Background: Hyaluronan accumulates in chronic demyelinated multiple sclerosis (MS) lesions.
Results: 4-Methylumbelliferone (4MU) inhibits HA synthesis, is protective in active and passive MS models, modulates T-cell responses, and prevents CXCL12 suppression within inflamed and non-inflamed CNS tissue.
Conclusion: Inhibition of hyaluronan synthesis protects against CNS inflammation.
Significance: Study data substantiate a link between hyaluronan and CNS inflammation.
Keywords: CXC Chemokine Receptor Type 4 (CXCR-4), Chemokine, Multiple Sclerosis, T-cell, Toll-like Receptor (TLR), 4-Methylumbelliferone, CXCL12, EAE, Hyaluronan
Abstract
Hyaluronan (HA) may have proinflammatory roles in the context of CNS autoimmunity. It accumulates in demyelinated multiple sclerosis (MS) lesions, promotes antigen presentation, and enhances T-cell activation and proliferation. HA facilitates lymphocyte binding to vessels and CNS infiltration at the CNS vascular endothelium. Furthermore, HA signals through Toll-like receptors 2 and 4 to stimulate inflammatory gene expression. We assessed the role of HA in experimental autoimmune encephalomyelitis (EAE), an animal model of MS by administration of 4-methylumbelliferone (4MU), a well established inhibitor of HA synthesis. 4MU decreased hyaluronan synthesis in vitro and in vivo. It was protective in active EAE of C57Bl/6 mice, decreased spinal inflammatory infiltrates and spinal infiltration of Th1 cells, and increased differentiation of regulatory T-cells. In adoptive transfer EAE, feeding of 4MU to donor mice significantly decreased the encephalitogenicity of lymph node cells. The transfer of proteolipid protein (PLP)-stimulated lymph node cells to 4MU-fed mice resulted in a delayed EAE onset and delayed spinal T-cell infiltration. Expression of CXCL12, an anti-inflammatory chemokine, is reduced in MS patients in CSF cells and in spinal cord tissue during EAE. Hyaluronan suppressed production of CXCL12, whereas 4MU increased spinal CXCL12 in naive animals and during neuroinflammation. Neutralization of CXCR4, the most prominent receptor of CXCL12, by administration of AMD3100 diminished the protective impact of 4MU in adoptive transfer EAE. In conclusion, hyaluronan exacerbates CNS autoimmunity, enhances encephalitogenic T-cell responses, and suppresses the protective chemokine CXCL12 in CNS tissue. Inhibition of hyaluronan synthesis with 4MU protects against an animal model of MS and may represent an important therapeutic option in MS and other neuroinflammatory diseases.
Introduction
Multiple sclerosis (MS)2 is a multifactorial disease with inflammatory and autoimmune components. Experimental autoimmune encephalomyelitis (EAE), an animal model of MS, recapitulates many pathological and clinical features of MS. It can be induced in various animal species by immunization with various myelin antigens or by transfer of activated CD4+ T-cells reactive to myelin components.
The glycosaminoglycan hyaluronan (HA) is ubiquitously present in the pericellular and extracellular matrix of vertebrate tissues. It is produced at the plasma membrane by three hyaluronan synthases (HAS1, -2, and -3) coupling glucuronic acid and N-acetylglucosamine into a linear polymer using the corresponding UDP-sugars as substrates. Hyaluronan is degraded by a family of enzymes called hyaluronidases. In humans, there are at least seven types of hyaluronidase-like enzymes (1).
HA exhibits diverse biological functions, including regulation of cell adhesion, cell proliferation, and diffusion of nutrients and growth factors (2), by its ability to interact with different proteins like CD44, brevican, aggrecan, versican, hyaluronectin, RHAMM, and neurocan (3, 4). Several studies suggest that hyaluronan may have proinflammatory roles in the context of CNS autoimmunity: 1) it accumulates in demyelinated CNS lesions in MS and EAE (5, 6); 2) HA production by dendritic cells and T-cells promotes antigen presentation and enhances T-cell activation and proliferation (2, 3, 7, 8); 3) HA-CD44 interactions at the CNS vascular endothelium facilitate lymphocyte binding to vessels and CNS infiltration (9–11); and 4) hyaluronan fragments signal through both Toll-like receptors (TLR) 2 and 4 and thereby stimulate inflammatory gene expression in immune cells (12).
In the present study, we assessed the effect of hyaluronan in EAE by inhibiting hyaluronan synthesis with 4-methylumbelliferone (4MU) administration (3, 13–17). 4MU inhibits hyaluronan synthesis in a dose-dependent manner by two mechanisms: depletion of cellular UDP-glucuronic acid and down-regulation of hyaluronan synthases 2 and 3 (14). Importantly, 4MU has no effect on the synthesis of any other glycosaminoglycan besides hyaluronan (15, 18). This agent facilitates analyses of the effects of HA inhibition on inflammatory responses in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Animals
Female Dark Agouti (DA) rats, 6–8 weeks old, were purchased from Harlan Laboratories (Indianapolis, IN) and female SJL/J and C57Bl/6 mice, 6–8 weeks old, from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in the animal facility of Roosevelt Hospital (New York) and were 8–10 weeks old when used for experiments. All procedures were conducted according to the protocols approved by the Institutional Animal Care and Use Committee of the Roosevelt Hospital.
Quantification of Hyaluronan in LN18 Cell Culture Supernatants
To determine HA cell culture levels, 100,000 LN18 cells were incubated overnight in a 12-well plate in DMEM containing 10% FBS. Subsequently, cells were washed with PBS and incubated for 2 days in DMEM in the absence of FBS. Varying amounts of 4MU dissolved in DMSO were added to the cultures. The final concentration of DMSO was 0.2%. Hyaluronan was quantified using the Hyaluronan DuoSet (R&D Systems).
Quantification of Hyaluronan in Serum and Liver Protein Extracts
Blood was collected from rat tail veins. Liver tissue was homogenized by sonication in radioimmune precipitation assay buffer. The BCA method was used to determine protein concentrations. Hyaluronan was quantified using the Hyaluronan DuoSet (R&D Systems).
Induction and Clinical Evaluation of EAE
For active EAE induction, C57Bl/6 mice were immunized with 200 μl of a suspension containing 200 μg of murine myelin oligodendrocyte glycoprotein (MOG) peptide (35MEVGWYRSPFSRVVHLYRNGK55, Pepceuticals, UK) and an equal volume of complete Freund's adjuvant supplemented with 400 μg of H37RA (DIFCO Laboratories, Detroit, MI) by subcutaneous injection.
To induce adoptive transfer EAE (atEAE) in SJL/J mice, donor mice were immunized subcutaneously with 200 μl of a suspension containing 200 μg of PLP peptide (139HSLGKWLGHPDKF151, Pepceuticals) and 100 μl of complete Freund's adjuvant. Ten days later, lymph node and spleen cells were harvested and restimulated in vitro for 4 days with 10 μg/ml PLP peptide. For EAE induction, female SJL/J mice were injected intraperitoneally with 2.5 × 107 preactivated PLP-specific cells. DA rats were immunized subcutaneously at the base of the tail with 65 Ag MOG1–125 emulsified in complete Freund's adjuvant containing 400 μg H37Ra in a total volume of 200 μl.
Animal weights and clinical scores were recorded routinely by a masked observer (0 = healthy, 1 = limp tail, 2 = partial hind limb weakness and/or ataxia, 3 = complete paralysis of at least one hind limb, 4 = severe forelimb weakness, and 5 = moribund or dead). The mean cumulative score for a treatment group was calculated as the sum of the clinical scores of all animals from day zero until the end of the experiment divided by the number of animals in the respective group.
Histopathological Analysis of Spinal Cord Infiltration
Mice were perfused transcardially with ice-cold saline followed by 4% paraformaldehyde. Spinal cord specimens were coded and cut into three pieces representing the cervical, thoracic, and lumbosacral levels. 8-μm paraffin sections were prepared. From each of the three rostrocaudal levels, five coronal sections spaced every 500 μm were stained with hematoxylin and eosin (H&E) to assess cellular infiltrates. Thus, 15 tissue sections from each animal were analyzed. Both the total area of each tissue section and the area occupied by inflammatory infiltrates were measured semiautomatically using ImageJ software (ImageJ 1.47e, National Institutes of Health, Bethesda, MD) (19) by an investigator masked for the clinical results. The percentage of spinal cord with inflammation was calculated as follows: area of the spinal cord infiltrated by inflammatory cells/total spinal cord area of the respective section.
