Abstract
ATP-sensitive potassium (KATP) channels are widely distributed in vasculatures, and play an important role in the vascular tone regulation. The KATP channels consist of 4 pore-forming inward rectifier K+ channel (Kir) subunits and 4 regulatory sulfonylurea receptors (SUR). The major vascular isoform of KATP channels is composed of Kir6.1/SUR2B, although low levels of other subunits are also present in vascular beds. The observation from transgenic mice and humans carrying Kir6.1/SUR2B channel mutations strongly supports that normal activity of the Kir6.1/SUR2B channel is critical for cardiovascular function. The Kir6.1/SUR2B channel is regulated by intracellular ATP and ADP. The channel is a common target of several vasodilators and vasoconstrictors. Endogenous vasopressors such as arginine vasopressin and α-adrenoceptor agonists stimulate protein kinase C (PKC) and inhibit the KATP channels, while vasodilators such as β-adrenoceptor agonists and vasoactive intestinal polypeptide increase KATP channel activity by activating the adenylate cyclase-cAMP-protein kinase A (PKA) pathway. PKC phosphorylates a cluster of 4 serine residues at C-terminus of Kir6.1, whereas PKA acts on Ser1387 in the nucleotide binding domain 2 of SUR2B. The Kir6.1/SUR2B channel is also inhibited by oxidants including reactive oxygen species allowing vascular regulation in oxidative stress. The molecular basis underlying such a channel inhibition is likely to be mediated by S-glutathionylation at a few cysteine residues, especially Cys176, in Kir6.1. Furthermore, the channel activity is augmented in endotoxemia or septic shock, as a result of the upregulation of Kir6.1/SUR2B expression. Activation of the nuclear factor-κB dependent transcriptional mechanism contributes to the Kir6.1/SUR2B channel upregulation by lipopolysaccharides and perhaps other toll-like receptor ligands as well. In this review, we summarize the vascular KATP channel regulation under physiological and pathophysiological conditions, and discuss the importance of KATP channel as a potentially useful target in the treatment and prevention of cardiovascular diseases.
Keywords: ATP-sensitive potassium channel, Kir6.1, SUR2B, protein phosphorylation, S-glutathionylation, nuclear factor-κB, sepsis, sudden infant death syndrome, J-wave syndrome
1 Introduction
The ATP-sensitive K+ (KATP) channel was firstly identified by Noma in cardiac myocytes [1]. The KATP channel differs from other inward rectifier K+ channels (Kir) in that it is sensitive to intracellular ATP concentration [1]. Besides in cardiac myocytes, KATP channels have also been found in skeletal myoctes [2] and pancreatic β cells[3]. In 1989, Standen et al. [4] recorded a novel KATP current in rabbit mesenteric arterial smooth muscle cells (SMCs). The current is enhanced by vasoactive intestinal polypeptide (VIP) and a KATP channel opener cromakalim, and suppressed by glibenclamide, similar to currents carried by other KATP channels identified previously. In the presence of pinacidil, the Kir6.1/SUR2B is stimulated by micromolar ATP and millimolar UDP or ADP, and higher doses (1–3 mmol/L) of ATP inhibits the channel, properties that lead to the name KNDP channel [5]. Subsequently, KATP channels were found to be broadly expressed in vasculatures[6, 7]. Cloning of Kir6.x and SUR subunits of KATP channels in the mid-1990s, and the generation of KATP channel transgenic mice in 2000s, have greatly extend our understanding in the biophysical features and physiological functions of vascular KATP channel. Recently, reports of KATP mutations in human patients start to reveal the role of KATP channel in pathophysiological conditions [8–10].
2 Molecular structures of vascular KATP channels
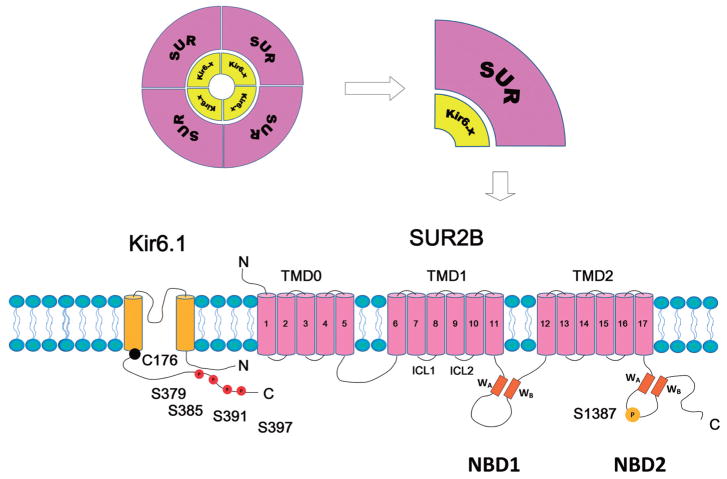
KATP channels are octameric protein complexes containing 4 pore-forming Kir6 subunits and 4 accessory sulfonylurea receptor (SUR) subunits (Fig. 1). To date, two Kir6.x genes (KCNJ8 for Kir6.1, and KCNJ11 for Kir6.2) and two SUR genes (ABCC8 for SUR1 and ABCC9 for SUR2A and SUR2B) have been identified. The Kir6.x share 40%–50% homology in amino acid sequence with other Kir channels. Structural studies suggest the Kir6.x subunit has 2 transmembrane helixes (M1 and M2), cytoplasmic N- and C-termini and a pore-forming loop with a glycine-phenylalanine-glycine signature motif for K+ selectivity [11]. In symmetrical 140 mmol/L K+ recording conditions, the unitary conductance of Kir6.1-containing channels is ~35 pS (Kir6.1/SUR2B) [12], whereas Kir6.2-containing channels is ~80 pS (Kir6.2/SUR2B) [13].
