Background: The properties of regulatory B cells are not fully understood.
Results: CX3CR1+ B cells do not express but contain αvβ6 and TGF-β and inhibit T cell proliferation.
Conclusion: CX3CR1+ B cells have immune suppressor properties.
Significance: CX3CR1+ B cells may be used to adjust skewed immune reactions.
Keywords: Allergy, Cytokine, Flow Cytometry, Lymphocyte, Western Blot
Abstract
The immune regulatory functions of B cells are not fully understood yet. The present study aims to characterize a subtype of B cells that expresses CX3CR1. In this study, peripheral blood samples were collected from patients with food allergies and healthy subjects. Peripheral B cells were analyzed by flow cytometry. T cell proliferation was assessed by carboxyfluorescein succinimidyl ester dilution assay. The results showed that the CX3CR1+ B cells were detected in the peripheral blood samples of healthy subjects and were significantly less in patients with food allergies. CX3CR1+ B cells expressed high levels of TGF-β and integrin αvβ6. CX3CR1+ B cells could efficiently suppress other effector CD4+ T cell activation. We conclude that human peripheral CX3CR1+ B cells have immune suppressor properties.
Introduction
Similar to T cells, B cells are one type of the major immune cells. The main function of B cells is to produce antibodies to fight against the invaded pathogens and foreign antigens to maintain the homeostasis of the body. On the other hand, skewed functional status of B cells may undermine body functions or injure the organs and tissue, such as the production of auto antibodies against islet cells to cause diabetes (1, 2). Recent reports indicate that B cells also play important roles in the immune regulation (3), such as in antigen-specific immunotherapy, fractions of B cells produce IL-10 (4), and/or transforming growth factor (TGF)-β (5), to suppress other effector T cell activities. Because IL-10 and TGF-β are immune tolerogenic cytokines (6), those fractions of B cells possess immune tolerogenic properties and may be the tolerogenic B cells. However, the conditions of induction of tolerogenic B cells remain to be further investigated.
The integrin family has several immune regulatory members, such as αvβ6 (7, 8). αvβ6 is produced by epithelial cells (9). Although αvβ6 is not a secretory molecule, it attaches to the plasma membrane and may be carried by exosomes to be released to the microenvironment to contact immune cells and regulate immune functions, such as modulating dendritic cell tolerogenic properties (10). Because B cells are one type of the antigen-presenting cells, αvβ6 may also regulate B cell tolerogenic properties. Whether B cells can capture the vascular endothelial cell-derived αvβ6 has not been reported yet.
CX3CR1 is a chemokine receptor; it is encoded by the CX3CR1 gene in human. The ligand of CX3CR1 is fractalkine (CX3CL1). It is proposed that CX3CR1/CX3CL1 plays an important role in immune tolerance, such as in survival of allograft transplantation (11). CX3CL1 can be produced by epithelial cells and endothelial cells (12, 13). The CX3CR1-expressing cells may be chemotracted to the direction of the endothelial/epithelial region to capture the released αvβ6 or αvβ6-laden exosomes (10). In the preliminary study, we observed that human vascular endothelial cells express both CX3CL1 and αvβ6. Based on the above information, we hypothesize that a fraction of CX3CR1-expressing B cells may capture the endothelial cell-released αvβ6 to differentiate into tolerogenic B cells. Thus, we collected human peripheral B cells to be analyzed by flow cytometry. The results showed that a fraction of B cells were both CX3CR1+ and αvβ6+. This fraction of B cells showed immune regulatory properties.
MATERIALS AND METHODS
Reagents
Antibodies of β6 (C-19), CX3CR1 (H-70), CX3CL1 (H-300), LAMP1 (C-20), LAP (T-17), TGF-β1 (d-12), and shRNA kits of CX3CL1 and β6 were purchased from Santa Cruz Biotechnology (Beijing, China). Fluorescence-labeled antibodies for flow cytometry were purchased from BD Bioscience. The ELISA kit of TGF-β was purchased from R&D Systems (Beijing, China). The reagents of real time RT-PCR were purchased from Invitrogen.
Study Subjects
Ten patients (10 male and 10 female; age was 25–58 years old, with an average of 33.4 years old) with egg sensitization, and 10 healthy subjects were recruited into the present study. The using human tissue in the research was approved by the Research Ethic Committee at China PLA General Hospital. An informed written consent was obtained from each human subject.
