Abstract
Behavioral learning is mediated by cellular plasticity such as changes in the strength of synapses at specific sites in neural circuits. The theory of cerebellar motor learning1,2,3 relies on movement errors signaled by climbing-fiber inputs to cause long-term depression of synapses from parallel fibers to Purkinje cells4,5. Yet, a recent review6 has called into question the widely-held view that the climbing fiber input is an “all-or-none” event. In anesthetized animals, there is wide variation in the duration of the complex-spike (CS) caused in Purkinje cells by a climbing fiber input7. Further, the duration of electrically-controlled bursts in climbing fibers grades the amount of plasticity in Purkinje cells8,9. The duration of bursts depends on the “state” of the inferior olive and therefore could be correlated across climbing fibers8,10. Here, we provide a potential functional context for these mechanisms during motor learning in behaving monkeys. The magnitudes of both plasticity and motor learning depend on the duration of the CS responses. Further, the duration of CS responses appears to be a meaningful signal that is correlated across the Purkinje cell population during motor learning. We suggest that during learning, longer bursts in climbing fibers lead to longer duration CS responses in Purkinje cells, more calcium entry into Purkinje cells, larger synaptic depression, and stronger learning. The same graded impact of instructive signals for plasticity and learning could occur throughout the nervous system.
We recorded the neural activity of single Purkinje cells in the floccular complex of the cerebellum in monkeys that had been trained to perform smooth pursuit eye movements11,12. We chose to study the floccular complex because its output drives pursuit eye movements13 as well as pursuit learning11,14 through a disynaptic pathway to extraocular motoneurons15.
During pursuit eye movements, floccular Purkinje cells show direction-tuning in simple-spike firing, which is driven by mossy fiber inputs to the cerebellum. To set the stage for learning experiments, we defined each neuron’s “on-direction” by the pursuit direction with the largest increases in simple-spike firing rate; pursuit in the opposite, or “off-direction”, was associated with decreases in simple-spike firing rate (Extended data Figure 1b and c). The CS responses driven by climbing-fiber inputs show direction tuning opposite that for the simple-spike responses (Extended data Figure 1).
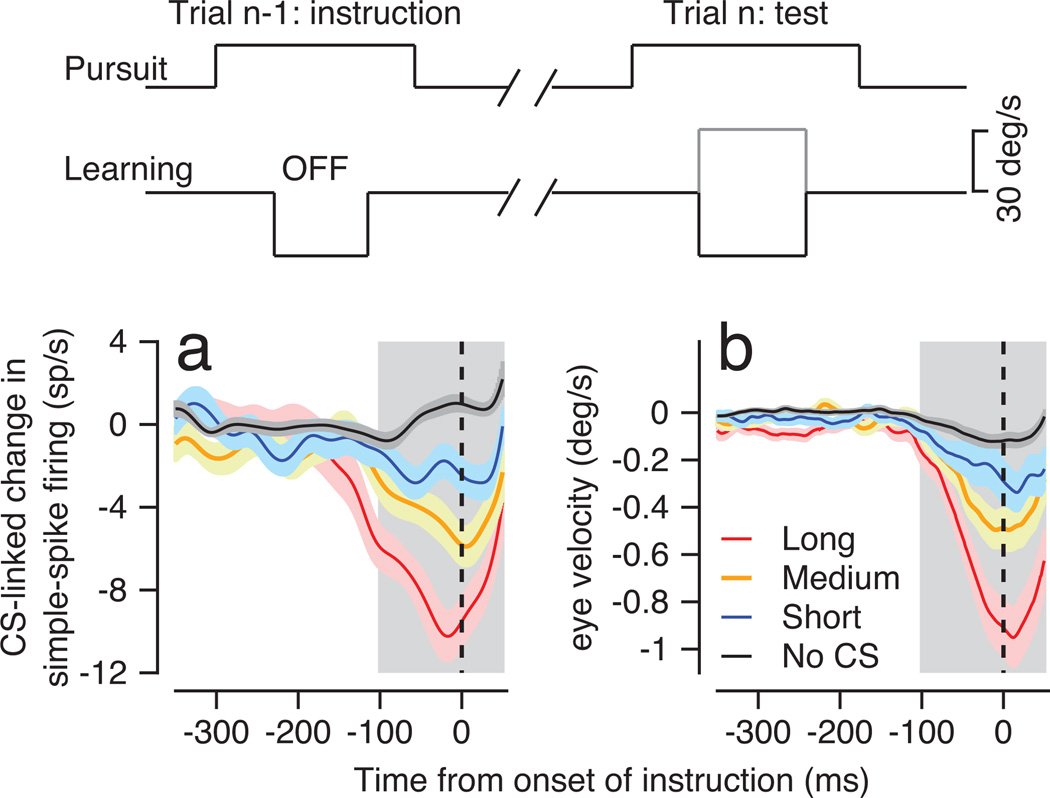
Motor learning occurred during a succession of “trials”. In each trial, monkeys tracked a target that first moved for 250 ms at 20 deg/s in a “pursuit” direction orthogonal to the on-direction of the Purkinje cell under study (horizontal in Figure 1b). Then, the target underwent an instructive change in direction that added orthogonal target motion at 30 deg/s for 400 ms. The “instruction” was in either the on- or off-direction for the simple-spike response of the PC under study (downward or upward in Figure 1b). After the instruction ended, the target continued to move in the pursuit direction for 200 ms and then stopped.
