Abstract
Aim
To assess whether the APOE4 genotype affects the relationship of long-term glycemic control with cognitive function in elderly with type 2 diabetes (T2D).
Methods
Participants were cognitively normal and pertained to a Diabetes Registry which provided access to HbA1c levels and other T2D related factors since 1998. Glycemic control was defined as the mean of all HbA1c measurements available (averaging 18 measurements) per subject. Four cognitive domains (episodic memory, semantic categorization, attention/working memory and executive function), based on factor analysis and an overall cognitive score (the sum of the 4 cognitive domains) were the outcome measures.
Results
The analysis included 808 subjects; 107 (11.9%) subjects had ≥1ApoE4 allele. In ApoE4 carriers, higher mean HbA1c level was significantly associated with lower scores on all cognitive measures except attention/working memory (p-values ranging from 0.047 to 0.003). In ApoE4 noncarriers, higher mean HbA1c level was significantly associated with lower scores on executive function, but not with other cognitive measures—despite the larger sample size. Compared to non- carriers, there were significantly stronger associations in ApoE4 carriers for overall cognition (p=0.02), semantic categorization (p=0.03) and episodic memory (p=0.02), and the difference for executive function approached statistical significance (p=0.06).
Conclusion
In this cross-sectional study of cognitively normal T2D subjects, higher mean HbA1c levels were generally associated with lower cognitive performance in ApoE4 carriers, but not in non-carriers, suggesting that ApoE4 affects the relationship between long-term glycemic control and cognition, so APOE4 carriers may be more vulnerable to the insults of poor glycemic control.
Keywords: ApoE, glycemic control, HbA1c, cognition, type 2 diabetes
Introduction
Type 2 Diabetes (T2D) has been consistently associated with cognitive decline and dementia(Luchsinger, 2010). Several mechanisms have been suggested to underlie this association(Ravona-Springer and Schnaider-Beeri, 2011), but no specific mechanism has been determined, hindering the development of dementia prevention strategies in T2D. Degree of glycemic control is a key determinant of micro and macrovascular complications of T2D. High Hemoglobin A1c (HbA1c) levels, the gold standard measure of poor glycemic control, are associated with poorer cognitive function(Yaffe et al., 2012) and brain volume(Launer et al., 2011), suggesting that good glycemic control may be beneficial in preventing cognitive decline and dementia in T2D. Nevertheless, this strategy cannot be universally implemented in all T2D subjects given the increased risk for morbidity and mortality observed in some patients(Gerstein et al., 2008). Additionally, when tested in T2D subjects in general, strict glycemic control has demonstrated only limited efficacy in preventing cognitive decline(Launer et al., 2011). It is therefore important to identify and characterize T2D subjects who are at particularly high risk for cognitive impairment, and therefore may most benefit from strategies to prevent cognitive decline and dementia.
ApoE4, a risk factor for cognitive decline and dementia(Liu et al., 2013) in its own right, has been shown to modify the relationship between T2D and cognitive performance, since the differences between T2D and non-T2D (i.e. poorer cognitive performance) were stronger for those who carried ≥1 APOE ε4 alleles, both cross-sectional(Dore et al., 2009) and longitudinally(Haan et al., 1999). Neuropathologically, the presence of APOE ε4 allele enhanced the differences between T2D and non-T2D subjects in the numbers of hippocampal and cortical neuritic plaques and neurofibrillary tangles (pathological hallmarks of AD)and in the load of cerebral amyloid angiopathy(Peila et al., 2002); these were higher in T2D subjects who were also ApoE4 carriers compared to subjects who carried only one or none of these risk factors.
To the best of our knowledge, within T2D, the contribution of the APOE4 genotype to the relationship of degree of glycemic control with cognitive function has not been studied. The aim of the present study was to analyze, cross-sectionally, the modifying effect of presence of an ApoE4 allele on the association between long-term glycemic control (expressed as HbA1c levels) and cognitive performance in a cognitively normal cohort of elderly T2D subjects.
Experimental procedures
The IDCD is collaboration among the Icahn School of Medicine at Mount Sinai, the Sheba Medical Center, and the Maccabi Healthcare Services (MHS). The study was approved by all three IRB committees.