Collection of Blood Samples
Blood samples were collected from the human subjects (20 ml/person). The peripheral blood mononuclear cells were isolated by gradient density centrifugation and cultured in RPMI1640 medium complemented with 10% fetal cowl serum, 2 mm l-glutamin, 1 mg/ml streptomycin, and 200 units/ml penicillin. The cells were used in further experiments.
Isolation of Immune Cells
The immune cells were isolated from peripheral blood mononuclear cells by magnetic cell sorting with commercial reagent kits following the manufacturer's instructions. The isolated immune cells were checked the purity (more than 95%) by flow cytometry before using in further experiments.
Human Umbilical Vein Endothelial Cell Culture and Purification of Exosomes
Human umbilical vein endothelial cells (HUVEC)2 were cultured in the HUVEC-specific culture medium. To purify the exosomes, the culture supernatant was collected and processed following published procedures (14). Briefly, the supernatant was subjected to three successive centrifugation steps at 12,000 × g for 1 h, 35,000 × g for 1 h, and 70,000 × g for 3 h at 4 °C. The supernatant was filtered sequentially through 10.0-, 0.45-, and 0.22-μm filters. The supernatant was then ultracentrifuged at 100,000 × g for 1 h at 4 °C. The pellet of exosomes was used for further experiments. The purified exosomes were lysed with lysing buffer; the proteins were analyzed by Western blotting to assess the levels of αvβ6 and the endosome marker LAMP1.
Flow Cytometry
For analysis by flow cytometry, brefildin A (10 μg/ml) was added to the culture medium for the last 3 h of culture. Cells were fixed with 2% paraformaldehyde (in case of the intracellular staining, 0.1% Triton X-100 was added to the fixatives) for 1 h. After washing, the cells were blocked by 5% skim milk. The cells were incubated with fluorescence-labeled antibodies as indicated in figures at concentrations of 0.5–1 μg/ml for 1 h at room temperature. The cells were analyzed by a flow cytometer (FACSCanto II; BD Bioscience). The gating was performed when necessary. Briefly, cells were stained with the indicated fluorescence-labeled antibodies. One positively stained cell population was gated first; by double clicking the gated cell population, a new window popped out for further analysis, and so on.
Test the Immune Suppressor Function of CX3CR1+ B Cells
CX3CR1+ B cells and CD4+ CD25− T cells (labeled with carboxyfluorescein diacetate, succinimidyl ester) were obtained from healthy subjects. The cells were cultured at a ratio of 1:5 (B cell:T cell) for 3 days (the treatment is denoted in Fig. 5). The cells were collected and analyzed by the carboxyfluorescein diacetate, succinimidyl ester dilution assay.
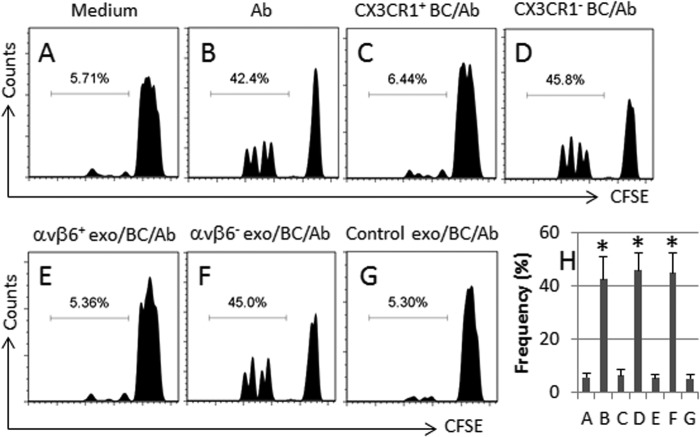
FIGURE 5.
The generated Tregs show immune suppressor function. The generated Tregs were cultured with carboxyfluorescein diacetate, succinimidyl ester (CFSE)-labeled T effector cells at graded ratios (denoted above histograms) in the presence of anti-CD3/CD28 antibodies (Ab) for 3 days. The cells were analyzed by flow cytometry. 100,000 cells were analyzed for each sample. A–G, the histograms indicate the frequency of proliferated T effector cells, which was summarized in H. The culture conditions are denoted above each histogram. exo, exosomes. The absolute numbers of the proliferated cells are 4240 ± 355 (A), 3280 ± 277 (B), 2290 ± 166 (C), and 5440 ± 434 (D). The data of F are presented as mean ± S.D. *, p < 0.01, compared with group A. The data represent three separate experiments.