Figure 1. Design of the learning paradigm and variation of CS duration.
a: Trajectories of target position during a random-order learning block. b: Trial-over-trial learning in an example pair of consecutive trials. From top to bottom, superimposed traces are horizontal and vertical eye velocity, and horizontal and vertical eye position. Red and blue traces show data for trials “n-1” and “n”. Dashed traces show target motion. The red zigzag between the position traces shows the trajectory of target position in two dimensions. c: Times of occurrence of CS responses and the probability of CS responses in 100 ms bins. d: Two example CS waveforms from an example Purkinje cell. e: Superimposed, statistically-different distributions of CS duration during fixation versus learning for the example Purkinje cell (two-tailed t-test, p<0.01). f: Distributions of mean CS duration for the “short”, “medium”, and “long” duration CS responses across the population of 34 Purkinje cells. g: Scatter plot shows average CS duration during fixation versus learning for all 34 Purkinje cells. The filled symbol indicates the example neuron in e.
We used a “random-direction learning paradigm”12 (Figure 1a) to study neural correlates of motor learning on the time scale of single behavioral trials. Each eye velocity response showed a small, transient, learned deflection in the direction of the instruction on the prior trial (Figure 1b, arrowhead on vertical eye velocity traces). The small upward deflection of vertical eye velocity in the nth trial (Figure 1b, blue traces) was caused by the upward instruction on the prior, n-1st trial (red traces). The small downward deflection at the same time on the n-1st trial (red traces) was caused by the downward instruction on the n-2nd trial. We call these brief deflections “trial-over-trial” behavioral learning.
For each of 34 individual Purkinje cell with well-isolated CS waveforms, we observed CS responses with a probability of 0.3 to 0.4 in the interval from 75 to 175 ms after an instruction (Figure 1c), but a wide range in the total duration of the extracellular CS waveform (Figure 1d) and in the number of spikelets7. We found a linear relationship between CS duration and the number of spikelets (Extended data Figure 2), and a strong correlation. We assume that the duration of the extracellular CS waveform is a valid probe for the duration of the depolarization in the Purkinje cell’s membrane potential8. Our results showing an effect of CS duration on plasticity support that assumption, with the caveat that it is difficult to be sure of intracellular events from extracellular recordings.
