Abstract
Synthetic biology has significantly advanced the design of mammalian trigger-inducible transgene-control devices that are able to programme complex cellular behaviour. Fruit-based benzoate derivatives licensed as food additives, such as flavours (e.g. vanillate) and preservatives (e.g. benzoate), are a particularly attractive class of trigger compounds for orthogonal mammalian transgene control devices because of their innocuousness, physiological compatibility and simple oral administration. Capitalizing on the genetic componentry of the soil bacterium Comamonas testosteroni, which has evolved to catabolize a variety of aromatic compounds, we have designed different mammalian gene expression systems that could be induced and repressed by the food additives benzoate and vanillate. When implanting designer cells engineered for gene switch-driven expression of the human placental secreted alkaline phosphatase (SEAP) into mice, blood SEAP levels of treated animals directly correlated with a benzoate-enriched drinking programme. Additionally, the benzoate-/vanillate-responsive device was compatible with other transgene control systems and could be assembled into higher-order control networks providing expression dynamics reminiscent of a lap-timing stopwatch. Designer gene switches using licensed food additives as trigger compounds to achieve antagonistic dual-input expression profiles and provide novel control topologies and regulation dynamics may advance future gene- and cell-based therapies.
INTRODUCTION
The rational assembly of mammalian synthetic transcription control switches to higher-order control networks that can programme cellular behaviour in a precise, predictable and reliable manner (1) has paved the way for the design of therapeutic devices that have the potential to specifically kill cancer cells (2,3), modulate the immune system (4,5) and correct metabolic disorders such as gouty arthritis (6), obesity (7,8) and diabetes (9,10). For optimal compatibility with host physiology, the trigger cues that fine-tune therapeutic transgene expression in future gene- and cell-based therapies need to be traceless (9,11,12), physiologically inert (13,14) or biocompatible such as vitamins (15,16), amino acids (17,18) or licensed food additives (14,19,20). Licensed food additives are particularly attractive trigger compounds for modulating heterologous transcription-control devices as long as they are used at a concentration that is above the one reached by standard dietary intake to prevent interference with meals and below the no observed adverse effect level (NOAEL) to prevent side effects.
Prokaryotic trigger-adjustable repressors that change their operator-binding affinity in response to a specific small-molecule compound have been a continuous source of molecular parts for the assembly of orthogonal mammalian transcription-control devices (1,14,19,21–24). Following a generic design blueprint, the prokaryotic repressors are fused to transactivation/transsilencing domains to produce synthetic transactivators/transsilencers that activate/repress specific target promoters containing transactivator/transsilencer-specific operator sites linked to minimal/constitutive mammalian promoters (1). The trigger compound typically reduces the affinity of the transactivator/transsilencer for the cognate promoter and switches target gene expression OFF/ON in a dose-dependent manner (1,25). Non-limiting prominent examples of prokaryotic repressor-derived trigger-inducible mammalian gene switches are responsive to pristinamycin (21), erythromycin (22), tryptophan (17) and uric acid (6) as well as γ-butyrolactone (23), phloretin (19) and vanillic acid (14); these have been successfully validated in mammalian cells and mice (see (1) for a comprehensive list). Most of these mammalian transgene-control systems are compatible with each other and can either be used for independent control of different sets of transgenes or functionally interconnected to provide higher-order control networks (26–29).
Chlorinated benzoic acids are a common class of pollutants introduced into the environment by the use of herbicides (30). The aerobic soil bacterium Comamonas testosteroni BR60 contains the plasmid pBRC60, which harbours the composite transposon Tn5271 encoding the chlorobenzoate catabolism pathway operon cbaABC (30). Catabolism of 3-chlorobenzoate (3CBA) is initiated by the binding of 3CBA to the 19.4 kDa MarR-type transcriptional repressor CbaR, which loses its affinity for the two operator sites (OCbaR) of the PCbaA promoter. This change results in full expression of the cbaABC operon, thereby producing the enzymatic components necessary for the sequential breakdown of 3CBA to acetyl-CoA (31).
Previous studies in C. testosteroni have shown that the inactivation of CbaR and the induction of cbaABC also occur in the presence of benzoic acid, a plant defence metabolite that is present at low levels in cranberries, prunes and apples (32). Benzoate is generally recognized as safe (GRAS) and is licensed as a food preservative by the US Food and Drug Administration (FDA) and the European Union (food additive approval number E210; E211–E213). The no observed adverse effect level (NOAEL) of dietary sodium benzoate was estimated to be 1400 mg/kg (33). Following food intake, benzoate rapidly reaches the bloodstream and is eliminated by renal clearance within 24 h (34). With its GRAS status (35), high NOAEL level (36), approval as food additive (37), optimal bioavailability (38) and clearance rate (34) as well as its metabolic inertness (35), benzoate can be considered an almost ideal orthogonal trigger compound to control transgene expression in mammalian cells and organisms.
Capitalizing on C. testosteroni's aromatic acid-catabolizing components, we have engineered a synthetic mammalian gene switch that is responsive to antagonistic input by the two food additives benzoate and vanillate and fine-tunes transgene expression in different mammalian cell lines as well as in mice. Synthetic gene switches with antagonistic dual-input control capacity add a new instrument to the synthetic biology toolbox and expand the genetic intervention portfolio that may foster novel advances in gene- and cell-based therapies.
MATERIALS AND METHODS
Plasmid design
Comprehensive design and construction details for all expression vectors are provided in Table 1. The assembly of some plasmids required annealing complementary oligonucleotides. For optimal annealing, 50 pmol of each oligonucleotide was mixed in 50 μl ddH2O-diluted 1x NEB Buffer 4 (New England Biolabs, Ipswich, MA, USA), heated for 10 min at 95°C, cooled down over 4 h to 22°C and incubated at 22°C for another 2 h prior to cloning into the corresponding vector backbone. All relevant genetic components have been confirmed by sequencing (Microsynth, Balgach, Switzerland).
Table 1. Plasmids designed and used in this study.
| Plasmid | Description | Reference |
|---|---|---|
| pSEAP2-Control | Constitutive mammalian SEAP expression vector (PSV40-SEAP-pA). | Clontech, CA |
| pUC57 | pUC19-derived prokaryotic expression vector. | GeneScript, NJ |
| pCK25 | Constitutive mUTS expression vector (PhEF1α-mUTS-pA). | (6) |
| pCK189 | Constitutive VanA4 expression vector (PSV40-VanA4-pA). | (14) |
| pCK191 | Vanillic acid-inducible SEAP expression vector (PVanON8-SEAP-pA). | (14) |
| pDA43 | Tetracycline-repressible GLuc expression vector (PhCMV*-1-GLuc-pA). | (25) |
| pKR38 | Constitutive mammalian KstR-VP16 expression vector (PSV40-KstR-VP16-pA). | Unpublished |
| pKR80 | Constitutive mammalian KstR-KRAB expression vector (PhEF1α-KstR-KRAB-pA). | Unpublished |
| pMF111 | Tetracycline-repressible SEAP expression vector (PhCMV*-1-SEAP-pA). | (44) |
| pMG10 | Phloretin-repressible SEAP expression vector (PTtgR1-SEAP-pA). | (19) |
| pMG11 | Constitutive TtgA1 expression vector (PSV40-TtgA1-pA). | (19) |
| pSAM200 | Constitutive tTA expression vector (PSV40-tTA-pA). | (44) |
| pWW124 | γ-butyrolactone (SCB1)-repressible SEAP expression vector (PSPA-SEAP-pA). | (23) |
| pMX34 | Constitutive CTS1 expression vector (PhEF1α-CTS1-pA; CTS1, KRAB-CbaR). | This work |
| CbaR was PCR-amplified from pMX43 using oligonucleotides OMX49 (5′-gaatgatctctgggcgcgcATGCTGGCCCGTGACCCCCGGAAAAG-3′) and OMX48 (5′-ggccctctagattaCTCCTGAGGGCTCTCCCGATGCCAATG-3′), restricted with BssHII/XbaI and cloned into the corresponding sites (BssHII/XbaI) of pCK25. | ||
| pMX38 | Benzoic and vanillic acid-responsive SEAP expression vector (PCTA-O2-SEAP-pA; PCTA-O2, (OCbaR)2-PhCMVmin). OCbaR was PCR-amplified from pWW124 using oligonucleotides OMX33 (5′-ccacctgacgtcgacacaagttgctaaaccaacaacatgtttgacacaagttgctaaaccaacaacatccgcaaatTCGAGCTCGGTACCCGGGTCGAGT-3’) and OMX24 (5′-CTTGAGCACATAGCCTGGACCGTTTCCGTA-3′), restricted with AatII/EcoRI and cloned into the corresponding sites (AatII/EcoRI) of pWW124. | This work |