Sample
This study consists of elderly (≥ 65 years old) T2D subjects, who are engaged in the IDCD, a longitudinal investigation assessing the relationship of long-term T2D characteristics and cognitive decline. The study is ongoing, but longitudinal follow up began recently, so the present results are based on baseline data only. Design and detailed methods have been published elsewhere(Ravona-Springer et al., 2013). Briefly, subjects were randomly selected from the approximately 11,000 T2D individuals that are in the diabetes registry of the MHS, the second largest HMO in Israel. The MHS diabetes registry collects detailed laboratory, medication and diagnosis information since 1998 and is an integral part of the MHS Electronic Patient Record (EPR) system. Entry criteria to the registry are any of the following: 1) HbA1c >7.25%, 2) Glucose >200mg/dl on two exams more than three months apart, 3) purchase of diabetic medication twice within three months supported by a HbA1c>6.5% or Glucose>125 mg/dl within half a year, 4) diagnosis of Type 2 diabetes (ICD9 code), supported by a HbA1c>6.5% or Glucose>125 mg/dl within half a year. These criteria have been validated by twenty physicians in MHS against their own practice records(Heymann et al., 2006).
Eligibility criteria for the study
Subjects were eligible for the IDCD study if they were listed in the MHS diabetes registry, living in the central area of Israel, aged ≥65 years, cognitively normal at baseline, not suffering from major medical or neuropsychiatric conditions that affect cognition, had ≥3HbA1c measurements in the diabetes registry, spoke Hebrew fluently and had contact with an informant for the study.
Subject recruitment process
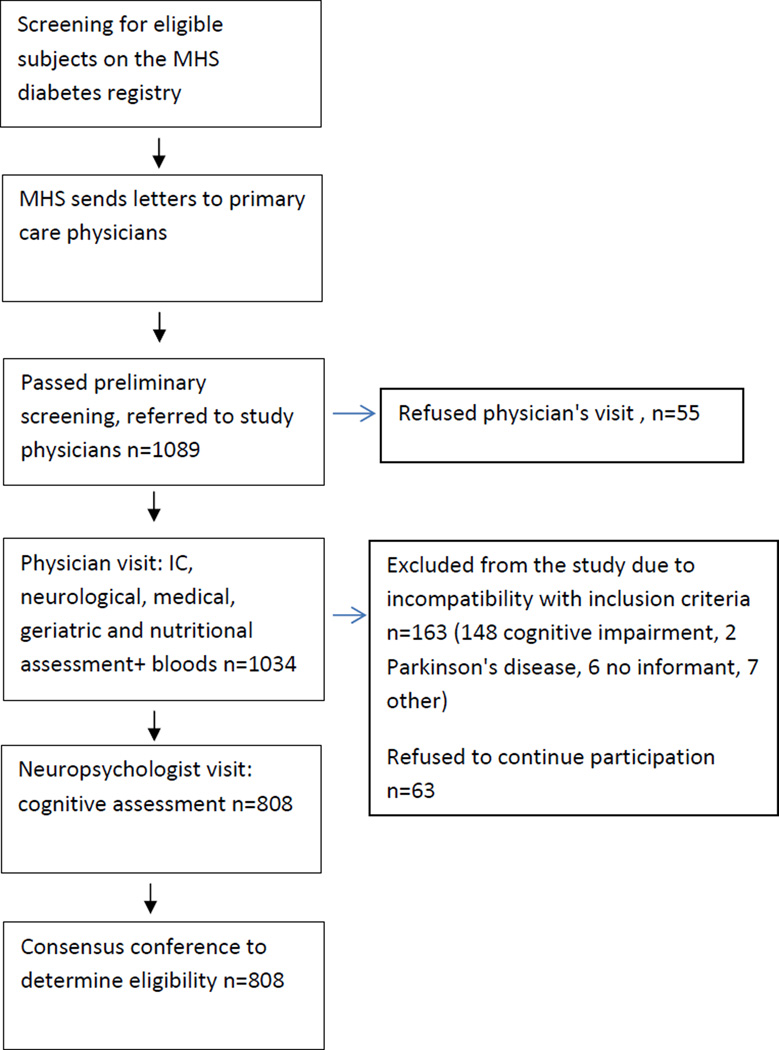
(Figure 1): The recruitment process has been described in detail elsewhere(Ravona-Springer et al., 2013). Shortly, the MHS Diabetes Registry was thoroughly reviewed, potential subjects were randomly selected, contacted by mail and then by phone and asked if they were willing to participate. Consenting subjects were assessed in two phases optimally two weeks apart. First they were visited by a study physician who obtained signed informed consent; performed medical, neurological, geriatric and nutritional assessments; and drew blood for inflammatory markers (Il-6, CRP), Haptoglobin, and APOE genotypes. In the second phase, the potential subjects were visited by a neuropsychologist who administered a cognitive battery, and questionnaires to the potential subject and informant for cognitive and functional impairment and for depression and behavioral disturbances characteristic of dementia. All the data collected was discussed by a multidisciplinary consensus conference team (which included a psychiatrist or a neurologist with expertise in dementia, and a neuropsychologist) in order to define the subjects' cognitive status (as cognitively normal, MCI, or dementia and their subtypes). For eligibility, subjects had to be cognitively normal at baseline.