Analysis of Th1, Th17, and Treg Cells in Lymph Nodes and Spinal Cords
Single cell suspensions of cells from inguinal lymph nodes and spinal cords of mice were established. Cells from spinal cord tissue were extracted by using a neural tissue dissociation kit (T) (Miltenyi Biotec, Germany). Cells were restimulated in vitro at a concentration of 106/ml with 100 ng/ml phorbol 12-myristate 13-acetate and ionomycin and 1 μl/ml BD GolgiPlug (BD Biosciences) for 4 h. Subsequently, cells were harvested and stained extracellularly with eFluor450 anti-mouse CD4 antibody (Clone RM4-5, eBioscience) and FITC anti-mouse CD25 (clone 7D4, BD Biosciences). Afterward, cells were washed, fixed, permeabilized with the BD Cytofix/Cytoperm kit (BD Biosciences), and stained with a phycoerythrin (PE) anti-mouse IL-17 antibody (Clone eBIO17B7, eBioscience), allophycocyanin (APC) anti-mouse IFNγ (clone XMG1.2, eBioscience), or APC anti-FoxP3 antibody (clone 150D, BioLegend). Flow cytometry analyses were performed to assess the proportions of Treg, Th17, and Th1 cells.
Western Blot
After transcardial perfusion with ice-cold saline, mouse spinal cords were isolated and homogenized by sonication. Total protein was extracted with a radioimmune precipitation assay buffer, separated by SDS-PAGE, and blotted onto nitrocellulose membranes (Schleicher & Schuell). A rabbit anti-mouse CD3e antibody (ab119332, Abcam) was used to detect CD3 and a rabbit anti-actin antibody (Thermo Scientific) for standardization. The secondary anti-rabbit antibody (Sigma-Aldrich) was conjugated to horseradish peroxidase. Bands were detected using ECLplus as substrate (Invitrogen), the Kodak Image Station 4000R Pro, and Carestream MI imaging software (Carestream).
Analysis of TLR Simulation
HEK-BlueTM mTLR2 cells (InvivoGen) were incubated with supernatants from U937 cells that had been incubated for 2 days with 0 or 200 μm 4MU in the presence of HEK-Blue detection medium. A stimulation of Toll-like receptor 2 triggers the expression of the secreted embryonic alkaline phosphatase, which results in a blue color development. After 8 h, the development of blue color was assessed using a Synergy HT plate reader (Bio-Tek).
Analysis Impact of Hyaluronan on CXCL12 Expression by Human Astroglioma LN18 Cell Line
150,000 LN18 cells were cultivated for 24 h in 12-well plates in 1 ml of DMEM containing 10% FBS. Cells were stimulated with ultra-low molecular weight and high molecular weight hyaluronan (R&D Systems) with LPS (EMD Millipore) or 4MU. 4MU was dissolved in DMSO. The final DMSO cell culture concentration was 0.2%.
RNA was isolated by using the RNeasy Plus micro kit (Qiagen) and reversely transcribed into cDNA by using the Sensiscript reverse transcription kit (Qiagen). CXCL12 expression was quantified using human CXCL12-specific TaqMan probes (Hs00171022_m1, Invitrogen) and normalized to respective 18S rRNA quantities also determined by using TaqMan probes.
Determination of CXCL12 Expression in Spinal Cord Tissue of DA Rats
DA rats were perfused with PBS. Total RNA was extracted from spinal cords using the RNeasy lipid tissue midi kit (Qiagen). cDNA was generated using the Sensiscript reverse transcription kit (Qiagen). CXCL12 expression was quantified using rat CXCL12-specific TaqMan probes (Rn00573260_m1, Invitrogen) and normalized to respective actin-b quantities (Rn00667869_m1).
Subcutaneous Administration of AMD3100
Alzet osmotic pumps (Alzet 1004, DURECT Corp.) were used to deliver AMD3100 (Sigma). Filled osmotic pumps were incubated overnight in sterile saline at 37 °C. For pump installation, mice were anesthetized with a mixture of ketamine (77 mg/kg) and xylazine (15 mg/kg) in saline. The volume and rate of delivery of PBS or AMD3100 by mini-pump were ∼100 nl/h continuously for 28 days. The AMD3100 concentration was 20 mg/ml.
Statistical Analyses
Statistical analyses were performed using SigmaStat 3.0 (SPSS Inc.). Comparisons of groups of normally distributed data were done by Student's t test or one-way ANOVA, respectively. For non-normally distributed data from EAE experiments, a Mann-Whitney rank-sum test was used for comparisons. Error bars represent standard deviation unless indicated otherwise.
RESULTS
4MU Feeding Inhibits Hyaluronan Production in Vitro and in Vivo
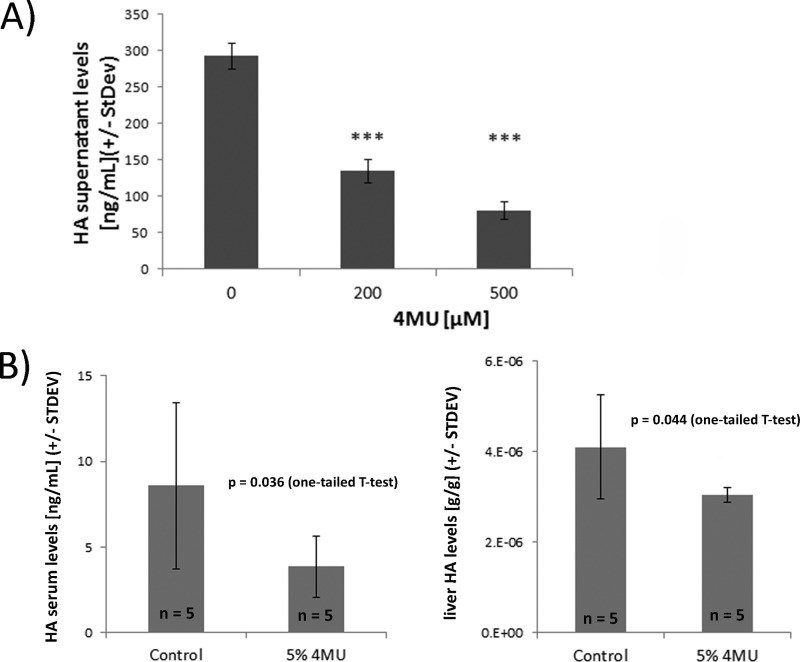
We used 4MU, a well established and specific inhibitor of hyaluronan production (3, 7, 13–17, 20), to modulate hyaluronan synthesis. First, we confirmed the inhibitory influence of 4MU on hyaluronan synthesis by in vitro experiments. 4MU strongly decreased hyaluronan supernatant levels in LN18 astroglioma cell cultures as determined by an ELISA-like hyaluronan assay (Fig. 1A).
FIGURE 1.
4MU decreases hyaluronan production in vitro and in vivo. A, LN18 cells were incubated for 2 days with 4MU dissolved in DMSO under serum-free conditions. The final DMSO concentration in cell cultures was 0.2%. Hyaluronan production was monitored by using an ELISA-like hyaluronan quantification assay (R&D Systems). ***, p < 0.001 compared with control-treated LN18 cultures (one-way ANOVA (Holm-Sidak method)). B, five naive DA rats mice were fed with 5% 4MU mixed into ground chow or with just ground chow for a total of 4 weeks. Hyaluronan concentrations in serum samples (left) and in liver protein extracts (right) were lower in 4MU-fed animals.
To modulate hyaluronan synthesis in vivo, 4MU was fed to animals ad libitum mixed within ground chow at a ratio of 5%. To confirm that 4MU reduces hyaluronan synthesis in vivo, it was fed to otherwise naive rats for 4 weeks. Subsequently, the hyaluronan levels in serum and liver protein extracts were quantified (Fig. 1B). 4MU reduced hyaluronan serum levels (13.5 ± 7.5 ng/ml (control) versus 6.2 ± 2.9 ng/ml (4MU)) and hyaluronan liver concentrations (3.9 × 10−6 ± 1.4 × 10−6 g hyaluronan/g protein (control) versus 3.2 × 10−6 ±3.4 × 10−7 g hyaluronan/g protein (4MU)). 4MU becomes glucuronidated, leading to an inhibition of hyaluronan synthesis by depleting UDP-glucuronic acid (14). Most of the 4MU glucuronidation happens in the liver, kidneys, and gastrointestinal tract (21), making the liver a likely site for observing an impact of 4MU on hyaluronan synthesis. In conclusion, we found significantly reduced levels of hyaluronan in peripheral blood and liver extracts derived from 4MU-fed rats, confirming that 4MU feeding inhibits hyaluronan synthesis in vivo.