| pMX39 | Benzoic and vanillic acid-responsive SEAP expression vector (PCTS1-SEAP-pA; PCTS1, PSV40-OCbaR). OCbaRwas introduced by PCR-amplification of pSEAP2-Control with oligonucleotides OMX36 (5′-tagtaataagcttgaaagttgctaaaccaacaacatccgCCACCATGCTGCTGCTGCTGCTGCTGCTGGG-3′) and OMX24 (5′-CTTGAGCACATAGCCTGGACCGTTTCCGTA-3′), restricted with HindIII/PstI and cloned into the corresponding sites (HindIII/PstI) of pSEAP2-Control. | This work |
| pMX40 | Benzoic and vanillic acid-responsive SEAP expression vector (PCTS2-SEAP-pA; PCTS2, PSV40-(OCbaR)2). (OCbaR)2 was introduced by PCR-amplification of pSEAP2-Control with oligonucleotides OMX35 (5′-tagtaatAagcttgaaagttgctaaaccaacaacatgtttgacacaagttgctaaaccaacaacatccgCCACCATGCTGCTGCTGCTGCTGCTGCTGGG-3’) and OMX24 (5′-CTTGAGCACATAGCCTGGACCGTTTCCGTA-3′), restricted with HindIII/PstI and cloned into the corresponding sites (HindIII/PstI) of pSEAP2-Control. | This work |
| pMX41 | Benzoic and vanillic acid-responsive SEAP expression vector (PCTS3-SEAP-pA; PCTS3, PSV40-(OCbaR)3). (OCbaR)3 was introduced by PCR-amplification of pSEAP2-Control with oligonucleotides OMX43 (5′-caaaaagcttgaaagttgctaaaccaacaacatgtttgacacaagttgctaaaccaacaacatgtttgacacaagttgctaaaccaacaacatccgCCACCATGCTGCTGCTGCTGC-3′) and OMX24 (5′-CTTGAGCACATAGCCTGGACCGTTTCCGTA-3’), restricted with HindIII/PstI and cloned into the corresponding sites (HindIII/PstI) of pSEAP2-Control. | This work |
| pMX42 | Benzoic and vanillic acid-responsive SEAP expression vector (PCTS-O3P2-SEAP-pA; PCTS-O3P2, (OCbaR)3-PSV40-(OCbaR)2). Oligonucleotides OMX44 (5′-CTTGAAAGTTGCTAAACCAACAACATGTTTGACACAAGTTGCTAAACCAACAACATGTTTGACACAAGTTGCTAAACCAACAACACTATCGATAGGTACCGAGCTCTTA-3′) and OMX45 (5′-cgcgTAAG AGCTCGGTACCTATCGATAGTGTTGTTGGTTTAGCAACTTGTGTCAAACATGTTGTTGGTTTAGCAACTTGTGTCAAACATGTTGTTGGTTTAGCAACTTTCAAGgtac-3′) were annealed and cloned into the KpnI/MluI-restricted pMX39. | This work |
| pMX43 | Constitutive CTA expression vector (PSV40-CTA-pA; CTA, CbaR-VP16). | This work |
| Custom-designed mammalian codon-optimized cbaR was excised from pUC57 with NotI/BssHII and cloned into the corresponding sites (NotI/BssHII) of pKR38. | ||
| pMX45 | Constitutive CTS2 expression vector (PhEF1α-CTS2-pA; CTS2, CbaR-KRAB). | This work |
| Custom-designed mammalian codon-optimized cbaR was excised from pUC57 with NotI/BssHII and cloned into the corresponding sites (NotI/BssHII) of pKR80. | ||
| pMX78 | Vanillic acid-inducible GLuc expression vector (PVanON8-GLuc-pA). GLuc was excised from pDA43 using EcoRI/FseI and cloned into the corresponding sites (EcoRI/FseI) of pCK191. | This work |
Oligonucleotides: Restriction endonuclease-specific sites are underlined, annealing base pairs are indicated in capital letters and the operator module OCbaR is shown in bold.
Abbreviations: CbaR, Comamonas testosteroni BR60 repressor of the 3-chlorobenzoate catabolism; CTA, CbaR-derived benzoic and vanillic acid-dependent transactivator (CbaR-VP16); CTS1, CbaR-derived benzoic and vanillic acid-dependent transsilencer variant 1 (KRAB-CbaR); CTS2, CbaR-derived benzoic and vanillic acid-dependent transsilencer variant 2 (CbaR-KRAB); GLuc, Gaussia princeps luciferase; HucR,Deinococcus radiodurans uric acid-dependent repressor; KRAB, Krueppel-associated box protein of the human kox-1 gene; KstR, Mycobacterium tuberculosis repressor of the cholesterol catabolism; mUTS, mammalian urate-dependent transsilencer (KRAB-HucR); OCbaR, CbaR-specific operator; OPapRI, ScbR-specific operator; OTtgR, TtgA1-specific operator; pA, polyadenylation site; PCTA-O2, CTA-specific benzoic and vanillic acid-responsive promoter ((OCbaR)2-PhCMVmin); PCTS1, benzoic and vanillic acid-responsive promoter (PSV40-OCbaR); PCTS2, benzoic and vanillic acid-responsive promoter (PSV40-(OCbaR)2); PCTS3, benzoic and vanillic acid-responsive promoter (PSV40-(OCbaR)3); PCTS-O3P2, benzoic and vanillic acid-responsive promoter ((OCbaR)3-PSV40-(OCbaR)2); PhEF1α, human elongation factor 1α promoter; PhCMV, human cytomegalovirus immediate early promoter; PhCMVmin, minimal version of PhCMV; PhCMV*-1, tetracycline-responsive promoter (tetO7-PhCMVmin); PSPA, γ-butyrolactone (SCB1)-repressible promoter (OPapRI-PhCMVmin); PSV40, simian virus 40 promoter; PTtgR1, phloretin-responsive promoter (OTtgR-PhCMVmin); PVanON8 vanillic acid-inducible promoter (PhCMV-VanO8); SEAP, human placental secreted alkaline phosphatase; SCB1, 2-(1′-hydroxy-6-methylheptyl)-3-(hydroxymethyl)-butanolide; ScbR, Streptomyces coelicolor γ-butyrolactone (SCB1)-specific quorum-sensing receptor; tetO, TetR-specific operator; TetR, Escherichia coli Tn10-derived tetracycline-dependent repressor of the tetracycline resistance gene; tTA, tetracycline-dependent transactivator (TetR-VP16); TtgA1, phloretin-dependent transactivator (TtgR-VP16); VanA4, VanR-derived vanillic acid-dependent transsilencer (VanR-KRAB); VanO, VanR-specific operator. VanR, vanillic acid-dependent repressor of the Caulobacter crescentus VanAB gene cluster; VP16, Herpes simplex virus-derived transactivation domain.
Cell culture and transfection
Human embryonic kidney cells (HEK-293T, ATCC: CRL-11268), human cervical adenocarcinoma cells (HeLa, ATCC: CCL-2) and telomerase-immortalized human mesenchymal stem cells (hMSCs (39)) were cultivated in 10-cm tissue culture dishes containing 10 ml Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Basel, Switzerland) supplemented with 10% foetal bovine serum (Sigma-Aldrich, Buchs, Switzerland, cat. no. F7524, lot no. 022M3395) and 1% (v/v) penicillin/streptomycin solution (Biowest, Nuaillé, France, cat. no. L0022–100) at 37°C in a humidified atmosphere containing 5% CO2. For maintenance (10-cm tissue culture dish), passaging (10-cm tissue culture dish) and transfection (6- and 48-well plates), cells of pre-confluent cultures were detached by incubation in 3 ml Trypsin-EDTA (Biowest, Nuaillé, France, cat. no. L0940) for 3 min at 37°C, collected in 10 ml cell culture medium, centrifuged for 3 min at 290 × g and resuspended in Dulbecco's modified Eagle's medium at standard cell densities (15 000 cells in 100 μl per well of a 96-well plate; 25 000 cells in 300 μl per well of a 48-well plate; 50 000 cells in 500 μl per well of a 24-well plate; 200 000 cells in 2 ml per well of a 6-well and 1 × 106 cells in 10 ml per 10 cm tissue culture dish). Cell concentrations and viability were profiled with a CASY® Cell Counter and Analyser System Model TT (Roche Diagnostics GmbH, Mannheim, Germany). For transfection, cells cultivated in 6-well culture dishes were incubated for 6 h with 400 μl (for 48-well plates: 50 μl) of a 1:3 (1:5, HeLa cells only) PEI:DNA mixture (w/w) (polyethyleneimine; MW 40 000, stock solution 1 mg/ml in ddH2O; Polysciences, Eppelheim, Germany; cat. no. 24765–2) containing 2.0 μg (for 48-well plates: 0.5 μg) of total DNA (preparation: 15 min at 22°C). After transfection, the culture medium was replaced by PEI-free medium (2 ml for 6-well and 300 μl for 48-well plates), and the engineered cells were used for a dedicated experiment.
Quantification of reporter gene expression
The production of human placental secreted alkaline phosphatase (SEAP) was quantified in culture supernatants according to a p-nitrophenylphosphate-based light absorbance time course (40). The SEAP levels of the serum were profiled using a chemiluminescence-based assay (Roche Diagnostics GmbH, Mannheim, Germany; cat. no. 11 779 842 001). Gaussia luciferase was quantified using the BioLux® Gaussia luciferase assay kit (New England Biolabs, MA, USA; cat. no. E3300S) and the EnVision 2104 multilabel plate reader (Envision 2104 Plate-reader, Perkin Elmer, MA, USA) by integrating the luminescence signal for 2 s per well (40).
qRT-PCR
Total RNA was isolated from transfected HeLa using the ZR RNA MiniPrep™ kit (Zymo Research, CA, USA; cat. no. R1064), treated with DNaseI (Thermo Scientific, cat. no. EN0521) and cDNA was synthesized using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Life Technologies, CA, USA; cat. no. 4368814). qRT-PCR-based quantification of SEAP transcripts was performed with the Eppendorf Realplex2 Mastercycler (Eppendorf GmbH, Hamburg, Germany), using Invitrogen SYBR® Green PCR Master Mix (Life Technologies, CA, USA; cat. no. 4309155) and the custom-designed primers (forward: 5′-GTCAGTGGGAGTGGTAACCA-3′, reverse: 5′-ACATGTACTTTCGGCCTCCA-3′) purchased from Sigma-Aldrich (Buchs, Switzerland). The following amplification parameters were used: 2 min at 50°C, 20 s at 95°C and 60 cycles of 1 s at 95°C, followed by 1 min at 60°C. The relative cycle threshold (CT) was determined and normalized against the endogenous human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene.
