Background: p47phox is a regulatory subunit of NADPH oxidase, which produces superoxide.
Results: We propose a molecular dynamics model of p47phox phosphorylation and assembly with p22phox in NADPH oxidase activation supported by biochemical experiments.
Conclusion: Ser-379 phosphorylation in the C-terminal tail is a molecular switch in p47phox activation.
Significance: This report provides novel structural and mechanistic information of p47phox activation.
Keywords: Computer Modeling, Endothelial Cell, Molecular Docking, Molecular Dynamics, NADPH Oxidase, Phosphorylation, Site-directed Mutagenesis
Abstract
Phagocyte superoxide production by a multicomponent NADPH oxidase is important in host defense against microbial invasion. However inappropriate NADPH oxidase activation causes inflammation. Endothelial cells express NADPH oxidase and endothelial oxidative stress due to prolonged NADPH oxidase activation predisposes many diseases. Discovering the mechanism of NADPH oxidase activation is essential for developing novel treatment of these diseases. The p47phox is a key regulatory subunit of NADPH oxidase; however, due to the lack of full protein structural information, the mechanistic insight of p47phox phosphorylation in NADPH oxidase activation remains incomplete. Based on crystal structures of three functional domains, we generated a computational structural model of the full p47phox protein. Using a combination of in silico phosphorylation, molecular dynamics simulation and protein/protein docking, we discovered that the C-terminal tail of p47phox is critical for stabilizing its autoinhibited structure. Ser-379 phosphorylation disrupts H-bonds that link the C-terminal tail to the autoinhibitory region (AIR) and the tandem Src homology 3 (SH3) domains, allowing the AIR to undergo phosphorylation to expose the SH3 pocket for p22phox binding. These findings were confirmed by site-directed mutagenesis and gene transfection of p47phox−/− coronary microvascular cells. Compared with wild-type p47phox cDNA transfected cells, the single mutation of S379A completely blocked p47phox membrane translocation, binding to p22phox and endothelial O2⨪ production in response to acute stimulation of PKC. p47phox C-terminal tail plays a key role in stabilizing intramolecular interactions at rest. Ser-379 phosphorylation is a molecular switch which initiates p47phox conformational changes and NADPH oxidase-dependent superoxide production by cells.
Introduction
NADPH oxidase is an O2⨪-generating enzyme expressed in a variety of mammalian cell types (1), and NADPH oxidase activation in phagocytes (oxidative burst) is essential for immune response against infection (2). However excessive and unabated reactive oxygen species (ROS)2 production by this enzyme causes chronic inflammation and multi-organ oxidative damage with severe consequences. Vascular endothelial cells also express constitutively NADPH oxidase, which generates consistently low levels of O2⨪ involved in redox-signaling under physiological conditions. However, under pathological conditions, NADPH oxidase is activated and excessive ROS production causes endothelial dysfunction, which is an early hallmark predisposing many diseases such as atherosclerosis and hypertension (1, 3). Insight into the regulatory mechanism of NADPH oxidase activation is essential for developing novel therapeutic strategies to treat these oxidative stress-related cardiovascular diseases without affecting the neutrophil oxidative response to infection (4).
The NADPH oxidase is a multi-component enzyme, which consists of a cytochrome b558 containing a catalytic subunit Nox2 (also called gp91phox) and a p22phox, and several cytosolic regulatory subunits i.e. p47phox, p67phox, p40phox, and Rac (5, 6). The phosphorylation of p47phox (a major regulatory subunit) has been recognized to be a prerequisite of NADPH oxidase activation (7–12). The p47phox consists of an N-terminal PX-domain which interacts with cell membrane phosphoinositides; two tandem Src homology 3 (SH3) domains forming a super-SH3 (sSH3) binding groove for binding to the proline rich region (PRR) of p22phox; a polybasic auto-inhibitory region (AIR), and a C-terminal PRR domain for interaction with other NADPH oxidase subunits (1, 6, 13). In the resting state, the sSH3 groove is masked by the AIR to keep p47phox in its autoinhibited conformation. Phosphorylation of serine residues i.e. Ser-303–304, Ser-310, Ser-315, Ser-320, and Ser-328 within the AIR results in AIR destabilization, which exposes the sSH3 groove for p22phox to bind and to activate NADPH oxidase (14, 15). However, p47phox has several serine phosphorylation sites outside the AIR toward the C terminus (14), yet their phosphorylation and the position of the C terminus tail (residues 341–390) in the p47phox global conformation remains unclear.
It had been shown previously that phosphorylation of Ser-379 is a key step required for p47phox membrane translocation and interactions with other proteins, and a single substitution of Ser-379 almost abolished leukocyte NADPH oxidase activity (16, 17), and TNFα-induced NADPH oxidase-dependent O2⨪ production in endothelial cells (18). However, the molecular mechanism of how a single serine (Ser-379) phosphorylation can promote NADPH oxidase activation is unknown.
In the current study, we have generated, for the first time, an in silico model of the complete p47phox protein structure and shown the importance of the C-terminal tail in stabilizing the p47phox structure at rest. We have demonstrated step by step the phosphorylation-induced p47phox conformational changes and protein/protein interactions with p22phox by molecular dynamics. The in silico results were further confirmed by site-directed mutagenesis and gene transfection of p47phox−/− cells. Our study has discovered a molecular switch in initiating p47phox activation and revealed an important mechanism for how p47phox becomes activated from partial to full opening of the sSH3 groove for p22phox binding and O2⨪ production by NADPH oxidase.
MATERIALS AND METHODS
Generation of a Full p47phox Protein Structural Model
The auto-inhibited p47phox (1–390 amino acids) model was generated based on three available crystal structures i.e. the N-terminal PX domain (residues 1–141, PDB: 1KQ6); the p47phox PRR domain (residues 359–390, PDB: 1K4U) (19); and the super-SH3 domain (sSH3) (residues 159–340, the 1NG2 crystallized structure) (14), which was kindly provided by Dr. Franca Fraternali, King's College London, UK. The missing linking segments (142-MKDGKSTATDITGPII-156 and 340-PGPQSPGSPLEEERQTQRSK-360) were generated using the web-based homology protein modeling server Phyre2 as described previously (20–22). Briefly, the short residue sequences were uploaded to the server, and underwent template identification and structure refinement. The subsequent Phyre2 models scored 50 and 90% structure confidences, respectively, which satisfied structural motifs predicted by SWISS-MODEL (23) and PHDsec (24). The generated Phyre2 linking peptides had 5 residue extensions at both the N- and C-terminal sides to superimpose with the corresponding ends of the protein crystal structures, and the protein backbones were joined together manually in molecular operating environment (MOE; Chemical Computing Group Inc., Canada). The final model of the full p47phox protein (a.a.1–390) was constructed using the protein homology modeling function in MOE, and was refined by energy minimization using the AMBER99 force field as described previously (25).
Structural Analysis
The secondary structure, residue contacts and water affinities of the energy minimized models were analyzed using the protein geometry function in MOE. The stereochemical qualities of the models were assessed by using Ramachandran plot analysis and structural analysis using the protein report function in MOE. This searches for disallowed bond angles, bond lengths and side chain rotamers. There were no unacceptable deviations in the models with less than 2 outliers in the Ramachandran plot.
In Silico p47phox Phosphorylation and Molecular Dynamics
The effect of phosphorylation-induced p47phox conformational changes was investigated in silico by adding a phosphate group (PO43−) to the side-chain of each serine residue of Ser-303, Ser-304, Ser-310, Ser-315, Ser-320, Ser-328, Ser-345, Ser-348, Ser-359, Ser-370, and Ser-379 using the molecular builder function of MOE. The phosphorylated p47phox model then underwent energy minimization for structural refinement to correct any potential disallowed bond angles, bond lengths and unfavorable torsion angles following PO43− addition.