Figure 1. Study Flow Chart.
Cognitive assessment
Clinical Dementia Rating (CDR) scale
assesses, through an interview with the subject and an informant, the existence and severity of cognitive and functional impairments.(Fillenbaum et al., 1996).
Mini Mental State Exam (MMSE)
This questionnaire assesses orientation, concentration, memory, and language(Folstein et al., 1975).
Outcome measures
were based on a comprehensive neuropsychological battery. All neuropsychological test scores were transformed into standardized scores. Factor analysis revealed four cognitive domains, which were then scored as totals of z scores of highly loading variables (reversed as necessary so that high values indicated good cognition): episodic memory (word list immediate and delayed recall, and recognition from the CERAD neuropsychological battery); semantic categorization (letter and category fluency, and similarities); attention/working memory (diamond cancellation test, digit span forward and backward), and executive factor (trails making A and B, and the digit symbol test). An overall cognitive score was the sum of the scores of all four domains(Ravona-Springer et al., 2013).
HbA1c
HbA1c values were extracted from the Diabetes Registry. The mean of all HbA1c measurements (averaging 18 assessments per subject since entry into the Diabetes Registry) were used in the present analysis.
ApoE4 analysis
DNA was extracted from blood taken during the physicians' assessment. APOE genotypes were determined at Polymorphic DNA Technologies (polymorphicdna.com, Alameda, CA).
Covariates
The socio-demographic covariates were age at the time of cognitive assessment, years of education, sex and number of follow up years in the registry (a surrogate for duration of T2D). The cardiovascular covariates were BMI, creatinine, total cholesterol, triglycerides, diastolic and systolic blood pressure, and anti-diabetes medications. These covariates were chosen based on previous findings regarding risk factors for cognitive decline and dementia. To reflect cardiovascular profile over years of T2D, we used the average of all the subject’s measurements available in the MHS Diabetes Registry for the cardiovascular measures. BMI was defined based on weight and height measured at the IDCD baseline (weight/height2). T2D medications were classified as no medications (never received anti-diabetic medications), treated with insulin only, treated with hypoglycemic medications only, and combination of insulin and hypoglycemic medications (not necessarily concomitantly)
Statistical Analyses
The present analysis assesses, cross-sectionally, the relationship of long-term glycemic control (mean (mean HbA1c) of all HbA1c measurements available at the Diabetes Registry) with cognitive function at the IDCD baseline visit stratified by Apo E4 status. For descriptive purposes, differences between ApoE4 carriers and non-carriers on socio-demographic and cardiovascular characteristics were evaluated by Student’s t-test and Pearson’s chi square. Differences on the five cognitive measures were evaluated by analysis of covariance, controlling for the socio-demographic and cardiovascular variables. Partial correlations of mean HbA1c with the cognitive measures, in the total sample and each ApoE4 status, also controlled for these covariates. Fisher’s z-transformation was used to compare the partial correlations in ApoE4 carriers and non-carriers.
Recently, variability in glycemic control over time has emerged as a risk factor for T2D complications(Sugawara et al., 2012). Thus, in secondary analysis, we took advantage of the numerous measurements of HbA1c that the IDCD has for each subject and repeated the analyses and examined the variability of HbA1c (calculated by the standard deviation of HbA1c for each subject) with cognitive outcomes, stratified by APOE4 genotype.
Results
Eight hundred and eight subjects were included in the analysis. The socio-demographic and cardiovascular characteristics of the sample are described in Table 1. The mean age of the sample was 71.98, the majority were males (60%), mean number of years in the registry was 10.48, mean HbA1c level was 6.78%, consistent with good overall glycemic control and mean score in MMSE is 28.00 consistent with a cognitively normal status. At least one ApoE4 allele was present in 107 (11.9%) subjects (4 homozygotes). The majority of subjects were treated by anti-diabetic medications; 77.1% with oral hypoglycemic medications only, 0.7%) with Insulin only and 8.7 with a combination of oral hypoglycemic medications and Insulin. ApoE4 carriers did not differ significantly (p <0.05) from non-carriers on any of the 12 demographic and cardiovascular variables tested (Table 1). ApoE 4 carriers performed better in the five cognitive domains, however, the difference in cognitive performance was not statistically significant after controlling for socio-demographic and cardio-vascular variables: overall cognitive score, p=0.17; semantic categorization, p=0.06; attention/working memory, p=0.58; executive function, p=0.09; and episodic memory, p=0.69.