Chemicals
Ethanol (EtOH; cat. no. 02860), dimethyl sulfoxide (DMSO; cat. no. D8418), benzoic acid (stock solution 1 M in EtOH; cat. no. 242381), sodium benzoate (stock solution 1.7 M in ddH2O; cat. no. B3420), vanillic acid (stock solution 200 mM in EtOH, cat. no. 94770), 3,4-dihydroxybenzoic acid (stock solution 200 mM in EtOH, cat. no. 37580), 3-hydroxybenzoic acid (stock solution 100 mM in EtOH, cat. no. H20008), 4-hydroxybenzoic acid (stock solution 100 mM in EtOH, cat. no. 240141), homo-vanillyl-alcohol (stock solution 50 mM in EtOH, cat. no. 148830), homo-vanillic-acid (stock solution 20 mM in EtOH, cat. no. H1252), tetracycline (stock solution 2mM in ddH2O, cat. no. T7660) and phloretin (stock solution 25mM in DMSO, cat. no. P7912) were purchased from Sigma-Aldrich (Buchs, Switzerland). Ethyl 3,4-dihydroxybenzoate (stock solution 200 mM in EtOH, cat. no. D0571) was purchased from Tokyo Chemical Industry (Tokyo, Japan).
Soft drinks
Ocean Spray® (0.01% benzoic acid, (41)), Guarana Antarctica® (<0.1% sodium benzoate, (37)) and Vanilla Coke® were purchased at local supermarkets, degassed by extensive shaking and directly administered to mice (4 × 200 μl). A vanillin syrup was produced by dissolving 13 g of vanillin sugar (Dr Oetker; Bielefeld, Germany) in 50 ml ddH2O.
Animal experiments
Intraperitoneal implants were produced by encapsulating transgenic HeLa cells into coherent alginate-poly-(l-lysine)-alginate beads (400 μm; 200 cells/capsule) using an Inotech Encapsulator Research Unit IE-50R (EncapBioSystems Inc.) set to the following parameters: 200 μm nozzle with a vibration frequency of 1025 Hz, 25-ml syringe operated at a flow rate of 410 units and 1.12 kV voltage for bead dispersion (8). Eight-week-old female OF1 mice (oncins France souche 1, Charles River Laboratory, Lyon, France) were intraperitoneally injected with 1 ml of phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO; pH 7.4) containing 2 × 106 encapsulated pMX34/pMX42-transgenic HeLa cells. One hour after implantation, the treated animals received 200 μl of the trigger compound-containing solutions while control groups received 200 μl of PBS or ddH2O. Blood samples were collected 24 h after treatment, and blood serum was isolated using microtainer SST tubes according to the manufacturer's instructions (centrifugation for 5 min at 10 000 x g; Becton Dickinson, Plymouth, UK; cat. no. 365967). All experiments involving animals were performed according to the directive of the European Community Council (2010/63/EU), approved by the French Republic (No. 69266310; project No. DR2013–01 (v2)) and carried out by Ghislaine Charpin-El Hamri at the Institut Universitaire de Technologie, IUT, F-69622 Villeurbanne Cedex, France.
RESULTS
Design and validation of the Comamonas testosteroni CbaR-derived synthetic mammalian transactivator (CTA)
By fusing the Herpes simplex virus-derived transactivation domain (VP16) to the C′-terminus of the Comamonas testosteroni repressor CbaR, we created the synthetic mammalian transcription factor CTA (CbaR-derived transactivator; pMX43, PSV40-CTA-pA; CTA, CbaR-VP16), whose expression did neither reduce the viability nor the metabolic capacity of mammalian cells (Supplementary Figure S1A). This transcription factor was able to bind to a chimeric promoter (PCTA-O2, (OCbaR)2-PhCMVmin) consisting of a CTA-specific CbaR-derived tandem operator module (OCbaR)2 5′ of a minimal version of the human cytomegalovirus immediate-early promoter (PhCMVmin) and activate expression of the reporter protein SEAP (human placental secreted alkaline phosphatase; pMX38, PCTA-O2-SEAP-pA) (Figure 1A). When cotransfecting pMX43 (PSV40-CTA-pA) and pMX38 (PCTA-O2-SEAP-pA) at different ratios into human embryonic kidney cells (HEK-293), the engineered cells produced and secreted SEAP at levels that depended on the relative abundance of the transfected CTA- and SEAP-encoding vectors (Figure 1B). Control experiments with isogenic populations lacking pMX43 (PSV40-CTA-pA) only showed basal SEAP levels, indicating that the binding of CTA to PCTA-O2 was indeed essential for transgene expression in mammalian cells (Figure 1B). Previous studies in C. testosteroni have shown that the binding of CbaR to OCbaR-containing promoters was derepressed by 3-chlorobenzoate as well as 3,4-dihydroxy-benzoate to initiate the catabolism of the compounds (30). However, CbaR's natural trigger compounds 3-chlorobenzoate and 3,4-dihydroxy-benzoate were too toxic for use in mammalian cells, and the chocolate catabolite 3-hydroxy-benzoate (42) was not able to modulate SEAP expression in mammalian cells at concentrations that are licensed for use as a food additive (Figure 1C and D).
Figure 1.
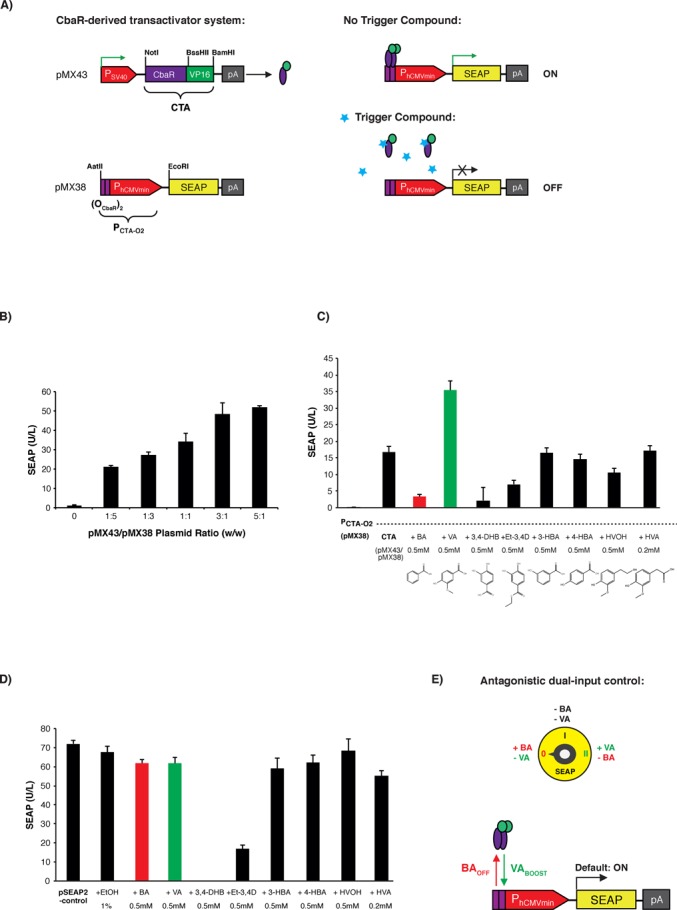
Comamonas testosteroni CbaR-derived transactivation-based mammalian gene switch. (A) Design and functionality of the CbaR-derived transactivation-based transgene control system. C′-terminal fusion of CbaR to the transactivation domain VP16 of the Herpes simplex virus results in a CbaR-derived transactivator CTA (pMX43, PSV40-CTA-pA; CTA, CbaR-VP16). Following constitutive expression by the Simian virus 40 promoter (PSV40), CTA binds and activates a chimeric promoter PCTA-O2 (pMX38, PCTA-O2-SEAP-pA; PCTA-O2, (OCbaR)2-PhCMVmin) containing a tandem CbaR/CTA-specific operator module (OCbaR)2 5′ of a minimal human cytomegalovirus immediate early promoter (PhCMVmin), which is set to drive expression of human placental secreted alkaline phosphatase (SEAP). In the presence of trigger compounds, CTA is released from PCTA-O2, and SEAP expression is shut down. (B) Validation of CTA-mediated PCTA-O2-driven SEAP expression in HEK-293 cells. HEK-293 cells were cotransfected with different ratios (w/w; 0.6 μg total DNA) of transactivator-encoding pMX43 and the SEAP reporter plasmid pMX38, and SEAP expression was scored in the culture supernatant after 48 h. The data are show as the mean ± SD, n = 3. (C) Impact of small-molecule compounds on CTA/PCTA-O2-controlled transgene expression. HEK-293 cells cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA) were reseeded at a standard cell density in a 96-well plate (15 000 cells in 100 μl per well) containing potential trigger compounds at the indicated concentrations (BA: benzoic acid; VA: vanillic acid; 3,4-DHB: 3,4-dihydroxybenzoic acid; Et-3,4D: ethyl 3,4-dihydroxybenzoate; 3-HBA: 3-hydroxy-benzoate; 4-HBA: 4-hydroxy-benzoate; HVOH: homo-vanillyl-alcohol; HVA: homo-vanillic-acid). pMX38-transfected cells were used as a basal expression control. The SEAP levels were profiled in the culture supernatant after 48 h. The data are shown as the mean ± SD, n = 3. (D) Cytotoxicity score of the control compounds shown in Figure 1C. Cytotoxicity was assessed by scoring the impact of control molecules on viability and metabolic integrity by profiling SEAP expression of HEK-293 cells transfected with the constitutive SEAP expression vector pSEAP2-control. pSEAP2-control-transfected HEK-293 cells cultivated in the absence of any compound and HEK-293 cells cultivated in the presence of 1% EtOH, which represents the final solvent concentration present in compound-containing cultures, were used as controls. The SEAP levels were profiled in the culture supernatant 48 h after transfection and compound addition. The data are shown as the mean ± SD, n = 3. (E) Schematic of the step switch-like control characteristic produced by the dual-input CbaR-derived gene switch. CTA transactivates PCTA-O2 while accepting both boosting (vanillic acid, VABOOST) and inactivating (benzoic acid, BAOFF) control inputs. These three distinct output levels (OFF, ON, BOOST) are reminiscent of an electrical stepping switch (0, I, II). (F) Dual-input control characteristic of the CTA/PCTA-O2 expression device. HEK-293, HeLa and hMSC cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 0.5 μg total DNA). The SEAP levels were profiled in the culture supernatant 48 h after cultivation in medium containing either benzoic acid (BA, 750 μM) or vanillic acid (VA, 750 μM). Compound-free and pMX38-transfected cultures were used as controls. The data are shown as the mean ± SD, n = 3. (G) Dose-dependent dual-input control of the CTA/PCTA-O2 gene switch. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 (w/w; 0.5 μg total DNA) ratio, and SEAP levels were profiled in the culture supernatant 48 h after cultivation of the cells in medium containing different concentrations (0–1000 μM) of either benzoic or vanillic acid. pMX38-transfected cells were used as a basal expression control. The data are shown as the mean ± SD, n = 3. (H) qRT-PCR analysis of SEAP mRNA levels. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA) and cultivated for 48 h in medium containing either benzoic acid (BA, 750 μM), vanillic acid (VA, 750 μM) or no control compound (CTA). The SEAP transcript levels were profiled relative to the basal expression control (cells transfected only with pMX38). The analysis was performed in triplicate. (I) Comparative analysis of different mammalian small molecule-repressible transcription-control systems. HeLa cells were cotransfected with the TET- (pSAM200/pMF111), PEACE- (pMG11/pMG10) and CbaR-derived (pMX43/pMX38) control components at a 1:1 ratio (w/w; 0.5 μg total DNA) and cultivated for 48 h in the presence (+, repression) or absence (−, induction) of the corresponding regulating (2 μM tetracycline, 40 μM phloretin and 750 μM benzoic acid) and boosting (750 μM vanillic acid) compounds before SEAP was quantified in the culture supernatant. Cells transfected exclusively with the reporter constructs (TET, pMF111; PEACE, pMG10; CbaR-derived gene switch, pMX38) were used as negative controls. The data are shown as the mean ± SD, n = 3. Fold-changes in SEAP expression were calculated by dividing absolute SEAP levels (induction, repression, boosted induction) by the basal SEAP levels produced by the reporter plasmid used as isogenic negative control. (J) Dose-dependent benzoic acid-mediated repression of vanillic acid boosted SEAP expression by the CTA/PCTA-O2 device. HeLa cells were cotransfected with pMX43 and pMX38 at a 1:3 ratio (w/w; 2 μg total DNA), collected in culture medium containing different concentrations of vanillic acid (20, 100 or 500 μM) and reseeded at standard concentration into the wells of a 96-well plate (15 000 cells in 100 μl per well) containing the indicated concentrations of benzoic acid. Forty-eight hours after the addition of benzoic acid, the SEAP levels were profiled in the culture supernatant. The data are shown as the mean ± SD, n = 3.
The food additives benzoic acid and vanillic acid modulate CTA-PCTA-O2-driven transgene expression
To identify non-toxic, bioavailable and cell-permeable trigger compounds modulating CTA's capacity to bind and activate PCTA-O2-driven transgene expression in mammalian cells, we operated the CTA-PCTA-O2 system as a mammalian cell-based screening platform (20,43). Therefore, pMX43/pMX38-transfected HEK-293 cultures were exposed to various aromatic acids at concentrations that are naturally present in edible plants and fruits (38). Then, SEAP levels were profiled in the culture supernatants to reveal the compounds’ control capacity (Figure 1C). Benzoic acid, 3,4-dihydroxybenzoic acid and ethyl 3,4-dihydroxybenzoate reduced SEAP levels of CTA-PCTA-O2-transgenic cultures, making them prime candidates to trigger the release of CTA from PCTA-O2 (Figure 1C). However, in parallel control assays using isogenic cultures transfected with the constitutive SEAP expression vector (pSEAP2-Control, PSV40-SEAP-pA), 3,4-dihydroxybenzoic acid and ethyl 3,4-dihydroxybenzoate actually reduced constitutive SEAP production due to their cytotoxicity (Figure 1D). Thus, the licensed food preservative benzoic acid was identified as a non-toxic control compound that abolishes the CTA-PCTA-O2 interaction and shuts down CTA-mediated PCTA-O2-driven transgene expression in mammalian cells.
Interestingly, the cell-based screening assay also revealed another licensed food additive, vanillic acid, as control compound. In contrast to benzoic acid, which triggers the release of CTA from PCTA-O2 and therefore represses transgene expression, vanillic acid seems to increase the affinity of CTA to PCTA-O2, thereby boosting the system's expression performance (Figure 1C). Therefore, CTA is a synthetic transcription factor that accepts both boosting (vanillic acid) and inactivating (benzoic acid) control inputs. This unique antagonistic dual-input control characteristic reminiscent of an electrical stepping switch (Figure 1E) was successfully validated in other human cell lines (Figure 1F) as well as in mice (Supplementary Figure S2), where both trigger compounds independently fine-tuned CTA-PCTA-O2-mediated SEAP expression in a dose-dependent manner (Figure 1G). qRT-PCR-based analysis of SEAP mRNA levels confirmed the dual-input control characteristics of the CbaR-derived gene switch (Figure 1H). Comparative analysis of the CbaR-derived gene switch with other established transcription-control devices such as the tetracycline- (TET; (44)) and phloretin- (PEACE; (19)) responsive expression systems revealed similar basic control performance while the expression mediated by the CbaR-derived gene switch could be boosted by vanillic acid to about three times the maximum levels of the TET and PEACE counterparts (Figure 1I).
Simultaneous control of CTA by benzoic acid and vanillic acid
Although we have observed that the CTA-PCTA-O2 device accepted opposing dose-dependent input by vanillate (expression boosting) and benzoate (expression inactivation), it remains to be shown what impact the presence of both control compounds has on the overall transgene expression output. We have therefore programmed SEAP expression of pMX43/pMX38-transgenic HeLa cells to defined levels by exposing the cultures to specific vanillate concentrations (20, 100 and 500 μM) and then scored benzoic-acid dose-dependent shut down of SEAP expression (Figure 1J). Interestingly, both compounds had independent control of CTA, and neither of them seemed to dominate CTA activity. Consequently, increased vanillate-boosted SEAP expression levels required higher benzoate concentrations to reverse the SEAP expression state. Thus, the overall SEAP expression level mediated by the CTA-PCTA-O2 device directly correlates with the relative concentrations of the two control compounds (Figure 1J).