The p47phox conformational changes associated with serine phosphorylation were studied by molecular dynamics (MD). Prior to MD simulation the protein was prepared by addition of missing hydrogen atoms which were unable to be located in low resolution protein x-ray structures. The hydrogen atoms were added at positions calculated using the Protonate 3-dimensional module of MOE. Next, the protein was solvated in a square-box of water, which was positioned randomly to occupy the volume using the solvate module in MOE. Finally, sodium ions were added to the simulation box to balance charges, and followed by energy minimization to remove any high energy interactions or van der Waals violations. The MD simulation was recorded every 0.5 picoseconds (ps) at a constant temperature of 310K for 500 ps, with a time step of 0.002 ps. The algorithm employed was the Nosé-Poincaré-Anderson with an NPT ensemble. The force field used was AMBER99 with a distance-dependent dielectric of 4 for the protein and 80 for the solvent. This resulted in a stable simulation after initial equilibration of the structure under calculated conditions. The simulation was considered to have been equilibrated after the first 100 ps.
The NPA Hamiltonian used in MOE is shown in Equations 1 and 2,
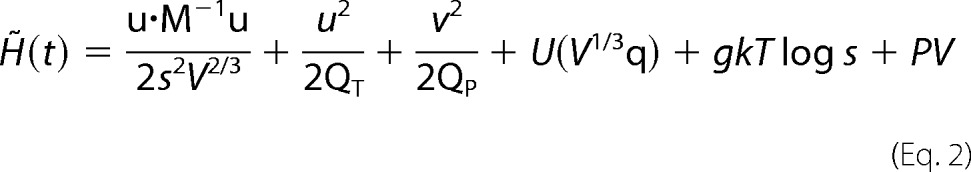 |
where g is the number of degrees of freedom in the atomic system plus one if V is not fixed. The s coordinate is a time scaling coordinate used to enforce constant temperature. The quantity H is conserved, and the equations of motion can be derived by differentiating H with respect to the conjugate coordinates and momenta to obtain the equations of motion.
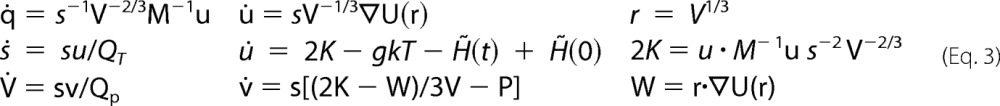 |
To solve these equations, the symplectic, time-reversible Generalized Leapfrog Algorithm is used. A Hamiltonian, H (p,q) in which q are the coordinates and p the conjugate momenta at time t can be integrated through a time step h with Equations 4–6,
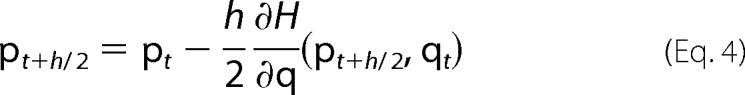 |
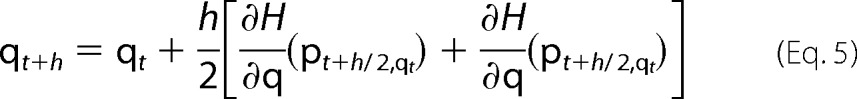 |
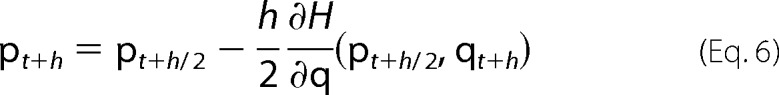 |
which is a concatenation of the Symplectic Euler method with its adjoint (its reverse time analog) (see the MOE user manual).
Molecular Docking
Protein docking is a well-established technique for probing the proteome or interactome (26). Protein-protein interaction between the p47phox sSH3 domain and the p22phox PRR domain was examined by molecular docking using MOE. A 1.80Å resolution x-ray structure of the p22phox (14) PRR was positioned near to the AIR region before and after phosphorylation and the molecular dynamics was re run for 500 ps to see if the p22phox peptide would migrate into the p47phox sSH3 binding groove. This dynamics simulation was followed by energy minimization to convergence. The resulting root mean squared deviation (RMSD) following MD when compared with the orientation of the crystallized p22phox peptide was 1.676Å, and the peptide interacted successfully with the p47phox in the sSH3 binding groove.
Site-directed Mutagenesis, Cell Culture, and Gene Transfection
The substitution of serine (S) by alanine (A) in human p47phox cDNA (GenBankTM: AF330627.1) was performed exactly as described previously using the QuikChange Multi Site-directed Mutagenesis kit (Agilent Technologies) (18). Briefly, Wild-type p47phox cDNA was cloned into pcDNA3.1/Zeovector (Invitrogen) and used for the mutagenesis. Mutated p47phox cDNAs were then sequenced and cloned into Escherichia coli, DH5α (Invitrogen) (18). The plasmids were purified for gene transfection.
The p47phox knock-out (KO) mice on a 129sv background were obtained from the European Mouse Mutant Archive, and backcrossed to C57BL/6J for 10 generations. All studies were performed in accordance with protocols approved by the Home Office under the Animals (Scientific Procedures) Act 1986 UK. The p47phox−/− coronary microvascular endothelial cells (CMEC) were isolated from the hearts of 10–12 week-old p47phox KO mice as described previously (18, 27) and were used at passage 2. The gene transfection was performed exactly as described previously (18). After 48 h of gene transfection, cells were treated with vehicle, or with phorbol 12-myristate 13-acetate (PMA, Sigma) 100 ng/ml for 30 min to activate the NADPH oxidase. Cells were used immediately for ROS measurement and cell membrane isolation.
Preparation of Cell Membrane Fraction
The cell membrane protein fraction was prepared in MOPS-KOH buffer (MOPS-KOH 20 mmol/liter, sucrose 250 mmol/liter, pH 7.4) containing PMSF (1 mmol/liter), EDTA (0.1 mmol/liter), sodium fluoride (50 mmol/liter), sodium vanadate (2 mmol/liter), leupeptin (2 μmol/liter), and pepstatin (2 μmol/liter). The fractions of cell nuclei, mitochondria, submitochondria, and small organelles were eliminated by sequential ultracentrifugation as described previously (11), and the final pellet of membrane fraction was obtained after 60 min of centrifugation at 100,000 × g. Membrane proteins were analyzed for NADPH-dependent oxidase activity by lucigenin chemiluminescence or used for immunoblotting.
Immunoprecipitation and Immunoblotting
Immunoprecipitation of p47phox was performed as described previously (18). Subsequent immunoblotting was performed using either phospho-serine specific monoclonal antibody for p47phox phosphorylation or antibody to p22phox for their association. For quantification of phos-p47phox or p22phox pulled-down with p47phox, the levels of total p47phox detected in the same sample were used as loading controls.
Measurement of O2⨪ Production
The O2⨪ production was measured by lucigenin (5 μmol/liter)-chemiluminescence as described previously (28, 29). NADPH (100 μm) was added into the cell homogenates as substrate for the detection of NADPH-dependent O2⨪ production. The specificity of O2⨪ detection was confirmed by tiron (5 mmol/liter, an O2⨪ scavenger). Potential enzymatic sources of O2⨪ production were verified using inhibitors: rotenone (100 μmol/liter), oxypurinol (100 μmol/liter), or diphenyleneiodonium (DPI, 20 μmol/liter). As an independent approach, the intracellular ROS production by adherent cells was examined by 5-(and 6)-chloromethyl-2′,7′-dichlorodrofluorescein diacetate (DCF, Invitrogen) (28). Briefly, cells cultured onto chamber slides were incubated with 5 μmol/liter of DCF in Hanks' buffer for 15 min at 37 °C with or without PMA stimulation. DCF fluorescence at an excitatory wavelength of 495 nm was immediately acquired using Olympus BX61 fluorescence microscopy. Fluorescence intensity was quantified from at least 3 random fields (269.7 × 269.2 μm) per chamber, >1000 cells assessed per cell culture experiment, and at least 3 separated cell cultures per condition. Tiron (10 mmol/liter), a cell membrane permeable non-enzymatic scavenger of O2⨪ was used to verify the detection of O2⨪ (29).