4MU Ameliorates Active EAE in C57Bl/6 Mice
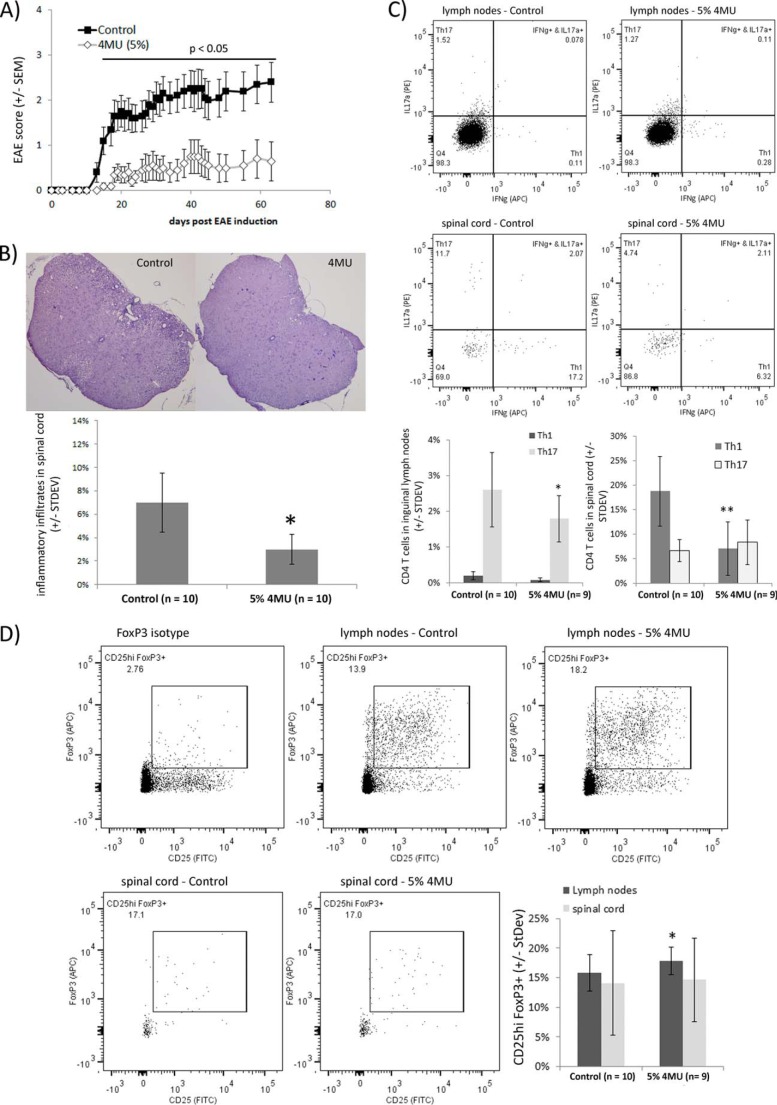
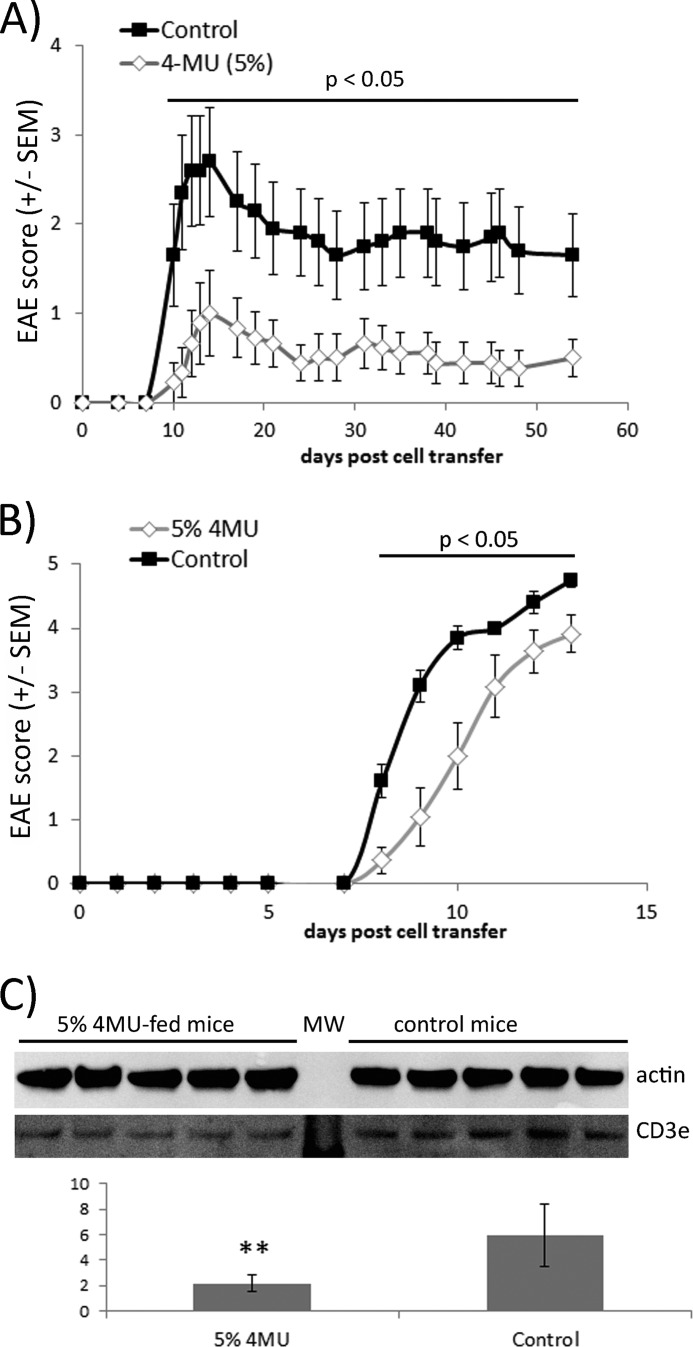
To investigate the role of hyaluronan in CNS autoimmunity, we examined the impact of 4MU feeding on the active EAE of female C57Bl/6 mice. EAE was induced in female C57Bl/6 mice by MOG peptide (amino acids 35–55) immunization. Animals were fed ad libitum with normal chow or ground chow containing 5% 4MU starting on 1 day before EAE induction. 4MU had a reproducible effect on the clinical disease course of EAE. EAE signs were more severe in control-fed mice (Fig. 2A). Both the average maximal and cumulative scores were lower in mice fed with 4MU than in control mice. In addition, 4MU delayed the onset of EAE disease (Table 1). At day 63 after EAE induction, mice were sacrificed and subsequently analyzed for inflammatory infiltrates, consisting typically of infiltrating mononuclear cells, by H&E staining of spinal cord sections. Inflammatory lesions were detectable in all mice showing clinical signs of EAE. The spinal cord area covered with inflammatory infiltrates was quantified by blinded semiautomatic image analysis and compared between groups based on the percentage of the spinal cord affected by inflammation. Mice fed with 4MU showed significantly less inflammation in spinal cord sections than control animals (Fig. 2B), and the extent of inflammation in individual mice correlated positively with the clinical score at the termination of the experiment (data not shown).
FIGURE 2.
4MU feeding inhibits development of active EAE. A, C57Bl/6 mice were fed with 5% 4MU mixed into ground chow or normal chow starting 1 day before EAE induction. Differences between the 4MU-fed and the control group were statistically significant throughout the whole experiment after day 13 (Mann-Whitney rank-sum test, p < 0.05). B, inflammatory lesions in spinal cord mice were reduced in 4MU-fed mice. Quantitative analysis of the amount of inflammation in spinal cord sections was done by H&E staining. Bars represent the average spinal cord area affected by inflammation. *, p < 0.05 (one-tailed t test). C, 9 mice fed with 5% 4MU and 10 control mice were sacrificed 20 days after EAE induction. Cells from inguinal lymph nodes and spinal cords were extracted and stimulated for 4 h with 100 ng/ml phorbol 12-myristate 13-acetate, ionomycin, and GolgiPlug. The numbers of Th1 and Th17 cells were determined by flow cytometry. *, p = 0.028 for significance of reduced Th17 cell number in lymph nodes of 4MU-fed mice **, p = 0.002 for significance of reduced Th1 cell number in spinal cords of 4MU-fed mice (one-tailed t test). D, mice analyzed in C were also analyzed for CD25hi FoxP3+ CD4+ regulatory T-cells in inguinal lymph nodes and spinal cords. *, p = 0.018 for significance of increased Treg cell number in inguinal lymph nodes of 4MU-fed mice compared with control fed mice (one-tailed t test).
TABLE 1.
