Background: CREPT/RPRD1B promotes tumor growth through up-regulating CYCLIN D1 expression.
Results: CREPT interacts with β-catenin in response to Wnt signaling and enhances the occupancy of β-catenin to promoters of Wnt-targeted genes, resulting in an up-regulation of expression.
Conclusion: CREPT functions as a co-activator for β-catenin·TCF4.
Significance: CREPT-regulated Wnt signaling implies an oncogenic role in promoting cell proliferation and invasion.
Keywords: β-Catenin (B-catenin), Cyclin D1, T-cell Factor (TCF), Transcription Coactivator, Wnt Pathway, CREPT, RPRD1B
Abstract
CREPT (cell cycle-related and expression elevated protein in tumor)/RPRD1B (regulation of nuclear pre-mRNA domain-containing protein 1B), highly expressed during tumorigenesis, was shown to enhance transcription of CCND1 and to promote cell proliferation by interacting with RNA polymerase II. However, which signaling pathway is involved in CREPT-mediated activation of gene transcription remains unclear. In this study, we reveal that CREPT participates in transcription of the Wnt/β-catenin signaling activated genes through the β-catenin and the TCF4 complex. Our results demonstrate that CREPT interacts with both β-catenin and TCF4, and enhances the association of β-catenin with TCF4, in response to Wnt stimulation. Furthermore, CREPT was shown to occupy at TCF4 binding sites (TBS) of the promoters of Wnt-targeted genes under Wnt stimulation. Interestingly, depletion of CREPT resulted in decreased occupancy of β-catenin on TBS, and over-expression of CREPT enhances the activity of the β-catenin·TCF4 complex to initiate transcription of Wnt target genes, which results in up-regulated cell proliferation and invasion. Our study suggests that CREPT acts as an activator to promote transcriptional activity of the β-catenin·TCF4 complex in response to Wnt signaling.
Introduction
CREPT2 (cell cycle-related and expression elevated protein in tumor) (Gene code 58490), also named RPRD1B or C20ORF77, is a recently identified gene localized to human chromosome 20, with a homolog named p15RS or RPRDB1A (1). A phylogenetic analysis revealed that yeast Rtt103 is an ortholog of the human CREPT and p15RS genes. In human, the CREPT gene contains 5 exons encoding a protein sequence of 326 amino acids, whereas the p15RS gene contains 7 exons encoding a sequence of 312 amino acids. Interestingly, whereas p15RS was reported to negatively regulate cell proliferation, migration, and invasion (2–4), we previously observed that CREPT was highly expressed in a variety of tumors and promoted cell proliferation (1). Recently, Jung et al. (5) found that CREPT, together with 7 other genes, was significantly elevated in SV40-immortalized cells, cancer cells, and NSCLC tissues. Wang et al. (6) reported that CREPT was significantly up-regulated in endometrial cancer tissues, promoted tumor growth, and accelerated cellular cell cycle, confirming our observations that CREPT is highly expressed in tumors and functions to promote tumorigenesis.
CREPT protein contains a RPR (regulation of nuclear pre-mRNA) domain (also named CTD-interacting domain, CID) and a coiled-coil terminus (CCT) domain. RPR was reported to be critical for the interaction with the C-terminal domain of Rbp1, the largest subunit of RNA polymerase II (RNAP II), which contains 26 (yeast) or 52 (mammals) heptapeptide repeats of a consensus sequence (Y1S2P3T4S5P6S7) (7). The phosphorylated status of CTD at Ser-2, Ser-5, as well as Ser-7 was present during the initiation, elongation, and termination of gene transcription (8–10). Recently, Ni et al. (11) reported that CREPT/RPRD1B, and also p15RS/RPRD1A and RPRD2, were co-purified with RNAP II. It appeared that CREPT/RPRD1B and p15RS/RPRD1A preferentially bound to the phosphorylated CTD of RNAP II, leading to decreased Ser-5 and Ser-7 phosphorylation of RNAP II at target gene promoters (11). Our previous study demonstrated that CREPT interacted with RNAP II via its RPR domain (1). Recently, we observed that both CREPT and p15RS, in a dimerized form, associate with Ser(P)-2 CTD of RNAP II (12). Intriguingly, our previous observations showed that CREPT bound to both the promoter region and the region before the Poly(A) signal in the termination region of CCND1 gene (1). This pattern was quite different from the binding behavior of its ortholog Rtt103, which bound only to the termination region after Poly(A) in several yeast genes (13). Therefore, we proposed a model where CREPT promotes chromatin loop formation, which seemed to facilitate the recycling of RNAP II in mammalian cells (1, 14).
Our previous study revealed that CREPT participated in the regulation of the transcription of CCND1, which encodes the Cyclin D1 protein (1). Cyclin D1 is a critical regulator for the cell cycle and is regulated by multiple signaling pathways including Wnt/β-catenin, STAT3, and NF-κB (15–18). Many studies demonstrate that several transcriptional factors individually bind to specific sites in the CCND1 promoter and activate gene transcription in response to different stimuli (15). In particular, TCF4, a critical factor in response to the Wnt/β-catenin signal, proved to bind to the promoter of the CCND1 gene (16).
TCF4 initiates target gene transcription through an interaction with β-catenin, which accumulates and translocates from the cytoplasm into the nucleus after Wnt stimulation (19, 20). TCF4 directly binds to DNA via a high mobility group domain (21). In the absence of Wnt stimulation, TCF4 is repressed by transcriptional suppressors including Groucho (22, 23) and HDAC (24). In the presence of Wnt, nuclear β-catenin replaces the suppressors and associates with TCF4 to recruit transcriptional co-activators such as Brg1, CBP/p300, Bcl9, and Pygopus, which initiates the transcription of Wnt-targeted genes including CCND1 and c-MYC (20, 25). By up-regulation of downstream gene expression, the Wnt/β-catenin signaling pathway plays a critical role in embryonic development, tissue homoeostasis, multipotential stem cells maintenance, and tumorigenesis (19, 20). In this study, we provide evidence that CREPT functions as a co-activator of the β-catenin·TCF4 complex to enhance the transcriptional activity of Wnt-targeted genes.
EXPERIMENTAL PROCEDURES
Plasmids and Reagents
Myc-CREPT, Myc-CREPT/RPR, Myc-CREPT/CCT, Wnt1, FLAG-Dvl2, FLAG-β-catenin, HA-TCF4, and LEF-l-luciferase constructs were constructed in this laboratory. SuperTop-luciferase reporter was a gift from Dr. Wei Wu (School of Life Science, Tsinghua University, China). Anti-β-catenin (E-5), anti-cyclin D1 (A-12), anti-Myc (9E10), anti-HA (F-7), and anti-GFP (FL) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, TX). Anti-c-Myc (9402) and anti-TCF4 (2953) antibodies were purchased from Cell Signaling Technology, Inc. Anti-β-Actin (AC-15) and anti-FLAG (M2) antibodies were from Sigma. Anti-β-catenin (610154) antibody used in ChIP assay was purchased from BD Transduction Laboratories (San Jose, CA). Anti-CREPT antibody was prepared by this laboratory. G418 (A1720) was from Sigma. Adenovirus over-expressing CREPT or GFP was from our laboratory. Wnt3a-conditioned medium (CM) was prepared according to ATCC (CRL-2647). siRNAs were synthesized from GenePharma (Shanghai GenePharma Co. Ltd., China) with the oligo sequence information as: si-CREPT #1, GCAAGAACGAAGUGUUAUTT; si-CREPT #2, GUCUGUUACUAGCAGAAUATT; negative control, UUCUCCGAAGUCACGUTT. The oligos were transfected into cells at a 20 nm final concentration using Lipofectamine® RNAi-MAX Reagent (Invitrogen, 13778). Kits of SuperReal PreMix Plus (SYBR Green) used for real-time PCR were purchased from Tiangen (Beijing, China). Human recombinant Wnt1 (catalog number 120-17) was from PEPROTECH Co. (Rocky Hill, NJ).