Fluorescence Microscopy
Immunofluorescence microscopy was performed as described previously (18). Antibody binding was detected by extravidin-FITC (green) or streptavidin-Cy3 (red). Normal rabbit or goat IgG (5 μg/ml) were used instead of primary antibody as negative controls. Images were acquired with an Olympus BX61 fluorescence microscope system as described previously (30).
Statistics
For the in vitro studies, data were presented as means±S.D. of results taken from at least 3 independent cell cultures/per condition. In the case of CMEC, each isolation used 6 mice/per group, and the data presented were the mean from at least 3 isolations. Comparisons were made by one-way ANOVA with Bonferroni test analysis, and p < 0.05 was considered statistically significant.
RESULTS
Computer Structural Model of Full p47phox Protein
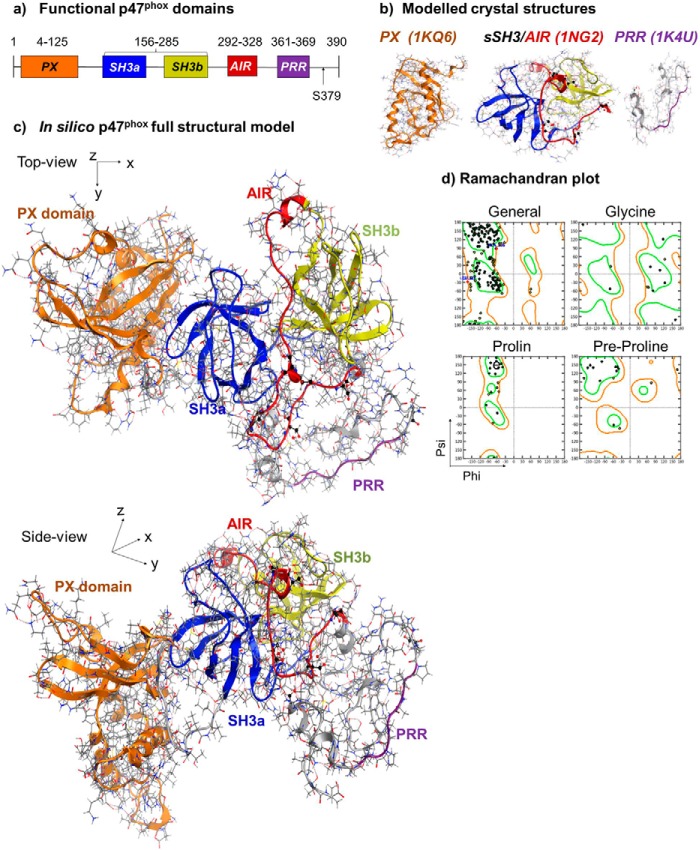
To produce a reliable structural model of the full p47phox protein, we initially generated computational models from three available crystal structures of the p47phox functional domains: the PX-domain (a.a 1–141, 1KQ6), the sSH3/AIR-domain (a.a. 159–340, 1NG2), and the PRR domain (a.a. 359–390, 1K4U) (Fig. 1, a and b). Two missing linking peptides (142-MKDGKSTATDITGPII-156 and 340-PGPQSPGSPLEEERQTQRSK-360) between these structures were generated using the homology modeling server Phyre2 with a 50 and 90% structural confidence, respectively. A full p47phox structure of the auto-inhibited form was generated using the in silico homology modeling technique (Fig. 1c). The structure has no distorted torsion angles or residues in disallowed regions as analyzed using a Ramachandran plot (Fig. 1d), and all internal hydrogen-bonds were optimally occupied as an independent measure of structural reliability. Furthermore, the generated p47phox structure is extended rather than globular, with a radius of gyration in the same order of magnitude that was published previously in a study employing small angle x-ray scattering (31).
FIGURE 1.
Computer model of the complete p47phox protein structure. a, illustration of p47phox functional domains. b, computer models of the crystal structures of p47phox functional domains shown in skeleton depiction with overlaid ribbons. Left panel: PX domain (orange color). Middle panel: Auto-inhibited sSH3 and AIR: SH3a (blue), SH3b (yellow) and AIR (red). Right panel: C-terminal tail (gray) with PRR (purple). c, computer model of complete autoinhibited p47phox with functional domains in the same color scheme as in b. d, representative Ramachandran plot for structural analysis of the p47phox protein model in autoinhibited form.
Molecular Dynamics (MD) Simulation and p47phox Phosphorylation-induced Conformational Changes
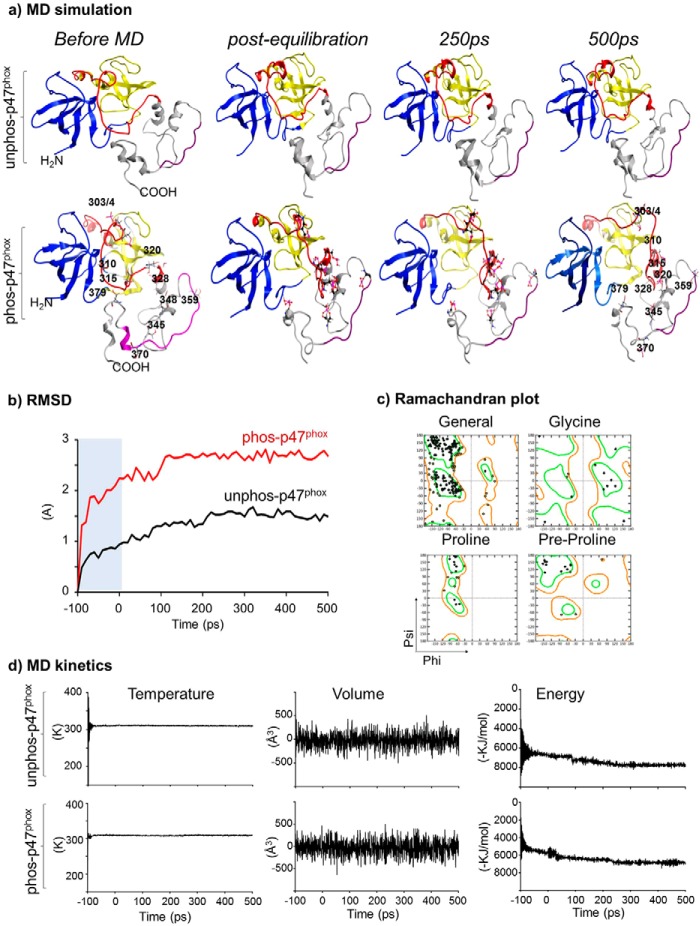
To examine the conformational changes in the p47phox structure toward the C terminus, the PX domain was omitted, and the rest of the structure (a.a. 156–390) was subjected to MD simulation (Fig. 2). We found that without phosphorylation, the auto-inhibited p47phox model remained stable over the MD time course (post-equilibration, 250 ps and 500 ps of MD) (Fig. 2a, upper panel). Importantly, there were no noticeable core structural changes between the pre-MD and after MD with a RMSD of the α-carbon backbone being 1.493 Å (Fig. 2b). The lack of gross structural changes throughout the MD simulation confirms the stability of the generated p47phox model used in this study.
