg folic acid, and an increase in red blood cell folate after supplementation with 400 μg.
ACKNOWLEDGEMENTS
This project was supported, in part, by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL058138).
We would like to thank our study volunteers for their contributions to this study. We also acknowledge the contributions of the staff from the University of Washington Office of Minority Affairs and the leadership of the Seattle Black Health Care Professionals group. Laboratory support was provided by the Clinical Nutrition Research Unit at the University of Washington.
List of abbreviations
- MTHFR
methylenetetrahydrofolate reductase gene
Footnotes
All authors contributed intellectually or practically to this work and take responsibility for the content of the manuscript, including the conception (SB), design (SB, AM, RK, ZF, SD), conduct (CA, SB, DM, JL, SD, AM), data analysis (CA, DM), writing of the manuscript (CA) and data interpretation (CA, SB, AM, DM).
CONFLICT OF INTEREST STATEMENT Drs. Anderson, Beresford, Lampe, Deeb, Feng and Motulsky, and Mr. McLerran declare they have no financial or personal relationships between themselves and others that might bias their work. There are no conflicts of interest.
REFERENCES
- 1.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 2.Molloy AM, Daly S, Mills JL, et al. Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: implications for folate intake recommendations. Lancet. 1997;349:1591–3. doi: 10.1016/S0140-6736(96)12049-3. [DOI] [PubMed] [Google Scholar]
- 3.Zittoun J, Tonetti C, Bories D, Pignon JM, Tulliez M. Plasma homocysteine levels related to interactions between folate status and methylenetetrahydrofolate reductase: a study in 52 healthy subjects. Metabolism. 1998;47:1413–8. doi: 10.1016/s0026-0495(98)90315-8. [DOI] [PubMed] [Google Scholar]
- 4.Kauwell GP, Wilsky CE, Cerda JJ, et al. Methylenetetrahydrofolate reductase mutation (677C-->T) negatively influences plasma homocysteine response to marginal folate intake in elderly women. Metabolism. 2000;49:1440–3. doi: 10.1053/meta.2000.16555. [DOI] [PubMed] [Google Scholar]
- 5.Homocysteine Lowering Trialists’ Collaboration Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ. 1998;316:894–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Homocysteine Lowering Trialists’ Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 7.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 8.Medical Research Council Vitamin Study Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- 9.Durga J, Verhoef P, Anteunis J, Schouten E, Kok F. Effects of Folic Acid Supplementation on Hearing in Older Adults: A Randomized, Controlled Trial. Ann Intern Med. 2006;146:1–9. doi: 10.7326/0003-4819-146-1-200701020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A., 3rd High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 11.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–43. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 12.Malouf M, Grimley EJ, Areosa SA. Folic acid with and without B12 for cognition and dementia. Cochrane Database Systematic Review. 2003;(4) doi: 10.1002/14651858.CD004514. CD004514. [DOI] [PubMed] [Google Scholar]
- 13.Brady CB, Gaziano JM, Cxypoliski RA, et al. Homocysteine lowering and cognition in CKD: The VA homocysteine study. Am J Kidney Dis. 2009;54(3):440–449. doi: 10.1053/j.ajkd.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norbye Wien T, Pike E, Wisloff T, et al. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ. doi: 10.1136/bmjopen-2011-000653. epub before print 2012 January 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst. 1993;85:875–84. doi: 10.1093/jnci/85.11.875. [DOI] [PubMed] [Google Scholar]
- 16.Kim YI, Baik HW, Fawaz K, et al. Effects of folate supplementation on two provisional molecular markers of colon cancer: a prospective, randomized trial. Am J Gastroenterol. 2001;96:184–95. doi: 10.1111/j.1572-0241.2001.03474.x. [DOI] [PubMed] [Google Scholar]
- 17.Clark R, Halsey J, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37, 485 individuals. Arch Intern Med. 170(18):1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 18.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 19.Ebbing M, Bleie O, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300(7):795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 20.Jamison RL, Hartigan P, Kaufman JS, Goldfarb DS, Warren SR, Guarino PD, Gaziano JM, Veterans Affairs Site Investigators Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298(10):1163–70. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 21.Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 22.Demuth K, Moatti N, Hanon O, Benoit MO, Safar M, Girerd X. Opposite effects of plasma homocysteine and the methylenetetrahydrofolate reductase C677T mutation on carotid artery geometry in asymptomatic adults. Arterioscler Thromb Vasc Biol. 1998;18:1838–43. doi: 10.1161/01.atv.18.12.1838. [DOI] [PubMed] [Google Scholar]