Cell Culture
HEK293T, NIH3T3, and HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS. The cells secreting active Wnt3a (ATCC: CRL-2647) and control L cells (ATCC: CRL-2648) were kindly provided by Dr. Xi He (Harvard Medical School, Boston, MA) and cultured with DMEM containing of 10% FBS. All the above cells were kept at 37 °C in a 5% CO2-containing atmosphere. SW480 cells were cultured in L-15 medium with 10% FBS and kept with 100% air at 37 °C. Medium and serum were purchased from Invitrogen.
Immunoprecipitation and Western Blotting
For interaction assays, FLAG-β-catenin or HA-TCF4 was co-transfected with Myc-CREPT into HEK293T cells. After 24 h of transfection, cells were lysed in cell lysis buffer (50 mm Tris-Cl, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1% SDS, pH 8.0) with protease inhibitors to prepare whole cell lysates. For endogenous interaction assays, the nuclear fraction of SW480 cells was incubated with the appropriate antibodies at 4 °C for 4 h, followed by the addition of protein G-agarose beads to pellet the immune complexes for 2 h. 5% of cell lysates and immunoprecipitants were analyzed by immunoblotting for the indicated proteins.
Immunofluorescence Staining
HeLa cells were seeded on coverslips. After starvation overnight, cells were treated with Wnt3a-conditioned or control medium for 4 h. The cells were fixed with 4% paraformaldehyde for 20 min and perforated with 0.3% Triton X-100 for 10 min. After blocking with 10% FBS for 50 min, cells were incubated with the indicated antibodies overnight at 4 °C, followed with incubation with the secondary antibodies conjugated with FITC (green) or TRITC antibody (Jackson Research Laboratories) for 1 h. Stained cells were visualized using a confocal laser scanning microscope (OLYMPUS FV10i-Oil) with co-localization of the two proteins indicated by a merged image.
Luciferase Assay
HEK293T or HeLa cells were transiently transfected with the indicated plasmids using Vigofect (Vigofect Inc., Beijing, China) according to the manufacturer's instructions. Briefly, 0.1 μg of reporter plasmids LEF-1-luc or superTop-luc together with 5 ng of an internal control plasmid pRL-TK were transfected into the cells cultured in a 24-well plate. For the expression of Wnt1, Dvl2, β-catenin, and TCF4, the indicated amounts of plasmids were co-transfected into the cells. An empty plasmid was used to balance the total amount of DNA. Luciferase activity was assayed after 24 h of transfection using a Dual Luciferase reporter assay system (Vigofect Inc.). The luciferase activity was normalized by firefly against Renilla luciferase activity and presented as mean ± S.D.
Nuclear and Cytoplasmic Protein Extraction
HEK293T cells were transfected with the indicated plasmids. After 24 h of transfection, cell pellets were re-suspended in 600 μl of ice-cold Buffer I (10 mm HEPES, 1.5 mm MgCl2, 10 mm KCl, and protease inhibitors, pH 8.0) and incubated on ice for 15 min. Nonidet P-40 (10%) was then added to a final concentration of 1%. After a 10-s vortex, the samples were centrifuged at top speed for 2–3 min. The supernatants were collected as the cytoplasmic fraction. The nuclear pellets were re-suspended in 220 μl of ice-cold Buffer II (20 mm HEPES, 1.5 mm MgCl2, 420 mm NaCl, 0.2 mm EDTA, 25% glycerol, and protease inhibitors, pH 8.0) and rotated vigorously at 4 °C for 30 min. Samples were centrifuged at 4 °C at top speed for 10 min, with the supernatant being the nuclear fraction.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed according to the Millipore Kit protocol with minor modification. Briefly, cells were fixed at 37 °C for 10 min with 1% formaldehyde for cross-linking and with 125 mm glycine for 5 min. The cells were re-suspended in 500 μl of ChIP SDS lysis buffer and mixed at 0 °C for 10 min and then sonicated for 10-s pulsed with a 30-s interval at 30% output power to yield DNA fragments of 200 to 1000 bp in size. The lysates containing of protein·DNA complexes were diluted 10-fold and immunoprecipitated with the indicated antibodies. After washing 5 times, the complexes were reversely cross-linked. The eluted DNAs were recovered with QIAquick columns (Qiagen) and used as templates for PCR or real-time PCR analyses. The input control was 1% of the supernatant before precipitation. The fragment corresponding to the TCF4 binding site in the CCND1 promoter was amplified by PCR using the primers: CACCTCCACCTCACCCCCTAAATCC and ACTCCCCTGTAGTCCGTGTGACGTT. c-MYC promoter was amplified with TTGCTGGGTTATTTTAATCAT and ACTGTTTGACAAACCGCATCC (26). Primers used for the CCND1 gene were reported previously (1).
Reverse Transcriptase-PCR (RT-PCR) and Quantitative PCR
Total cellular RNA was prepared from cells using TRIzol reagent (Invitrogen). 2 μg of total mRNA was reverse transcribed using a Quantscript RT Kit (TIANGEN Biotech, Beijing, China). Real-time PCR was performed using SuperReal PreMix Plus (SYBR Green). Primers used for real-time PCR analyses for the human c-MYC gene were: 5′-TGGTCGCCCTCCTATGTTG-3′ and 5′-CCGGGTCGCAGATGAAACTC-3′; for mouse c-Myc, 5′-ATGCCCCTCAACGTGAACTTC-3′ and 5′-CGCAACATAGGATGGAGAGCA-3′; for the human CCND1 gene, 5′-CCGAGAAGCTGTGCATCTACAC-3′ and 5′-AGGTTCCACTTGAGCTTGTTCAC-3′; for mouse Ccnd1 gene, 5′-GCGTACCCTGACACCAATCTC-3′ and 5′-CTCCTCTTCGCACTTCTGCTC-3′; for human CREPT gene, 5′-CACGCGGGACCCATCGTCTC-3′ and 5′-AGCCTTCATCTGCCTCTCTGGCA-3′; for human β-Actin gene, 5′-TCGTCGACAACGGCTCCGGCATGT-3′ and 5′-CCAGCCAGGTCCAGACGCAGGAT-3′; for mouse β-actin, 5′-GTCCCTCACCCTCCCAAAAG-3′ and 5′-GCTGCCTCAACACCTCAACCC-3′. Gene expression levels were presented as relative values. All the experiments were performed in triplicate.