Table 1.
demographic and cardiovascular characteristics of the sample by Apo E4 status.
| ApoE4+* Mean (SD) |
ApoE4− * Mean (SD) |
Total sample |
P* | |
|---|---|---|---|---|
| n | 107 | 701 | 808 | |
| Age | 72.28 (4.72) |
71.94 (4.68) |
71.98 (4.69) |
0.48 |
| Sex (percent females) |
42 | 39 | 40 | 0.58 |
| Years of education |
13.68 (3.49) |
13.05 (3.43) |
13.13 (3.44) |
0.08 |
| Years of follow up in the registry |
10.26 (1.86) |
10.51 (1.28) |
10.48 (1.37) |
0.08 |
| BMI* | 28.73 (4.53) |
28.24 (4.37) |
28.31 (4.38) |
0.29 |
| Creatinine | 0.99 (0.19) | 0.99 (0.25) |
0.99 (0.25) |
0.99 |
| Diastolic BP* |
85.55 (15.35) |
82.98 (15.34) |
83.32 (15.35) |
0.11 |
| Systolic BP | 166.01 (9.75) |
165.79 (9.64) |
165.82 (9.65) |
0.83 |
| Cholesterol | 180.93 (27.48) |
180.32 (24.40) |
180.40 (24.81) |
0.82 |
| Triglycerides | 159.24 (96.47) |
156.29 (57.32) |
156.68 (63.80) |
0.66 |
| Diabetes medications |
1.93 (0.06) | 2.06 (0.70) |
2.04 (0.68) |
0.80 |
| HbA1c (%) | 6.75 (0.74) | 6.79 (0.78) |
6.79 (0.77) |
0.58 |
BMI= Body mass index, BP= blood pressure, HbA1c=Hemoglobin A1c, p for difference between Apo E4+ and Apo E4 −
The relationship between mean HbA1c and cognition differed significantly between ApoE4 carriers and non-carriers. In ApoE4 carriers, higher mean HbA1c level was significantly associated with lower scores on all cognitive measures except attention/working memory (Table 2). In ApoE4 non-carriers, higher mean HbA1c level was significantly associated with lower scores on executive function, but was not significantly associated with other cognitive measures—despite the much larger sample size. The associations were significantly larger in magnitude among ApoE4 carriers for overall cognition (p=0.02), semantic categorization (p=0.03) and episodic memory (p=0.02), and the difference for executive function approached statistical significance (p=0.06), compared to non-carriers (Table 2).
Table 2.
partial correlations between mean HbA1c and cognitive scores by Apo E4 status*
| Apo E status | ApoE4+ df=89, n=107 |
Apo E4− df=652, n=701 |
Total df=744, n=808 |
Difference |
|---|---|---|---|---|
| Cognitive domain | r (p) | r (p) | r (p) | P** |
| Overall cognitive score |
−0.30 (0.003) | −0.07 (0.07) | −0.115 (0.002) | 0.02 |
| Semantic categorization |
−0.27 (0.01) | −0.06 (0.15) | −0.080 (0.029) | 0.03 |
| Attention/working memory |
−0.09 (0.41) | −0.02 (0.53) | −0.047 (0.20) | 0.29 |
| Executive function |
−0.28 (0.007) | −0.11 (0.004) | −0.15 (<0.0001) | 0.06 |
| Episodic memory | −0.21 (0.047) | 0.01 (0.75) | −0.031 (0.399) | 0.02 |
Controlling for: Sex, age, years of education, BMI, creatinine, triglycerides, cholesterol, diastolic blood pressure, systolic blood pressure, years of follow up in the Diabetes Registry, anti-diabetes medication (none/ only oral hypoglycemic medications/ only Insulin/ combination of oral hypoglycemic medications and insulin)
Significance of differences between correlations of Apo E4 carriers and non-carriers
Discussion
In cognitively normal elderly T2D subjects, higher mean HbA1c levels—based on repeated HbA1c measurements over years—are associated with lower cognition scores in ApoE4 carriers compared to non-carriers in all cognitive outcomes but attention/working memory. These differences suggest that ApoE4 modifies the association between long-term glycemic control and cognition, making carriers of this allele more vulnerable to the insults of worse glycemic control.