Design and validation of synthetic mammalian transsilencers (CTS) with benzoate-inducing and vanillate-repressing dual-input responsiveness
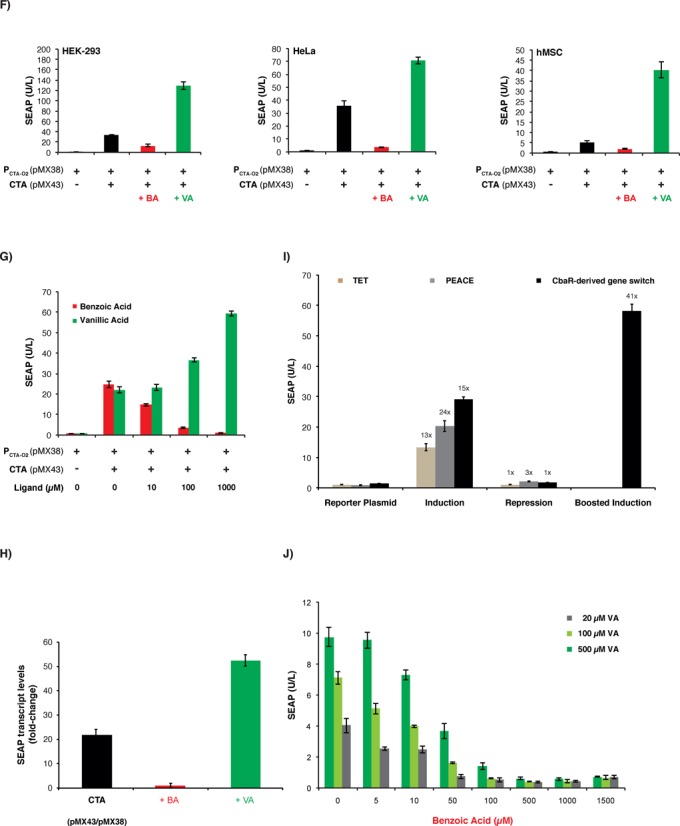
To engineer a benzoic acid-inducible and vanillic acid-repressible dual-input control device, we designed synthetic transsilencers (CTS) by fusing the Krueppel-associated box (KRAB) domain of the human kox-1 gene either to the N′-(pMX34, PhEF1α-CTS1-pA; CTS1, KRAB-CbaR) or the C′-terminus (pMX45, PhEF1α-CTS2-pA; CTS2, CbaR-KRAB) of CbaR. CTS1/2 bind and silence chimeric promoters (PCTS) consisting of a constitutive promoter (PSV40) containing one (pMX39, PCTS1-SEAP-pA; PCTS1, PSV40-OCbaR), two (pMX40, PCTS2-SEAP-pA; PCTS2, PSV40-(OCbaR)2) or three (pMX41, PCTS3-SEAP-pA; PCTS3, PSV40-(OCbaR)3) 3′ OCbaR operator modules or multiple flanking OCbaR's (pMX42; PCTS-O3P2-SEAP-pA; PCTS-O3P2, (OCbaR)3-PSV40-(OCbaR)2) (Figure 2A). A direct comparison of both CTS configurations revealed that the N′-terminal fusion of KRAB to CbaR (CTS1, KRAB-CbaR) resulted in tighter repression of the target promoter (Figure 2B and C). Constitutive expression of CTS1 did neither reduce the viability nor the metabolic capacity of mammalian cells (Supplementary Figure S1B). Furthermore, the PCTS-O3P2 promoter showed the lowest basal SEAP expression level and the highest SEAP induction profile. Therefore, the CTS1/PCTS-O3P2 device was chosen for all further experiments (Figure 2C). Exposure of pMX34/pMX42-transgenic HeLa cells to increasing concentrations of benzoic acid triggers the release of CTS1 from PCTS-O3P2, resulting in reversible (Figure 2D) and dose-dependent derepression of SEAP expression (Figure 2E). In contrast, vanillate increased the affinity of CTS1 for PCTS-O3P2, thereby decreasing benzoate-inducible transgene expression and improving overall tightness and the dynamic range of the gene switch (Figure 2E). Therefore, CTS1 retained the dual-input characteristics of CTA because transgene expression induced to high levels by benzoate could be programmed to specific intermediate levels by the addition of the repressing input vanillate (Figure 2F). Furthermore, a kinetic analysis of benzoate-induced pMX34/pMX42-transgenic HeLa cells revealed that SEAP expression could be stopped at any point in time by the addition of different vanillic acid concentrations (Figure 2G and H).
Figure 2.
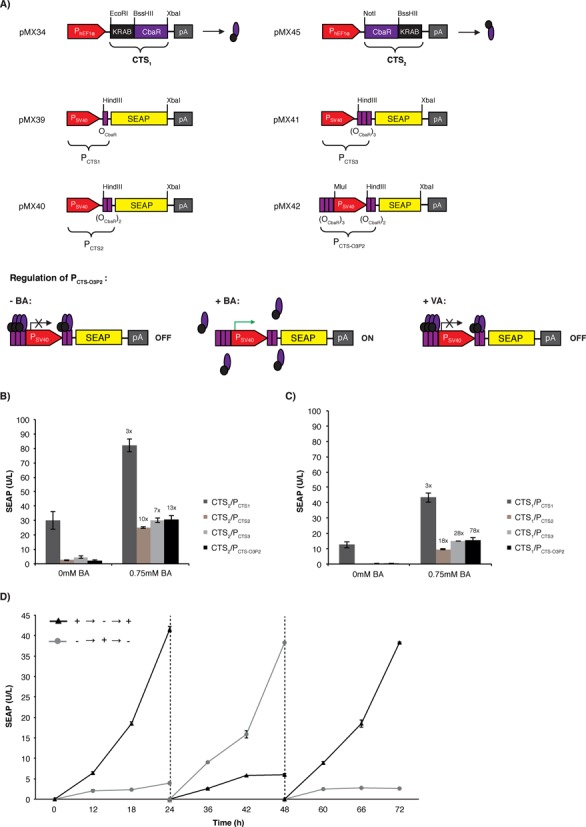
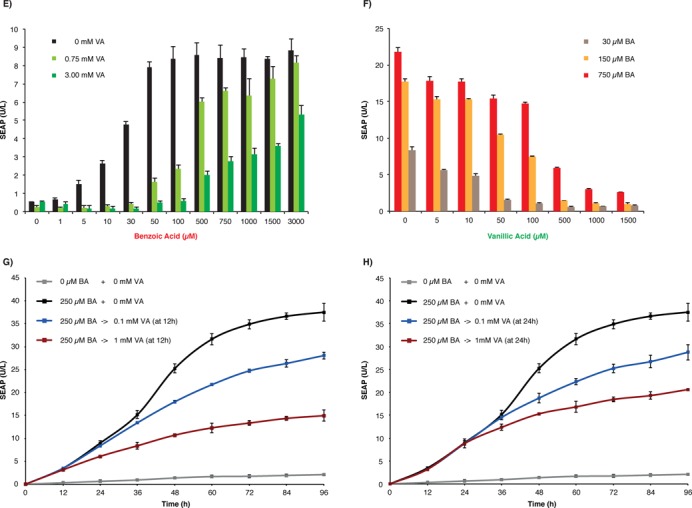
Comamonas testosteroni CbaR-derived transsilencing-based gene switch. (A) Design and functionality of the CbaR-derived transsilencing-based transgene control system. N-terminal (pMX34, PhEF1α-CTS1-pA; CTS1, KRAB-CbaR) or C′-terminal (pMX45, PhEF1α-CTS2-pA; CTS2, CbaR-KRAB) fusion of CbaR to the transsilencing Krueppel-associated box (KRAB) domain of the human kox-1 gene resulted in the CbaR-derived transsilencers CTS1/2. Following constitutive expression by the human elongation factor 1 alpha promoter (PhEF1α), CTS1/2 bind and silence chimeric promoters consisting of the constitutive Simian virus 40 promoter (PSV40) linked to one (pMX39, PCTS1-SEAP-pA; PCTS1, PSV40-OCbaR), two (pMX40, PCTS2-SEAP-pA; PCTS2, PSV40-(OCbaR)2) and three (pMX41, PCTS3-SEAP-pA; PCTS3, PSV40-(OCbaR)3) 3′ CbaR/CTS1/2-specific operator modules (OCbaR) or flanked by multiple OCbaR's (pMX42; PCTS-O3P2-SEAP-pA; PCTS-O3P2, (OCbaR)3-PSV40-(OCbaR)2). All promoters were set to drive the expression of human placental secreted alkaline phosphatase (SEAP). The presence of benzoic acid triggers the release of CTS1/2 from their cognate PCTS promoters, resulting in the activation of SEAP expression. The presence of vanillic acid increases the affinity of CTS1/2 for PCTS and thus inhibits benzoate-inducible transgene expression. (B, C) Validation of CTS2- (B) and CTS1 (C)-mediated PCTS1/2/PCTS1/CTS2/CTS3/CTS-O3P2-driven SEAP expression in HeLa cells. HeLa cells were cotransfected with the indicated combinations of CTS and PCTS-SEAP-encoding expression vectors at a 1:1 ratio (w/w; 0.6 μg total DNA) and cultivated for 48 h in the presence (+) or absence (−) of 0.75 mM benzoic acid before SEAP expression was scored in the culture supernatant. The data are shown as the mean ± SD, n = 3. Fold-changes in SEAP expression of all CTS/PCTS combinations were calculated by dividing BA-induced SEAP expression levels (0.75mM benzoic acid) by basal SEAP levels (0mM benzoic acid). (D) Reversibility of CTS1/PCTS-O3P2-driven SEAP expression. pMX34/pMX42-cotransfected HeLa cells were cultivated for 72 h while alternating the presence (+) and absence (−) of benzoic acid (BA, 50 μM) and scoring SEAP levels in the culture supernatant every 12 h. The data are shown as the mean ± SD, n = 3. (E) Dose-dependent benzoic acid-induced SEAP expression by the CTS1/PCTS-O3P2 device in the presence of different CTS1-inactivating vanillic acid concentrations. HeLa cells were cotransfected with pMX34 and pMX42 at a 1:1 ratio (w/w; 2 μg total DNA), collected in culture medium containing different vanillic acid concentrations (0 mM, 0.75 mM or 3 mM) and reseeded into the wells of a 96-well plate (15 000 cells in 100 μl per well) containing the indicated concentrations of benzoic acid. The SEAP levels were profiled in the culture supernatant after 48 h. The data are shown as the mean ± SD, n = 3. (F) Dose-dependent vanillic acid-repressed SEAP expression by the CTS1/PCTS-O3P2 device in the presence of different CTS1-inducing benzoic acid concentrations. HeLa cells were cotransfected with pMX34 and pMX42 at a 1:1 ratio (w/w; 2 μg total DNA), collected in culture medium containing different benzoic acid concentrations (30 μM, 150 μM or 750 μM) and reseeded into the wells of a 96-well plate (15 000 cells in 100 μl per well) containing the indicated concentrations of vanillic acid. The SEAP levels were profiled in the culture supernatant after 48 h. The data are shown as the mean ± SD, n = 3. (G, H) Control kinetics of the CTS1/PCTS-O3P2 device. HeLa cells were cotransfected with pMX34 and pMX42 at a 1:1 ratio (w/w; 2 μg total DNA) and cultivated for 96 h in the presence (+) or absence (−) of 250 μM benzoic acid (BA). At (G) 12 h and (H) 24 h, vanillic acid was added at different concentrations. The SEAP levels were profiled in the culture supernatant every 12 h. The data are shown as the mean ± SD, n = 3.