Clinical characteristics of active EAE experiments
*, p < 0.05; **, p < 0.01; for comparison of mice fed with 4MU with control mice (one-tailed t test). dpi, days post-injection.
| EAE incidence | Mortality | Day of onset ± S.D. | Maximal score ± S.D. | Cumulative score ± S.D. | |
|---|---|---|---|---|---|
| Active EAE in C57Bl/6 micea | |||||
| Control | 9/10 | 0 | 14.6 ± 1.3 | 2.9 ± 1.5 | 58.3 ± 34 |
| 5% 4MU | 5/10 | 0 | 17.0 ± 1.9** | 1.4 ± 1.4** | 14 ± 24** |
| 4MU administration starting at dpi 8 in active EAE in C57Bl/6 miceb | |||||
| Control | 10/10 | 0 | 12.2 ± 1.8 | 2.9 ± 1.5 | 32.9 ± 10.8 |
| 5% 4MU | 10/10 | 0 | 17.1 ± 7.0* | 3.2 ± 1.3 | 16.3 ± 16.3* |
EAE is an animal model of MS driven by IFNγ-producing Th1 and IL-17a-producing Th17 cells (22). We addressed the influence of 4MU feeding on generation of Th1 and Th17 cells as well as their infiltration into spinal cord tissue during active MOG peptide EAE in C57Bl/6 mice. Animals were sacrificed on day 20 after active EAE induction, and CD4+ T-cells in inguinal lymph nodes and spinal cord tissue were analyzed by flow cytometry. 4MU did not modulate Th1 but decreased Th17 cell numbers in inguinal lymph nodes, suggesting that 4MU interfered with differentiation or stimulation of encephalitogenic T-cells. On the other hand, 4MU significantly reduced the numbers of Th1 cells in spinal cord tissue (Fig. 2C). In addition, the numbers of CD25hi FoxP3+ regulatory T-cells were slightly increased in inguinal lymph nodes of 4MU-fed mice (Fig. 2D). The percentage of regulatory T-cells in spinal cord tissue was characterized by a high variability and did not differ between control and 4MU-fed mice.
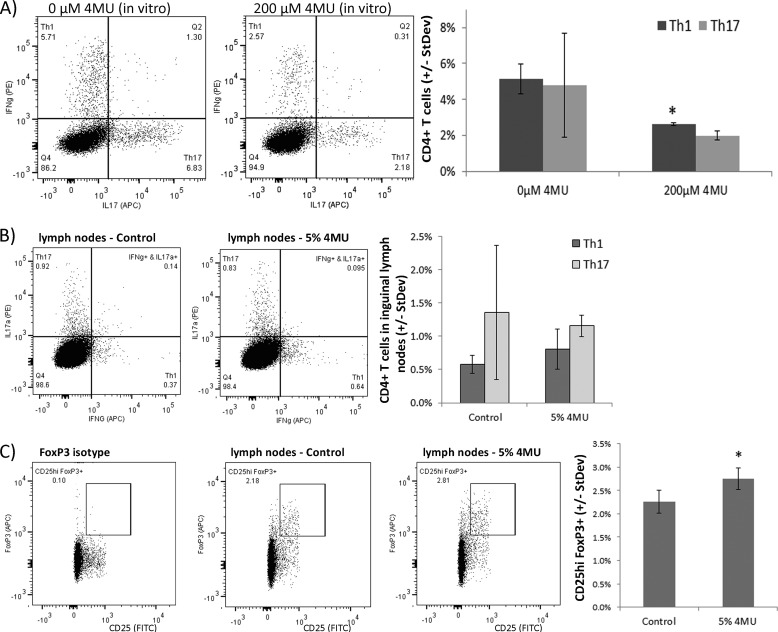
To determine whether 4MU affects the differentiation of proinflammatory Th1 and Th17 in vitro, lymph node cells isolated from MOG-immunized C57Bl/6 mice were restimulated in vitro with MOG peptide in the presence or absence of 200 μm 4MU dissolved in DMSO. A 4-day incubation with 4MU resulted in a significant reduction of Th1 cell differentiation, corroborating the anti-inflammatory impact of 4MU on T-cells (Fig. 3A).
FIGURE 3.
4MU modulates proinflammatory T-cell responses in vitro and in in vivo. A, C57Bl/6 mice were immunized with MOG peptide. Inguinal lymph node cells (LNC) were extracted on day ten and restimulated in vitro with MOG in the presence or absence of 200 μm 4MU dissolved in DMSO. Numbers of Th1 and Th17 cells were determined by flow cytometry. *: p < 0.05 for significance of reduced Th1 cell numbers (one-sided t test). B and C, inguinal LNC were extracted from naive C57Bl/6 mice which were fed with 4MU for 7 days or with normal chow. LNC were treated in vitro with 100 ng/ml phorbol 12-myristate 13-acetate and 100 ng/ml ionomycin and GolgiPlug. B, quantification of Th1 and Th17 cells by flow cytometry. C, quantification of regulatory T-cells. *: p < 0.05 for significance of increased number of regulatory T-cells in mice fed with 5% 4MU (Student's t test).
Impact of 4MU on T-cells in Naive Mice
In non-immunized naive C57Bl/6 mice fed for 7 days with 4MU, we found no difference in the number of Th1 and Th17 cells but a significant increase in the number of regulatory T-cells in inguinal lymph nodes compared with control mice (Fig. 3). In conclusion, the inhibition of hyaluronan synthesis by 4MU decreased the differentiation and CNS infiltration of encephalitogenic T-cells and enhanced the generation of regulatory T-cells.
Therapeutic 4MU Administration
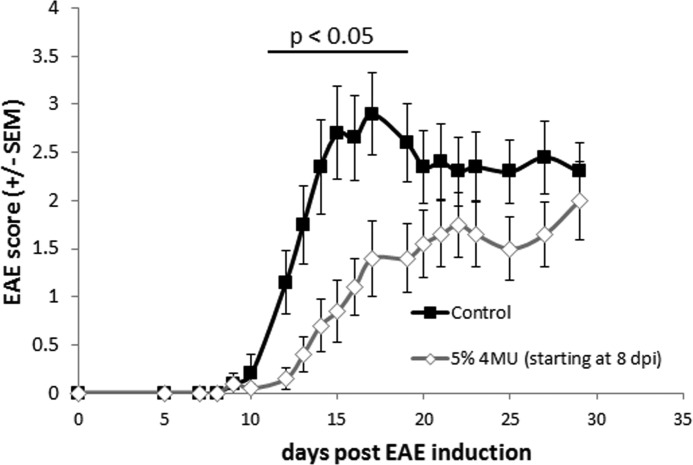
To clarify whether 4MU has any therapeutic efficacy after disease induction, 4MU was administered beginning on day 8 after MOG immunization. 4MU had a fast acting, protective effect on EAE. 4MU-fed mice had significantly lower EAE disease scores between days 12 and 19 (Fig. 4 and Table 1) as well as a significantly delayed disease onset and cumulative disease score at the end of experiment on day 29 (Table 1).
FIGURE 4.
Delayed onset of 4MU feeding (8 days post-injection (dpi)) provides transient protection within active EAE of C57Bl/6 mice. C57Bl/6 mice were fed with 5% 4MU mixed into ground chow or normal chow starting 8 days after EAE induction. Differences between the 4MU-fed and the control group were statistically significant between days 12 and 19 (Mann-Whitney rank-sum test, p < 0.05).
Influence of 4MU on atEAE of SJL/J Mice
Hyaluronan is known to be an important factor in the activation and proliferation of T-cells (2, 3, 7). Hence, a likely mechanism by which 4MU ameliorates EAE is interference with the encephalitogenicity of T-cell by inhibition of hyaluronan synthesis. However, 4MU was able to protect against EAE even when its administration was started on day 8 after MOG immunization. In other words, 4MU still protected against EAE, even when its administration started at a time point at which encephalitogenic T-cell responses were already formed (23, 24).
We designed atEAE-based experiments to investigate 4MU modulation of encephalitogenic T-cell responses independent from its effects on other potential pathological mechanisms leading to disability progression in EAE. First, we specifically targeted the 4MU influence on T-cell encephalitogenicity. Donor SJL/J mice were fed with 5% 4MU or control chow followed 1 day later by PLP peptide immunization. After 10 days lymph node and spleen cells were harvested and restimulated in vitro for 4 days with PLP. 2.5 × 107 cells were subsequently transferred by intraperitoneal injections to otherwise naive mice for EAE induction and evaluation of the 4MU impact on T-cell encephalitogenicity. Cells from 4MU-fed mice caused a significantly milder disease course compared with cells from control-fed mice, paralleled by reduced mean maximal and cumulative EAE disease scores (Fig. 5A and Table 2). This finding demonstrates that the 4MU protective influence in active EAE relies at least partially on modulation of T-cell responses.
FIGURE 5.