Assessment of the Viability of the Cultured Cells
The viability of the cells was checked by trypan blue exclusion assay before each experiment, which was more than 95%.
Quantitative RT-PCR
HUVEC were purchased from ATCC and cultured with the HUVEC culture medium (Sigma-Aldrich). The total RNA was extracted using TRIzol reagents. The cDNA was synthesized using a reverse transcription kit. The quantitative PCR was performed on a MiniOpticon real time PCR device using the SYBR Green Master Mix. The results were calculated with the 2−ΔΔCt method. The primers using in this study include integrin β6 (forward, gaaggggtgactgctactgt; reverse, tgcacacacattcaccacag) and β-actin (forward, cgcaaagacctgtatgccaa; reverse, cacacagagtacttgcgctc).
Western Blotting
The total proteins were extracted from cells. An equal volume (50 μg/well) of the proteins was added to each well, fractioned by SDS-PAGE, and transferred onto a nitrocellulose membrane. The membrane was blocked by 1% BSA for 1 h and incubated with the primary antibodies (0.5–1 μg/ml) overnight at 4 °C, followed by incubation with the second antibodies (conjugated with horseradish peroxidase) for 1 h at room temperature. The immune complex on the membrane was developed by ECL. The results were recorded with x films.
Statistics
The data were presented as means ± S.D. The differences between the two groups were analyzed by analysis of variance, and the means were compared by Student-Newman-Keuls test. p < 0.05 was set as a significant criterion.
RESULTS
Frequency of CX3CR1+ B Cells Is Decreased in Patients with FA
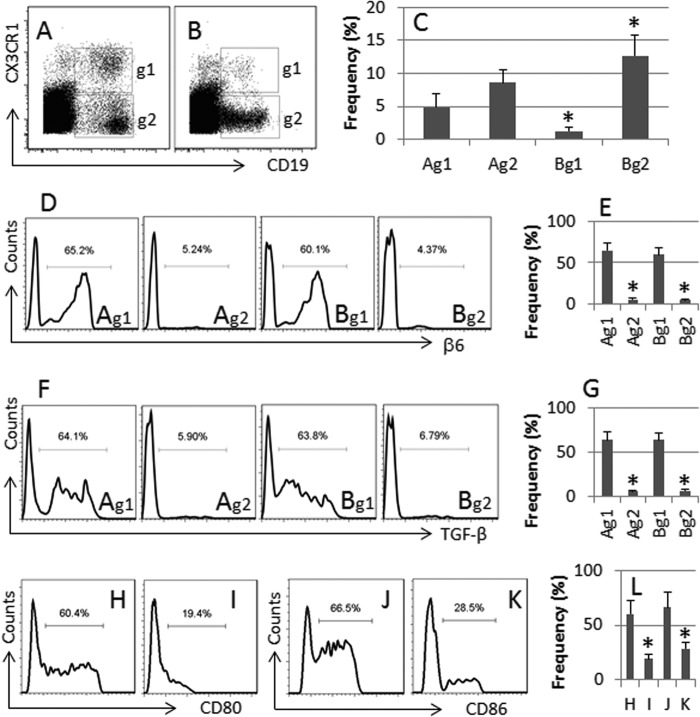
Published data indicate that the CX3CR1+ B cells are involved in immune regulation (15); whether their amounts or functions are affected in immune diseases, such as in food allergy (FA), is unclear. In this study, we collected the peripheral blood samples from 20 FA patients (10 male, 10 female; age, 34.5 ± 5.5 years) sensitized to egg, and 20 healthy subjects (10 male, 10 female; age, 35.6 ± 5.8 years). The cell samples were analyzed by flow cytometry. The results showed that CX3CR1+ B cells were detected in the peripheral blood samples of healthy subjects (Fig. 1, A and C), which were significantly less in FA patients (Fig. 1, B and C).
FIGURE 1.