A synthetic gene network with a vanillate control interface
To demonstrate the compatibility of the CTS1/PCTS-O3P2 device with other transgene control modalities and to assess its potential for the assembly of higher-order control circuits, we combined the dual-input benzoate-inducible and vanillic acid-repressible designer switch set to control SEAP expression with the previously established VACON system (pCK189, PSV40-VanA4-pA; pMX78, PVanON8-GLuc-pA) (14). The VACON system allows exclusive vanillic acid-inducible Gaussia princeps luciferase (GLuc) expression in mammalian cells (Figure 3A). When combining the CTS1/PCTS-O3P2 device (pMX34/pMX42) with the VACON (pCK189/pMX78) system in the same cell, vanillic acid provides a functional control link between the two systems and enables mutually exclusive expression switches of the two reporter proteins, SEAP (controlled by the CTS1/PCTS-O3P2 device) and GLuc (controlled by VACON). In particular, the combined transgene control network enables dose-dependent benzoate-inducible SEAP expression that could be stopped at any point in time by the addition of vanillic acid, which concomitantly triggers VACON-mediated GLuc expression. Thus, a single trigger compound such as vanillic acid is able to simultaneously stop (CTS1/PCTS-O3P2-driven SEAP expression) and turn on (VACON-driven GLuc expression) the expression of different sets of transgenes (Figure 3B). These control dynamics are reminiscent of a lap-timing stop watch in which benzoic acid represents the ‘start button’ and vanillic acid defines the ‘lap button’ that holds the time value of the first lap (SEAP expression) while initiating the recording of the second lap (GLuc expression) (Figure 3C).
Figure 3.
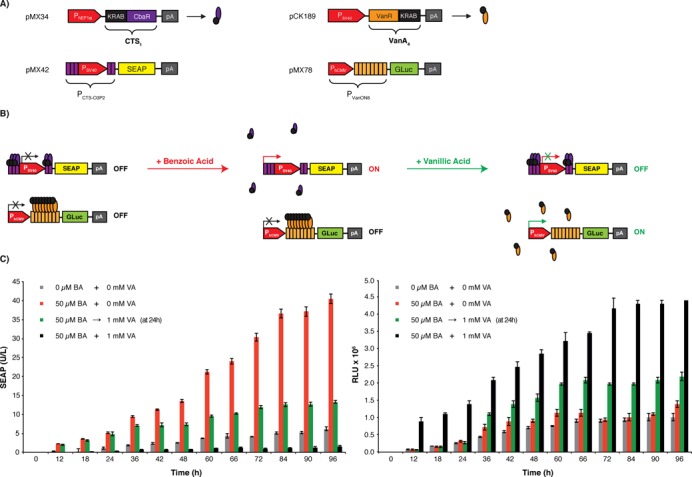
A synthetic gene network with a vanillate control interface. (A) Componentry of the higher-order control network combining two different orthogonal gene switches with antagonistic responses to vanillic acid, the CTS1/PCTS-O3P2 (pMX34, PhEF1α-CTS1-pA; pMX42, PCTS-O3P2 -SEAP-pA) driving SEAP expression and the VACON system (pCK189, PSV40-VanA4-pA; pMX78, PVanON8-GLuc-pA) controlling the expression of GLuc (Gaussia princeps luciferase). The VACON system consists of a vanillic acid-dependent transsilencer VanA4, a fusion of the Caulobacter crescentus repressor VanR and KRAB, which binds and represses PVanON8, a chimeric promoter containing an octameric VanA4-specific operator module (VanO8) 3′ of the human cytomegalovirus immediate early promoter (PhCMV). (B) Operation of the vanillic acid-responsive designer network providing vanillic acid-triggered mutually exclusive expression switches from SEAP to GLuc expression. At the same time as vanillic acid attenuates benzoate-induced CTS1/PCTS-O3P2-driven SEAP expression, it turns on VanA4/PVanON8-driven GLuc expression. (C) Vanillic acid-programmed SEAP and GLuc expression kinetics. HeLa cells were cotransfected with pMX34, pMX42, pCK189 and pMX78 (10:10:1:0.5 ratio [w/w]; 2 μg total DNA) and cultivated for 96 h in the presence or absence of vanillic acid (VA, 1 mM) and benzoic acid (BA, 50 μM). For one experimental configuration, vanillic acid (1 mM) was added after 24 h to a culture containing benzoic acid (50 μM). SEAP as well as GLuc production were profiled every 12 h. The data are shown as the mean ± SD, n = 3.
Validation of benzoate-inducible transgene expression in mice
To assess the benzoate-inducible CTS1/PCTS-O3P2 device in vivo, we microencapsulated pMX34/pMX42-transgenic HeLa cells into coherent, semi-permeable and immunoprotective alginate-poly-(l-lysine)-alginate beads (Figure 4A) and implanted them into the peritoneum of mice where they become vascularized and connected to the bloodstream (7). The mice were offered to drink water containing different concentrations of benzoate (0–1000 mg/kg) and correlating SEAP levels in the bloodstream showed benzoate dose-dependent transgene activation and confirmed the precise expression adjustment by this food additive in mice (Figure 4Bi). Likewise, treated animals that drank CTS1/PCTS-O3P2-activating benzoate concentrations but received different vanillate concentrations (0–500 mg/kg) showed dose-dependent inhibition of SEAP expression (Figure 4Bii), which confirms the unique dual-input characteristic of CTS1 in vivo. When feeding mice with commercial soft drinks such as Ocean Spray (cranberry juice) or Guarana Antarctica (guarana lemonade), which are known for their high natural and spiked benzoate concentrations, respectively (<0.1% (w/w) by FDA), no increased blood SEAP levels could be detected (Figure 4C). Also, mice receiving high vanillate-containing drinks such as Vanilla Coke® or a saturated aqueous vanillin-sugar solution (260 g/l) did not show any changes of SEAP levels in their bloodstream (Figure 4C). Collectively, these data suggest that activation of the CTS1/PCTS-O3P2 device in vivo is rapid (Supplementary Figure S3) but remains insensitive to standard dietary intake (Figure 4C) while responding exclusively to higher benzoate concentrations (≤1000 mg/kg) within its NOAEL range.
Figure 4.
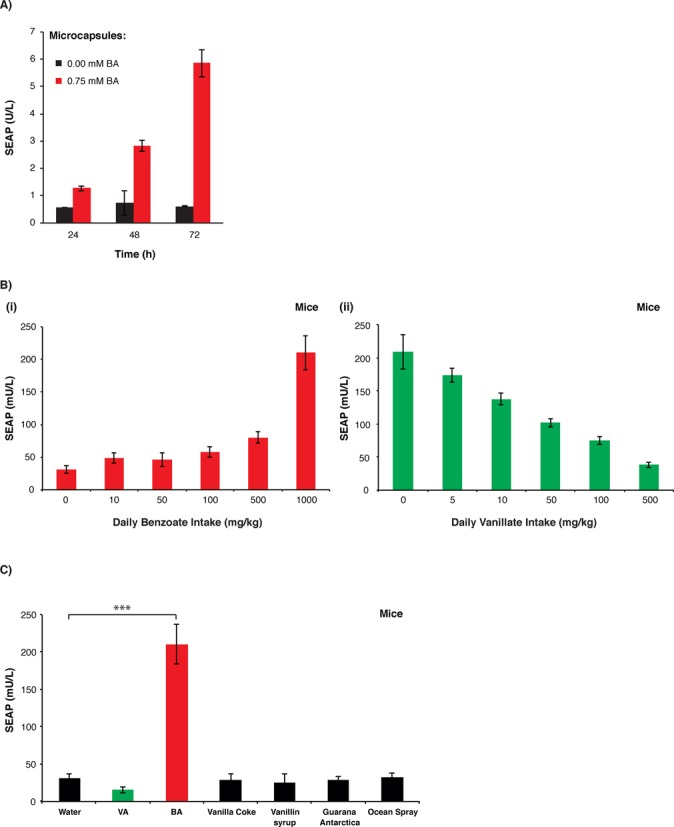
Benzoate-inducible transgene expression in mice. (A) SEAP induction profiles of microencapsulated CTS1/PCTS-O3P2-transgenic HeLa cells in vitro. In total, 2 × 106 pMX34/pMX42-transgenic microencapsulated HeLa cells (10 000 capsules, 200 cells/capsule) were cultivated per well of a 6-well plate containing 3 ml of standard medium with or without 0.75 mM sodium benzoate. SEAP expression was profiled in the culture supernatant every 24 h. The data are shown as the mean ± SD, n = 3. (B) Antagonistic dual-input control of the CTS1/PCTS-O3P2in vivo. In total, 2 × 106 pMX34/pMX42-transgenic microencapsulated HeLa cells (10 000 capsules, 200 cells/capsule) were intraperitoneally implanted into mice. (i) Treated mice drank 4 × 200 μl H2O containing different concentrations of sodium benzoate (total daily intake: 0–1000 mg/kg). (ii) Treated mice drank 4 × 200 μl sodium benzoate-containing H2O (total daily intake: 1000 mg/kg) and received (2 × 100 μl) different concentrations of vanillic acid concentrations (total daily intake: 0–500 mg/kg). The SEAP levels in the bloodstream of the treated animals were profiled after 24 h. The data are shown as the mean ± SEM, n = 8 mice. (C) Insensitivity of the CTS1/PCTS-O3P2-device to dietary intake of standard benzoate- and vanillate-containing food. In total, 2 × 106 pMX34/pMX42-transgenic microencapsulated HeLa cells (10 000 capsules, 200 cells/capsule) were intraperitoneally implanted into mice that drank 4 × 200 μl of either commercial soft drinks such as Guarana Antarctica, Ocean Spray cranberry juice or Vanilla Coke®, or a custom-made vanillin syrup (260 g/l of vanillin). Control mice either drank 4 × 200 μl sodium benzoate-containing H2O (total daily intake: 1000 mg/kg) or received (2 × 100 μl) of vanillic acid (total daily intake: 500 mg/kg). The SEAP levels in the bloodstreams of the treated animals were profiled after 24 h. The data are shown as the mean ± SEM; statistics by two-tailed t-test; n = 6 mice. ***P < 0.001 versus control.