Cell Proliferation
Cell proliferation was measured through a MTT assay. HeLa cells were transfected with a mixture of two siRNAs against CREPT or a non-target siRNA as a negative control. After 24 h transfection, cells were re-seeded and transfected with the Wnt1 plasmid. The cells were digested and seeded into a 96-well plate at a density of 1000 cells/well. When cells were cultured for the indicated times, MTT was added. Cells were incubated for another 4 h before measurement. The MTT-produced formazan was dissolved in 150 μl of dimethyl sulfoxide and the absorbance at 530 nm was measured with a reference filter of 630 nm using a spectrophotometer (model 680, Bio-Rad). Three independent experiments were performed.
Clone Formation Assay
HeLa cells with depletion of CREPT were transfected with the Wnt1 plasmid. After 24 h of transfection, the cells were re-seeded into a 6-well plate at a density of 1000 cells/well. After being cultured for 2 weeks, the cells were fixed with methanol for 10 min, followed with staining using 0.1% crystal violet for 10 min. Cell clones were counted and presented as mean ± S.D. from three individual experiments.
Cell Invasion Assay
Transwell assays were performed according to a previous report (27). The in vitro invasive abilities of cells were evaluated using a transwell chamber (Millipore Corp., Bedford, MA) coated with 100 μl of Matrigel (BD Biosciences, 1:10 diluted). A total of 5 × 104 cells in 250 μl of serum-free medium containing of 100 ng/ml of human recombinant Wnt1 were introduced into the upper chamber, and 700 μl of medium with 20% FBS was introduced into the lower chamber. Cells were allowed to invade the Matrigel for 24 h. The invaded cells were fixed by methanol and stained with 0.1% crystal violet (Sigma). The number of invaded cells was counted under a phase-contrast microscope. Cells in five different fields of each well were averaged.
Statistical Analysis
All experiments were repeated at least 3 times. Data were presented as mean ± S.D. Significant differences between groups were determined using the Student's t test.
RESULTS
CREPT Enhances the Transcriptional Activity of Wnt/β-Catenin Pathway
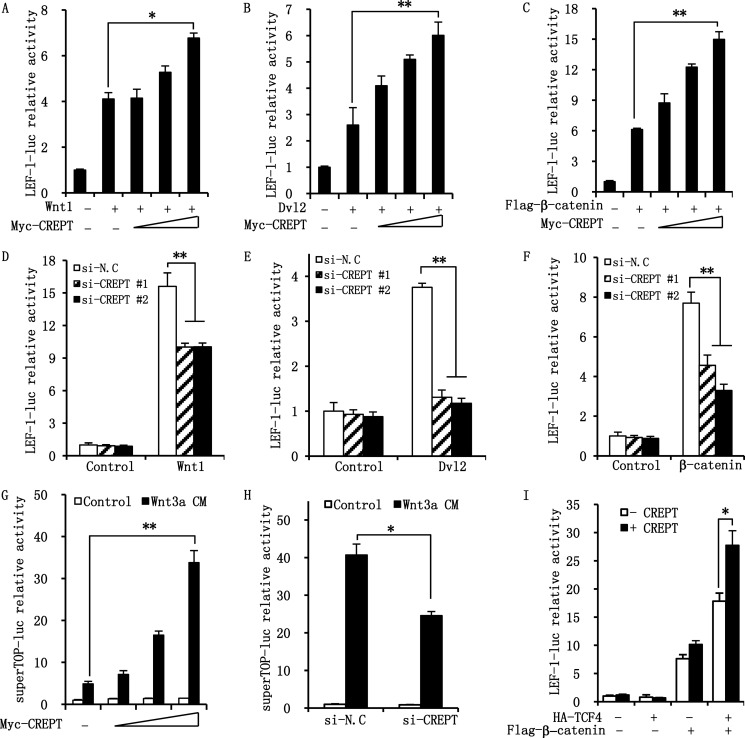
Based on our previous study that CREPT enhances the expression of CCND1 (1), a direct target gene of Wnt/β-catenin signaling, we questioned whether CREPT participates in the regulation of Wnt/β-catenin signaling. To this end, we investigated the effect of CREPT on the Wnt/β-catenin transcriptional activity by a luciferase reporter assay. LEF-1-luciferase, a Wnt-responding reporter, was co-expressed in the presence of Wnt1, Dvl2, or β-catenin in HEK293T cells. The results show that although Wnt1 activated the reporter activity, over-expression of CREPT significantly elevated the effect of Wnt1 stimulation in a dose-dependent manner (Fig. 1A). In parallel, over-expression of CREPT dramatically enhanced the activities stimulated by over-expressed Dvl2 (Fig. 1B) and β-catenin (Fig. 1C). To further confirm the role of endogenous CREPT on Wnt/β-catenin-mediated gene transcription, we performed a luciferase assay in HeLa cells using two specific siRNAs against CREPT. The results indicate that depletion of endogenous CREPT impairs the activation of luciferase in response to Wnt1 stimulation (Fig. 1D) or under over-expression of Dvl2 (Fig. 1E) or β-catenin (Fig. 1F). To confirm these results, we performed a luciferase assay using another Wnt-response reporter, superTOP-luciferase, stimulated by Wnt3a CM. The results show that over-expression of CREPT significantly increased Wnt3a-mediated activation of the reporter (Fig. 1G), whereas depletion of endogenous CREPT significantly impairs activity (Fig. 1H). Taken together, these results indicate that CREPT promotes the transcriptional activity of the Wnt/β-catenin pathway.
FIGURE 1.
CREPT enhances the transcriptional activity of the Wnt/β-catenin pathway. A–C, over-expression of CREPT promotes LEF-1-luciferase activity activated by Wnt1 (A), Dvl2 (B), and β-catenin (C) in a dose-dependent manner. LEF-1-luciferase reporter and pRL-TK plasmids were co-transfected into HEK293T cells with Wnt1 (A), Dvl2 (B), and β-catenin (C) plasmids as well as different dosages of the Myc-CREPT plasmid. The activity was expressed as fold-changes, normalized by an internal control (Renilla). Results were from three independent repeats and are presented as mean ± S.D. *, p < 0.05. D–F, depletion of CREPT resulted in a decreased transcriptional activity of Wnt/β-catenin pathway. HeLa cells were transfected with siRNAs against CREPT (si-CREPT #1 and #2). The cells were re-seeded and further transfected with Wnt1 (D), Dvl2 (E), and β-catenin (F). The luciferase activity was examined as in A and B. A nonspecific siRNA (si-N.C) was used as a control. **, p < 0.01. G, over-expression of CREPT enhances Wnt3a-mediated Wnt signaling. Super-TOP and pRT-TK reporter were transfected into HEK293T cells with dosages of Myc-CREPT. Before harvest, the cells were treated with Wnt3a or control medium for overnight and the luciferase activity were detected. **, p < 0.01. H, depletion of CREPT inhibits Wnt3a-mediated Wnt signaling. HEK293T cells were transfected with two siRNAs against CREPT (si-CREPT #1 and #2) and super-TOP reporter. After transfection and treated with Wnt3a CM overnight, the luciferase activity were examined. *, p < 0.05. I, CREPT accelerates the β-catenin·TCF4 transcriptional activity. HEK293T cells were co-transfected with LEF-1-luc, pRL-TK, and the indicated plasmids. After 24 h for transfection, the luciferase activity was examined. Each experiment was performed in triplicate. The relative luciferase activity is showed as mean ± S.D. *, p < 0.05.