Peripheral CX3CR1+ B cells show αvβ6+ and TGF-β+. Human peripheral mononuclear cells were collected from 10 healthy subjects of 10 FA patients and analyzed by flow cytometry. A and B, the dot plots indicate the frequency of CX3CR1+ CD19+ B cells (g1) and CX3CR1− CD19+ B cells (g2) in healthy subjects (A) and FA patients (B). C, the bars indicate the summarized data of A and B. D and F, the flow cytometry histograms indicate the frequency of αvβ6+ cells (D) and TGF-β+ cells (F) in the gated cells of A and B (the gates are designated g1 and g2). E and G, the bars indicate the summarized data of D and F. H–K, the histograms indicate the frequency of CD80+ (H and I) and CD86+ (J and K) B cells from LPS-treated B cells (H and J) and CX3CR1+ B cells (I and K). L, the bars indicate the summarized data of H–K. The data presented as bars are means ± S.D. *, p < 0.01, compared with group A (C, E, and G) or group H (L). Samples from individual subjects were processed separately.
Human Peripheral B Cells Contain αvβ6 and TGF-β
Integrin αvβ6 and TGF-β are immune regulatory molecules (16). To elucidate whether the CX3CR1+ B cells contained αvβ6 and TGF-β, using the gating technique, we further analyzed the αvβ6+ and TGF-β+ cells in the gated CX3CR1+ CD19+ B cells and CX3CR1− CD19+ B cells of Fig. 1 (A and B). The results showed that ∼60% CX3CR1+ CD19+ B cells were αvβ6+ and TGF-β+ in both healthy subjects and FA patients (Fig. 1, D–G). We also measured the levels of αvβ6 and TGF-β in CX3CR1− CD19+ B cells; the results showed significantly less αvβ6 and TGF-β were detected in CX3CR1− CD19+ B cells (Fig. 1, D–G). Compared with the LPS-treated B cells, CX3CR1+ B cells expressed much lower levels of CD80 and CD86 (Fig. 1, H–L).
CX3CR1+ B Cells Do Not Express, but Contain, αvβ6
Because the αvβ6 is usually expressed by epithelial cells, we wondered whether the detected αvβ6 in the B cells was originally expressed by the B cells. Thus, we isolated CX3CR1+ B cells from the peripheral blood mononuclear cells of healthy subjects. The cells were analyzed by quantitative RT-PCR and Western blotting. The results showed that although the αvβ6 was detected in the CX3CR1+ B cells, the levels of αvβ6 mRNA were below the detectable levels (Fig. 2). The results implicate that the αvβ6 in CX3CR1+ B cells may be captured from extrinsic sources.
FIGURE 2.
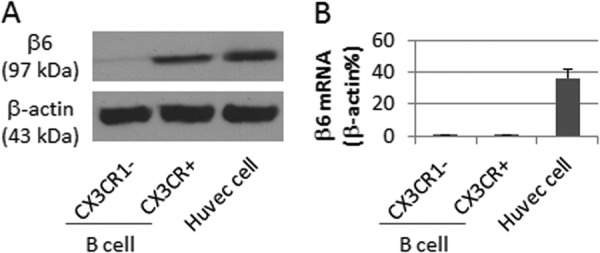
Human CX3CR1+ B cells do not express but contain αvβ6. Human CX3CR1− and CX3CR1+ B cells or HUVEC (positive controls) were analyzed by Western blotting and quantitative RT-PCR. A, the immune blots indicate the contents of αvβ6. B, the bars indicate the mRNA levels of αvβ6 (means ± S.D.). The data represent three separate experiments.
CX3CR1+ B Cells Capture αvβ6-containing Endothelial Cell-derived Exosomes
Previous data indicate that αvβ6 can be released to the microenvironment from epithelial cells via the exosome system (10). The αvβ6 in the B cells may be absorbed from the blood stream. To test the inference, we measured the levels of αvβ6 in the sera from healthy subjects and FA patients. However, the levels of αvβ6 in the sera were below the detectable levels (data not shown). Then we took an alternative approach. Based on the fact of the HUVEC express αvβ6 (Fig. 2), we purified exosomes from the culture supernatant of HUVEC; the extracts of exosomes were analyzed by Western blotting. The results showed that CX3CL1 (the ligand of CX3CR1; Fig. 3A), αvβ6 (Fig. 3B), and LAMP1 (a marker of exosomes; Fig. 3C) were detected in the cellular extracts, indicating that the endothelial cell-derived exosomes carry CX3CL1 and αvβ6. We further cultured HUVEC in the inserts of Transwells and BC-3 cells in the basal chambers. Twenty-four hours later, the cells were analyzed by flow cytometry. The results showed 61.1% BC-3 cells containing αvβ6+ (Fig. 3D), indicating that these cells captured αvβ6 in the culture. To clarify whether the detected αvβ6 was attaching to the surface of the cells, some BC-3 cells were processed via the surface staining approach, which resulted in only 1.25% αvβ6+ BC-3 cells (Fig. 3E).