Influence of 4MU feeding on atEAE. A, influence of 4MU on T-cell encephalitogenicity. SJL/J mice fed with or without 4MU were immunized with PLP peptide. Ten days later their spleen/lymph node cells were harvested and rest7imulated for 4 days with PLP. 2.5 × 107 cells were transferred into naive mice for EAE induction. Differences between the mice receiving cells from 4MU-fed mice and those receiving cells from control mice were statistically significant throughout the whole experiment after day 10 (Mann-Whitney rank-sum test, p < 0.05). B, influence of 4MU on mice receiving encephalitogenic T-cells. SJL/J mice fed with 5% 4MU or normal chow were injected with 2.5 × 107 PLP-specific lymph node/spleen cells for EAE induction. Differences between the 4MU-fed mice and the control mice were statistically significant throughout the whole experiment after day 8 (Mann-Whitney rank-sum test, p < 0.05). C, spinal cords were dissected from 4MU-fed and control mice 8 days after injection of PLP-stimulated lymph node/spleen cells. At this time point 2 of 5 control mice showed EAE disease symptoms while all 4MU-fed mice were healthy. Spinal cord tissue was homogenized and used for CD3e Western blot. Results represent the mean of CD3e-specific signal normalized to actin signals. **, p < 0.01 for differences between CD3e signals derived from 4MU-fed mice and control mice (Student's t test).
TABLE 2.
Clinical characteristics of atEAE experiments
*, p < 0.05; ***, p < 0.001; for comparison of 4MU-fed group with control group (one-tailed t test).
| EAE incidence | Mortality | Day of onset ± S.D. | Maximal score ± S.D. | Cumulative score ± S.D. | |
|---|---|---|---|---|---|
| atEAE in SJL/J mice − 4MU feeding of donor micea | |||||
| Control | 9/10 | 1 | 11.9 ± 4.9 | 3.0 ± 1.8 | 44.4 ± 36 |
| 5% 4MU | 4/9 | 0 | 12.0 ± 1.4 | 1.2 ± 1.4* | 13.0 ± 16* |
| atEAE in SJL/J mice − 4MU feeding of mice receiving MOG-stimulated cellsb | |||||
| Control | 10/10 | 6 | 8.1 ± 0.3 | 4.75 ± 0.4 | 21.4 ± 2.1 |
| 5% 4MU | 10/10 | 1 | 9.8 ± 1.5*** | 3.9 ± 1.0* | 13.4 ± 6.5*** |
| Passive EAE in C57Bl/6 mice with or without 50 μg/day AMD3100c | |||||
| PBS | 8/8 | 2 | 7.6 ± 0.8 | 4.1 ± 0.7 | 47.9 ± 12.4 |
| PBS/5% 4MU | 7/8 | 1 | 9.2 ± 1.4* | 3.1 ± 1.8 | 33.8 ± 22.1 |
| AMD3100 | 8/8 | 2 | 7.6 ± 0.7 | 4.3 ± 0.5 | 51.6 ± 10.3 |
| AMD3100/5% 4MU | 8/8 | 2 | 8.4 ± 1.8 | 3.8 ± 1.8 | 42.1 ± 17.4 |
To examine whether 4MU interferes with additional pathogenic processes, we tested the 4MU impact on a complimentary atEAE experiment. 4MU-fed SJL/J mice were injected with cells from PLP-stimulated cell cultures established from the spleens and lymph nodes of mice immunized with PLP 10 days earlier. Importantly, the donor mice were not exposed to 4MU at all. Although the resulting disease course was very severe, feeding 4MU delayed the disease onset and significantly reduced the mortality and mean maximal and cumulative disease scores (Fig. 5B and Table 2).
To quantitatively assess early T-cell infiltration into the CNS of 4MU-fed and control mice, spinal cords were dissected 8 days after the transfer of PLP-stimulated encephalitogenic T-cells. Tissue homogenates were analyzed by semiquantitative Western blot for CD3e protein as a marker for T-cell infiltration. At that time point, mice showed only minimal clinical disease; none of the 4MU-fed mice and two of five control mice had developed mild disease symptoms. Control mice had greater than 2-fold more CD3e protein in spinal cord protein extracts than the 4MU-fed animals (Fig. 5C). In conclusion, 4MU protects against encephalitogenic myelin-specific T-cells, generated in the absence of 4MU, most likely by reducing early T-cell infiltration into CNS tissue.
4MU Ameliorates EAE by Interfering with Suppression of CXCL12 by Hyaluronan
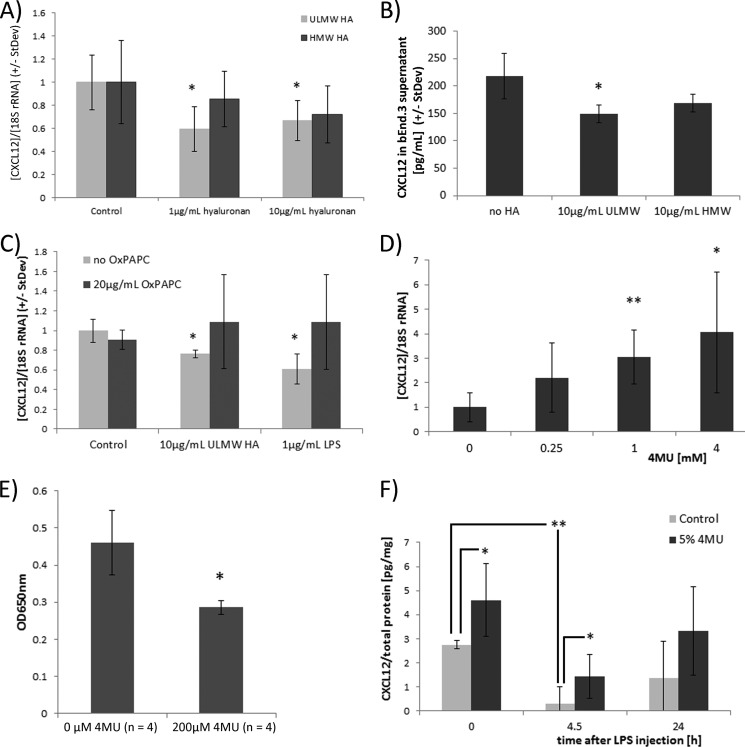
To identify the mechanism by which the inhibition of hyaluronan synthesis was protective in atEAE, we stimulated cell lines representative of CNS-resident cells with hyaluronan. Human astroglioma LN18 cells were incubated for 24 h in DMEM containing 10% FBS with ultra-low molecular weight (ULMW, 4000–8000) and high molecular weight (HMW, >950,000) hyaluronan. ULMW, but not HMW hyaluronan significantly suppressed the chemokine CXCL12 (Fig. 6A). Suppression of CXCL12 by ULMW HA was independent of the presence of FBS in the culture medium, because treating LN18 cells for 1 day with 10 μg/ml ULMW HA in serum-free medium decreased CXCL12 supernatant levels significantly (285.7 ± 55 pg/ml CXCL12 (control cultures) versus 194.1 ± 43 pg/ml CXCL12 (ULMW HA-treated cultures), n = 4, p = 0.024; one-sided t test, data not shown). Murine brain endothelial bEnd.3 cell cultures stimulated with ULMW had lower CXCL12 supernatant levels (Fig. 6B) corroborating the theory that ultra-low molecular weight hyaluronan suppresses CXCL12 in CNS-resident cells.
FIGURE 6.
Inhibition of hyaluronan synthesis by 4MU increases expression of CXCL12 in the CNS. A, LN18 cells were incubated in DMEM containing 10% FBS for 24 h with indicated amounts of ULMW and HMW hyaluronan. CXCL12 was significantly suppressed by ULMW HA. n = 4/group; *, p < 0.05 (one-way ANOVA (Holm-Sidak method)). B, 250,000 bEnd.3 cells (40) were incubated for 48 h in 2 ml of DMEM containing 10% FBS in the presence of 10 μg/ml ULMW HA or HMW HA. CXCL12 cell culture supernatant levels were determined by ELISA (mouse CXCL12 DuoSet, R&D Systems). n = 4/group; *, p < 0.05 for significance of reduced CXCL12 levels in ULMW-treated cell cultures (one-Way ANOVA (Holm-Sidak method)). C, LN18 cells were incubated for 24 h in FBS-containing DMEM with the indicated amounts of ULMW hyaluronan or LPS in the presence or absence of 20 μg/ml TLR2 and -4 inhibitor OxPAPC. *, p < 0.05 comparing LN18 cells stimulated with ULMW HA or LPS in the absence of OxPAPC with untreated LN18 cells (one-way ANOVA (Holm-Sidak method)). D, LN18 cells incubated for 24 h in FBS-containing DMEM with the indicated concentration of 4MU dissolved in DMSO (final DMSO concentration, 0.2%). *, p < 0.05; **, p < 0.01 comparing cells treated with particular 4MU concentration with control-treated cells (one-way ANOVA (Holm-Sidak method)). E, 200,000 HEK-BlueTM hTLR2 cells (InvivoGen) were incubated in 1 ml of serum-containing DMEM for 8 h in the presence of HEK-Blue detection medium. 200 μl of cell culture supernatants from U937 cells was added; this was initially incubated without 4MU or 200 μm 4MU for 2 days. TLR2 stimulation correlates with blue color development. 4MU was dissolved in DMSO with a final DMSO concentration of 0.1% (v/v). F, mice were fed with 4MU for 5 days, LPS was injected intraperitoneally at a concentration of 5 mg/kg bodyweight, and CXCL12 was quantified by ELISA in spinal protein extracts. *, p < 0.05 comparing CXCL12 spinal cord levels of 4MU-fed with control-fed mice. **, p = 0.0011 for reduced levels of CXCL12 4.5 h after LPS injection (one-way ANOVA).