Although over-expression of TCF4 showed no activity on the transcription, over-expression of CREPT still retained basal luciferase activity (Fig. 1I). We reasoned that TCF4 per se could not activate gene transcription, as reported by others (28). Intriguingly, when β-catenin is co-expressed with TCF4, the luciferase activity was dramatically boosted and CREPT further increased the transcriptional activity (Fig. 1I, last column), suggesting that CREPT promotes transcriptional activity of the β-catenin·TCF4 complex.
CREPT Interacts with β-Catenin and TCF4
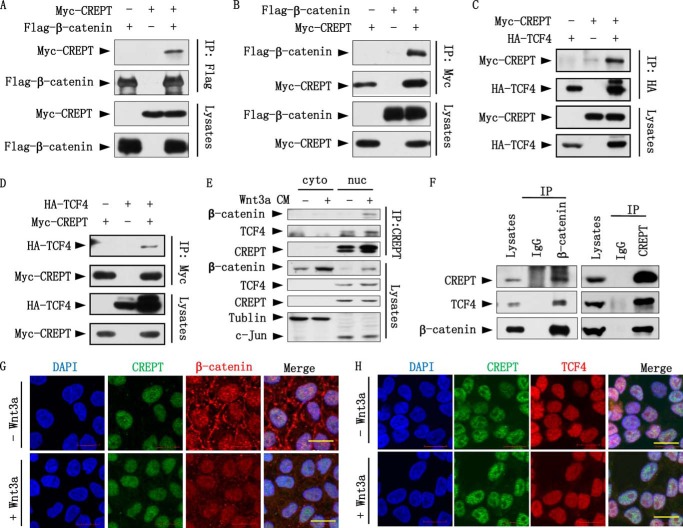
To reveal the mechanism by which CREPT promotes the transcriptional activity of Wnt/β-catenin signaling, we examined whether CREPT interacts with nuclear components of the Wnt/β-catenin signaling pathway, because CREPT is localized to the nucleus (1). Reciprocal immunoprecipitation experiments indicated that Myc-CREPT and FLAG-β-catenin interact with each other in HEK293T cells, as precipitated by either an anti-FLAG (Fig. 2A) or an anti-Myc (Fig. 2B) antibody. These results suggest that CREPT forms a complex with β-catenin. Because β-catenin interacts with TCF4 in the activation of Wnt signaling, we questioned whether CREPT is associated with TCF4. An immunoprecipitation experiment demonstrated that HA-TCF4 precipitated down Myc-CREPT (Fig. 2C). Reciprocally, we observed that Myc-CREPT precipitated down HA-TCF4 (Fig. 2D). These results suggested that CREPT also associates with TCF4.
FIGURE 2.
CREPT interacts with β-catenin and TCF4. A and B, Myc-CREPT interacts with FLAG-β-catenin. Myc-tagged CREPT (Myc-CREPT) and FLAG-tagged β-catenin (Flag-β-catenin) co-expressed in HEK293T cells. Cell lyses were IP by an anti-FLAG antibody (A) or an anti-Myc antibody (B). The immunoprecipitates were subjected to Western blots using the indicated antibodies. C and D, Myc-CREPT interacts with HA-TCF4. HA-tagged TCF4 was co-expressed with Myc-CREPT in HEK293T cells. The IP assay was performed using an anti-HA (C) or anti-Myc antibody (D). E, endogenous CREPT interacts with β-catenin and TCF4 in nucleus. After treated with Wnt3a CM for 4 h, the cytoplasmic and nuclear fraction of HeLa cells were extracted and incubated with anti-CREPT antibody for IP experiment. F, endogenous CREPT interacts with β-catenin and TCF4. Nuclear fraction of SW480 cells was incubated with anti-β-catenin antibody (left panel) or anti-CREPT antibody (right panel) for the IP experiments. Mouse IgG was used as a negative control. The immunoprecipitates were analyzed by Western blotting. G, CREPT co-localizes with β-catenin upon Wnt3a treatment. HeLa cells were treated with Wnt3a CM or a control medium for 4 h, followed with immunostaining using an anti-CREPT antibody (rabbit original) and an anti-β-catenin antibody (mouse original). Cells were incubated with an anti-rabbit IgG conjugated with FITC and an anti-mouse IgG conjugated with TRITC. H, CREPT co-localizes with TCF4. After treatment with Wnt3a CM for 4 h, HeLa cells were immnuostained with mouse anti-CREPT antibody and rabbit anti-TCF4 antibody. Cells were then incubated with anti-mouse IgG conjugated with FITC and an anti-rabbit IgG conjugated with TRITC. Scale bar represents 20 μm.
To determine whether endogenous CREPT interacts with β-catenin and TCF4, we performed an immunoprecipitation experiment using both the cytoplasmic and nuclear components of HeLa cells treated with Wnt3a CM. The results show that CREPT interacts with both β-catenin and TCF4 in the nucleus (Fig. 2E). In another IP experiment using the nuclear extract from SW480 cells, where β-catenin is constitutively activated and localized into the nucleus due to the APC mutation (29), we observed that an antibody against β-catenin precipitated both CREPT and TCF4 (Fig. 2F, left panel) and an antibody against CREPT precipitated both β-catenin and TCF4 (Fig. 2F, right panel), suggesting that CREPT associates with β-catenin and/or TCF4 in the nucleus. Taken together, all of these results indicate that CREPT interacts with β-catenin and TCF4 in vivo.
Furthermore, we performed an immunostaining assay to investigate the co-localization of CREPT with β-catenin or TCF4. The results show that β-catenin co-localized in the nucleus with CREPT after treatment with Wnt3a CM for 4 h in HeLa cells (Fig. 2G). However, it appeared that CREPT always co-localizes with TCF4 in the nucleus (Fig. 2H). The results indicate that CREPT interacts with β-catenin or TCF4 in the nucleus.
The RPR Domain of CREPT Is Responsible for Its Interaction with TCF4
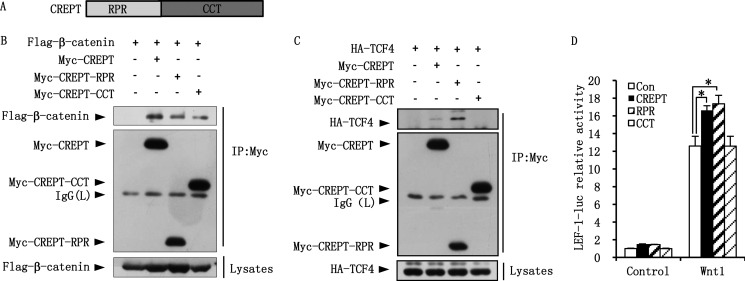
CREPT consists of the RPR and CCT domains (Fig. 3A). To map the region of CREPT responsible for the interaction with β-catenin and TCF4, we performed an immunoprecipitation assay using different domains of CREPT. The results show that both RPR and CCT domains interact with FLAG-β-catenin, with an interaction that is slightly weaker than that of full-length CREPT (Fig. 3B). On the other hand, the RPR domain shows a strong interaction with HA-TCF4 but the CCT domain fails to interact with HA-TCF4 (Fig. 3C). Interestingly, interaction of the RPR domain with TCF4 appears much stronger than that of the full-length CREPT protein (Fig. 3C, compare lanes 3 with 2). The role of the different domains of CREPT on the β-catenin·TCF4 complex was further revealed by a luciferase reporter experiment. The results indicate that the RPR domain maintained the role of full-length CREPT in enhancing the luciferase activity induced by Wnt1. However, the CCT domain had no effect on the luciferase activity (Fig. 3D). This result, together with the IP results, suggests that the interaction of CREPT with TCF4 via the RPR domain is critical for promoting the transcription activity of the β-catenin·TCF4 complex.