Fig. 1.
Molecular structure of the vascular KATP channel. KATP channels are octameric complexes formed by 4 Kir6 subunits (Kir6.x) and 4 accessory sulfonylurea receptor (SUR) subunits. The Kir6.x subunit has 2 transmembrane helixes. SUR subunit has 3 transmembrane domains (TMD0, 1 and 2). There are two intracellular loops linking the adjacent TMDs. Each intracellular loop contains a nucleotide binding domain (NBD1 and NBD2, respectively). A Walker A motif (WA), a Walker B motif (WB), and a linker region are located within the NBDs. The Ser379, Ser385, Ser391, and Ser397 at the distal C-terminus of Kir6.1 are PKC phosphorylation residues. The Ser1387 is PKA phosphorylation residue. The Cys176 in the transmembrane domain of Kir6.1 is the major residue accounting for the channel’s oxidant sensitivity.
Functional expression of KATP channel requires co-expression of SUR subunit [14], which is under the category of ATP-binding cassette transporter (ABCC) family. SUR1 is dominantly expressed in pancreatic β cells. SUR2 has two variants: SUR2A and SUR2B, which are produced by alternative splicing of exon 38 in ABCC9 [13, 15]. They are different in the last 42 amino acids in the C terminus. SUR2A is mainly expressed in myocardium and skeletal muscles, whereas SUR2B is generally distributed in smooth muscles. SUR subunit has 3 transmembrane domains (Fig. 1): TMD1 and TMD2 with 6 transmembrane segments in each, plus TMD0, an N-terminal transmembrane domain with 5 transmembrane segments. There are two large intracellular loops connecting the adjacent TMDs (Fig. 1). Each intracellular loop contains a nucleotide binding domain (NBD1 and NBD2, respectively). A Walker A motif (WA), a Walker B motif (WB), and a linker region are located within the NBDs and are critical for nucleotide binding [11].
The main body of SUR2B (TMD1, 2 and NBD1, 2) shares many properties with other transmembrane AB-CCs. Thereof it can be modeled with the bacteria ATP binding protein SAV1866 [16]. The TMDs and NBDs display a twisted interaction: TMD1 mainly interacts with NBD2 and TMD2 mainly with NBD1[16]. This intercrossed interaction might have a significant effect on protein kinase A (PKA) phosphorylation and regulation, for a phosphorylation site has been found in NBD2 domain (see below for detail).
3 Evidence of vascular KATP channel function from transgenic mouse models and human diseases
KATP channels have been demonstrated to play a substantial role in vascular tone regulation by using traditional pharmacological approaches, including the application of KATP channel openers, such as pinacidil, diazoxide and cromakalim, and KATP channel blockers, such as sulfonylureas (tolbutamide, glibenclamide) and PNU-37883A. Transgenic mouse models provide more specific strategies to understand the impacts of KATP channels on cardiovascular system. In general, both Kir6.1 and SUR2 knockout mice exhibit coronary arterial spasm and sudden early death, with EKG showing frequently spontaneous ST segment elevation [17, 18]. In addition, Kir6.1-null mice are more sensitive to endotoxemia, suggesting functional KATP channel is important for survival from sepsis [19, 20]. Since KATP channel is distributed in vascular endothelial cells as well as SMCs, both of which contribute to vascular tone regulation, the function of endothelial KATP channel has been noticed recently. In a transgenic animal model, SUR2B expression is selectively knocked in in SMCs. These SUR2-null mice remain to show coronary vasospasm similar to Kir6.1 knockout mice, suggesting that KATP channel in vascular smooth muscle (VSM) is not enough for vascular tone regulation[21]. In another study, transgenic mice expressing dominant negative Kir6.1 subunits exclusively in endothelium exhibit an elevated endothelin-1 (ET-1) release and an increase in coronary resistance[22]. These observations thus suggest that endothelial KATP channel is important for coronary circulation.
Recently, several mutations of vascular KATP channels were found in human patients. A missense mutation in exon 3 (S422L) of KCNJ8 was identified in a patient presenting massive accentuation of the early repolarization and recurrent ventricular fibrillation in EKG with normal coronary angiography [8]. The S422L mutation was also found by another group in 2 patients presenting J-wave syndrome[9]. The current density of Kir6.1 S422L/SUR2A channel heterologously expressed in COS-1 cells is increased by ~65%. Two other KCNJ8 mutations (an in-frame deletion E332del and a missense mutation V346I) located at Kir6.1’s C-terminus were found in sudden infant death syndrome (SIDS) patients. Patch clamping shows that the pinacidil-stimulated KATP currents are reduced by ~ 50% in E332del and V346I. The loss-of-function KCNJ8 mutations may result in a maladaptive cardiac response to systemic metabolic stimulators leading to SIDS[10].