The duration of CS responses varied widely even in a given behavioral condition and differed between behavioral conditions, i.e. fixation versus learning (Figure 1e, g). To study the effects of CS duration, we divided the distribution for each neuron into thirds by trisecting it at the mean±0.44 standard deviations; we categorized the duration of the CS in instruction trials as “short”, “medium”, or “long”. The mean CS durations in the three groups were 6.59 ms, 8.12 ms, and 9.84 ms across our sample of Purkinje cells (Figure 1f).
Our data-analysis monitors plasticity linked to the CS responses driven by natural sensory stimuli during behavioral learning. We broke the string of learning trials into pairs of “instruction” and “test” trials (Figure 2, schematic). We included only pairs with an instruction in off-direction for simple-spike firing in the first trial; instructions in the on-direction seldom caused a CS response (Extended data Figure 1b). To demonstrate the trial-over-trial effects linked to the duration of a CS response, we grouped the pairs of trials according to whether the duration of the CS was “short”, “medium”, “long”, or absent. Within each group, we then averaged the trial-over trial change in firing rate between the instruction and test trial.
Figure 2. Effect of CS duration on trial-over-trial depression and learning.
Schematic at the top shows the sequence of instruction and test trials. The pursuit stimulus was the same in both trials, and the instruction provided an off-direction target motion. Plots show trial-over-trial changes in simple-spike firing rate (a) or eye velocity (b). Red, yellow, blue, and black traces show data when the CS response to the instruction had a long, medium, or short duration, or was absent. Ribbons around the traces show standard errors of firing rate averaged across Purkinje cells. Vertical dashed line shows the time of the instruction; gray shading shows the analysis interval used for Figure 3.
Simple-spike firing rate underwent trial-over-trial depression that was related to the presence11,16 and duration of the CS response in the instruction trial (Figure 2a). The trial-over-trial depression was largest after a long-duration CS (red trace), smallest after a short-duration CS (blue trace), and intermediate after a medium-duration CS (yellow trace). Trial-over-trial changes of simple-spike firing rate showed a small potentiation that was not significantly different from zero (two-sided Wilcoxon signed rank test, p>0.05) if the off-direction instruction failed to evoke a CS response (Figure 2a, black trace)11,16.
CS duration also affected the trial-over-trial learning in eye velocity (Figure 2b). Learning was strongest if the CS duration was long, smaller in a graded way when CS duration was medium or short, and very small in the absence of a CS response to the off-direction instruction. In contrast, the duration of the post-CS pause in simple-spike firing rate did not affect the magnitude of trial-over-trial depression in simple-spike firing rate or trial-over-trial learning in eye velocity (Extended data Figure 3).
The data from all 34 Purkinje cells show a consistent trend of downward slopes as a function of whether the CS response is short, medium, or long (Figure 3a, c). For statistical analysis, we plotted the magnitude of the trial-over-trial effects in each neuron as a function of the mean of CS duration in that neuron’s “short”, “medium”, and “long” groups (Figure 3b, d). Regression analysis yielded negative slopes. The slope for firing rate was −0.70 spikes/s for each millisecond of CS duration (95% confidence limits on slope −1.13 to −0.27). The slope for eye velocity was −0.07 deg/s for each millisecond of CS duration (95% confidence limits on slope −0.11 to −0.04).
Figure 3. Quantitative analysis of the effects of CS duration for all individual Purkinje cells.
a, b: Trial-over-trial change in simple-spike firing rate. c, d: Trial-over-trial learning in eye velocity. Different colors indicate the absence or duration of the CS responses to an off-direction instruction. In a and c, gray lines connect four symbols for each neuron; black symbols show means across the population. In b and d, CS-linked trial-over-trial changes are plotted as a function of the mean duration of the CS waveforms for each group. The continuous lines were obtained by linear regression. The horizontal dashed lines show the averages for the No-CS group.