FIGURE 3.
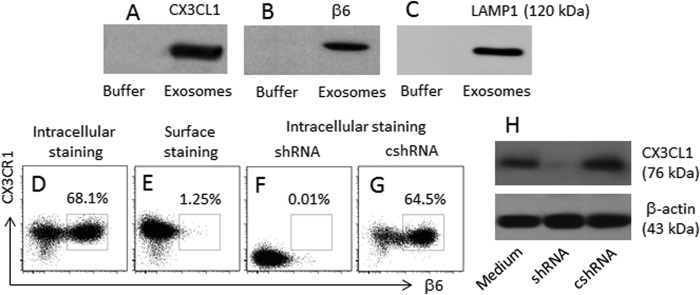
B cells capture HUVEC-derived αvβ6. Exosomes were purified from the HUVEC culture supernatant. Proteins were extracted from the exosomes. A–C, the immune blots indicate the proteins of CX3CL1 (A), αvβ6 (B), and LAMP1 (C) in the extracts of exosomes. D–G, BC-3 cells were cultured in the basal chambers of Transwells. HUVEC were cultured in the inserts for 24 h. The BC-3 cells were analyzed by flow cytometry. The dot plots indicate the frequency of αvβ6+ CX3CR1+ B cells; the treatment is denoted above each subpanel. H, the CX3CL1 gene in HUVEC of F are knocked down by RNAi. shRNA, HUVEC were treated with shRNA of CX3CL1. cshRNA, control shRNA. The data represent three separate experiments.
Because CX3CL1 was detected in the HUVEC-derived exosomes (Fig. 3A) and CX3CR1+ BC-3 cells captured the exosomes (Fig. 3D), we inferred that the interaction of CX3CL1/CX3CR1 was required in the capturing of the αvβ6-containing exosomes. BC-3 cells were cultured with CX3CL1-null HUVEC in the Transwell system with similar procedures above. Indeed, the αvβ6 capturing by BC-3 cells was abolished (Fig. 3, F–H).
αvβ6-carrying Exosomes Activate Latent TGF-β in B Cells
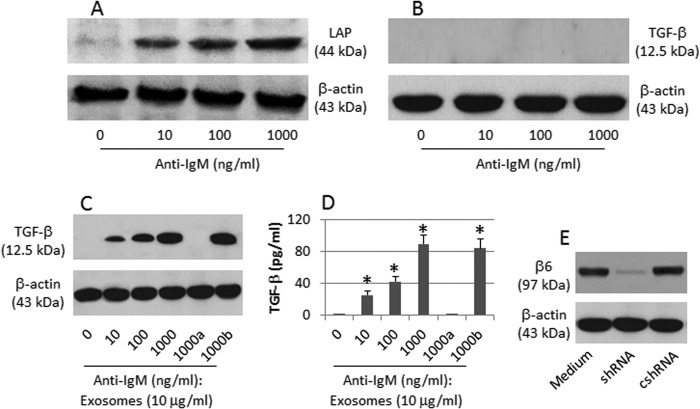
Based on the data of Fig. 1 indicating that CX3CR1+ B cells are TGF-β+ but the CX3CR1− B cells are not, we inferred that the endocytic αvβ6 activated the latent TGF-β (LTGF-β) in B cells. To this end, we treated CX3CR1− B cells with anti-IgM. Indeed, the levels of LTGF-β in the B cells increased in an anti-IgM dose-dependent manner (Fig. 4A), but the TGF-β was still below the detectable levels (Fig. 4B). Considering that the HUVEC-derived exosomes contain αvβ6 (Fig. 3B), which can convert LTGF-β to TGF-β, we carried out this experiment again in Transwells where HUVEC or αvβ6-null HUVEC were cultured in the inserts and B cells were cultured in the basal chambers. The results showed that TGF-β was increased in the presence of αvβ6-sufficient HUVEC, but not in the presence of αvβ6-null HUVEC (Fig. 4C). The results indicate that HUVEC-derived αvβ6 converts LTGF-β to TGF-β in B cells.