To the best of our knowledge, this is the first study to report, within T2D subjects, the contribution of the APOE4 genotype to the relationship of long-term glycemic control and cognitive performance. Previous studies assessing the modifying effect of Apo E genotype on the relationship of T2D, irrespective of degree of glycemic control, with cognition or dementia, demonstrated that dementia-free subjects who had both T2D and ApoE4 allele, were at substantially higher risk of cognitive decline (Haan et al., 1999) and dementia (Peila et al., 2002) at follow up compared to non-diabetic ApoE4 non-carriers. Apo E4 allele exacerbated the effect of midlife diabetes on rate of decline in several cognitive domains- verbal memory, attention and visuospatial abilities(Bangen et al., 2013). The cognitive domains affected were similar to those affected in the present study (except for visuospatial abilities- not assessed in the present study). In non T2D subjects (Barnes et al., 2013; Caselli et al., 2011; Praetorius et al., 2013), memory was the cognitive domain most impaired in ApoE4 genotype carriers. This may suggest that in T2D subjects, ApoE4 has a different, perhaps more extensive—for example increased amount of cerebral amyloid angiopathy in addition to higher burden on neurofibrillary tangles and amyloid plaques (Peila et al., 2002)—role in brain pathology, in addition to its effect on mid-temporal AD-related neuropathology.
HbA1c levels have been shown to be associated with increased risk for worse cognitive function and dementia(Yaffe et al., 2012). Improvement in glycemic control in elderly T2D subjects has been shown to improve cognitive performance in some(Meneilly et al., 1993), but not all previous studies(Hewer et al., 2003). These discrepancies may be partially explained by differential effects of the APOE4 status on the relationships of glycemic control and cognition suggesting heterogeneous vulnerability of T2D subjects to the effects of worse glycemic control on cognition.
Hyperglycemia is associated with accelerated formation of advanced glycation end products (AGEs), which decrease the solubility and increase resistance of several proteins present in neurofibrillary tangles (NFTs) and senile plaques(Smith et al., 1994). ApoE4 allele was associated with a greater burden of senile plaques, NFTs and amyloid angiopathy in the brains of T2D subjects(Peila et al., 2002). ApoE4 may further exacerbate T2D neuropathology due to reduced ability to repair neuronal damage and decrease antioxidant activity(Liu et al., 2013).
Surprisingly, ApoE4 was not significantly associated with worse cognitive performance(Bangen et al., 2013). A possible explanation is that subjects were included in the present study after several screening procedures that ensured they are cognitively normal and thus, they may carry factors that protect their cognition, offsetting APOE4 deleterious effects. Our finding, however, is consistent with findings from others that included only cognitively intact subjects who were at increased risk for dementia by being nonagenerians(Carrion-Baralt et al., 2009).
Previous studies have demonstrated that within cognitively normal subjects, cognitive performance in the lower, albeit normal, range, is associated with increased risk for dementia(Weinstein et al., 2013). Thus, although the contributions of the interrelationships of HbA1c and Apo E status to dementia will become clearer only when the longitudinal follow-up assessments of the IDCD become available, the present results provide preliminary evidence for the role of Apo E and degree of long-term glycemic control in the identification of T2D subjects who may be at particularly higher risk for dementia.
There are several limitations to the present report. First, it is based on cross-sectional data and thus causality cannot be inferred. The possibility that an incipient dementia process is underlying poor self-care leading to worsening HbA1c levels in APOE4 carriers, who are at higher risk for dementia, cannot be ruled out. Brain imaging was not performed in this study, thus limiting our ability to evaluate the contribution of cerebrovascular abnormalities to the present findings. Women are slightly underrepresented in the study. Strengths of this study include the large sample, validated T2D diagnosis for each subject, an average of 18 HbA1c measurements and other diabetes-related characteristics, strong validity for risk factor levels and medical diagnosis, and a thorough cognitive evaluation.
Acknowledgments
This study was supported by the American Federation for Aging Research (AFAR), Young investigator award 2011 and NIRG-11-205083 Alzheimer’s Association, 2012 for Dr. Ravona-Springer, NIA grant R01 AG034087 for Dr. Beeri and P50 AG05138 for Dr. Sano as well as the Helen Bader Foundation, the Irma T. Hirschl Scholar Award, and the Leroy Schecter Foundation for Dr. Beeri.
Role of funding source:
The above mentioned organizations had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to report
Contributors
Ramit Ravona-Springer : collaborated in study design, data collection, data research and manuscript writing.