Low molecular weight hyaluronan is known to stimulate TLR2 and -4 (12). We next determined that ULMW HA suppressed CXCL12 in LN18 cells via TLR stimulation, because incubating LN18 cells with ULMW hyaluronan or the TLR2 and -4 stimulant LPS inhibited CXCL12 expression. In contrast, the addition of OxPAPC, an inhibitor of TLR2 and -4 signaling (25), to LPS- and ULMW hyaluronan-stimulated cells reversed the CXCL12 suppression by ULMW HA (Fig. 6C).
On the other hand, the inhibition of hyaluronan synthesis in LN18 cells by 4MU resulted in a concentration-dependent increase of CXCL12 expression (Fig. 6D). To determine whether 4MU interferes with TLR2 signaling, we incubated the HEK-Blue mTLR2 reporter cell line (InvivoGen) with 4MU. The stimulation of TLR2 induces the expression of alkaline phosphatase in these cells that can be followed by a blue color development. The addition of supernatants from 4MU-treated U937 cell cultures to HEK-Blue mTLR2 cells reduced the blue color development (Fig. 6E), indicating that 4MU is able to interfere with TLR2 stimulation by inhibiting hyaluronan synthesis and thereby overcome the inhibition of CXCL12.
We subsequently investigated the in vivo impact of 4MU on CNS levels of CXCL12. Proteins were extracted from spinal cord tissue of control-fed mice and of mice fed with 5% 4MU for 5 days. Quantification of CXCL12 protein by ELISA revealed that 4MU-fed mice had higher spinal CXCL12 levels (Fig. 6F). Intraperitoneal LPS injections decreased CXCL12 spinal cord levels within 4.5 h, suggesting that systemic inflammation decreases CXCL12 expression in the CNS. After 24 h the CXCL12 levels in spinal cord protein extracts were closer to the original levels prior to LPS injection. 4MU feeding resulted in higher CXCL12 spinal cord levels at 4.5 h after LPS injection (Fig. 6F).
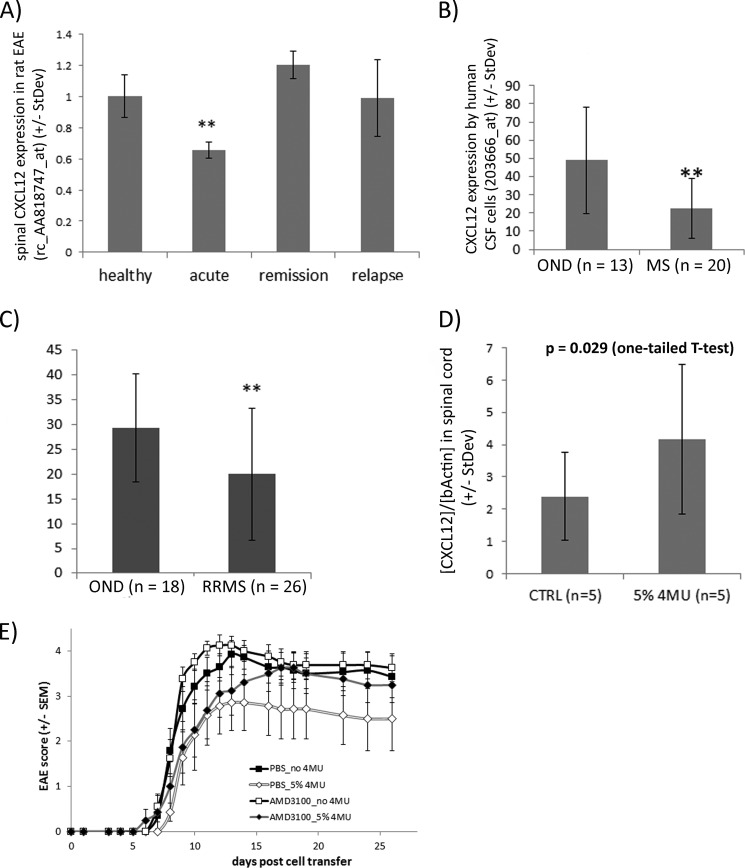
To corroborate the inhibitory influence of CNS inflammation on CXCL12 expression, we looked into recent microarray studies. CXCL12 was significantly suppressed in spinal cord tissue during the acute disease phase of the MOG-induced EAE of DA rats (Fig. 7A, ArrayExpress database E-MEXP-1025 (26). Moreover, cerebrospinal fluid cells isolated from MS patients had a lower expression of CXCL12 than CSF cells from control patients (Fig. 7B (27)). The reduced expression of CXCL12 in the CSF cells from MS patients conformed to data from a comparable transcriptomic analysis in which the CSF cell transcriptome of relapsing-remitting MS patients was analyzed and compared with profiles from control patients (Fig. 7C (28)).
FIGURE 7.
CXCL12 is suppressed in inflamed CNS, and neutralizing CXCL12 signaling reduces the protective effect of 4MU on atEAE. A, CXCL12 expression in spinal cord tissue is significantly decreased during active MOG-induced EAE of DA rats compared with naive rats (26). **, p < 0.01 (one-way ANOVA (Holm-Sidak method)). B, expression of CXCL12 is lower in CSF cells of untreated MS patients than in untreated patients with other neurological diseases (27). Data are derived from hybridization of HG U133 plus 2.0 Affymetrix microarrays (probe set 203666_at). **, p = 0.0011 (one-way ANOVA). C, decreased expression of CXCL12 by CSF cells of relapsing-remitting MS (RRMS) patients compared with patients with other neurological diseases (OND). Data are derived from a microarray study published in 2010 (ArrayExpress data set E-MTAB-69, probe set 203666_at (28)). **, p < 0.01 (one-tailed t test). D, female DA rats were sacrificed 10 days after MOG immunization (before disease onset). Half of the rats were fed with 4MU. CXCL12 expression was quantified by real-time PCR. *, p < 0.029 (one-tailed t test). CTRL, control. E, SJL/J mice were injected with 20 × 106 encephalitogenic lymph node/spleen cells for EAE induction. CXCR4 inhibitor AMD3100 (∼50 μg/day) was delivered subcutaneously by osmotic pumps starting 2 days before EAE induction. After day 22, the disease scores of 4MU-fed mice were significantly lower than of those of control mice in the absence of AMD3100. 4MU was not protective in mice that received AMD3100 at any time point (Mann-Whitney rank-sum test).
To determine whether 4MU feeding modulated the expression of CXCL12 in CNS tissue during MOG-induced EAE of DA rats, we analyzed the expression of CXCL12 before disease onset at day 10 after MOG immunization. Real-time PCR analyses showed higher CXCL12 expression in spinal cord tissue of 4MU-fed rats (Fig. 7D). We concluded that CNS inflammation suppresses CXCL12 within the CNS in EAE and MS and that this suppression can be overcome by hyaluronan synthesis inhibition achieved by oral administration of 4MU.
Currently three independent studies provide evidence that CXCL12 has a protective role in EAE (29–31). Therefore, we investigated whether the modulation of CXCL12 expression contributed to the protective impact of 4MU on EAE by inhibiting its most prominent receptor, CXCR4, in vivo by administration of AMD3100, a highly selective inhibitor of CXCR4 (32). AMD3100 was administered subcutaneously by osmotic pumps starting 2 days before the initiation of adoptive transfer EAE in SJL/J mice; the control mice received PBS instead. EAE was induced by transfer of lymph node/spleen cells from PLP-immunized SJL/J mice as described previously. Feeding mice with 4MU reduced the disease severity and delayed its onset significantly, as already shown in Fig. 5B, but in AMD3100-treated mice it neither decreased disease scores nor delayed the disease onset (Fig. 7E and Table 2). This implies that inhibition of hyaluronan synthesis through 4MU administration is not only protective by modulation of T-cell responses but also by increasing CXCL12 expression in the CNS.