FIGURE 3.
The RPR domain of CREPT is responsible for TCF4. A, a graphic representation of the CREPT protein structure. CREPT consisted of the RPR and CCT domains. B, both RPR and CCT domains interact with β-catenin. FLAG-β-catenin and Myc-CREPT full-length and RPR and CCT domains were co-expressed in HEK293T cells. The cell lysates were incubated with anti-Myc antibody for IP assay. C, the RPR domain of CREPT interacts with HA-TCF4. HA-TCF4 and Myc-CREPT full-length and RPR and CCT domains were co-expressed in HEK293T cells. Cell lysates were incubated with anti-Myc antibody for IP experiment. D, the RPR domain is critical for promoting the Wnt signaling of CREPT. LEF-1-luciferase and pRL-TK reporters were co-expressed with Myc-CREPT full-length, RPR and CCT domains as well as Wnt1. Luciferase activity was detected after 24 h transfection.
CREPT Enhances the Formation of β-Catenin·TCF4 Complex
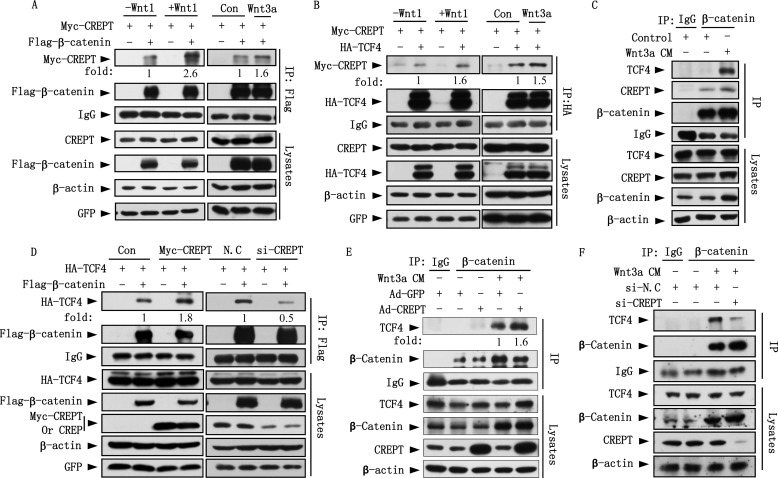
We questioned whether the interaction of CREPT with β-catenin or TCF4 is regulated by Wnt stimulation. For this aim, Myc-CREPT was co-expressed with FLAG-β-catenin or HA-TCF4 in HEK293T cells in the presence of over-expressed Wnt1 or with the addition of Wnt3a CM. An immunoprecipitation experiment demonstrated that the interaction of Myc-CREPT and FLAG-β-catenin was increased upon Wnt1 and Wnt3a stimulations (Fig. 4A). Similarly, Wnt treatments also enhanced the interaction of Myc-CREPT with HA-TCF4 (Fig. 4B). Furthermore, we show that Wnt3a treatment enhances the interaction of endogenous CREPT or TCF4 with β-catenin (Fig. 4C). These results suggest that Wnt stimulation enhances the interaction of CREPT with β-catenin and TCF4.
FIGURE 4.
CREPT is required for β-catenin·TCF4 complex formation. A, Wnt stimuli enhance the interaction of CREPT and β-catenin. Myc-CREPT and FLAG-β-catenin were co-expressed in HEK293T cells together with or without Wnt1 or Wnt3a. The IP experiments were performed using an anti-FLAG antibody. The complex of CREPT and β-catenin was quantitated by the band density and presented as fold-change. B, the interaction of CREPT and TCF4 increased upon Wnt stimulation. Myc-CREPT and HA-TCF4 were co-expressed in HEK293T cells in the presence of Wnt1 or Wnt3a. The IP experiments were performed using an anti-HA antibody. C, Wnt3a enhances the interaction of endogenous CREPT or TCF4 with β-catenin. HEK293T cells were starved with serum-free medium overnight and then cultured by feeding with Wnt3a CM or control medium for 4 h. Extracted nucleus proteins were used for IP experiment using an anti-β-catenin antibody. D, CREPT enhances the interaction of β-catenin and TCF4. HA-TCF4 and FLAG-β-catenin were co-expressed with Myc-CREPT (left panel) or siRNAs against endogenous CREPT (right panel) in HEK293T cells. The cell lysates were subjected to IP with an anti-FLAG antibody. E, over-expression of CREPT enhances the interaction of endogenous β-catenin and TCF4. HeLa cells were infected with Ad-CREPT or Ad-GFP for 24 h. The nuclear fractions were incubated with an anti-β-catenin antibody for the IP experiment. IgG was used as a negative control. F, depletion of CREPT attenuates interaction of endogenous β-catenin and TCF4. The nuclear fractions of HeLa cells depleted of CREPT were subjected to an IP experiment with anti-β-catenin antibody.
To address whether CREPT regulates complex formation of β-catenin and TCF4 upon Wnt stimulation, we examined the interaction of β-catenin and TCF4 under over-expression or depletion of CREPT. Immunoprecipitation results show that the interaction of FLAG-β-catenin with HA-TCF4 increased when Myc-CREPT was co-expressed (Fig. 4D, left panel), and was impaired when CREPT was depleted (Fig. 4D, right panel). Furthermore, an immunoprecipitation experiment using endogenous proteins demonstrated that the interaction of β-catenin and TCF4 was significantly increased by over-expression of CREPT (Fig. 4E) and decreased by depletion of CREPT (Fig. 4F). Taken together, these results suggest that CREPT enhances the formation of the β-catenin·TCF4 complex.