DISCUSSION
Continuous expansion of the genetic components portfolio is required to design highly sophisticated devices that programme cellular behaviour and provide novel and sustainable solutions for global challenges in renewable energy (45), food security (46) and biomedicine (28,47–49). The availability of synthetic gene switches that allow trigger-adjustable expression of therapeutic transgenes is particularly important to provide the dynamic control behaviour required to functionally interface with host metabolic activities (6–9,50). To guarantee optimal interference-free operation in multicellular organisms, trigger molecules that fine-tune the expression of heterologous transgene expression are of key importance. Optimal inducer compounds are non-toxic, enable reversible and dose-dependent transgene expression and should be orally applicable for maximum administration compliance. Trigger molecules should be bioavailable to quickly reach concentrations suitable for efficient control in the bloodstream or elsewhere in the body yet should be readily eliminated by renal clearance to allow rapid reversal of the intervention. Additionally, inducer compounds should be metabolically inert to enable perfect orthogonal control without any interference with the host metabolism. Therefore, food additives seem to be particularly suitable trigger compounds because they are generally regarded as safe (GRAS), are licensed for dietary intake over a wide concentration range and have a long and global history of complication-free use. However, the major challenge associated with food additive-responsive gene switches is that they should remain insensitive to standard dietary intake and be exclusively controlled by the intentional uptake of higher levels of the food additive within its NOAEL concentration range. Benzoate meets these high standards and therefore is an ideal trigger compound. It is a natural plant defence metabolite present in different fruits, such as cranberries, prunes and apples (32), and has a long history as a licensed food preservative (51). Benzoate is known to be metabolically inert (35), rapidly reaches the bloodstream (34) and is renally cleared within 24 h (34). Benzoate also is the best trigger compound that reversibly adjusts CbaR-derived gene switches in a variety of configurations in mammalian cells as well as in mice. Most importantly, implanted CbaR-derived gene switches remained insensitive when treated animals drank Ocean Spray cranberry juice and Guarana Antarctica lemonade, which are among the beverages that contain the highest natural and spiked benzoate concentrations, respectively. This result indicates that the CbaR-derived gene switch is not deregulated by standard dietary intake of benzoate (0–5 mg/kg body weight (37)) and remains exclusively sensitive to higher concentrations (500–1000 mg/kg) within the NOAEL range (33,37). Therefore, the benzoate sensitivity range of the CbaR-derived gene switch may be compatible with future gene- and cell-based therapies.
Currently available gene switches typically accept a single type of control input that either induces or represses the gene switch and modulates the expression of a single or set of target genes (1,6,14,19,21–24). Daisy-chain assembly of compatible trigger-controlled repressor proteins to multipartite transcription factors has recently been shown to provide multi-input devices that are able to address different sets of promoters in a trigger-repressible manner (25). Asymmetries in the binding affinity of the individual repressor moieties of the multipartite transcription factors to their respective promoters exhibited double-pole double-throw (DPDT) relay switch characteristics, a unique control topology that allows the composite transcription factor to move from one synthetic promoter to the next one in a trigger-inducible manner, thereby sequentially and exclusively switching on a specific target promoter by a particular inducer compound. In contrast, the CbaR-derived transactivators CTA and transsilencers CTS are the first synthetic mammalian transcription factors that antagonistically fine-tune a single target promoter in response to two different trigger molecules, such as benzoic acid and vanillic acid, in a reversible, dose-dependent and antagonistic manner: Benzoate reduces and vanillate increases the CbaR-OCbaR interaction of the CTA/PCTA-O2 and CTS/PCTS device variants. In the absence of benzoate, vanillate can be used to boost the expression capacity of CTA- and improve the tightness of CTS-mediated transgene control, which increases the dynamic range of both gene switches in a trigger-controlled manner. Interestingly, benzoate and vanillate seem to have equal access to CTA and CTS, as neither compound is dominant. Therefore, neither compound can lock the activity of the transactivator or transsilencer in a particular state: when primed by one compound, the device remains sensitive to the other. The relative concentrations of the two compounds always determine the final expression level of the device. The antagonistic control impact allows OFF-to-ON and ON-to-OFF switches to be actively controlled by a specific compound. In contrast, classic single-input gene switches require degradation or active removal to achieve reversibility. Another unique feature of the CTS/PCTS device is that it can be functionally combined with the VACON system via vanillic acid, which stops benzoate-induced CTS/PCTS -driven transgenes and simultaneously turns on VACON-driven transgenes. This genetic lap-timing stopwatch feature may be of interest for the design of lineage-control networks for stem cells which require timely and transient expression of specific differentiation factors on their way to the desired terminally differentiated cell phenotype (52). As a new addition to the synthetic biology toolbox, the dual-input CTA/PCTA-O2 and CTS/PCTS systems, which are responsive to two different food additives, hold great promise for the design of novel and the most compact gene network topologies that may foster advances in gene- and cell-based therapies.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We thank Katrin Rössger for providing plasmids pKR38 and pKR80 and Marc Folcher, Hui Wang, Pratik Saxena and Taeuk Kim for generous advice.
Footnotes
Present address:
Haifeng Ye, Shanghai Key Laboratory of Regulatory Biology, Institute of Biomedical Sciences and School of Life Sciences, East China Normal University, Dongchuan Road 500, 200241 Shanghai, China.
FUNDING
European Research Council (ERC) advanced grant [321381]; INTERREG IV A.20 tri-national research programme and the Gutenberg Chair awarded [to M.F.] (in part).
Conflict of interest statement. None declared.