FIGURE 2.
MD simulation of the p47phox structure containing sSH3, AIR, and C-terminal tail. a, structural changes of p47phox shown in ribbon depiction. Upper panel: unphosphorylated form. Lower panel: phosphorylated form. Phospho-serines at 303, 304, 310, 315, 320, 328, 345, 348, 359, 370, and 379 are shown in pink skeleton representation. b, kinetic changes of the RMSD over the time course of MD simulation in comparison to their respective pre-MD structures. Shaded region denotes the pre-equilibration stage of the simulation. c, representative Ramachandran plot of phosphorylated p47phox structure at 500 ps of MD for structural reliability. d, MD simulation temperature (left panels), volume (middle panels), and energy (right panels) of both unphosphorylated (upper panel) and phosphorylated p47phox (bottom panel).
We then added a phosphate (PO43−) group to the side-chains of Ser-303, Ser-304, Ser-310, Ser-315, Ser-320, Ser-328, Ser-345, Ser-348, Ser-359, Ser-370, and Ser-379 (Fig. 2a, lower panel). The addition of phosphate groups before MD had no significant effect on the core structural stability of the p47phox because the radius of convergence of molecular mechanics used in this way was quite small and the local structural energy was accordingly minimized to accommodate the changes to keep the structural stability. The sSH3 remained completely masked by the AIR (Fig. 2a, lower panel, left image). However, when the structure was subjected to MD simulation, the interaction between the C-terminal tail, AIR, and sSH3 underwent rapid dissociation that released AIR in less than 100 ps of MD. There was a time-dependent sequential structural change of the AIR during the course of MD simulation with the movement of the phosphorylated double serines (Ser-303/4) as the final step to fully open the sSH3 groove (Fig. 2a, lower panel at 500 ps). Compared with the un-phosphorylated structure, the p47phox α-carbon backbone RMSD kinetics in the phosphorylated form increased rapidly during the equilibration period (<100 ps) and plateaued afterward during the MD time course (Fig. 2b). The phosphorylated p47phox structure after MD had no distorted torsion angles or residues in disallowed regions as analyzed using a Ramachandran plot (Fig. 2c). There was no significant difference in the experimental settings of unphosphorylated and phosphorylated forms in terms of MD simulation parameters, i.e. temperature and volume (Fig. 2d).
Crucial Role of Ser-379 Phosphorylation in Initiating AIR Instability and p47phox Conformational Change
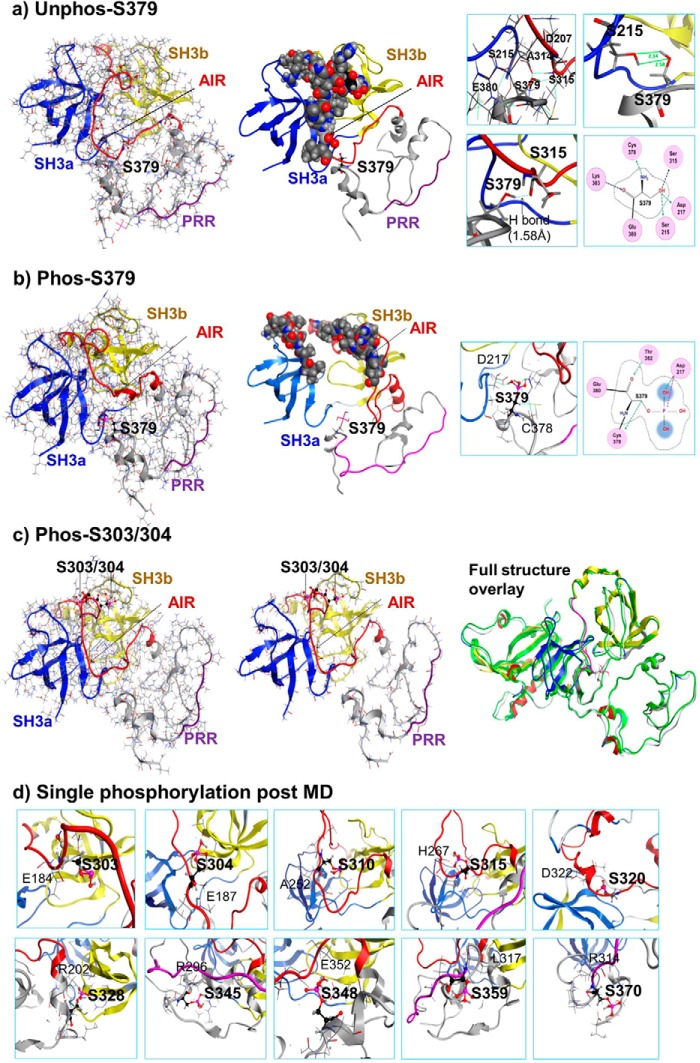
Our MD data (Fig. 2a) revealed an unexpected discovery that the movement of the phosphorylated C-terminal tail occurred before AIR destabilization. There are at least three known phosphorylation sites of PKC (Ser-359, Ser-370, and Ser-379) located in p47phox C-terminal tail. The phosphorylation of Ser-359 and Ser-370 had been found to have no significant effect on p47phox interaction with other phox subunits (16). However, the phosphorylation of Ser-379 was found to be essential for TNFα-induced ROS production by NADPH oxidase and redox-signaling in endothelial cells, although the molecular mechanism remained unknown (32). To find out how a single Ser-379 phosphorylation could affect p47phox function, we performed in silico single Ser-379 phosphorylation and MD simulation. Before phosphorylation, Ser-379 was in close proximity to both sSH3 and the AIR (Fig. 3a), and formed a H-bond with Ser-215 located in the hinge region of sSH3 and another H-bond between Ser-379 side chain and the main chain of Ser-315 located in the central part of the AIR, which tied AIR into the tandem SH3 groove (Fig. 3a, residue interaction images in the right panel), MD simulation (500 ps) of un-phosphorylated Ser-379 had no significant effect on the autoinhibited structure of p47phox (Fig. 3a, middle panel). When Ser-379 was phosphorylated and subjected to MD simulation, the negative charge generated broke the previous links immediately during the equilibration period and caused the C-terminal tail to move (Fig. 3A, left panel). After 500 ps of MD simulation, new H-bonds were formed between phosphorylated Ser-379 and Thr-382 located in the C-terminal tail and between the phosphate group of Ser-379 and the Asp-217 as a way to neutralize the local environment charges (Fig. 3c, right panel). The AIR was free, and the sSH3 groove was partially exposed (Fig. 3b, middle panel).
FIGURE 3.
Structure analysis of single serine phosphorylation at post-equilibration and at 500 ps of MD simulation. Phosphorylated serine is shown in pink skeleton. a, unphosphorylated Ser-379 in ribbon overlaid skeleton structure. Left panel: post-equilibration. Middle panel: post-MD (500ps). AIR in the sSH3 groove is presented in atomic volume balls. Right panel: Ser-379 local residue interaction maps. Hydrogen bonds are shown in green with distance labeled. b, phosphorylated Ser-379. Left panel: post-equilibration. Middle panel: post-MD (500 ps). Right panel: phospho-Ser-379 local residue interaction maps. Hydrogen bonds are shown in green. c, phosphorylated Ser-303/4. Left panel: post-equilibration. Middle panel: post-MD (500 ps). Right panel: Superposed images of p47phox unphosphorylated (in green color) and Ser-303/4 phosphorylated at 500 ps of MD (same image as in the middle panel). d, local residue interaction maps of single individual serine phosphorylation (as indicated in the map) at 500 ps of MD.