FIGURE 4.
αvβ6 converts LTGF-β to TGF-β in B cells. A and B, CX3CR1− B cells were exposed to anti-IgM in the culture for 48 h. The immune blots indicate the levels of LTGF-β (A) and TGF-β (B) in the cellular extracts. C, CX3CR1− B cells were exposed to anti-IgM and αvβ6-containing exosomes (or αvβ6-null exosomes (a); the b indicates the HUVEC were treated with control shRNA) in the culture for 48 h. The immune blots indicate the contents of TGF-β of the cellular extracts. D, the bars indicate the levels of TGF-β in the culture supernatant (by ELISA; mean ± S.D.; *, p < 0.01, compared with group 0). E, shRNA (or cshRNA), the exosomes were collected from the culture supernatants of HUVEC treated with αvβ6 shRNA (or control shRNA). The data represent three separate experiments.
CX3CR1+ B Cells Show Immune Suppressor Properties
Published data indicate that TGF-β plays a critical role in the differentiation of Tregs (10). Because CX3CR1+ B cells express TGF-β, these cells may have immune regulatory ability. Thus, we cultured healthy human CX3CR1+ B cells with CD4+ CD25− T cells (Fig. 4). Using the proliferation as a reference of activation of T cells, we observed that 5.71% T cells proliferated in culturing with medium alone (Fig. 5, A and H); exposure to anti-CD3/CD28 induced 42.4% proliferated T cells (Fig. 5, B and H), which was significantly suppressed in the presence of CX3CR1+ B cells (Fig. 5, C and H) but could not be suppressed by the presence of CX3CR1− B cells (Fig. 5D, H). After priming with HUVEC-derived exosomes, BC-3 cells could suppress T cell proliferation (Fig. 5, E and H), the latter was not suppressed by those BC-3 cells primed by αvβ6-null exosomes (Fig. 5, F–H). The data indicate that human CX3CR1+ B cells have immune suppressor properties.
DISCUSSION
The present study has revealed a subfraction of B cells, the CX3CR1+ B cells, in the human peripheral blood system. Such a fraction of B cells contains αvβ6 and TGF-β, expresses less costimulatory molecules, and has immune suppressor properties. FA patients have a low frequency of CX3CR1+ B cells in the peripheral blood system.
Several integrins are involved in immune regulation. αvβ6 can convert the LTGF-β to the active form of TGF-β (17). Because TGF-β is a critical molecule in immune regulation, the lack of TGF-β compromises immune tolerance and causes severe autoimmune disorders (18). Other investigators report that αvβ6 contributes to the development of immune tolerance (7, 19). Our data indicate that a fraction of B cells also contains αvβ6 and has an immune suppressor feature. The present data indicate that B cells contribute immune regulation, apart from via releasing IL-10 (20, 21), and a fraction of αvβ6-containing B cells may contribute to immune tolerance. Similar to the tolerogenic dendritic cells, the CX3CR1+ B cells also express lower levels of costimulatory molecules as shown by the present data.
αvβ6 is produced by epithelial cells or endothelial cells; it attaches to the plasma membrane and is not a secretory protein. Thus, it is not expected to detect αvβ6 in the body fluid. However, it can be carried by exosomes; the latter can be captured by immune cells. For example, Chen et al. (10) reported that intestinal epithelial cell-derived exosomes carried αvβ6 that could be captured by dendritic cells. Our data also show that human vascular endothelial cell line expresses the αvβ6 and CX3CL1. It seems that CX3CR1+ B cells capture the epithelial cell-derived αvβ6 via the interaction of CX3CR1/CX3CL1. The expression of CX3CL1 by epithelial cells is also reported by others (22–24). There are some other immune regulatory cells, such as regulatory T cells, in the body that play important roles in the maintenance of the homeostasis. We propose that the CX3CR1+ B cells, compared with regulatory T cells, may be more, or at least equally, important in the immune regulation of the mucosa because the ligand of CX3CR1 (the CX3CL1) is expressed by epithelial cells; the chemotactic force makes CX3CR1+ B cells move to the subepithelial region to fulfill the immune regulatory task.