4 Sepsis susceptibility
In a genome-wide association study using N-ethyl-N-nitrosourea in-vivo mutagenesis, Beutler and his colleagues screened a large population of mice and identified 4 strains that were highly susceptible to multiple infectious pathogens, including cytomegalovirus, lipopolysaccharides (LPS), synthetic Toll-like receptor 3 (TLR3) ligand polyinosine: polycytidylic acid and TLR9 agonists CpG oligodeoxynucleotides[19]. They have found that the high sepsis susceptibility is due to Kcnj8, as disruptions of the locus containing Kcnj8 are present in the homozygous form in all the 4 strains of mice. Their mutagenesis study suggests that the LPS hypersensitivity phenotype is not suppressed by mutations in Myd88, Trif, Tnf, Tnfrsf1a, Ifnb, Ifng or Stat1 as well as several other genes known to contribute to inflammation responses. The investigators believe that their forward genetic approaches also can exclude tumor necrosis factor (TNF), type-I interferon (IFN), and type-II IFN as essential lethal factors, because mice that lacked these receptor genes succumbed to low doses of LPS. These control studies strongly suggest that the sepsis hypersusceptibility is not a result of these genes and pro-inflammation cytokines. Consistent with these observations, Kcnj8-knockout mice show severe survival disadvantages in response to septic pathogens, with progressive deterioration in cardiac activity, ischemic myocardial damage, and myocardial contractile dysfunction [20]. Since genetic disruption of KATP channels is not lethal in mice, these studies indicate that activation of the KATP channels is crucial for the systemic response to sepsis by retaining myocardial perfusion.
Studies by Beutler, Hoffman and their colleagues indicate that the KATP channel also functions in antiviral activity in Drosophila. Knockout of the dSUR gene increases the lethality of Drosophila after infection with the cardiotrophic flock house virus (FHV) [19]. Similar effects were observed by knockdown of both Ir and Ir2 genes [23]. In the KATP mutant flies, FHV causes rapid viremia and death, likely to be mediated by modulating the antiviral RNA interference in the heart, while flies treated with the KATP agonist pinacidil are protected against the viral infection [23].
5 Regulation of vascular KATP channels
The regulation of vascular KATP channels is composed by an immediate and a delayed phase. The immediate regulation by most of metabolites, hormones and neurotransmitters is through channel gating, whereas transcriptional mechanisms contribute to the delayed regulation.
5.1 Metabolites
5.1.1 ATP/ADP
KATP channels are subject to a direct and fast regulation by intracellular ATP and ADP. Such modulations directly link the cellular metabolic states to membrane electric activities. ATP reduces the vascular KATP channel activity; however, the inhibitory effects of ATP are variable in different reports[24]. Due to a relatively high intracellular ATP concentration in physiological condition (1–11.7 mmol/L), the vascular KATP channels usually display a low activity at rest[25]. In comparison, intracellular ADP concentration ranges between 0.1 and 3 mmol/L [25], and exhibits stimulatory effect on KATP channel. According to this characteristic, vascular KATP channel was once termed as KNDP channel[5, 26].
5.1.2 pH
pH changes in local tissues are very common in heavy exercise, hypoxia, ischemia, and severe diabetes. Hypercapnia and acidosis relax blood vessels, especially cerebral arterioles, and increase regional blood flow in circulation[27, 28]. Hypercapnic acidosis induces vasodilation through activation of KATP channels in VSMs, with maximal effect at pH 6.5 to 6.8 [29]. Blockade of KATP channels attenuates the vasodilation, which is observed in cerebral arterioles, basil artery, coronary artery, mesenteric artery or internal mammary artery. The modulations of KATP channel activity by H+, ATP and ADP are mediated via direct ligand binding to Kir6.x or SUR, leading to alternation in the channel gating [30–32].
5.1.3 Nitric oxide (NO)
NO is released by endothelial cells and causes vasodilation. It is reported NO hyperpolarizes SMCs in rabbit mesenteric arteries through increasing cGMP and activating KATP channels[33]. NO released from skeletal muscle vasculatures during excise may activate vascular KATP channels, and antagonizes sympathetic vaso-constriction, providing a delicate mechanism to regulate blood flow in exercising skeletal muscles[34]. Lactate, an important metabolic product in retina, relaxes retinal arterioles through activation of nitric oxide synthase (NOS) and guanylyl cyclase, and opening of KATP channel [35]. However, it is also reported that NO donor sodium nitroprusside fails to activate KATP currents isolated from rabbit mesenteric arterial SMCs and pig coronary arterial SMCs [36, 37]. Therefore, the exact role of NO in regulating vascular KATP channels is still debatable.
5.1.4 Eicosanoids
Epoxyeicosatrienoic acids (EETs) are cytochrome P-450 metabolites of arachidonic acid synthesized in endothelial cells[38]. Since EETs participate in vasodilation by hyperpolarizing cell membrane, some groups classified them in endothelium-derived hyperpolarizing factors (EDHFs) [39, 40]. Both 11, 12-EET and 14, 15-EET induce dose-dependent vasodilation in isolated small mesenteric arteries through activation of KATP channels [41, 42], but the underlying mechanisms seem to be different: 11, 12-EET activates mesenteric SMC KATP channels through PKA [42], whereas the stimulation of 14, 15-EET depends on ADP-ribosylation of Gs [41].