Although our data strongly indicate that Ser-379 phosphorylation is an early key event in p47phox activation, we need to confirm if it is also linked with any other single serine phosphorylation. The p47phox has a double serine phosphorylation site (Ser-303/4) located within the AIR, therefore, we checked firstly the Ser-303/4 phosphorylation. There was no significant structural change associated with Ser-303/4 phosphorylation at post-equilibration (Fig. 3c, left panel) and at 500 ps of MD (Fig. 3c, middle panel). Overlapping images of the unphosphorylated and phosphorylated structures overlaid further confirmed no significant structural change (Fig. 3c, right panel). We then examined one by one the single phosphorylation plus 500 ps of MD of Ser-303, Ser-304, Ser-310, Ser-315, Ser-328, Ser-345, Ser-348, Ser-359, and Ser-370, and confirmed that there was no significant structural change associated with these single serine phosphorylations. The local residue interaction maps taken after phosphorylation and 500ps of MD (Fig. 3d) remained the same as the maps taken before phosphorylation.
Interaction Kinetics of Phosphorylated p47phox with p22phox
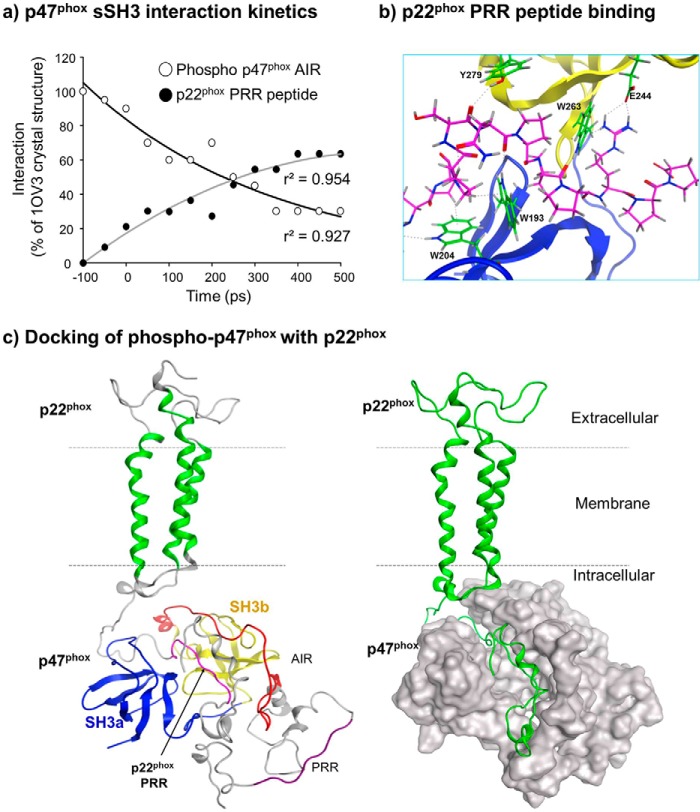
It is well documented that the cytosolic subunits of the NADPH oxidase anchor at membranes through an interaction between the p47phox sSH3 groove and p22phox PRR (33). However due to the lack of the entire protein structure, the interaction dynamics between p47phox phosphorylation, AIR dissociation, and binding to p22phox remains unclear. Using a MD-based docking technique we examined the interaction kinetics of phosphorylation-induced dissociation between AIR and sSH3 of the p47phox and the binding of p47phox sSH3 with the p22phox PRR peptide (sequence: QPPSNPPPRPP) (Fig. 4, a and b). We found a time-dependent decrease in the intramolecular interactions between the p47phox AIR and sSH3 (r2 = 0.954), and this was accompanied with a time-dependent increase in the interaction between the p22phox PRR peptide and the p47phox sSH3 (r2 = 0.927) (Fig. 4a). At 500 ps of MD simulation, the p22phox peptide migrated completely into the p47phox sSH3 binding groove in contact with the key p47phox residues as shown in the crystallized structure (1OV3) (14) except for the residue of Asp-243 (Fig. 4B). We also examined the protein-protein interaction between our phosphorylated p47phox model and the consensus p22phox model published previously (25) (Fig. 4C). We found that both the p22phox and p47phox models remained stable throughout the MD simulations and bound together successfully through the interaction between p47phox sSH3 and p22phox PRR to form a p47phox/p22phox protein complex, which for the first time provides mechanistic insight into the global association of these two proteins.
FIGURE 4.
Interaction between phosphorylated p47phox and p22phox. a, interaction kinetics of p47phox sSH3 with AIR (open circles) or with p22phox PRR peptide (filled circles) during MD simulation. b, interactions of both p47phox SHa (blue) and SHb (yellow) with p22phox-PRR peptide (pink skeleton). Green skeletons represent p47phox residues interacting with p22phox PRR peptide. c, models of global structural interactions between p47phox and p22phox following MD simulation. Left panel: Docking of phosphorylated p47phox and p22phox in ribbon presentation. p22phox PRR is in pink color. Right panel: docking of p47phox (silver space-filled presentation) with p22phox (green).
The Effect of Single p47phox Serine to Alanine Mutation on Acute PMA Stimulation-induced O2⨪ Production by p47phox−/− Endothelial Cells after Gene Transfection
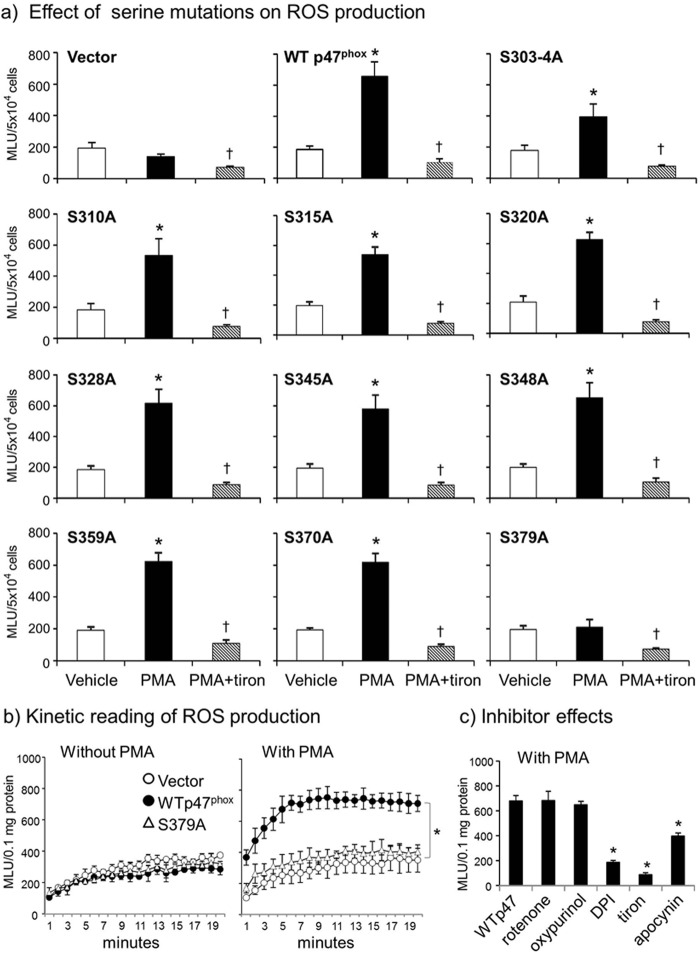
To confirm the in silico findings where Ser-379 phosphorylation is shown to be crucial for p47phox activation, we performed site-directed mutagenesis by replacing eleven serines individually in the human p47phox cDNA with alanine which could not be phosphorylated. An empty vector (PcDNA3.1/Zeo) was used as a negative control and the wild-type p47phox cDNA was used as a positive control. Mutated and wild-type p47phox cDNA constructs were used to transfect the primary CMEC isolated from the p47phox−/− mice. The cells were then stimulated with either vehicle or a potent protein kinase C activator, PMA (100 ng/ml, for 30 min) to induce p47phox phosphorylation, and then examined for NADPH oxidase activity by detecting the levels of NADPH-dependent O2⨪ production (Fig. 5a). Tiron (an O2⨪ scavenger) was used to confirm the assay specificity. We found that there was no significant difference in the basal (without PMA) levels of O2⨪ production by p47phox−/− cells after gene transfection with different constructs. However, when the cells were stimulated with PMA and compared with cells transfected with an empty vector, cells transfected with any constructs i.e. wild-type (WT) p47phox cDNA, S303–4A, S310A, S315A, S320A, S328A, S345A, S349A, S359A, and S370A increased significantly the levels of O2⨪ production, except the cells transfected with S379A. It was obvious that Ser-379 phosphorylation had a key role in initiating p47phox phosphorylation-dependent O2⨪ production by the NADPH oxidase.