After synthesis, TGF-β exists as a precursor, the LTGF-β; the latter needs to be converted to the active form, TGF-β, to fulfill the immune regulatory function. αvβ6 can remove the latency-associated peptide from TGF-β precursor (25). Our data are consistent with the previous studies (10); the αvβ6-containing CX3CR1+ B cells are also TGF-β-positive. It is reported that exogenous TGF-β can induce Tregs (26). The present data suggest that the CX3CR1+ B cells are one of the immune regulatory cells in the body. The inference is supported by further data showing that CX3CR1+ B cells efficiently suppressed T cell proliferation. Supporting data have been reported by Liu et al. (27), who show that CX3CR1+ B cells facilitate the generation of regulatory T cells. In summary, the present data define a subfraction of B cells expressing CX3CR1 that contain αvβ6 and TGF-β; the cells have immune suppressor properties.
This work was supported by the clinical research program of Beijing Scientific Foundation through Grant 131107002213040.
- HUVEC
- human umbilical vein endothelial cell(s)
- FA
- food allergy.
REFERENCES
- 1. Culina S., Brezar V., Mallone R. (2013) Mechanisms in endocrinology: insulin and type 1 diabetes: immune connections. Eur. J. Endocrinol. 168, R19–R31 [DOI] [PubMed] [Google Scholar]
- 2. Ozkurede V. U., Franchi L. (2012) Immunology in clinic review series: focus on autoinflammatory diseases: role of inflammasomes in autoinflammatory syndromes. Clin. Exp. Immunol. 167, 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chorny A., Puga I., Cerutti A. (2012) Regulation of frontline antibody responses by innate immune signals. Immunol. Res. 54, 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van de Veen W., Stanic B., Yaman G., Wawrzyniak M., Söllner S., Akdis D. G., Rückert B., Akdis C. A., Akdis M. (2013) IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J. Allergy Clin. Immunol. 131, 1204–1212 [DOI] [PubMed] [Google Scholar]
- 5. Natarajan P., Singh A., McNamara J. T., Secor E. R., Jr., Guernsey L. A., Thrall R. S., Schramm C. M. (2012) Regulatory B cells from hilar lymph nodes of tolerant mice in a murine model of allergic airway disease are CD5+, express TGF-β, and co-localize with CD4+Foxp3+ T cells. Mucosal Immunol. 5, 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oberg H. H., Juricke M., Kabelitz D., Wesch D. (2011) Regulation of T cell activation by TLR ligands. Eur. J. Cell Biol. 90, 582–592 [DOI] [PubMed] [Google Scholar]
- 7. Liu T., Liang X., Li T. L., Ma J., Yang J. F., Yang P. C. (2012) Staphylococcal enterotoxin B compromises the immune tolerant status in the airway mucosa. Clin. Exp. Allergy 42, 375–382 [DOI] [PubMed] [Google Scholar]
- 8. Feng B. S., Chen X., Li P., Zheng P. Y., Chong J., Cho D. B., He S. H., Tang S. G., Yang P. C. (2009) Expression of integrin αvβ6 in the intestinal epithelial cells of patients with inflammatory bowel disease. N. Am. J. Med. Sci. 1, 200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheppard D. (2005) Integrin-mediated activation of latent transforming growth factor b. Cancer Metastasis Rev. 24, 395–402 [DOI] [PubMed] [Google Scholar]
- 10. Chen X., Song C. H., Feng B. S., Li T. L., Li P., Zheng P. Y., Chen X. M., Xing Z., Yang P. C. (2011) Intestinal epithelial cell-derived integrin aβ6 plays an important role in the induction of regulatory T cells and inhibits an antigen-specific Th2 response. J. Leukoc. Biol. 90, 751–759 [DOI] [PubMed] [Google Scholar]
- 11. Louvet C., Heslan J. M., Merieau E., Soulillou J. P., Cuturi M. C., Chiffoleau E. (2004) Induction of Fractalkine and CX3CR1 mediated by host CD8+ T cells in allograft tolerance induced by donor specific blood transfusion. Transplantation 78, 1259–1266 [DOI] [PubMed] [Google Scholar]