CREPT Affects the Occupancy of β-Catenin to Gene Promoters
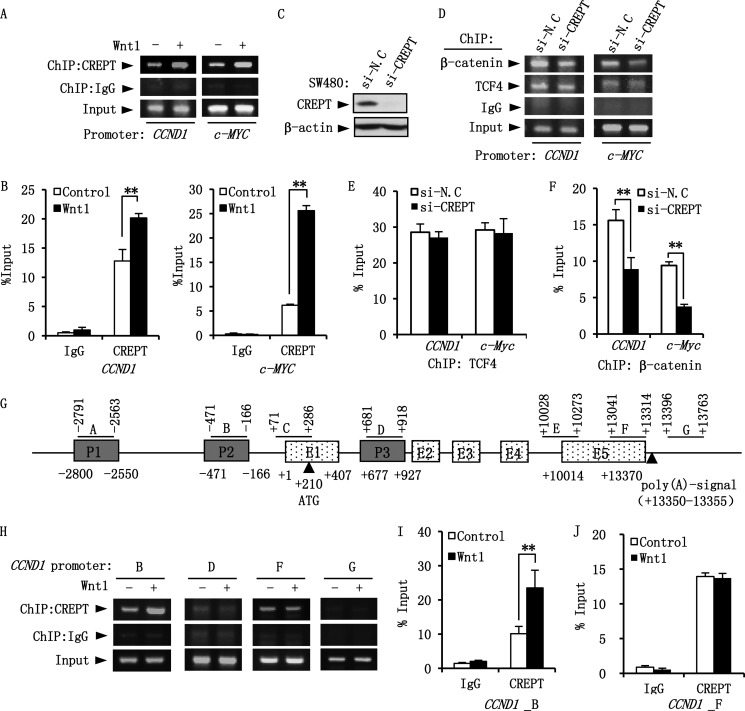
To address whether CREPT enhances the formation of the β-catenin·TCF4 complex at the promoters of the Wnt-targeted genes, we performed ChIP assays using a TCF4 binding sequence (TBS) from CCND1 (30) and c-MYC (31) promoters. The results show that CREPT localizes to the TBS in both CCND1 and c-MYC promoters and Wnt stimulation enhances occupancy (Fig. 5, A and B). Interestingly, we observed that depletion of CREPT (Fig. 5C) impairs the occupancy of β-catenin to the promoters (Fig. 5D). However, depletion of CREPT had no effect on the binding of TCF4 to the promoters (Fig. 5, D and E), but significantly decreased the occupancy of β-catenin (Fig. 5F). All of these data suggest that CREPT stabilizes the occupancy of β-catenin to the promoters of targeted genes.
FIGURE 5.
CREPT is critical for the DNA binding ability of β-catenin·TCF4 complex. A, Wnt enhances the occupancy of CREPT on the TBS of CCND1 and c-MYC promoters. HEK293T cells were transfected with the Wnt1 plasmid and starved overnight before harvest. The cells were cross-linked and subjected to a ChIP assay using an anti-CREPT antibody. The co-immunoprecipitated DNA was examined by a PCR using specific primers for TBS. B, relative amounts of CREPT on CCND1 and c-MYC promoters. The amount of ChIPed DNA from A were quantitated by real-time PCR and presented as percent of input. C, depletion of CREPT by siRNA. A mixture of two siRNAs against CREPT (si-CREPT) was transfected into SW480 cell. Western blot shows the effect of the siRNA in regulation of CREPT expression. A nonspecific siRNA (si-N.C) was used as a control. D, depletion of CREPT attenuates the binding of β-catenin to TBSs. SW480 cells were transfected with a mixture of two siRNAs against CREPT (si-CREPT) or a negative control (si-N.C) for 48 h. ChIP assay was performed using an anti-TCF4 and anti-β-catenin antibody. The ChIPed DNA was examined by PCRs using primers for TBS of CCND1 and c-MYC gene. E and F, quantification of TCF4 and β-catenin binding on the CCND1 and c-MYC promoters. The quantity of ChIPed DNA in D was examined through real-time PCR and presented as the fold-changes of TCF4 (E) or β-catenin (F) binding on the promoters based on the input. G, a graphic representation of the CCND1 genomic structure, modified from Ref. 1. Fragments detected by PCR are shown as A to G. H, Wnt enhances the presence of CREPT at the promoter region, but not termination region of CCND1 gene. ChIP assay was performed as in A and the complex was examined by PCRs using primers showed as B, D, F, and G. I and J, relative amounts of CREPT on the CCND1 gene. The graph is a quantification of ChIPed DNA from H examined by real-time PCR. *, p < 0.05.
Our previous study showed that CREPT is present at both the promoter and termination regions of the CCND1 gene (1). To reveal whether Wnt promotes the presence of CREPT to different regions of the gene, we performed a ChIP assay using different pairs of primers along the gene (Fig. 5G). The results show that whereas CREPT indeed associates with the promoter (marked B) and the termination region (marked F) (Fig. 5H), Wnt1 significantly enhances the presence of CREPT at the promoter region (Fig. 5I) but not at the termination region (Fig. 5J). All of these results suggest that Wnt promotes the association of CREPT to the promoters of targeted genes.
CREPT Promotes Cell Proliferation and Invasion
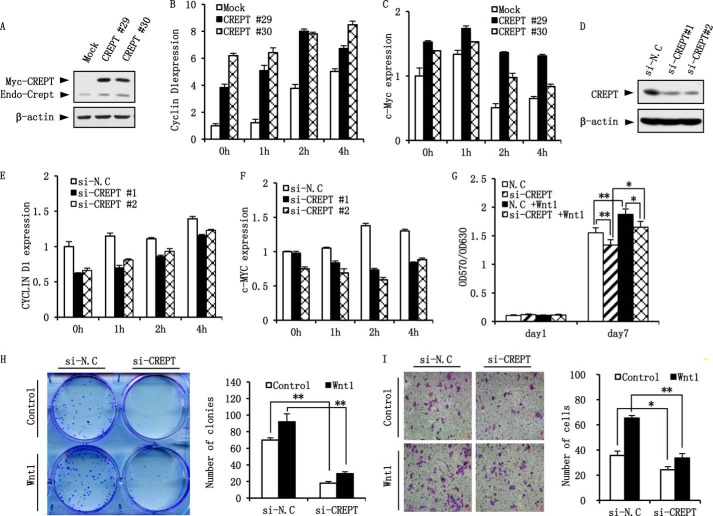
To examine the role of CREPT on Wnt target gene expression, we stably over-expressed CREPT in NIH3T3 cells where the endogenous CREPT level was relatively low (Fig. 6A). After stimulation with Wnt3a-conditioned media for different times, total RNA was extracted and subjected into a real-time PCR analysis. The results show that mRNA levels of Cyclin D1 (Fig. 6B) and c-Myc (Fig. 6C) were increased after treatment with Wnt3a in NIH3T3 mock cells, and over-expression of CREPT elevated the levels dramatically. In contrast, depletion of endogenous CREPT in HeLa cells (Fig. 6D) resulted in decreased expression of CYCLIN D1 (Fig. 6E) and c-MYC (Fig. 6F) in both Wnt3a-treated and untreated cells.
FIGURE 6.