5.1.5 Hydrogen sulfide (H 2S)
H2S is a product from L-cysteine metabolism catalyzed by cystathionine-γ-lyase and cystathionine-β-synthase in mammalian tissues. Endogenous H2S has been detected in various vascular tissues (e.g. aorta, tail, and mesenteric arteries) [43]. H2S in physiological concentrations (nearly 45 μmol/L) induces vasodilation in rat aorta and transient reduction of blood pressure through activation of KATP channels [44, 45]. Pinacidil- and H2S-induced vasorelaxation are compromised in cerebral arterioles of SUR2-null mice[46]. Patch clamping studies demonstrate that exogenous H2S activates KATP channels and hyperpolarizes cell membrane in SMC isolated from rat mesenteric artery [47] and piglet cerebral arterioles [46]. In addition, aortic rings seem to be more sensitive to H2S than pulmonary arterial rings. The reason could be due to the increased SUR2B expression in aorta [48]. Recently, a slow-releasing hydrophilic H2S compound GYY4137 has been demonstrated to display vasorelaxing effect in rat endothelium-intact aortic rings and perfused rat renal vasculature through stimulation of vascular KATP channels[49]. Because GYY4137 reduces blood pressure in hypertensive rats without changing heart rate or contracting force in vitro, it could be a promising drug for anti-hypertension therapy in future.
5.2 Hormones and neurotransmitters
Vascular KATP channels are regulated by many hormones and neurotransmitters (Table 1). Based on vaso-active functions, these endogenous vasoactive substances are classified into two groups: vasoconstrictors and vasodilators. The receptors of these substances are coupled to Gq and Gs respectively. Gq activation stimulates phospholipase C (PLC), which converts membrane phospholipids to diacylglycerol (DAG) and inositol triphosphate (IP3). DAG subsequentially activates protein kinase C (PKC). Gs activation stimulates adenylyl cyclase, which catalyzes ATP to produce cAMP. The elevated cAMP in turn binds to regulatory subunits of PKA, leading to PKA dissociation and release of the catalytic subunits. Vascular KATP channels are substrates of both PKC and PKA (see below).
Table 1.
Summary of vasoactive substances targeting vascular KATP channels
| Vasoactive substances | Receptor | Distributions | References | |
|---|---|---|---|---|
| Vasoconstrictors | Noradrenaline | α2 | Rat tail artery | [55] |
| Endothelin-1 | N/A | Rabbit coronary and pulmonary arteries | [56] | |
| Anginotension II | N/A | Rat mesenteric artery | [57] | |
| AVP | V1a | Rat mesenteric artery | [54] | |
| Neuropeptide Y | NPY1 | Rabbit mesenteric artery, dog coronary artery | [58, 59] | |
| Serotonin | 5-HT2 | Rabbit mesenteric artery | [58] | |
| Histamine | H1 | Rabbit mesenteric artery | [58] | |
| Vasodilators | Adenosine | A2 | Rat mesenteric artery, guinea pig coronary artery | [60, 61] |
| VIP | VPAC1 | Rat mesenteric artery | [62] | |
| Glucagon-like peptide-1 | GLP-1 | Rat aorta | [63] | |
| CGRP | N/A | Rabbit mesenteric artery, pig coronary artery | [36, 37] |
N/A, not reported.
Understanding the molecular mechanism underlying how these vasoactive substances regulate KATP channel is important. As an example, arginine vasopression (AVP, 0.01–0.04 U/min) was recommended for management of sepsis to avoid using high concentration of catecholamines (e.g. norepinephrine, dopamine) [50] and improve insufficiency of AVP secretion in septic patients[51, 52]. However, a dose higher than 0.03 U/min is not recommend since it may induce coronary vasoconstriction and impair cardiac function[53]. Studies using exogenous expression system show that vascular KATP channel activity is inhibited by AVP [54]. AVP binds to V1a receptor and activates PKC leading to a reduced channel open probability. Therefore, it is likely that AVP elevates blood pressure by inhibiting KATP channels located in peripheral blood vessels, and decreases coronary perfusion through inhibiting coronary arterial KATP channels.
5.3 Post-translational regulation
5.3.1 PKC pathway
As shown in Table 1, most vasoconstrictors inhibit Kir6.1/SUR2B channel activity though Gq protein coupled receptor stimulation which results in PKC activation. Patch clamp recording shows that Kir6.1/SUR2B channel rather than Kir6.2/SUR2B channel is sensitive to PKC activation, suggesting Kir6.1 subunit is the target of PKC[64]. Further investigation shows that a motif containing Ser379, Ser385, Ser391 and Ser397 at the distal C-terminus of Kir6.1 is phosphorylated by PKC (Fig. 1) [64]. The 4 serine residues display a repeated pattern (SXRR/KXN) where the underlined S are the phosphorylation sites. The channel inhibition by PKC is slightly reduced when either of the serine residues is mutated to unphosphorylable alanine. The PKC effect is gradually diminished when more serines are mutated. The PKC inhibition is almost completely eliminated when all the 4 residues are mutated. The additive effects of the repeating PKC sites may allow the channel activity to be elaborately regulated according to the levels of PKC stimulation. PKC activation may also result in caveolin-1 dependent internalization of vascular KATP channel [65], which might occur after the immediate response of channel inhibition and act as a long-term effect of PKC modulation.