FIGURE 5.
The effect of serine to alanine mutations on PMA-induced O2⨪ production by p47phox−/− CMEC after gene transfection. NADPH-dependent O2⨪ production was detected by lucigenin (5 μm) chemiluminescence in cell homogenates. a, individual serine to alanine mutations. *, p < 0.05 for PMA values versus vehicle values in the same group. †, p < 0.05 for indicated values versus PMA values in the same group. n = 3 independent CMEC isolations and gene transfection experiments. b, S379A mutation. Left panel: kinetic measurements of basal O2⨪ production (without PMA stimulation); right panel: kinetic measurements of PMA-stimulated O2⨪ production; c, effects of different enzyme inhibitors on PMA-stimulated O2⨪ production. *, p < 0.05 for indicated values versus WT p47phox cDNA transfected value in the same panel.
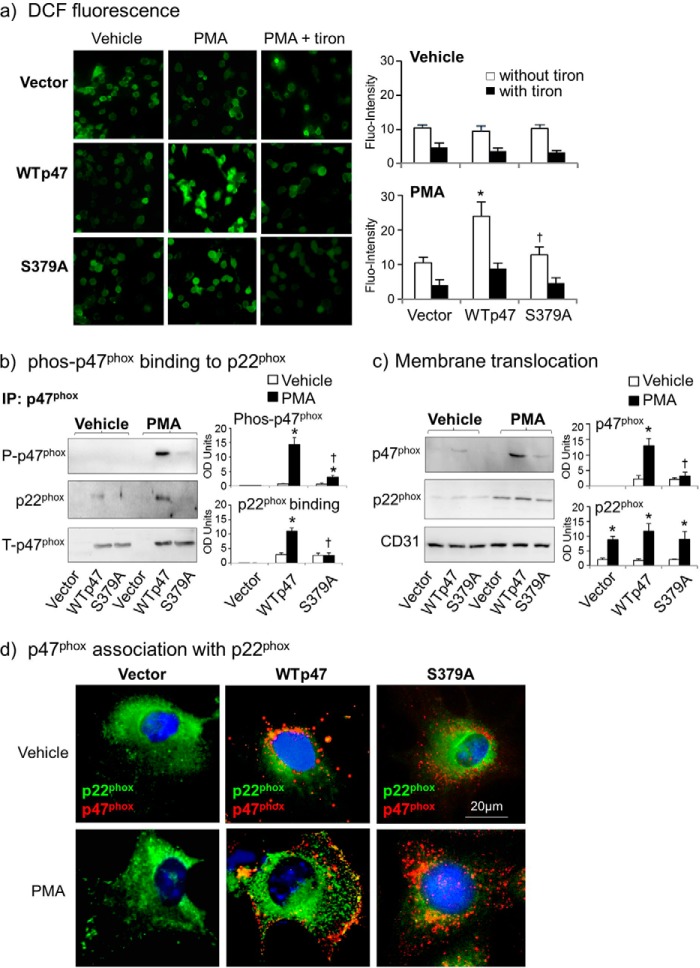
The effect of S379A on acute PMA-induced O2⨪ production by endothelial cells was further investigated in detail (Fig. 5b). Without PMA stimulation, there was no significant difference in the basal levels of O2⨪ production between cells transfected with an empty vector and cells transfected with wild-type p47phox cDNA or with S379A mutated construct (Fig. 5b, left panels). However, when the cells were stimulated with PMA, WT p47phox cDNA transfected cells significantly increased O2⨪ production, which was completely inhibited back to the vector control levels in S379A-transfected cells (p < 0.05) (Fig. 5b, middle panel). The enzymatic source of PMA-induced O2⨪ production by WT p47phox cDNA transfected cells was examined using different enzyme inhibitors. It was abolished by tiron, an O2⨪ scavenger, significantly inhibited by DPI (a flavo-protein inhibitor) and apocynin (a NADPH oxidase inhibitor), but not by inhibitors to the mitochondrial complex-1 enzymes (rotenone) or to xanthine oxidase (oxypurinol) (Fig. 5b, right panel). As an independent approach, the effect of S379A on PMA-induced O2⨪ production by intact adherent CMEC was double confirmed by tiron-inhibitable DCF fluorescence (Fig. 6a).
FIGURE 6.
Effects of S379A mutation on PMA-induced cell ROS production, p47phox binding to p22phox and membrane translocation in p47phox−/− CMEC after gene-transfection. WTp47 represents wild-type p47phox cDNA. a, DCF detection of intracellular ROS production by intact cells. *, p < 0.05 for indicated values versus vector values in the same group. †, p < 0.05 for indicated values versus WTp47 values in the same treatment group. n = 3 independent CMEC isolations and gene transfection experiments. b, p47phox was pulled down by antibody-bound beads (IP) and detected by Western blot for the levels of p47phox serine phosphorylation and binding to p22phox. The optical densities (OD) of protein bands were quantified digitally and normalized to the levels of total p47phox detected in the same samples. c, CMEC membrane fractions were detected for the levels of expression of p47phox and p22phox. The OD of protein bands were quantified digitally and normalized to the levels of CD31 detected in the same samples. *, p < 0.05 for PMA values versus vehicle values in the same group. †, p < 0.05 for indicated values versus PMA values of WT p47phox cDNA-transfected cells. n = 3 independent CMEC isolations and gene transfection experiments. d, fluorescence microscopic images of p47phox−/− CMEC after gene transfection. The p47phox is in red color and p22phox is in green color, the yellow fluorescence indicates the co-localization of p47phox with p22phox.
The Effects of S379A Mutation on PMA-induced p47phox Phosphorylation, Membrane Translocation, and Binding to p22phox in Cells
To confirm if Ser-379 phosphorylation was indeed required for the chain reaction of PMA-induced p47phox phosphorylation, membrane translocation, and complex formation with p22phox, we immune-pulled-down (IP) total p47phox protein and detected for the levels of serine phosphorylation and the levels of p22phox pulled-down together with p47phox (Fig. 6b). In vehicle-stimulated cells, the levels of p47phox phosphorylation and binding to p22phox were not detected in vector-transfected cells, and were just detectable in cells transfected with wild-type p47phox or S379A. However, when the cells were stimulated with PMA, WT p47phox cDNA-transfected cells significantly increased the levels of p47phox phosphorylation and the association with p22phox, and these were inhibited back to vector-transfected control levels in S379A construct transfected cells.