- 23.Crider KS, Zhu JH, et al. MTHFR C677CT genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplements. Am J Clin Nutr. 2011;93(6):1365–1372. doi: 10.3945/ajcn.110.004671. [DOI] [PubMed] [Google Scholar]
- 24.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 25.Kang S, Wong PW, Susmano A, Sora J, Norusis M, Ruggie N. Thermolabile methylenetetrahydrofolate reductase: An inherited risk factor for coronary artery disease. Am J Hum Genet. 1991;48:536–545. [PMC free article] [PubMed] [Google Scholar]
- 26.Kang S, Wong PW, Zhou J, et al. Themolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism. 1988;37:611–613. doi: 10.1016/0026-0495(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 27.Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–8. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- 28.Rydlewicz A, Simpson JA, Taylor RJ, Bond CM, Golden MH. The effect of folic acid supplementation on plasma homocysteine in an elderly population. QJM. 2002;95:27–35. doi: 10.1093/qjmed/95.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Bailey LB. Folate status assessment. J Nutr. 1990;120(Suppl 11):1508–11. doi: 10.1093/jn/120.suppl_11.1508. [DOI] [PubMed] [Google Scholar]
- 30.Kohlmeier L. Future of dietary exposure assessment. Am J Clin Nutr. 1995;61:702S–9S. doi: 10.1093/ajcn/61.3.702S. [DOI] [PubMed] [Google Scholar]
- 31.Zettner A, Boss GR, Seegmiller JE. A long-term study of the absorption of large oral doses of folic acid. Ann Clin Lab Sci. 1981;11:516–24. [PubMed] [Google Scholar]
- 32.Rader JI. Folic acid fortification, folate status and plasma homocysteine. J Nutr. 2002;132:2466S–70S. doi: 10.1093/jn/132.8.2466S. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer CM, Johnson CL, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988-2004. Am J Clin Nutr. 2007;86(3):718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 34.Neuhouser ML, Beresford SA, Hickok DE, Monsen ER. Absorption of dietary and supplemental folate in women with prior pregnancies with neural tube defects and controls. J Am Coll Nutr. 1998;17:625–30. doi: 10.1080/07315724.1998.10718812. [DOI] [PubMed] [Google Scholar]
- 35.Johansson M, Witthoft CM, Bruce A, Jagerstad M. Study of wheat breakfast rolls fortified with folic acid. The effect on folate status in women during a 3-month intervention. Eur J Nutr. 2002;41:279–86. doi: 10.1007/s00394-002-0388-9. [DOI] [PubMed] [Google Scholar]
- 36.Tucker KL, Olson B, Bakun P, Dallal GE, Selhub J, Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr. 2004;79:805–11. doi: 10.1093/ajcn/79.5.805. [DOI] [PubMed] [Google Scholar]
- 37.O’Keefe CA, Bailey LB, Thomas EA, et al. Controlled dietary folate affects folate status in nonpregnant women. J Nutr. 1995;125:2717–25. doi: 10.1093/jn/125.10.2717. [DOI] [PubMed] [Google Scholar]
- 38.Venn BJ, Mann JI, Williams SM, et al. Assessment of three levels of folic acid on serum folate and plasma homocysteine: a randomised placebo-controlled double-blind dietary intervention trial. Eur J Clin Nutr. 2002;56:748–54. doi: 10.1038/sj.ejcn.1601388. [DOI] [PubMed] [Google Scholar]
- 39.Houghton LA, Gray AR, et al. Long-term effect of low dose folic acid intake: potential effect of mandatory fortification on the prevention of neural tube defects. Am J Clin Nutr. 2011;94(1):136–141. doi: 10.3945/ajcn.110.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hurthouse NA, Gray AR, et al. Folate status of reproductive age women and neural tube defect risk: the effect of long-term folic acid supplementation at doses of 140 microg and 400 microg per day. Nutrients. 2011;3(1):49–62. doi: 10.3390/nu3010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao L, Yang QH, et al. Folate status and homocysteine response to folic acid doses and withdrawal among young Chinese women in a large-scale randomized double-blind trial. Am J Clin Nutr. 2008;88(2):448–457. doi: 10.1093/ajcn/88.2.448. [DOI] [PubMed] [Google Scholar]
- 42.Brouwer IA, van Dusseldorp M, Duran M, et al. Low-dose folic acid supplementation does not influence plasma methionine concentrations in young non-pregnant women. Br J Nutr. 1999;82:85–9. doi: 10.1017/s0007114599001221. [DOI] [PubMed] [Google Scholar]
- 43.Ashfield-Watt PA, Pullin CH, Whiting JM, et al. Methylenetetrahydrofolate reductase 677C-->T genotype modulates homocysteine responses to a folate-rich diet or a low-dose folic acid supplement: a randomized controlled trial. Am J Clin Nutr. 2002;76:180–6. doi: 10.1093/ajcn/76.1.180. [DOI] [PubMed] [Google Scholar]
- 44.de Bree A, Verschuren WM, Bjorke-Monsen AL, et al. Effect of the methylenetetrahydrofolate reductase 677C-->T mutation on the relations among folate intake and plasma folate and homocysteine concentrations in a general population sample. Am J Clin Nutr. 2003;77:687–93. doi: 10.1093/ajcn/77.3.687. [DOI] [PubMed] [Google Scholar]
- 45.Nelen WL, Blom HJ, Thomas CM, Steegers EA, Boers GH, Eskes TK. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr. 1998;128:1336–41. doi: 10.1093/jn/128.8.1336. [DOI] [PubMed] [Google Scholar]