CREPT enhances cell proliferation and invasion. A, establishment of stable cell lines over-expressing CREPT. Two clones of NIH3T3 cells with over-expressed Myc-CREPT (CREPT #29, #30) were selected. The protein level of CREPT was detected by Western blot. B and C, over-expression of CREPT promotes Cyclin D1 and c-Myc expression. NIH3T3 cells over-expressing Myc-CREPT cells and mock cells were starved for overnight and treated with Wnt3a CM or control medium for indicated times before harvest. The expression of Cyclin D1 (B) and c-Myc (C) were examined by real-time PCR. D, depletion of CREPT by siRNA. Two siRNAs against CREPT (#1, #2) were transfected into HeLa cells. Western blot shows the effect of the siRNAs in regulation of CREPT expression. A nonspecific siRNA (si-N.C) was used as a control. E and F, depletion of CREPT results in a decreased expression of CYCLIN D1 and c-MYC. HeLa cells were transfected with two siRNAs against CREPT (si-CREPT #1, #2) and a nonspecific siRNA (si-N.C) as a negative control. Cells were starved for overnight and treated with Wnt3a CM or control medium for the indicated times. The mRNA level of CYCLIN D1 (E) and c-MYC (F) in the cells were examined by real-time PCR. G, depletion of CREPT inhibits cell proliferation. HeLa cells transfected with a mixture of two siRNAs against CREPT (si-CREPT) and a nonspecific siRNA (si-N.C) were seeded into 96-well plate at a density of 1000 cells/well. MTT assay was used to measure cell proliferation. *, p < 0.05; **, p < 0.01. H, depletion of CREPT inhibits cell clone formation. A clone formation assay was performed using HeLa cells depleted CREPT. The cells were transfected with the Wnt1 plasmid and re-seeded into a 6-well plate at a density of 1000 cells/well. The cell clones were photographed (left panel). The clone numbers were presented as mean ± S.D. from three individual experiments (right panel). **, p < 0.01. I, knocking down CREPT inhibits the cell invasion. A transwell assay was performed with HeLa cells. 5 × 104 cells in serum-free medium containing of 100 ng/ml of Wnt1 were added into upper chamber. 20% FBS medium was added into lower chamber. After a 24 h culture, invaded cells were fixed with methanol and stained with 0.1% crystal violet. A representative picture was showed in the left panel (scale bar, 100 um). The invaded cells from five fields were counted and the statistical result was shown in the right panel. *, p < 0.05; **, p < 0.01.
To probe the physiological function of CREPT, we performed a MTT assay using HeLa cells. The results show that the cells with the depletion of CREPT grew more slowly than mock-si cells. Although Wnt treatment accelerates cell proliferation, cells with depletion of CREPT show slower growth than mock-si cells (Fig. 6G). To confirm the result, we performed a clone formation experiment. The results show that depletion of CREPT led to fewer cell clones (Fig. 6H), consistent with the results from the MTT assay. These results indicate that CREPT positively regulates cell proliferation, especially under Wnt stimulation.
Finally, we investigated the function of CREPT on cell invasion. HeLa cells with depletion of CREPT were cultured in a chamber coated with Matrigel for 24 h undergoing Wnt1 treatment or not. These results show that depletion of CREPT resulted in a decreasing number of infiltrated cells. When cells were treated with Wnt1, a similar result was obtained (Fig. 6I). Taken together, all these results demonstrate that CREPT promotes Wnt signaling, which results in accelerated cell proliferation and invasion.
DISCUSSION
Cyclin D1 controls the G1/S transition of the cell cycle and is tightly regulated by activators or repressors, which together keep cells growing normally. Amplification or over-expression of Cyclin D1 has been observed in the development of cancers, caused by dysfunction or abnormal expression of many regulators (32). In our previous study, we identified that CREPT, a novel oncoprotein, promoted transcription of Cyclin D1 and was highly expressed in several cancers (1). We, and others, have found that CREPT is associated with RNAP II (1, 11, 12) and that CREPT promotes the formation of a chromatin loop during the transcription of Cyclin D1 (1). However, which signaling pathway was involved in the process of CREPT-regulated transcription remains unknown. In this study, we found that the Wnt/β-catenin pathway regulates CCND1 or c-Myc transcription through an interaction of β-catenin with CREPT. It turned out that CREPT stabilizes a β-catenin·TCF4 complex and enhances their binding ability to the promoters of these genes. We propose that CREPT acts as a co-activator to promote the Wnt/β-catenin pathway.
In our previous study, we observed that CREPT was present at both the promoter and terminator regions to mediate the CCND1 gene forming a loop (1). In the current study, whereas we confirmed the presence of CREPT to the promoter and terminator regions of the CCDN1 gene, we found that Wnt stimulation enhanced the occupancy of CREPT in the promoter region of the CCND1 gene, but not the terminator region. We reasoned that this is because TCF4 recognizes the promoter sequence of the gene and the complex of β-catenin, TCF4, and CREPT functions to initiate the gene transcription upon Wnt signaling.
The molecular mechanism by which CREPT enhances the transcriptional activity of the β-catenin·TCF4 complex requires further study. It was reported that β-catenin is acetylated by CBP at Lys-49 (33) and p300 at Lys-345 (34). This modification leads to an increased binding affinity of β-catenin with TCF4 and an up-regulated transcriptional activity of the complex. Therefore the stable association of β-catenin with TCF4 is critical for the transcription of Wnt-targeted genes. We envisioned that CREPT may function as a co-factor to stabilize the complex formation of β-catenin with TCF4 and then enhance the transcriptional activity of the β-catenin·TCF4 complex. Because CREPT binds to the CTD domain of RNAP II, we speculate that CREPT may form a complex with both β-catenin·TCF4 and RNAP II. As β-catenin has a transcription activation domain, we propose that CREPT may bridge the association of the domain with the CTD domain of RNAP II. Further experiments are needed to examine our hypothesis.
The biological function of CREPT on the regulation of Cyclin D1 expression was reflected by its role in the regulation of cell proliferation (Fig. 6). Interestingly, we observed that CREPT also regulates the expression of c-Myc. CREPT is also important for the occupancy of β-catenin and TCF4 on the promoter of c-Myc (Fig. 5). These results suggested that CREPT is a general factor to enhance the transcription of cell growth-related genes. Previously, we showed that CREPT regulated the expression of Cyclin D1, CDK2/6, and Cyclin E but not p27 and p19 (1). In this study, we added evidence that CREPT, through regulation of Wnt signaling, promoted c-Myc expression. Our results explained the role of CREPT on cell proliferation because both c-Myc and Cyclin D1 are major regulators of cell growth. On the other hand, our results also explained the role of CREPT in tumorigenesis as Wnt signaling has been reported to regulate tumorigenesis by up-regulating both cell growth and migration ability. In our previous report, we observed that the level CREPT was correlated with tumor patient survival (1). We speculated that over-activated Wnt signaling by highly expressed CREPT in tumors is a reason for the poor survival of the patients, either through promoting cell proliferation or enhancing the migration and invasion ability. Indeed, in this study, we showed that depletion of CREPT dramatically decreased tumor clone formation and gel filtration ability (Fig. 6), implying that CREPT regulates tumor cell invasion and metastasis.
We have shown that CREPT regulates Wnt-targeted gene expression by promoting a complex formation of β-catenin and TCF4 in the promoters related genes. However, how CREPT maintains its specificity to regulate oncogene gene expression remains a question to be addressed. We speculate that CREPT might also recruit different factors to define its specificity in recognizing specific promoters. Identification of such kinds of factors will provide new insights in understanding the specificity of the different signals on gene expression under normal or tumor conditions.
In conclusion, we provide evidence that CREPT functions as a co-activator to enhance transcriptional activity of the β-catenin·TCF4 complex activated by Wnt stimulation. This study extends our understanding of the regulation of Wnt signaling by co-activating regulators in tumorigenesis.
Acknowledgments
We thank Dr. Xi He for the gifts of the Wnt3a-secreted cell lines and helpful discussions during the course of this work. We also appreciate Dr. Wei Wu for kindly providing plasmids. We thank Prof. David Irwin from the University of Toronto for editing of the manuscript.
This work was supported by 973 Project Grant 2011CB910502, National Natural Science Foundation of China Grants 81230044 and 81372167, Tsinghua Science Foundation Grant 20121080018, Natural Science Foundation of Beijing Grant 511003, Major Program Grant 2013ZX08011-006, and 863 project Grants 2014AA020802 and 2012AA021703 in China.