Next, we evaluated the mechanisms for the effect of CS duration on trial-over-trial depression of simple-spike firing rate. We averaged absolute simple-spike firing rate as a function of time for different groups of trials (Figure 4). The traces all start with an increase in firing rate of 10–15 spikes/s at about −150 ms (Figure 4a, arrow). This increase is related to the onset of eye velocity in the pursuit direction, which is orthogonal to the on-direction; the response to pursuit in the on-direction would have been much larger. The key interval for probing CS-linked trial-over-trial simple-spike depression is later, in the gray shaded area from 100 ms before to 50 ms after the onset of the instruction. CS responses would have occurred still later, after the end of the traces.
Figure 4. Evidence that CS duration affects the magnitude of CS-linked plasticity.
All graphs plot simple-spike firing rate as a function of time from the onset of the instruction. a: Test trials that followed instruction trials with CS responses of different durations. The diagonal arrow points out the increase in firing rate associated with the initiation of pursuit in the pursuit direction. b: Instruction trials with long, short, or absent CS responses. c: Pairs of trials that have the same simple-spike firing rates in the instruction trials but differ in the presence versus absence of a CS response to the instruction. d: Pairs of trials that have the same simple-spike firing rates in the instruction trials but differ in the duration of the CS response to the instruction.
Evidence for plasticity of simple-spike firing comes from the observation that a long-duration CS on the instruction trials drove the simple-spike firing rate on the subsequent test trials below the baseline established by trials without a CS (Figure 4a, red versus black traces). Short-duration CS responses also drove simple-spike firing rate below the baseline (Figure 4a, blue versus black traces). Thus, trial-over-trial depression must represent an active change, and cannot be due simply to the loss of a slight enhancement of simple-spike firing rate at the time of a CS response16.
The elevated simple-spike firing rate on instruction trials with a CS of long-duration (Figure 4b, red dashed trace versus blue or black) raises the possibility that the higher simple-spike firing rate on the instruction trial could be the causal agent that drives trial-over-trial depression of simple-spike firing rate17. We rejected this possibility by comparing two groups of instruction-test pairs that had equal simple-spike firing rates, but differed in the presence, duration, or absence of a CS in response to the instruction (Figure 4c, d). Simple-spike firing rate showed trial-over-trial depression only if there was a CS on the instruction trial (Figure 4c), and the magnitude of the depression depended on the duration of the CS (Figure 4d). Simple-spike firing rate on the instruction trial controls neither CS duration nor plasticity.
It is the context of the effects we demonstrate that is most newsworthy. We have been able to monitor cellular events that actually occur during learning. Our approach has analyzed the effect of natural variation in CS duration on trial-over-trial changes in simple-spike firing rate and behavioral performance. Of course, longer-duration CS responses should allow more calcium to enter a Purkinje cell and promote greater plasticity18, as demonstrated by electrical stimulation in the inferior olive9. In the present study, we take the next step by showing that CS duration matters for plasticity of simple-spike firing rate during motor learning driven by natural sensory stimuli.
The effect of CS duration on trial-over-trial learning in eye velocity has important implications for the organization of motor learning in the cerebellum. Learned eye velocity is a measure of the combined plasticity of simple-spike firing rate of all Purkinje cells in the floccular complex. If CS duration and the magnitude of plasticity varied randomly across the population of Purkinje cells on a given trial, then the trial-over-trial change in eye velocity would not depend on CS duration. Because learning is much larger when the Purkinje cell under study has a long CS, CS duration seems to be coordinated across the population of Purkinje cells.
Coordinated modulation of CS duration across the population of Purkinje cells could control the amount of learning induced by a given motor error. Descending influences on the inferior olive or local Purkinje cell membrane excitability could mediate the modulation. We lean toward modulation at the level of the inferior olive. The number of spikes in a climbing-fiber burst reads out the state of the inferior olive8,10, and is one determinant of CS duration8. Thus, intentional modulation of the state of the inferior olive could correlate the durations of bursts across a large group of climbing fibers. Modulation could arise either from the cerebral cortex, or from the cerebellum through in the deep cerebellar nuclei that inhibit the inferior olive19.