REFERENCES
- 1.Ausländer S., Fussenegger M. From gene switches to mammalian designer cells: present and future prospects. Trends Biotechnol. 2013;31:155–168. doi: 10.1016/j.tibtech.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Nissim L., Bar-Ziv R. H. A tunable dual-promoter integrator for targeting of cancer cells. Mol. Syst. Biol. 2010;6:444. doi: 10.1038/msb.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Z., Wroblewska L., Prochazka L., Weiss R., Benenson Y. Multi-input RNAi-based logic circuit for identification of specific cancer cells. Science. 2011;333:1307–1311. doi: 10.1126/science.1205527. [DOI] [PubMed] [Google Scholar]
- 4.Wei P., Wong W. W., Park J. S., Corcoran E. E., Peisajovich S. G., Onuffer J. J., Weiss A., Lim W. A. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature. 2012;488:384–388. doi: 10.1038/nature11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culler S. J., Hoff K. G., Smolke C. D. Reprogramming cellular behavior with RNA controllers responsive to endogenous proteins. Science. 2010;330:1251–1255. doi: 10.1126/science.1192128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemmer C., Gitzinger M., Daoud-El Baba M., Djonov V., Stelling J., Fussenegger M. Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nat. Biotechnol. 2010;28:355–360. doi: 10.1038/nbt.1617. [DOI] [PubMed] [Google Scholar]
- 7.Rössger K., Charpin-El-Hamri G., Fussenegger M. A closed-loop synthetic gene circuit for the treatment of diet-induced obesity in mice. Nat. Commun. 2013;4:2825. doi: 10.1038/ncomms3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye H., Charpin-El Hamri G., Zwicky K., Christen M., Folcher M., Fussenegger M. Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2013;110:141–146. doi: 10.1073/pnas.1216801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye H., Daoud-El Baba M., Peng R.W., Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science. 2011;332:1565–1568. doi: 10.1126/science.1203535. [DOI] [PubMed] [Google Scholar]
- 10.Ausländer D., Ausländer S., Charpin-El-Hamri G., Sedlmayer F., Müller M., Frey O., Hierlemann A., Stelling J., Fussenegger M. A synthetic multifunctional mammalian pH Sensor and CO2 transgene-control device. Mol. Cell. 2014 doi: 10.1016/j.molcel.2014.06.007. in press. [DOI] [PubMed] [Google Scholar]
- 11.Müller K., Engesser R., Metzger S., Schulz S., Kämpf M. M., Busacker M., Steinberg T., Tomakidi P., Ehrbar M., Nagy F., et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 2013;41:e77. doi: 10.1093/nar/gkt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley S. A., Gagner J. E., Damanpour S., Yoshida M., Dordick J. S., Friedman J. M. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science. 2012;336:604–608. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J. S., Rhau B., Hermann A., McNally K. A., Zhou C., Gong D., Weiner O. D., Conklin B. R., Onuffer J., Lim W. A. Synthetic control of mammalian-cell motility by engineering chemotaxis to an orthogonal bioinert chemical signal. Proc. Natl. Acad. Sci. U.S.A. 2014;111:5896–5901. doi: 10.1073/pnas.1402087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitzinger M., Kemmer C., Fluri D.A., Daoud-El Baba M., Weber W., Fussenegger M. The food additive vanillic acid controls transgene expression in mammalian cells and mice. Nucleic Acids Res. 2011;40:e37. doi: 10.1093/nar/gkr1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber W., Lienhart C., Daoud-El Baba M., Fussenegger M. A biotin-triggered genetic switch in mammalian cells and mice. Metab. Eng. 2009;11:117–124. doi: 10.1016/j.ymben.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Weber W., Bacchus W., Daoud-El Baba M., Fussenegger M. Vitamin H-regulated transgene expression in mammalian cells. Nucleic Acids Res. 2007;35:e116. doi: 10.1093/nar/gkm466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacchus W., Weber W., Fussenegger M. Increasing the dynamic control space of mammalian transcription devices by combinatorial assembly of homologous regulatory elements from different bacterial species. Metab. Eng. 2013;15:144–150. doi: 10.1016/j.ymben.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Hartenbach S., Daoud-El Baba M., Weber W., Fussenegger M. An engineered L-arginine sensor of Chlamydia pneumoniae enables arginine-adjustable transcription control in mammalian cells and mice. Nucleic Acids Res. 2007;35:e136. doi: 10.1093/nar/gkm652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gitzinger M., Kemmer C., Daoud-El Baba M., Weber W., Fussenegger M. Controlling transgene expression in subcutaneous implants using a skin lotion containing the apple metabolite phloretin. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10638–10643. doi: 10.1073/pnas.0901501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber W., Schoenmakers R., Keller B., Gitzinger M., Grau T., Daoud-El Baba M., Sander P., Fussenegger M. A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9994–9998. doi: 10.1073/pnas.0800663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fussenegger M., Morris R.P., Fux C., Rimann M., von Stockar B., Thompson C.J., Bailey J.E. Streptogramin-based gene regulation systems for mammalian cells. Nat. Biotechnol. 2000;18:1203–1208. doi: 10.1038/81208. [DOI] [PubMed] [Google Scholar]
- 22.Weber W., Fux C., Daoud-El Baba M., Keller B., Weber C.C., Kramer B.P., Heinzen C., Aubel D., Bailey J.E., Fussenegger M. Macrolide-based transgene control in mammalian cells and mice. Nat. Biotechnol. 2002;20:901–907. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- 23.Weber W., Schoenmakers R., Spielmann M., Daoud-El Baba M., Folcher M., Keller B., Weber C.C., Link N., van de Wetering P., Heinzen C., et al. Streptomyces-derived quorum-sensing systems engineered for adjustable transgene expression in mammalian cells and mice. Nucleic Acids Res. 2003;31:e71. doi: 10.1093/nar/gng071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacchus W., Fussenegger M. Engineering of synthetic intercellular communication systems. Metab. Eng. 2013;16:33–41. doi: 10.1016/j.ymben.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Folcher M., Xie M., Spinnler A., Fussenegger M. Synthetic mammalian trigger-controlled bipartite transcription factors. Nucleic Acids Res. 2013;41:e134. doi: 10.1093/nar/gkt405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausländer S., Ausländer D., Müller M., Wieland M., Fussenegger M. Programmable single-cell mammalian biocomputers. Nature. 2012;487:123–127. doi: 10.1038/nature11149. [DOI] [PubMed] [Google Scholar]
- 27.Deans T.L., Cantor C.R., Collins J.J. A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell. 2007;130:363–372. doi: 10.1016/j.cell.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Khalil A.S., Collins J.J. Synthetic biology: applications come of age. Nat. Rev. Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nandagopal N., Elowitz M.B. Synthetic biology: integrated gene circuits. Science. 2011;333:1244–1248. doi: 10.1126/science.1207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Providenti M.A., Wyndham R.C. Identification and functional characterization of CbaR, a MarR-like modulator of the cbaABC-encoded chlorobenzoate catabolism pathway. Appl. Environ. Microbiol. 2001;67:3530–3541. doi: 10.1128/AEM.67.8.3530-3541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmona M., Zamarro M.T., Blázquez B., Durante-Rodríguez G., Juárez J.F., Valderrama J.A., Barragán M.J., García J.L., Díaz E. Anaerobic catabolism of aromatic compounds: a genetic and genomic view. Microbiol. Mol. Biol. Rev. 2009;73:71–133. doi: 10.1128/MMBR.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen I., Shandil A., Shrivastava V.S. Determination of benzoic acid residue from fruit juice by gas chromatography with mass spectrometry detection technique. Arch. Appl. Sci. Res. 2011;3:245–252. [Google Scholar]
- 33.Onodera H., Ogiu T., Matsuoka C., Furuta K., Takeuchi M., Oono Y., Kubota T., Miyahara M., Maekawa A., Odashima S. [Studies on effects of sodium benzoate on fetuses and offspring of Wistar rats (authors’ translation), Article in Japanese] Eisei Shikenjo Hokoku. 1978;96:47–55. [PubMed] [Google Scholar]
- 34.Kristensen N.B., Nørgaard J.V., Wamberg S., Engbaek M., Fernández J.A., Zacho H.D., Poulsen H.D. Absorption and metabolism of benzoic acid in growing pigs. J Anim Sci. 2009;87:2815–2822. doi: 10.2527/jas.2009-2003. [DOI] [PubMed] [Google Scholar]
- 35.Adams T.B., Cohen S.M., Doull J., Feron V.J., Goodman J.I., Marnett L.J., Munro I.C., Portoghese P.S., Smith R.L., Waddell W.J., Expert Panel of the Flavor and Extract Manufacturers Association The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem Toxicol. 2005;43:1207–1240. doi: 10.1016/j.fct.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Scientific Committee for Food. Opinion on benzoic acid and its salts. Reports of the Scientific Committee for Food, Thirty-Fifth Series. CEC, Luxembourg: 1994. pp. 33–39. [Google Scholar]
- 37.JECFA. Evaluation of certain food additives and contaminants. Forty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives 1996. WHO Technical Report Series 868. Geneva: World Health Organisation; 1997. [PubMed] [Google Scholar]
- 38.Khadem S., Marles R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. Molecules. 2010;15:7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonsen J.L., Rosada C., Serakinci N., Justesen J., Stenderup K., Rattan S.I., Jensen T.G., Kassem M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 40.Müller M., Ausländer S., Ausländer D., Kemmer C., Fussenegger M. A novel reporter system for bacterial and mammalian cells based on the non-ribosomal peptide indigoidine. Metab. Eng. 2012;14:325–335. doi: 10.1016/j.ymben.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Hughes B. G., Lawson L. D. Nutritional content of cranberry products. Am. J. Hosp. Pharm. 1989;46:1129. [PubMed] [Google Scholar]
- 42.Williamson G., Clifford M.N. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br. J. Nutr. 2010;104(Suppl 3):S48–S66. doi: 10.1017/S0007114510003946. [DOI] [PubMed] [Google Scholar]
- 43.Aubel D., Morris R., Lennon B., Rimann M., Kaufmann H., Folcher M., Bailey J.E., Thompson C.J., Fussenegger M. Design of a novel mammalian screening system for the detection of bioavailable, non-cytotoxic streptogramin antibiotics. J. Antibiot. (Tokyo) 2001;54:44–55. doi: 10.7164/antibiotics.54.44. [DOI] [PubMed] [Google Scholar]
- 44.Fussenegger M., Mazur X., Bailey J.E. A novel cytostatic process enhances the productivity of Chinese hamster ovary cells. Biotechnol. Bioeng. 1997;55:927–939. doi: 10.1002/(SICI)1097-0290(19970920)55:6<927::AID-BIT10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Beisel C.L., Smolke C.D. Design principles for riboswitch function. PLoS Comput. Biol. 2009;5:e1000363. doi: 10.1371/journal.pcbi.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philp J.C., Ritchie R.J., Allan J.E. Synthetic biology, the bioeconomy, and a societal quandary. Trends Biotechnol. 2013;31:269–272. doi: 10.1016/j.tibtech.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Folcher M., Fussenegger M. Synthetic biology advancing clinical applications. Curr. Opin. Chem. Biol. 2012;16:345–354. doi: 10.1016/j.cbpa.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Ye H., Aubel D., Fussenegger M. Synthetic mammalian gene circuits for biomedical applications. Curr. Opin. Chem. Biol. 2013;17:910–917. doi: 10.1016/j.cbpa.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Weber W., Fussenegger M. Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 2012;13:21–35. doi: 10.1038/nrg3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ausländer S., Wieland M., Fussenegger M. Smart medication through combination of synthetic biology and cell microencapsulation. Metab. Eng. 2011;14:252–260. doi: 10.1016/j.ymben.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Hazan R., Levine A., Abeliovich H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2004;70:4449–4457. doi: 10.1128/AEM.70.8.4449-4457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller M., Hafner M., Sontag E., Davidsohn N., Subramanian S., Purnick P.E., Lauffenburger D., Weiss R. Modular design of artificial tissue homeostasis: robust control through synthetic cellular heterogeneity. PLoS Comput. Biol. 2012;8:e1002579. doi: 10.1371/journal.pcbi.1002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