5.3.2 PI3K-Akt pathway
The inhibitory effect of PKC on vascular KATP channel could be reduced by activation of phosphatidylinositol 3-kinases (PI3K)-Akt pathway. Phenylephrine, an α adrenergic receptor agonist that stimulates PKC, increases Akt phosphorylation in rat endothelium-denuded aorta. A PI3K inhibitor LY294002 enhances levcromakalim-induced vasodilation in aortic rings after pre-exposure to phenylephrine. The levcromakalim-induced KATP currents in the presence of phenylephrine are enhanced by an α adrenergic receptor blocker phentolamine and LY294002[66]. Therefore, PI3K-Akt pathway may provide a negative feedback to regulate vascular KATP channel upon PKC activation. The PI3K-Akt pathway may also be involved in the high glucose-induced vascular KATP channel dysfunction. Human endothelium-denuded omental artery treated with D-glucose (20 mmol/L) shows increased membrane expression of PI3K p85 α subunit and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase subunits (p47phox, p22phox, and Rac-1), and elevated Akt phosphorylation as well as intracellular superoxide (O2 ) production. High glucose impairs levcromakalim-induced vasorelaxation and cell membrane hyperpolarization, but can be antagonized by LY294002 or O2 generation inhibitors (tiron and apocynin) [67].
5.3.3 PKA pathway
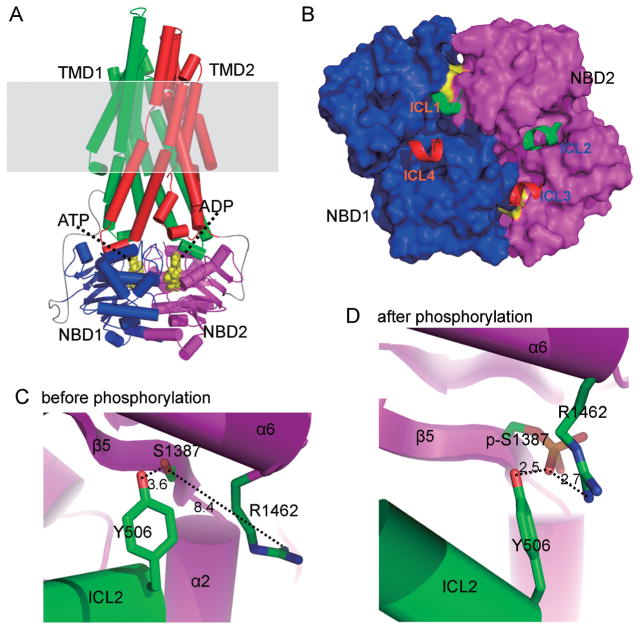
Most vasodilators increase Kir6.1/SUR2B channel activity by stimulating Gs protein coupled receptors leading to cAMP elevation and PKA activation. Quinn et al. have showed that PKA directly phosphorylates Kir6.1/SUR2B channels at 3 residues (Kir6.1 S385, SUR2B T633 and S1465) [68]. Our recent data have showed that 2 different serine residues (Ser1351 and Ser1387) located in the NBD2 of SUR2B are necessary for the channel activation. The Ser1387 is phosphorylated in an in vitro phosphorylation assay (Fig. 1) [69]. Further SUR2B modeling study based on the TMD topology of ABC protein SAV1866 suggests that Ser1387 is located on the interface of NBD2 with TMD1 and physically interacted with Tyr506 in TMD1 [16]. A positively charged residue (Arg1462) in NBD2 is close to Ser1387. The three residues produce compact triad upon PKA phosphorylation on Ser1387, leading to reshaping of the NBD2 interface and interdomain movement of NBD2 and TMD1 (Fig. 2). Mutation in any of the three residues diminishes PKA-dependent channel activation. Therefore, Ser1387 phosphorylation increases the NBD-TMD coupling efficiency, and opens the channel.
Fig. 2.
Model of SUR2B core domains. A: SUR2B core domains (TMD1, 2 and NBD1, 2) are modeled using SAV1866 as a template. Shaded region is plasmic membrane. B: The interface between TMDs and NBDs are highlighted. Intracellular loop-1 (ICL1) of TMD1 physically interacts with NBD1 and NBD2. ICL2 of TMD1 only interacts with NBD2. Thus TMD1 mainly interact with NBD2. Similarly, TMD2 mainly interacts with NBD1. C and D: The three critical residues involved in PKA phosphorylation are highlighted. Note the side chain of Arg1462 is far from phosphorlation residue Ser1387 before phosphorylation. It is attracted by phosphorylated Ser1387 (p-Ser1387) after PKA phosphorylation and forms a tight triad with p-Ser1387 and Tyr506. The figure is modified fromJournal of Biological Chemistry with permission [16].
cAMP also stimulates exchange protein directly activated by cAMP (Epac) which co-localizes with vascular KATP channel subunits and inhibit the channel activity via Ca2+-sensitive protein phosphatase 2B (calcineurin). The concentration of cAMP required to stimulate Epac is higher than PKA [70]. Therefore, cAMP shows two phases of regulation on vascular KATP channels: cAMP at low concentration activates the KATP channel via PKA, while a high concentration of cAMP inhibits channel activity through Epac.