Local modulation in the cerebellar cortex could affect CS duration by controlling the excitability of the Purkinje cell membrane20,21. If modulation occurs locally, then correlations in CS duration across the population could arise through neuron-neuron correlations in simple-spike firing rate22, or through coordinated sensory inputs23. We do not know whether simple-spike firing rate is a good index of Purkinje cell membrane excitability in vivo. If it is, then our data argue against local control of CS duration because CS duration grades simple-spike plasticity even when instruction trials are selected so that simple-spike firing rate is equated (Figure 4d).
Intentional modulation of CS duration could be important in cerebellar function. Purkinje cell output may modulate the duration of its own CS inputs and restrict its own learning range16,24–26, limiting short-term learning in the cerebellar cortex, and enabling transfer of plasticity to the deep cerebellar nucleus27. Modulation of the amount of learning induced by a given instructive stimulus might facilitate cerebellar learning under conditions that are particularly important to the organism (“Coach” is screaming at you during practice), and prevent learning when it would be counter-productive (in the middle of a crucial play in a game). Indeed, we observed that learning occurs more quickly and the durations of CS responses are generally longer in the first 30 trials of a learning block when the same instruction occurs in 100 consecutive trials. Such a situation was suggested before on theoretical grounds28.
The question of what happens in our brains while we learn is answered in the most detail for cerebellar motor learning. But the principles of cerebellar learning almost certainly will apply to other learning systems. Perhaps long-term potentiation in the hippocampus can be graded by modulation from the cerebral cortex. In the cerebellum, the duration of the climbing fiber input is particularly accessible to top-down control, but the same effect could occur in other systems through inhibition, for example. Thus, the details of motor learning in the cerebellum may lead to principles that apply in all learning systems in the brain.
Methods
We performed experiments on two adult male rhesus monkeys using techniques that have been described in detail before (refs 11, 12, and 16). The data in this paper are drawn from a single dataset that also provides the data for two other papers that cover different aspects of the results (refs 16 and 29). All procedures followed a protocol that had been approved in advance by the Institutional Animal Care and Use Committee at UCSF, where the experiments were performed. All procedures were in accordance with the NIH Guild for the Care and Use of Laboratory Animals.
We used sterile procedure under surgical levels of gas anesthesia to perform survival surgery to prepare each monkey for experiments. After each surgery, monkeys received several days of treatment with systemic analgesics. In the first two surgeries, we implanted a socket on the skull to allow restraint of the monkey’s head, and an eye coil on one eye to allow precise monitoring of eye position. Monkeys then were trained to fixate and track targets that appeared on a video screen in front of them. They received fluid rewards for accurate tracking, which involved keeping eye position within a small invisible rectangular window that moved with the target. Monkeys would normally work for ~2,000 rewards daily. After monkeys were fully experienced in the behavioral tasks, we performed one more surgery to make a craniotomy and implant a sealable recording cylinder to allow access to the cerebellum with microelectrodes.
An experiment consisted of discreet target motions presented in what we call “trials”. Each trial started when the monkey fixated a 0.3 deg white spot at the center of the video screen. After he had established stable fixation, the spot expanded to 0.5 deg, displaced by a few degrees to a new location and started to move at constant speed. It then underwent one or two more changes in direction and speed before stopping at an eccentric location. The details of the target motion were selected to match the preferred direction of the Purkinje cell under study, as well as the exact goals of each experiment (see full text of paper and Extended data Figure 1).
In each daily experiment, we introduced a microelectrode into the floccular complex of the cerebellum, isolated the extracellular potentials from an individual Purkinje cells, and recorded from that Purkinje cell during many repetitions of a few carefully chosen target motions. We took special care to obtain excellent isolation of the CS and simple-spike waveforms. Data were recorded for analysis after the experiment. After the experiment, we displayed the data from each behavioral trial on a video screen and used homemade software to identify each simple-spike and CS. We obtained records of simple-spike firing rate for individual trials with an inverse interspike-interval algorithm followed by smoothing with a Gaussian function (δ=15ms).