Heymann Antony: searched the data, contributed to the discussion.
Schmeidler James,: performed statistical analysis, reviewed the manuscript.
Sano Mary: reviewed the manuscript, contributed to the discussion.
Preiss Rachel: searched the data.
Koifman Keren: searched the data.
Hoffman Hadas: searched the data.
Silverman Jeremy M.: reviewed the manuscript, contributed to the discussion
Schnaider- Beeri Michal: collaborated in study design, data collection, data research and supervised manuscript writing.
References
- Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, Bondi MW, Seshadri S, Wolf PA, Au R. APOE Genotype Modifies the Relationship between Midlife Vascular Risk Factors and Later Cognitive Decline. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Arvanitakis Z, Yu L, Kelly J, De Jager PL, Bennett DA. Apolipoprotein E and change in episodic memory in blacks and whites. Neuroepidemiology. 2013;40:211–219. doi: 10.1159/000342778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion-Baralt JR, Melendez-Cabrero J, Rodriguez-Ubinas H, Schmeidler J, Beeri MS, Angelo G, Sano M, Silverman JM. Impact of APOE epsilon4 on the cognitive performance of a sample of non-demented Puerto Rican nonagenarians. J Alzheimers Dis. 2009;18:533–540. doi: 10.3233/JAD-2009-1160. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Locke DE, Sabbagh MN, Ahern GL, Rapcsak SZ, Baxter LC, Yaari R, Woodruff BK, Hoffman-Snyder C, Rademakers R, Findley S, Reiman EM. Cerebrovascular risk factors and preclinical memory decline in healthy APOE epsilon4 homozygotes. Neurology. 2011;76:1078–1084. doi: 10.1212/WNL.0b013e318211c3ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GA, Elias MF, Robbins MA, Elias PK, Nagy Z. Presence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine-Syracuse Study. Diabetologia. 2009;52:2551–2560. doi: 10.1007/s00125-009-1497-2. [DOI] [PubMed] [Google Scholar]
- Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer's Disease. Aging (Milano) 1996;8:379–385. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J.Psychiatr.Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- Hewer W, Mussell M, Rist F, Kulzer B, Bergis K. Short-term effects of improved glycemic control on cognitive function in patients with type 2 diabetes. Gerontology. 2003;49:86–92. doi: 10.1159/000067947. [DOI] [PubMed] [Google Scholar]
- Heymann AD, Chodick G, Halkin H, Karasik A, Shalev V, Shemer J, Kokia E. The implementation of managed care for diabetes using medical informatics in a large Preferred Provider Organization. Diabetes Res Clin Pract. 2006;71:290–298. doi: 10.1016/j.diabres.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Sullivan M, Horowitz KR, Ding J, Marcovina S, Lovato LC, Lovato J, Margolis KL, O'Connor P, Lipkin EW, Hirsch J, Coker L, Maldjian J, Sunshine JL, Truwit C, Davatzikos C, Bryan RN. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA. Diabetes related conditions and dementia. J Neurol Sci. 2010;299:35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol. 1993;48:M117–M121. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- Praetorius M, Thorvaldsson V, Hassing LB, Johansson B. Substantial effects of apolipoprotein E epsilon4 on memory decline in very old age: longitudinal findings from a population-based sample. Neurobiol Aging. 2013;34:2734–2739. doi: 10.1016/j.neurobiolaging.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Ravona-Springer R, Heymann A, Schmeidler J, Guerrero-Berroa E, Sano M, Preiss R, Koifman K, Hoffman H, Levy A, Silverman JM, Schnaider-Beeri M. Haptoglobin 1-1 genotype is associated with poorer cognitive functioning in the elderly with type 2 diabetes. Diabetes Care. 2013;36:3139–3145. doi: 10.2337/dc12-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravona-Springer R, Schnaider-Beeri M. The association of diabetes and dementia and possible implications for nondiabetic populations. Expert Rev Neurother. 2011;11:1609–1617. doi: 10.1586/ern.11.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, Hirasawa R, Shimano H, Yamazaki K, Sone H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012;55:2128–2131. doi: 10.1007/s00125-012-2572-7. [DOI] [PubMed] [Google Scholar]
- Weinstein G, Beiser AS, Decarli C, Au R, Wolf PA, Seshadri S. Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham Heart Study. Stroke. 2013;44:2787–2794. doi: 10.1161/STROKEAHA.113.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, Cauley JA, Rosano C, Launer LJ, Strotmeyer ES, Harris TB. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]