Another interesting aspect is that the PKA-dependent Kir6.1/SUR2B channel activation can be antagonized by calcineurin [71]. The mechanisms may be due to: (1) calcineurin reduces PKA activity leading to an indirect channel inhibition; (2) calcineurin directly dephosphorylates the channel. However, it is unknown if calcineurin can modulate the PKA phosphorylation sites at SUR2B. Nevertheless, calcineurin seems to balance the effects of cAMP/PKA on vascular KATP channels.
5.3.4 Reactive oxygen species (ROS) and S-glutathionylation
An overproduction of ROS is one of the characteristics of oxidative stress which contributes to the development of many types of diseases, such as diabetes, atherosclerosis and sepsis [72]. The effect of ROS on KATP channel has been noticed recently. Cerebral arterioles treated with O2− show a less vasorelaxation response to cromakalim [73]. A pre-exposure of isolated mesenteric arterial rings to H2O2 also attenuates the KATP channel-mediated vasorelaxation [74]. In a diabetic rat model, pinacidil-induced vasodilation in cerebral arterioles is impaired, but is completely restored by treatments with superoxide dismutase (SOD) and catalase[75]. Similar impaired vascular responses to KATP channel openers are also observed in diabetic patients[76]. Therefore, the ROS overproduction in oxidative stress disrupts vascular KATP activity.
Recent studies have showed that H2O2 induces a glutathione (GSH) dependent Kir6.1/SUR2B channel inhibition, which can be mimicked by oxidized glutathione (GSSG) or thiol-modulating reagents[74]. The oxidant-mediated channel suppression is rescued by the reducing agent dithiothreitol (DTT) and the specific deglutathionylation agent glutaredoxin-1 (Grx1), suggesting S-glutathionylation is a mechanism underlying the channel modulation in oxidative stress. Moreover, it has been identified that Cys43 in N-terminus, Cys120 and Cys176 in the transmembrane domain of Kir6.1, accounts for the oxidant sensitivity. Among the 3 residue, Cys176 makes a major contribution (Fig. 1). Using structural modeling, how the addition of GSH to the channel protein can affect the gating of KATP channel has been studied. Simulation modeling suggests that after binding residue Cys176, the GSH moiety occupies a space between the slide helix and two transmembrane helices. Since the gating of KATP channel requires the movement of inner transmembrane helix, the addition of GSH in this critical location limits the conformational changes of the inner transmembrane helix, thus impairs channel gating and retains the channel in its closed state (Fig. 3) [77].
Fig. 3.
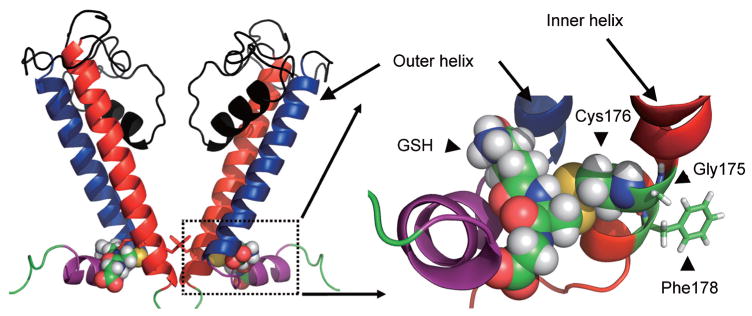
Structural modeling of Kir6.1 protein with the incorporation of GSH. The overall structural model of two opposing Kir6.1 monomers (out of four for clarity) was displayed. Boxed area was enlarged and showed the GSH associated area. The GSH moiety occupies a space between the inner and outer helix. The addition of GSH therefore impairs the movement of inner helix, which is necessary for the channel opening. The figure is modified fromJournal of Biological Chemistry with permission [77].
5.4 Transcriptional regulation
The expression of KATP channel subunits could be altered in some medical conditions and diseases. For instance, a declined SUR2B mRNA instead of Kir6.1 and Kir6.2 is observed in SMCs dissociated from diabetic rat aorta[78]. The Kir6.1 and SUR2B expression (mRNA and protein) in aortic SMCs, as well as the isolated KATP current density, are also reduced in obese rats[79]. Flow stress elevates the expression of Kir6.2 (both mRNA and protein) in rat pulmonary microvascular endothelial cells[80].
The KATP channel activity may also be subject to chronic hypoxic regulation. Glibenclamide reduces vasorelaxation of pial arterioles during hypoxia in vivo[81]. The diazoxide-induced vasodilation in near-term pregnant uterine arteries is compromised after long-term exposure to hypoxia at high altitude[82]. The molecular mechanism underlying the causal relationship between hypoxia and vascular KATP channel is not well examined. However, the mRNA and protein expression of Kir6.1 and Kir6.2 are up-regulated under venous hypoxemia in right atrium of patients with tetralogy of Fallot or ventricular septal defects. The study shows that Kir6.1 mRNA transcription is Forkhead box (FOX) O1 dependent, whereas FOXO1 transcription is hypoxia-inducible factor-1α (HIF-1α) dependent. Moreover, the study on cultured rat atrial myocytes also confirmed the causal relationships among hypoxia, HIF-1α, FOXO1, and Kir6.1 [83].