Four techniques require extra explanation.
We needed to select Purkinje cell recordings for analysis on the basis of how well the complex-spike responses were isolated. It is a challenge to maintain excellent isolation of complex-spike responses through ~400 trials of a random-direction learning block. As a result, we were able to identify the CS responses in 118 Purkinje cells, but we were able to measure the duration of the waveforms throughout the recordings in only 34 Purkinje cells. The selection of Purkinje cells for analyses was performed on the basis of the CS waveforms alone, before we had attempted any analysis of an effect of CS duration. The subjective procedure we used to select the Purkinje cells for analysis was analogous to the subjective procedures used in all extracellular single unit recordings from awake-behaving animals. Before including data in an analysis, it is always necessary to decide whether or not the isolation of unitary spikes is good enough to allow meaningful conclusions.
The veracity of our observations depends on the objectivity and accuracy of our measurements of CS duration. The measurements were made manually by one of the authors. As detailed in Figure 1e and Extended data Figure 2, CS duration was measured from the beginning of the first deflection of the extracellular potential to the time of the return to baseline potential (downward arrows on each trace). The investigator who analyzed the data was blind to whether the CS under measurement occurred during fixation or in response to an instruction. Also, all measurements of CS duration were made before a given neuron’s simple-spike firing rate and the associated eye velocities were analyzed at all. The observer was blind to simple-spike firing rate and eye velocity in the current trial or the next trial. Thus, there was no chance of unconscious bias. To be safe, we also asked two lab members to measure CS duration for several representative Purkinje cells. Their measurements of CS duration agreed well with those of the primary observer. We also note that random errors in measuring CS duration should dilute the effects we report here, and that shuffling the CS durations eliminated any effects of CS duration on trial-over-trial changes in simple-spike firing rate or trial-over-trial learning in eye velocity.
Our main data analysis approach was to evaluate the trial-over-trial changes in either simple-spike firing rate or eye velocity between pairs of trials. In each pair, we considered the first trial to be the “instruction” trial and the second trial to be the “test” trial. The time between the two trials was ~2.5 seconds. We selected only the pairs of trials with instructions on the first trial that were in the “off-direction” for simple-spike firing of the Purkinje cell under study. We then divided the pairs into groups according to the presence, duration, or absence of a CS response on the instruction trial. For each pair, we calculated the difference between the simple-spike firing rate (eye velocity) on the instruction trial and on the test trial for each millisecond from 350 ms before to 50 ms after the time of the instruction. We then averaged across the trials in each group of pairs for each Purkinje cell to obtain estimates of the trial-over-trial change in the time varying simple-spike firing rate or eye velocity. For many of our analyses, we then averaged across Purkinje cells as well.
For the analysis in Figures 4c and d, we needed to select pairs of trials. We did so to achieve the specific criterion of matching the simple-spike firing rates in groups of instruction trials that differed according to the duration or presence versus absence of a CS response. To create the required match, we analyzed each Purkinje cell separately and used the full sample of pairs of trials with “long” CS duration. From the pairs of trials with no CS or short CS duration in the instruction trial in each neuron, we selected a subset according to the simple-spike firing rate in the instruction trial. We included pairs of trials from that group only if simple-spike firing rate in the instruction trial was within the range defined by the mean plus or minus one standard deviation of the simple-spike firing rate in instruction trials with a long CS duration. We then created averages of simple-spike firing rate in the instruction and test trials that met our selection criteria for each neuron, and averaged the results across Purkinje cells. This allowed us to assess whether CS presence and duration had effects that were independent of simple-spike firing rate on the instruction trial.
Supplementary Material
Acknowledgements
We thank Court Hull, Lindsey Glickfeld, and Rich Mooney for helpful comments, and Mati Joshua and Joonyeol Lee for “blind” tests of our measurements of complex-spike duration. Research supported by the Howard Hughes Medical Institute and by the National Eye Institute of the National Institutes of Health under Award Number R01-EY003878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author contributions: YY performed all experiments and data analysis. YY and SGL designed and interpreted experiments and wrote the manuscript.