DISCUSSION
In the current study, we investigated the role of the glycosaminoglycan hyaluronan in the pathogenesis of EAE, an animal model of multiple sclerosis. Orally administered 4MU interferes with HA synthesis by becoming glucuronidated, primarily in the liver, kidneys, and gastrointestinal tract (21). Thereby it leads to the depletion of glucuronic acid, one of two components of HA, resulting in the inhibition of hyaluronan synthesis. We found that 4MU treatment reduced hyaluronan levels in vitro and in vivo. 4MU feeding had a protective effect on disability accumulation in active murine and rat EAE models. The protection correlated with reduced inflammatory spinal cord lesions and a decrease of proinflammatory IFNγ-producing Th1 cell numbers in spinal cord tissue and of Th17 cells in inguinal lymph nodes. This suggests that the inhibition of hyaluronan synthesis by 4MU lessens proinflammatory T-cell responses in the periphery, leading to a subsequent inhibition of CNS inflammation and occurrence of EAE-related disabilities.
To further determine that the protection mediated by 4MU feeding relies on modulation of T-cell responses, we used the adoptive transfer model of EAE. Transferring PLP-specific T-cells from 4MU-fed mice caused a less severe EAE disease course than transferring cells from control mice. This confirmed that the protective impact of 4MU on disability accumulation in EAE acted via the modulation of T-cell responses. Possible mechanisms by which hyaluronan can stimulate T-cells have already been discussed by others (i) 4MU-mediated inhibition of hyaluronan synthesis in mitogen-stimulated T-cell cultures suppresses the production of the cytokine IL-2 that plays a crucial role in T-cell proliferation and differentiation (7). (ii) Hyaluronan produced and displayed on the surface of dendritic cells enhances antigen-specific T-cell activation by stabilizing T-cell receptor-APC interactions through functioning as an intracellular “glue” between T-cells and antigen-presenting cells (2, 3).
We next examined the impact of 4MU feeding of mice receiving already activated PLP-specific lymph node/spleen cells for EAE induction. Encephalitogenic T-cell responses were formed in the absence of 4MU in this experiment. 4MU provided a significant benefit in this setting. It reduced mortality and disability accumulation and delayed the onset of EAE. Hence, 4MU protection in EAE may not rely solely on the reduction of T-cell encephalitogenicity. Other pathogenic mechanisms besides T-cell responses must have been interrupted by 4MU as well. It may interfere with infiltration of lymphocytes into the CNS, because hyaluronan can act as an anchor molecule for CD44+ lymphocytes facilitating their adherence to the CD44+ endothelial cells (33) required for subsequent lymphocyte rolling, adhesion, and diapedesis through the blood-brain barrier (10, 11). We showed by semiquantitative Western blot analysis that spinal cord tissue extracted from 4MU-fed mice on day 8 after encephalitogenic T-cell transfer had lower CD3e protein amounts in comparison with that from control mice. This suggests a delayed infiltration of T-cells into the CNS in 4MU-fed mice, substantiating the possible influence of 4MU on lymphocyte extravasation in EAE.
A possible inhibitory influence of 4MU on lymphocyte infiltration was corroborated by a significant decrease of EAE disease scores in mice fed with 4MU starting on 8 days after active EAE induction. In this experiment, encephalitogenic MOG-specific immune responses were already formed at the time the 4MU administration started (23, 24). Feeding of 4MU resulted in a very rapid protection in EAE. Disease scores were significantly lower in 4MU-fed mice than in control mice as soon as day 4 after onset of 4MU administration.
The mechanism by which 4MU has anti-inflammatory effects on experimental demyelination may not be solely mediated by T-cells. We detected reduced spinal cord levels of the chemokine CXCL12 under neuroinflammatory conditions caused by systemic LPS injection (34), paralleled by a reduced expression of CXCL12 in the spinal cords of EAE rats compared with healthy rats and by CSF cells of MS patients compared with patients diagnosed with other neurological diseases.
Oral 4MU administration raised spinal CXCL12 protein levels in non-inflamed CNS tissue and in inflamed CNS tissue of LPS-injected mice. It also increased spinal CXCL12 expression in MOG-immunized rats, indicating that hyaluronan regulates the production of CXCL12 in CNS. This conclusion was corroborated by in vitro experiments in which CXCL12 was suppressed in astroglioma and brain endothelial cells stimulated with low-molecular weight hyaluronan. The suppressive impact of hyaluronan on CXCL12 was mediated by TLR2 or -4 triggering, because the TLR2 and -4 inhibitor OxPAPC diminished hyaluronan-mediated suppression of CXCL12.
Although CXCL12 is a proinflammatory chemokine that attracts CXCR4+ T-cells to sites of inflammation and acts as a costimulator in T-cell activation (35), recent findings associate CXCL12 with anti-inflammatory properties, especially in context of neuroinflammation. (i) CXCL12 is up-regulated in spinal cord tissue after MOG immunization in EAE-resistant albino Oxford rats. Administration of the CXCL12 antagonist AMD3100 made these rats susceptible to EAE, suggesting that CXCL12 is able to suppress autoimmune processes within the CNS (31). (ii) In MOG peptide-induced EAE of C57Bl/6 mice, a strong basolateral expression of CXCL12 by CNS endothelial cells is necessary to limit the CNS infiltration of mononuclear cells. Inhibition of the interactions of CXCL12 and its receptor CXCR4 by AMD3100 is associated with widespread parenchymal invasion of mononuclear cells in EAE (30). (iii) CXCL12 attracts immature dendritic cells with anti-inflammatory properties into the CNS (36). (iv) Finally, CXCL12 is able to redirect already differentiated Th1 cells into anti-regulatory T-cells (29). We showed that inhibition of CXCR4 by the application of AMD3100 neutralized the protective impact of 4MU on passive EAE in C57Bl/6 mice, suggesting that hyaluronan augments CNS autoimmunity by suppressing CXCL12 production in the CNS.
CXCL12 also serves as an important factor for survival, differentiation, and migration of neuronal and oligodendroglial progenitor cells (37) and has been shown to be essential for remyelination in the cuprizone-induced demyelination model (38). Hyaluronan accumulates in chronic demyelinated MS lesions and may contribute substantially to remyelination failure by preventing the maturation of oligodendroglial progenitor cells (5). We showed that hyaluronan suppresses CXCL12 in astrocytes and brain endothelial cells. This may represent a new mechanism by which hyaluronan in astroglial scars contributes to the lack of lesion repair associated with chronic MS (39).
4MU is a natural compound and is abundant in many edible plants; moreover, 4MU is already used in patients as a musculotrophic smooth muscle relaxant to treat nonspecific abdominal pain and as a cholagogue (Cholspasmin). Furthermore, 4MU (Heparvit) is in phase II of a clinical study in which it is being evaluated for its benefit in chronic hepatitis B and C. Administration of 4MU to patients has been shown to be safe, implying that it could be tested for efficacy in MS patients.
In conclusion, feeding of 4MU, a highly specific hyaluronan synthesis inhibitor, ameliorated the development of active EAE in mice and rats, delayed development of adoptive transfer EAE, and suppressed the generation of encephalitogenic T-cell responses. In addition, hyaluronan suppressed CXCL12. This finding represents a novel disease mechanism that promotes CNS inflammation and diminishes CNS repair processes that could be targeted by modulation of hyaluronan synthesis.
Acknowledgments
We thank Hana Conlon and Esther Jun for great technical assistance, Kathleen Barrett for reviewing the manuscript, and Wei Chao (Roosevelt Hospital, New York) for supporting the radioactive experiments.
Footnotes
- MS
- multiple sclerosis
- EAE
- experimental autoimmune encephalomyelitis
- atEAE
- adoptive transfer EAE
- HA
- hyaluronan
- TLR
- Toll-like receptor
- 4MU
- 4-methylumbelliferone
- DMSO
- dimethyl sulfoxide
- OxPAPC
- oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine
- ANOVA
- analysis of variance
- PLP
- proteolipid protein
- ULMW
- ultra-low molecular weight
- HMW
- high molecular weight
- MOG
- myelin oligodendrocyte glycoprotein.