KATP channel exhibits a high channel activity in sepsis. Glibenclamide has been tested in several septic animal models and shows to raise blood pressure through increasing systemic vascular resistance[84, 85]. PNU-37883A, an KATP channel inhibitor targeting the pore region of Kir6 subunit, displays more potent inhibitory effect on Kir6.1/SUR2B than Kir6.2/SUR2B[86], and provides better outcomes to reverse LPS-induced vascular hyporeactivity to circulating epinephrine [87]. The high channel activity is mainly due to upregulation of KATP channel expression. Both mRNA and protein levels for Kir6.1 are increased in the diaphragm of rats treated with LPS, with the mRNA level increased by 4-fold in 48 h, whereas protein levels augmented 9-fold after 24 h[88]. Moreover, Kir6.1 expression in colonic smooth muscle is enhanced by 22-fold, the mRNA level for SUR2B is decreased by 3-fold in experimental colitis[89]. We reveal that an overnight LPS treatment hyperpolarizes aortic SMCs. Whole cell patch clamping shows that KATP current density is elevated in aortic SMCs exposed to LPS, but not changed in HEK293 cells heterologously expressing Kir6.1/SUR2B. The increased protein surface expression is due to nuclear factor (NF)-κB-dependent Kir6.1 and SUR2B mRNA expression[90]. Such an upregulation increases KATP channel activity, and may lead to excessive vasodilation during sepsis. A recent study using a rat septic shock model induced by peritonitis shows that septic vascular hyporeactivity is improved by PNU-37883A but not high-conductance Ca2+-activated K+ (BKCa) channel blocker IbTX. In consistent, sepsis increases mRNA and protein expression of Kir6.1 and SUR2B subunits, but does not change expression of BKCa channels. The elevated aortic NO release, NF-κB activation, and KATP channel upregulation are inducible NOS dependent[91]. These observations again suggest that a transcriptional mechanism underlying the KATP channel regulation during sepsis, and indicate that selectively inhibiting vascular KATP channel could offer promising therapeutic approaches to manage septic shock.
5.5 Non-sulfonylurea anti-diabetic agents
Some anti-diabetic agents that are not categorized in sulfonylurea family have been demonstrated to affect vascular KATP channel activity recently. Rosiglitazone (RSG) reduces blood glucose level by increasing insulin sensitivity and glucose uptake in skeletal muscle and adipose tissues. Our recent study have showed that RSG is not only able to inhibit the Kir6.1/SUR2B channels in a membrane-delimited manner, but also attenuate the adrenergic mediated coronary vasodilation[92]. The vasoactive effect is Kir6.1 dependent, because the isolated hearts from Kir6.1 knockout mice show less response to RSG. Phenformin is a biguanide that used to treat type II diabetes mellitus. It is more selective to block Kir6.1/SUR2B channel, with a 90% reduction of open probability in inside out patch, and also inhibit the KATP current in native vascular SMCs[93]. These results suggest that RSG and phenformin not only reduce blood glucose, but also act as vascular KATP channel inhibitors, and may potentially reduce coronary response to circulating vasodilators and metabolic stress.
6 Summary
Ample evidence from experimental animal models and the identification of KATP channel mutations in patients indicate that KATP channel plays a critical role in vascular tone regulation, and likely contributes to the pathogenesis of many cardiovascular diseases. It remains challenging to design therapeutic modalities based on an intervention to the KATP channel. For an example, LPS-induced vascular KATP channel upregulation may be a myocardial protective mechanism because it increases coronary blood flow and reduces myocardial depression during sepsis. However, an excessively up-regulated vascular KATP channel will cause severe peripheral vasodilation leading to lethal hypotension and organ failure. The two contradictory effects of the KATP channel on coronary and systemic circulations hinder the administration of KATP channel blockers and openers in sepsis in which both the reasonable controls of systemic vascular contractility and the maintenance of coronary circulation are necessary. All of these depend on the understanding of the molecular mechanisms underlying the vascular KATP channel regulation.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant (No. HL-067890), National Institutes of Health Grant (No. HD060959), and the American Heart Association (No. 09GRNT2010037).