- CREPT
- cell cycle-related and expression elevated protein in tumor
- RPR
- regulation of nuclear pre-mRNA
- RNAP II
- RNA polymerase II
- TCF
- T cell-specific factor
- CM
- conditioned medium
- TBS
- TCF4-binding sites
- CCT
- coiled-coil terminus
- TRITC
- tetramethylrhodamine isothiocyanate
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IP
- immunoprecipitation.
REFERENCES
- 1. Lu D., Wu Y., Wang Y., Ren F., Wang D., Su F., Zhang Y., Yang X., Jin G., Hao X., He D., Zhai Y., Irwin D. M., Hu J., Sung J. J., Yu J., Jia B., Chang Z. (2012) CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell 21, 92–104 [DOI] [PubMed] [Google Scholar]
- 2. Wu Y., Zhang Y., Zhang H., Yang X., Wang Y., Ren F., Liu H., Zhai Y., Jia B., Yu J., Chang Z. (2010) p15RS attenuates Wnt/β-catenin signaling by disrupting β-catenin·TCF4 interaction. J. Biol. Chem. 285, 34621–34631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X., Cao Q., Liu X., Liu S., Wang J., Sun S., Wang O., Tian Z., Liu H., Kuang J., Zhang W. (2012) Cellular and molecular evidence for malignancy inhibitory functions of p15RS. Cell Cycle 11, 1988–1998 [DOI] [PubMed] [Google Scholar]
- 4. Liu J., Liu H., Zhang X., Gao P., Wang J., Hu Z. (2002) Identification and characterization of P15RS, a novel P15(INK4b) related gene on G1/S progression. Biochem. Biophys. Res. Commun. 299, 880–885 [DOI] [PubMed] [Google Scholar]
- 5. Jung H. M., Choi S. J., Kim J. K. (2009) Expression profiles of SV40-immortalization-associated genes upregulated in various human cancers. J. Cell. Biochem. 106, 703–713 [DOI] [PubMed] [Google Scholar]
- 6. Wang Y., Qiu H., Hu W., Li S., Yu J. (2014) RPRD1B promotes tumor growth by accelerating the cell cycle in endometrial cancer. Oncol. Rep. 31, 1389–1395 [DOI] [PubMed] [Google Scholar]
- 7. Hsin J. P., Manley J. L. (2012) The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buratowski S. (2009) Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapman R. D., Heidemann M., Hintermair C., Eick D. (2008) Molecular evolution of the RNA polymerase II CTD. Trends Genet. 24, 289–296 [DOI] [PubMed] [Google Scholar]
- 10. Corden J. L. (2007) Transcription: seven ups the code. Science 318, 1735–1736 [DOI] [PubMed] [Google Scholar]
- 11. Ni Z., Olsen J. B., Guo X., Zhong G., Ruan E. D., Marcon E., Young P., Guo H., Li J., Moffat J., Emili A., Greenblatt J. F. (2011) Control of the RNA polymerase II phosphorylation state in promoter regions by CTD interaction domain-containing proteins RPRD1A and RPRD1B. Transcription 2, 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mei K., Jin Z., Ren F., Wang Y., Chang Z., Wang X. (2014) Structural basis for the recognition of RNA polymerase II C-terminal domain by CREPT and p15RS. Sci. China Life Sci. 57, 97–106 [DOI] [PubMed] [Google Scholar]
- 13. Kim M., Krogan N. J., Vasiljeva L., Rando O. J., Nedea E., Greenblatt J. F., Buratowski S. (2004) The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432, 517–522 [DOI] [PubMed] [Google Scholar]
- 14. Mischo H. E., Proudfoot N. J. (2013) Disengaging polymerase: terminating RNA polymerase II transcription in budding yeast. Biochim. Biophys. Acta 1829, 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein E. A., Assoian R. K. (2008) Transcriptional regulation of the cyclin D1 gene at a glance. J. Cell Sci. 121, 3853–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., Ben-Ze'ev A. (1999) The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsumura I., Kitamura T., Wakao H., Tanaka H., Hashimoto K., Albanese C., Downward J., Pestell R. G., Kanakura Y. (1999) Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 18, 1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guttridge D. C., Albanese C., Reuther J. Y., Pestell R. G., Baldwin A. S., Jr. (1999) NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol. Cell. Biol. 19, 5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reya T., Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 20. Clevers H., Nusse R. (2012) Wnt/β-catenin signaling and disease. Cell 149, 1192–1205 [DOI] [PubMed] [Google Scholar]
- 21. Castrop J., van Norren K., Clevers H. (1992) A gene family of HMG-box transcription factors with homology to TCF-1. Nucleic Acids Res. 20, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cavallo R. A., Cox R. T., Moline M. M., Roose J., Polevoy G. A., Clevers H., Peifer M., Bejsovec A. (1998) Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395, 604–608 [DOI] [PubMed] [Google Scholar]
- 23. Roose J., Molenaar M., Peterson J., Hurenkamp J., Brantjes H., Moerer P., van de Wetering M., Destrée O., Clevers H. (1998) The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395, 608–612 [DOI] [PubMed] [Google Scholar]
- 24. Chen G., Fernandez J., Mische S., Courey A. J. (1999) A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 13, 2218–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anastas J. N., Moon R. T. (2013) WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13, 11–26 [DOI] [PubMed] [Google Scholar]
- 26. Ito K., Lim A. C., Salto-Tellez M., Motoda L., Osato M., Chuang L. S., Lee C. W., Voon D. C., Koo J. K., Wang H., Fukamachi H., Ito Y. (2008) RUNX3 attenuates β-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 14, 226–237 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y., Ren F., Wang Y., Feng Y., Wang D., Jia B., Qiu Y., Wang S., Yu J., Sung J. J., Xu J., Zeps N., Chang Z. (2014) CHIP/Stub1 functions as a tumor suppressor and represses NF-κB-mediated signaling in colorectal cancer. Carcinogenesis 35, 983–991 [DOI] [PubMed] [Google Scholar]
- 28. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang J., Zhang W., Evans P. M., Chen X., He X., Liu C. (2006) Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 281, 17751–17757 [DOI] [PubMed] [Google Scholar]
- 30. Lin S. Y., Xia W., Wang J. C., Kwong K. Y., Spohn B., Wen Y., Pestell R. G., Hung M. C. (2000) β-Catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. U.S.A. 97, 4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. (1998) Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 32. Musgrove E. A., Caldon C. E., Barraclough J., Stone A., Sutherland R. L. (2011) Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 11, 558–572 [DOI] [PubMed] [Google Scholar]
- 33. Wolf D., Rodova M., Miska E. A., Calvet J. P., Kouzarides T. (2002) Acetylation of β-catenin by CREB-binding protein (CBP). J. Biol. Chem. 277, 25562–25567 [DOI] [PubMed] [Google Scholar]
- 34. Lévy L., Wei Y., Labalette C., Wu Y., Renard C. A., Buendia M. A., Neuveut C. (2004) Acetylation of β-catenin by p300 regulates β-catenin-Tcf4 interaction. Mol. Cell. Biol. 24, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]