The p47phox membrane translocation was examined in membrane fractions isolated from p47phox−/− CMEC after gene transfection (Fig. 6c). The p47phox was absent in the membrane fraction of vector transfected cells. After PMA stimulation, p47phox was highly detected in the membrane fraction of WT p47phox cDNA transfected cells, and this was significantly reduced in S379A-transfected cells (Fig. 6c). The inhibitory effect of S379A on p47phox membrane translocation and association with p22phox was further confirmed by immunofluorescence (Fig. 6d). The p47phox was labeled by a goat polyclonal antibody and detected by Cy3 (red color), and p22phox was labeled by a rabbit polyclonal antibody and detected by FITC (green color). In vector-transfected cells, p22phox was detected across the entire cells. PMA stimulation induced p22phox clustering and membrane localization. The p47phox was detected in wild-type p47phox cDNA and S379A construct transfected cells. PMA stimulation induced p47phox plasma membrane translocation and association with p22phox as indicated by the yellow fluorescence seen only in WT p47phox cDNA-transfected cells, but not in S379A mutation transfected cells.
DISCUSSION
Rapid NADPH oxidase activation in neutrophils is crucial for immune response to pathogen invasion (16, 17). However inappropriate activation of NADPH oxidase causes inflammation and oxidative damage to cells with severe consequences (1). Insight into the mechanism of NADPH oxidase activation is important for new drug development and disease prevention. p47phox phosphorylation has been recognized as a prerequisite for NADPH oxidase assembly and activation (14, 15, 34, 35). However, due to the lack of complete protein three-dimensional structural information, the molecular mechanisms that govern the p47phox phosphorylation cascade, assembly with cytochrome b558 and O2⨪ production by NADPH oxidase remain unclear. In the current study we have, for the first time, presented a complete in silico model of the full p47phox protein structure in both auto-inhibited and activated forms. Our structural model is generated by a combination of the existing x-ray crystal structures of p47phox functional domains, and is stable under MD simulation. There is no evidence of disordered contacts or residues in our model as shown by the Ramachandran plot, and internal hydrogen bonds were optimally occupied, which is an independent measure of structural reliability. Our model provides a powerful tool for further investigation of the function of the p47phox and the NADPH oxidase.
Although crystal structures of several functional domains of p47phox and their role in p47phox activation had been reported previously (14–16, 36, 37), these structures remain as disconnected pieces and none of these previous studies had examined the location and a possible role of p47phox C-terminal tail in p47phox activation. A peptide of the p47phox C-terminal tail was reported previously to bind to the SH3 pocket of other phox proteins regardless of phosphorylation (19). The novelty of the current study is that it not only provides a structural model of the full p47phox protein but also revealed an important mechanism as to how these functional domains i.e. sSH3, AIR, and C-terminal tail interact with each other and regulate p47phox function in its three-dimensional form. Using a combination of in silico phosphorylation, MD simulation, and protein/protein docking we demonstrated for the first time that the C-terminal tail plays a key role in connecting AIR to sSH3 and maintaining p47phox structural stability at rest and in the regulation of p47phox activation. During the time course of phosphorylation and MD simulation, there was a swift disconnection (<100 ps of MD) between the C-terminal tail and the AIR and the sSH3, and the movement of the phosphorylated C-terminal tail occurred before the AIR phosphorylation. The entire AIR phosphorylation process was a well organized chain reaction with the movement of phosphorylated double serine, Ser-303/4 as the final step to fully open the sSH3 binding site. This is important novel information on p47phox regulation, and may have a broad application to other SH3 domain-containing proteins.
The p47phox has multiple serine phosphorylation sites within and outside the AIR toward its C terminus. Previously we only knew that extensive p47phox phosphorylation of AIR is required for p47phox activation, but we did not know the molecular switch of the AIR phosphorylation cascade. An important contribution of the current study is that we have discovered Ser-379 phosphorylation as a molecular switch to initiate the AIR phosphorylation cascade. At rest, unphosphorylated Ser-379 forms H-bonds with the hinge of sSH3 and the AIR, which connects the sSH3 and AIR together and stabilizes p47phox in its autoinhibited form. When the Ser-379 is phosphorylated, it disrupts the links with the sSH3 and AIR, and in turn frees AIR for subsequent phosphorylation. The unique role of Ser-379 phosphorylation as a molecular switch in p47phox activation has been further confirmed by extensive experimental approaches of single serine phosphorylation and MD simulation one by one of eleven potential phosphorylation sites. It is clear that none of the other single serine phosphorylation sites or even the double serine phosphorylation sites (Ser-303/4) can cause significant conformational change of AIR.
Previously, the p22phox was reported to interact with the p47phox N-terminal SH3a domain rather than the C-terminal SH3b domain (37). In this study we found that both SH3 domains interact with p22phox PRR. We have presented successfully a global complex structure model through the protein/protein docking of the p22phox PRR and the sSH3 of p47phox protein, which for the first time provides a conformational model of the two phox proteins together. The ability to decipher the molecular opening of the p47phox binding site to p22phox is crucial for specific inhibitor design that control partial or full activation of NADPH oxidase in different cell types. Endothelial cell NADPH oxidase shares the same molecular structure with neutrophil NADPH oxidase. However, NADPH oxidase in resting endothelial cells is already partially activated (34, 38). Insight into how NADPH oxidase activity is differentially regulated in different cell types is important for novel drug discovery to treat oxidative stress-related diseases without affecting the neutrophil oxidative burst response to pathogen invasion. Our three-dimensional structural model of the entire p47phox protein and models of step by step phosphorylation dynamics fill the gap in current Nox research and provide valuable insight for future investigation.
The in silico discovery was further confirmed by site-directed mutagenesis and gene transfection of the p47phox knock-out CMEC. By acute stimulation of cells with PMA, a powerful pan-PKC activator, we showed that 30 min stimulation by PMA was able to induce significant O2⨪ production in cells transfected with any of the serine to alanine mutated constructs in p47phox protein, except the S379A mutation. S379A mutation completely blocked acute PMA stimulation-induced: (i) p47phox binding to p22phox, as evidenced by immuno-pull-down assay and Western blot; (ii) p47phox membrane translocation, as evidenced by cell membrane fractionation followed by Western blot and immunofluorescence; and (iii) O2⨪ production by NADPH oxidase, as examined by both lucigenin-chemiluminescence in cell homogenates and DCF fluorescence in intact cells. These in vitro data provide strong evidence in support of our in silico models that Ser-379 phosphorylation is a molecular switch of the p47phox phosphorylation cascade in response to agonist stimulation.
In summary, we have presented for the first time an in silico three-dimensional model of the entire p47phox protein and revealed an important mechanism of p47phox activation. The molecular insight of how Ser-379 phosphorylation in the C-terminal tail initiates p47phox activation and how phosphorylation dynamically controls partial or full opening of the p47phox binding site can be further exploited for developing specific NADPH oxidase inhibitors to treat oxidative stress-related diseases.
Acknowledgment
We thank Dr. Franca Fraternali for kindly allowing us to use her initial p47phox model.
This work was funded by the University of Surrey Seed Fund and the Wellcome Trust (Grant 07863/Z/05/Z).
- ROS
- reactive oxygen species
- AIR
- autoinhibitory region
- CMEC
- coronary microvascular endothelial cells
- DCF
- 5-(and 6)-chloromethyl-2′,7′-dichlorodrofluorescein diacetate
- DPI
- diphenyleneiodonium
- KO
- knockout
- MD
- molecular dynamics
- MOE
- molecular operating environment
- PMA
- phorbol 12-myristate 13-acetate
- PRR
- proline-rich region
- ps
- picoseconds
- RMSD
- root mean squared deviation
- SH3
- Src homology 3
- sSH3
- super-SH3.