The authors declare no competing financial interests.
References
- 1.Marr D. A theory of cerebellar cortex. J Physiol. (Lond.) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 3.Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972;40:81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- 5.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33:253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 6.Najafi F, Medina JF. Beyond “all-or-nothing” climbing fibers: graded representation of teaching signals in Purkinje cells. Front Neural Circuits. 2013;7 doi: 10.3389/fncir.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maruta J, Hensbroek RA, Simpson JI. Intraburst and interburst signaling by climbing fibers. J Neurosci. 2007;27:11263–11270. doi: 10.1523/JNEUROSCI.2559-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Hausser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen A, Jirenhed D-A, Zucca R, Johansson F, Svensson P, Hesslow G. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33:13436–13440. doi: 10.1523/JNEUROSCI.1527-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazzigaluppi P, De Gruijl JR, van der Geissen RS, Khosrovani S, De Zeeuw CI, de Jeu MT. Olivary subthreshold oscillations and burst activity revisited. Front Neural Circuits. 2012;6:91. doi: 10.3389/fncir.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Lisberger SG. Learning on multiple timescales in smooth pursuit eye movements. J Neurophysiol. 2010;104:2850–2862. doi: 10.1152/jn.00761.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46:878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- 14.Kahlon M, Lisberger SG. Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements. J Neurophysiol. 2000;84:2945–2960. doi: 10.1152/jn.2000.84.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Highstein SM. Synaptic linkage in the vestibulo-ocular and cerebello-vestibular pathways to the VIth nucleus in the rabbit. Exp Brain Res. 1973;17:301–314. doi: 10.1007/BF00234668. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Lisberger SG. Interaction of plasticity and circuit organization during the acquisition of cerebellum-dependent motor learning. eLife. 2013;2(0):e01574. doi: 10.7554/eLife.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartell N. Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron. 1996;16:601–610. doi: 10.1016/s0896-6273(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 18.Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum. 2006;5:7–14. doi: 10.1080/14734220500462757. [DOI] [PubMed] [Google Scholar]
- 20.Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15:2777–2787. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura K, Hausser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J Neurosci. 2011;31:10847–10858. doi: 10.1523/JNEUROSCI.2525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27:6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najafi F, Giavannucci A, Wang SS-H, Medina JF. Sensory driven enhancement of calcium signals in individual Purkinje cell dendrites of awake mice. Cell Reports. 2014 doi: 10.1016/j.celrep.2014.02.001. http://dx.doi.org/10.1016/j.celrep.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn Mem. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- 25.Kenyon GT, Medina JF, Mauk MD. A mathematical model of the cerebellar-olivary system I: self-regulating equilibrium of climbing fiber activity. J Comput Neurosci. 1998;5:17–33. doi: 10.1023/a:1008874209991. [DOI] [PubMed] [Google Scholar]
- 26.Miall RC, Keating JG, Malkmus M, Thach WT. Simple spike activity predicts occurrence of complex spikes in cerebellar Purkinje cells. Nat Neurosci. 1998;1:13–15. doi: 10.1038/212. [DOI] [PubMed] [Google Scholar]
- 27.Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. J Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert PFC. A theory of memory that explains the function and structure of the cerebellum. Brain Res. 1974;70:1–18. doi: 10.1016/0006-8993(74)90208-x. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Lisberger SG. Role of plasticity at different sites across the time course of cerebellar motor learning. J. Neurosci. 2014 doi: 10.1523/JNEUROSCI.0017-14.2014. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiz J, Karakossian MH, Pakaprot N, Robleto K, Thompson RF, Otis TS. Prolonging the postcomplex spike pause speeds eyeblink conditioning. Proc Natl Acad Sci USA. 2012;109:16726–16730. doi: 10.1073/pnas.1214274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.