- 12. Zhou R., Gong A. Y., Chen D., Miller R. E., Eischeid A. N., Chen X. M. (2013) Histone deacetylases and NF-κB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. PLoS One 8, e65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rius C., Company C., Piqueras L., Cerdá-Nicolás J. M., González C., Servera E., Ludwig A., Morcillo E. J., Sanz M. J. (2013) Critical role of fractalkine (CX3CL1) in cigarette smoke-induced mononuclear cell adhesion to the arterial endothelium. Thorax 68, 177–186 [DOI] [PubMed] [Google Scholar]
- 14. Luketic L., Delanghe J., Sobol P. T., Yang P., Frotten E., Mossman K. L., Gauldie J., Bramson J., Wan Y. (2007) Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J. Immunol. 179, 5024–5032 [DOI] [PubMed] [Google Scholar]
- 15. Corcione A., Ferretti E., Bertolotto M., Fais F., Raffaghello L., Gregorio A., Tenca C., Ottonello L., Gambini C., Furtado G., Lira S., Pistoia V. (2009) CX3CR1 is expressed by human B lymphocytes and meditates CX3CL1 driven chemotaxis of tonsil centrocytes. PLoS One 4, e8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saban D. R., Bock F., Chauhan S. K., Masli S., Dana R. (2010) Thrombospondin-1 derived from APCs regulates their capacity for allosensitization. J. Immunol. 185, 4691–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giacomini M. M., Travis M. A., Kudo M., Sheppard D. (2012) Epithelial cells utilize cortical actin/myosin to activate latent TGF-β through integrin αvβ6-dependent physical force. Exp. Cell Res. 318, 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoki C. A., Borchers A. T., Li M., Flavell R. A., Bowlus C. L., Ansari A. A., Gershwin M. E. (2005) Transforming growth factor β (TGF-β) and autoimmunity. Autoimmun. Rev. 4, 450–459 [DOI] [PubMed] [Google Scholar]
- 19. Yang S. B., Du Y., Wu B. Y., Xu S. P., Wen J. B., Zhu M., Cai C. H., Yang P. C. (2012) Integrin αvβ6 promotes tumor tolerance in colorectal cancer. Cancer Immunol. Immunother. 61, 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong Y. I., Hong S. H., Cho S. H., Lee W. J., Lee S. E. (2012) Induction of IL-10-producing CD1d high CD5+ regulatory B cells following Babesia microti infection. PLoS One 7, e46553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang M., Deng J., Liu Y., Ko K. H., Wang X., Jiao Z., Wang S., Hua Z., Sun L., Srivastava G., Lau C. S., Cao X., Lu L. (2012) IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am. J. Pathol. 180, 2375–2385 [DOI] [PubMed] [Google Scholar]
- 22. Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Nakajima M., Goto-Koshino Y., Fujino Y., Tsujimoto H. (2012) Increased expression of fractalkine and its receptor CX3CR1 in canine inflammatory bowel disease and their possible role in recruitment of intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 148, 226–235 [DOI] [PubMed] [Google Scholar]
- 23. Morimura S., Sugaya M., Sato S. (2013) Interaction between CX3CL1 and CX3CR1 regulates vasculitis induced by immune complex deposition. Am. J. Pathol 182, 1640–1647 [DOI] [PubMed] [Google Scholar]
- 24. Sung M. J., Kim D. H., Davaatseren M., Hur H. J., Kim W., Jung Y. J., Park S. K., Kwon D. Y. (2010) Genistein suppression of TNF-α-induced fractalkine expression in endothelial cells. Cell Physiol. Biochem. 26, 431–440 [DOI] [PubMed] [Google Scholar]
- 25. Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T. A. (2011) Latent TGF-β structure and activation. Nature 474, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dons E. M., Raimondi G., Cooper D. K., Thomson A. W. (2012) Induced regulatory T cells: mechanisms of conversion and suppressive potential. Hum. Immunol. 73, 328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Z. Q., Wu Y., Song J. P., Liu X., Liu Z., Zheng P. Y., Yang P. C. (2013) Tolerogenic CX3CR1+ B cells suppress food allergy-induced intestinal inflammation in mice. Allergy 68, 1241–1248 [DOI] [PubMed] [Google Scholar]