REFERENCES
- 1. Bedard K., Krause K.-H. (2007) The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 2. Babior B. M., Lambeth J. D., Nauseef W. (2002) The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397, 342–344 [DOI] [PubMed] [Google Scholar]
- 3. Li J.-M., Shah A. M. (2004) Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R1014–R1030 [DOI] [PubMed] [Google Scholar]
- 4. Drummond G. R., Selemidis S., Griendling K. K., Sobey C. G. (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 10, 453–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lambeth J. D., Cheng G., Arnold R. S., Edens W. A. (2000) Novel homologs of gp91phox. Trends Biochem. Sci. 25, 459–461 [DOI] [PubMed] [Google Scholar]
- 6. Nauseef W. M. (2004) Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 122, 277–291 [DOI] [PubMed] [Google Scholar]
- 7. Perisic O., Wilson M. I., Karathanassis D., Bravo J., Pacold M. E., Ellson C. D., Hawkins P. T., Stephens L., Williams R. L. (2004) The role of phosphoinositides and phosphorylation in regulation of NADPH oxidase. Adv. Enzyme Regul. 44, 279–298 [DOI] [PubMed] [Google Scholar]
- 8. Sumimoto H., Miyano K., Takeya R. (2005) Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 338, 677–686 [DOI] [PubMed] [Google Scholar]
- 9. El-Benna J., Dang P. M.-C., Gougerot-Pocidalo M.-A. (2008) Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 30, 279–289 [DOI] [PubMed] [Google Scholar]
- 10. Fontayne A., Dang P. M., Gougerot-Pocidalo M. A., El-Benna J. (2002) Phosphorylation of p47phox sites by PKCα, βII, δ and ζ: Effect on binding to p22phox and on NADPH oxidase activation. Biochemistry 41, 7743–7750 [DOI] [PubMed] [Google Scholar]
- 11. Li J.-M., Fan L. M., Christie M. R., Shah A. M. (2005) Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol. Cell. Biol 25, 2320–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J.-M., Shah A. M. (2003) Mechanism of endothelial cell NADPH oxidase activation by angiotensin II: Role of the p47phox subunit. J. Biol. Chem. 278, 12094–12100 [DOI] [PubMed] [Google Scholar]
- 13. McPhail L. C. (1994) SH3-dependent assembly of the phagocyte NADPH oxidase. J. Exp. Med. 180, 2011–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. (2003) Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 [DOI] [PubMed] [Google Scholar]
- 15. Autore F., Pagano B., Fornili A., Rittinger K., Fraternali F. (2010) In silico phosphorylation of the autoinhibited form of p47phox: Insights into the mechanism of activation. Biophys. J. 99, 3716–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massenet C., Chenavas S., Cohen-Addad C., Dagher M. C., Brandolin G., Pebay-Peyroula E., Fieschi F. (2005) Effects of p47phox C terminus phosphorylation on binding interaction with p40phox and p67phox: Structural and functional comparison of p40phox and p67phox SH3 domains. J. Biol. Chem. 280, 13752–13761 [DOI] [PubMed] [Google Scholar]
- 17. Faust L. R., el Benna J., Babior B. M., Chanock S. J. (1995) The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. J. Clin. Invest. 96, 1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teng L., Fan L. M., Meijles D., Li J.-M. (2012) Divergent effects of p47phox phosphorylation at S303–4 or S379 on tumor necrosis factor-α signaling via TRAF4 and MAPK in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 32, 1488–1496 [DOI] [PubMed] [Google Scholar]
- 19. Kami K., Takeya R., Sumimoto H., Kohda D. (2002) Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67phox, Grb2 and Pex13p. EMBO J. 21, 4268–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jefferys B. R., Kelley L. A., Sternberg M. J. (2010) Protein folding requires crowd control in a simulated cell. J. Mol. Biol. 397, 1329–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelley L. A., Sternberg M. J. (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 [DOI] [PubMed] [Google Scholar]
- 22. Shen H. D., Tam M. F., Huang C. H., Chou H., Tai H. Y., Chen Y. S., Sheu S. Y., Thomas W. R. (2011) Homology modeling and monoclonal antibody binding of the Der f 7 dust mite allergen. Immunol. Cell Biol. 89, 225–230 [DOI] [PubMed] [Google Scholar]
- 23. Schwede T., Kopp J., Guex N., Peitsch M. C. (2003) SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31, 3381–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rost B., Sander C. (1994) Conservation and prediction of solvent accessibility in protein families. Proteins 20, 216–226 [DOI] [PubMed] [Google Scholar]
- 25. Meijles D., Howlin B. J., Li J.-M. (2012) Consensus in silico computational modelling of the p22phox subunit of the NADPH oxidase. Comput. Biol. Chem. 39, 6–13 [DOI] [PubMed] [Google Scholar]
- 26. Wass M. N., Fuentes G., Pons C., Pazos F., Valencia A. (2012) Towards the prediction of protein interaction partners using physical docking. Mol. Syst. Biol. 7, 469- 10.1038/msb.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J.-M., Mullen A. M., Shah A. M. (2001) Phenotypic properties and characteristics of superoxide production by mouse coronary microvascular endothelial cells. J. Mol. Cell. Cardiol. 33, 1119–1131 [DOI] [PubMed] [Google Scholar]
- 28. Fan L. M., Li J. M. (2014) Evaluation of methods of detecting cell reactive oxygen species production for drug screening and cell cycle studies. J. Pharmacol. Toxicol. Methods 70, 40–47 [DOI] [PubMed] [Google Scholar]
- 29. Fan L. M., Teng L., Li J.-M. (2009) Knockout of p47phox uncovers a critical role of p40phox in reactive oxygen species production in microvascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29, 1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du J., Fan L. M., Mai A., Li J.-M. (2013) Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptor and endothelium in dietary obesity of middle-aged mice. Br. J. Pharmacol. 170, 1064–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durand D., Cannella D., Dubosclard V., Pebay-Peyroula E., Vachette P., Fieschi F. (2006) Small-angle X-ray scattering reveals an extended organization for the autoinhibitory resting state of the p47(phox) modular protein. Biochemistry 45, 7185–7193 [DOI] [PubMed] [Google Scholar]
- 32. Lambeth J. D. (2007) Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Rad. Biol. Med. 43, 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogura K., Nobuhisa I., Yuzawa S., Takeya R., Torikai S., Saikawa K., Sumimoto H., Inagaki F. (2006) NMR solution structure of the tandem Src homology 3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. J. Biol. Chem. 281, 3660–3668 [DOI] [PubMed] [Google Scholar]
- 34. Li J.-M., Wheatcroft S., Fan L. M., Kearney M. T., Shah A. M. (2004) Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2⨪ production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109, 1307–1313 [DOI] [PubMed] [Google Scholar]
- 35. El-Benna J., Dang P. M., Gougerot-Pocidalo M. A., Marie J. C., Braut-Boucher F. (2009) p47phox, the phagocyte NADPH oxidase/Nox2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 41, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burritt J. B., DeLeo F. R., McDonald C. L., Prigge J. R., Dinauer M. C., Nakamura M., Nauseef W. M., Jesaitis A. J. (2001) Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J. Biol. Chem. 276, 2053–2061 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe Y., Tsuboi H., Koyama M., Kubo M., Del Carpio C. A., Broclawik E., Ichiishi E., Kohno M., Miyamoto A. (2006) Molecular dynamics study on the ligand recognition by tandem SH3 domains of p47phox, regulating NADPH oxidase activity. Comput. Biol. Chem. 30, 303–312 [DOI] [PubMed] [Google Scholar]
- 38. Li J.-M., Shah A. M. (2002) Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J. Biol. Chem. 277, 19952–19960 [DOI] [PubMed] [Google Scholar]