REFERENCES
- 1. Tesar B. M., Jiang D., Liang J., Palmer S. M., Noble P. W., Goldstein D. R. (2006) The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transplant. 6, 2622–2635 [DOI] [PubMed] [Google Scholar]
- 2. Bollyky P. L., Evanko S. P., Wu R. P., Potter-Perigo S., Long S. A., Kinsella B., Reijonen H., Guebtner K., Teng B., Chan C. K., Braun K. R., Gebe J. A., Nepom G. T., Wight T. N. (2010) Th1 cytokines promote T-cell binding to antigen-presenting cells via enhanced hyaluronan production and accumulation at the immune synapse. Cell. Mol. Immunol. 7, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mummert M. E., Mummert D., Edelbaum D., Hui F., Matsue H., Takashima A. (2002) Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J. Immunol. 169, 4322–4331 [DOI] [PubMed] [Google Scholar]
- 4. Hoare K., Savani R. C., Wang C., Yang B., Turley E. A. (1993) Identification of hyaluronan-binding proteins using a biotinylated hyaluronan probe. Connect. Tissue Res. 30, 117–126 [DOI] [PubMed] [Google Scholar]
- 5. Back S. A., Tuohy T. M., Chen H., Wallingford N., Craig A., Struve J., Luo N. L., Banine F., Liu Y., Chang A., Trapp B. D., Bebo B. F., Jr., Rao M. S., Sherman L. S. (2005) Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966–972 [DOI] [PubMed] [Google Scholar]
- 6. Sloane J. A., Batt C., Ma Y., Harris Z. M., Trapp B., Vartanian T. (2010) Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl. Acad. Sci. U.S.A. 107, 11555–11560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahaffey C. L., Mummert M. E. (2007) Hyaluronan synthesis is required for IL-2-mediated T cell proliferation. J. Immunol. 179, 8191–8199 [DOI] [PubMed] [Google Scholar]
- 8. Maeshima N., Poon G. F., Dosanjh M., Felberg J., Lee S. S., Cross J. L., Birkenhead D., Johnson P. (2011) Hyaluronan binding identifies the most proliferative activated and memory T cells. Eur. J. Immunol. 41, 1108–1119 [DOI] [PubMed] [Google Scholar]
- 9. Winkler C. W., Foster S. C., Matsumoto S. G., Preston M. A., Xing R., Bebo B. F., Banine F., Berny-Lang M. A., Itakura A., McCarty O. J., Sherman L. S. (2012) Hyaluronan anchored to activated CD44 on CNS vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis. J. Biol. Chem. 287, 33237–33251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brocke S., Piercy C., Steinman L., Weissman I. L., Veromaa T. (1999) Antibodies to CD44 and integrin α4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc. Natl. Acad. Sci. U.S.A. 96, 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brennan F. R., O'Neill J. K., Allen S. J., Butter C., Nuki G., Baker D. (1999) CD44 is involved in selective leucocyte extravasation during inflammatory central nervous system disease. Immunology 98, 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang D., Liang J., Noble P. W. (2011) Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshioka Y., Kozawa E., Urakawa H., Arai E., Futamura N., Zhuo L., Kimata K., Ishiguro N., Nishida Y. (2013) Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 65, 1160–1170 [DOI] [PubMed] [Google Scholar]
- 14. Kultti A., Pasonen-Seppänen S., Jauhiainen M., Rilla K. J., Kärnä R., Pyöriä E., Tammi R. H., Tammi M. I. (2009) 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and down-regulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 315, 1914–1923 [DOI] [PubMed] [Google Scholar]
- 15. Nakamura T., Takagaki K., Shibata S., Tanaka K., Higuchi T., Endo M. (1995) Hyaluronic-acid-deficient extracellular matrix induced by addition of 4-methylumbelliferone to the medium of cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 208, 470–475 [DOI] [PubMed] [Google Scholar]
- 16. Aarvak T., Chabaud M., Miossec P., Natvig J. B. (1999) IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 162, 1246–1251 [PubMed] [Google Scholar]
- 17. Guo N., Li X., Mann M. M., Funderburgh M. L., Du Y., Funderburgh J. L. (2010) Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor β. J. Biol. Chem. 285, 32012–32019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura T., Funahashi M., Takagaki K., Munakata H., Tanaka K., Saito Y., Endo M. (1997) Effect of 4-methylumbelliferone on cell-free synthesis of hyaluronic acid. Biochem. Mol. Biol. Int. 43, 263–268 [DOI] [PubMed] [Google Scholar]
- 19. Schneider C. A., Rasband W. S., Eliceiri K. W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piccioni F., Malvicini M., Garcia M. G., Rodriguez A., Atorrasagasti C., Kippes N., Piedra Buena I. T., Rizzo M. M., Bayo J., Aquino J., Viola M., Passi A., Alaniz L., Mazzolini G. (2012) Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 22, 400–410 [DOI] [PubMed] [Google Scholar]
- 21. Mulder G. J., Brouwer S., Weitering J. G., Scholtens E., Pang K. S. (1985) Glucuronidation and sulfation in the rat in vivo: the role of the liver and the intestine in the in vivo clearance of 4-methylumbelliferone. Biochem. Pharmacol. 34, 1325–1329 [DOI] [PubMed] [Google Scholar]
- 22. Rostami A., Ciric B. (2013) Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 333, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy A. C., Lalor S. J., Lynch M. A., Mills K. H. (2010) Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 24, 641–651 [DOI] [PubMed] [Google Scholar]
- 24. Skundric D. S., Kim C., Tse H. Y., Raine C. S. (1993) Homing of T cells to the central nervous system throughout the course of relapsing experimental autoimmune encephalomyelitis in Thy-1 congenic mice. J. Neuroimmunol. 46, 113–121 [DOI] [PubMed] [Google Scholar]
- 25. Erridge C., Kennedy S., Spickett C. M., Webb D. J. (2008) Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 283, 24748–24759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller A. M., Pedré X., Stempfl T., Kleiter I., Couillard-Despres S., Aigner L., Giegerich G., Steinbrecher A. (2008) Novel role for SLPI in MOG-induced EAE revealed by spinal cord expression analysis. J. Neuroinflammation 5, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müller A. M., Jun E., Conlon H., Sadiq S. A. (2012) Cerebrospinal hepatocyte growth factor levels correlate negatively with disease activity in multiple sclerosis. J. Neuroimmunol. 251, 80–86 [DOI] [PubMed] [Google Scholar]
- 28. Brynedal B., Khademi M., Wallström E., Hillert J., Olsson T., Duvefelt K. (2010) Gene expression profiling in multiple sclerosis: a disease of the central nervous system, but with relapses triggered in the periphery? Neurobiol. Dis. 37, 613–621 [DOI] [PubMed] [Google Scholar]
- 29. Meiron M., Zohar Y., Anunu R., Wildbaum G., Karin N. (2008) CXCL12 (SDF-1α) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J. Exp. Med. 205, 2643–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCandless E. E., Wang Q., Woerner B. M., Harper J. M., Klein R. S. (2006) CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J. Immunol. 177, 8053–8064 [DOI] [PubMed] [Google Scholar]
- 31. Miljkovic D., Stanojevic Z., Momcilovic M., Odoardi F., Flugel A., Mostarica-Stojkovic M. (2011) CXCL12 expression within the CNS contributes to the resistance against experimental autoimmune encephalomyelitis in albino Oxford rats. Immunobiology 216, 979–987 [DOI] [PubMed] [Google Scholar]
- 32. Hatse S., Princen K., Bridger G., De Clercq E., Schols D. (2002) Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 527, 255–262 [DOI] [PubMed] [Google Scholar]
- 33. DeGrendele H. C., Estess P., Siegelman M. H. (1997) Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 278, 672–675 [DOI] [PubMed] [Google Scholar]
- 34. Qin L., Wu X., Block M. L., Liu Y., Breese G. R., Hong J. S., Knapp D. J., Crews F. T. (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nanki T., Hayashida K., El-Gabalawy H. S., Suson S., Shi K., Girschick H. J., Yavuz S., Lipsky P. E. (2000) Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 165, 6590–6598 [DOI] [PubMed] [Google Scholar]
- 36. Ambrosini E., Remoli M. E., Giacomini E., Rosicarelli B., Serafini B., Lande R., Aloisi F., Coccia E. M. (2005) Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 64, 706–715 [DOI] [PubMed] [Google Scholar]
- 37. Li M., Ransohoff R. M. (2008) Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog. Neurobiol. 84, 116–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel J. R., McCandless E. E., Dorsey D., Klein R. S. (2010) CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc. Natl. Acad. Sci. U.S.A. 107, 11062–11067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hagemeier K., Brück W., Kuhlmann T. (2012) Multiple sclerosis: remyelination failure as a cause of disease progression. Histol. Histopathol. 27, 277–287 [DOI] [PubMed] [Google Scholar]
- 40. Montesano R., Pepper M. S., Möhle-Steinlein U., Risau W., Wagner E. F., Orci L. (1990) Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 62, 435–445 [DOI] [PubMed] [Google Scholar]