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316(6030):736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- 3.Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- 4.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 5.Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110(2):573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 7.Winquist RJ, Heaney LA, Wallace AA, Baskin EP, Stein RB, Garcia ML, Kaczorowski GJ. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248(1):149–156. [PubMed] [Google Scholar]
- 8.Haissaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kaab S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20(1):93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac KATP channel Kir6. 1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7(10):1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tester DJ, Tan BH, Medeiros-Domingo A, Song C, Makielski JC, Ackerman MJ. Loss-of-function mutations in the KC-NJ8-encoded Kir6. 1 KATP channel and sudden infant death syndrome. Circ Cardiovasc Genet. 2011;4(5):510–515. doi: 10.1161/CIRCGENETICS.111.960195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JD, Sansom MS, Ashcroft FM. Potassium channel regulation. EMBO Rep. 2003;4(11):1038–1042. doi: 10.1038/sj.embor.7400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6. 1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499 (Pt 3):715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6. 2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271(40):24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 14.Ammala C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, Sakura H, Coles B, Ashcroft SJ, Ashcroft FM. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996;379(6565):545–548. doi: 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- 15.Chutkow WA, Simon MC, Le Beau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45(10):1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Jiang C, Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem. 2008;283(12):7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110(2):203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6. 1. Nat Med. 2002;8(5):466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 19.Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet. 2007;39(12):1453–1460. doi: 10.1038/ng.2007.25. [DOI] [PubMed] [Google Scholar]
- 20.Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knock-out of the KCNJ8-encoded Kir6. 1 KATP channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20(13):2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98(5):682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 22.Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, Hendricks-Munoz KD, Coetzee WA. Transgenic expression of a dominant negative KATP channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21(9):2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- 23.Eleftherianos I, Won S, Chtarbanova S, Squiban B, Ocorr K, Bodmer R, Beutler B, Hoffmann JA, Imler JL. ATP-sensitive potassium channel (KATP)-dependent regulation of cardiotropic viral infections. Proc Natl Acad Sci U S A. 2011;108(29):12024–12029. doi: 10.1073/pnas.1108926108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77(4):1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 25.Randak CO, Welsh MJ. ADP inhibits function of the ABC transporter cystic fibrosis transmembrane conductance regulator via its adenylate kinase activity. Proc Natl Acad Sci U S A. 2005;102(6):2216–2220. doi: 10.1073/pnas.0409787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the KNDP subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87(2):112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 27.Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8(3):358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- 28.Tian R, Vogel P, Lassen NA, Mulvany MJ, Andreasen F, Aalkjaer C. Role of extracellular and intracellular acidosis for hypercapnia-induced inhibition of tension of isolated cerebral arteries. Circ Res. 1995;76(2):269–275. doi: 10.1161/01.res.76.2.269. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wu J, Li L, Chen F, Wang R, Jiang C. Hypercapnic acidosis activates KATP channels in vascular smooth muscles. Circ Res. 2003;92(11):1225–1232. doi: 10.1161/01.RES.0000075601.95738.6D. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo M, Tanabe K, Kioka N, Amachi T, Ueda K. Different binding properties and affinities for ATP and ADP among sulfonylurea receptor subtypes, SUR1, SUR2A, and SU. J Biol Chem. 2000;275(37):28757–28763. doi: 10.1074/jbc.M004818200. [DOI] [PubMed] [Google Scholar]
- 31.Dabrowski M, Tarasov A, Ashcroft FM. Mapping the architecture of the ATP-binding site of the KATP channel subunit Kir6. 2. J Physiol. 2004;557(Pt 2):347–354. doi: 10.1113/jphysiol.2003.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Cui N, Yang Z, Wu J, Giwa LR, Abdulkadir L, Sharma P, Jiang C. Direct activation of cloned KATP channels by intracellular acidosis. J Biol Chem. 2001;276(16):12898–12902. doi: 10.1074/jbc.M009631200. [DOI] [PubMed] [Google Scholar]
- 33.Murphy ME, Brayden JE. Nitric oxide hyperpolarizes mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486 (Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506 (Pt3):817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47(2):693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- 36.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosolowsky M, Campbell WB. Synthesis of hydroxyeicosatetraenoic (HETEs) and epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Biochim Biophys Acta. 1996;1299(2):267–277. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 39.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107(5):769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 40.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49(3):590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 41.Ye D, Zhou W, Lu T, Jagadeesh SG, Falck JR, Lee HC. Mechanism of rat mesenteric arterial KATP channel activation by 14, 15-epoxyeicosatrienoic acid. Am J Physiol Heart Circ rat Physiol. 2006;290(4):H1326–1336. doi: 10.1152/ajpheart.00318.2005. [DOI] [PubMed] [Google Scholar]
- 42.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11, 12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2005;288(1):H358–H364. doi: 10.1152/ajpheart.00423.2004. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287(5):R2B.H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- 44.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283(2):H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 45.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol. 2011;300(6):H2088–H2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang G, Wu L, Liang W, Wang R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68(6):1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Tang CS, Jin HF, Du JB. The vasorelaxing effect of hydrogen sulfide on isolated rat aortic rings versus pulmonary artery rings. Acta Pharmacol Sin. 2011;32(4):456–464. doi: 10.1038/aps.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 50.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30(4):536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 51.Landry DW, Levin HR, Gallant EM, Ashton RC, Jr, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 52.Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25(8):1279–1282. doi: 10.1097/00003246-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Holmes CL, Walley KR, Chittock DR, Lehman T, Russell JA. The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. 2001;27(8):1416–1421. doi: 10.1007/s001340101014. [DOI] [PubMed] [Google Scholar]
- 54.Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6. 1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R191–199. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- 55.Tan JH, Al Abed A, Brock JA. Inhibition of KATP channels in the rat tail artery by neurally released noradrenaline acting on postjunctional α2-adrenoceptors. J Physiol. 2007;581(2):757–765. doi: 10.1113/jphysiol.2007.129536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park WS, Ko EA, Han J, Kim N, Earm YE. Endothelin-1 acts via protein kinase C to block KATP channels in rabbit coronary and pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2005;45(2):99–108. doi: 10.1097/01.fjc.0000150442.49051.f7. [DOI] [PubMed] [Google Scholar]
- 57.Kubo M, Quayle JM, Standen NB. Angiotensin II inhibition of ATP-sensitive K+ currents in rat arterial smooth muscle cells through protein kinase C. J Physiol. 1997;503(Pt 3):489–496. doi: 10.1111/j.1469-7793.1997.489bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108(4):315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka E, Mori H, Chujo M, Yamakawa A, Mohammed MU, Shinozaki Y, Tobita K, Sekka T, Ito K, Nakazawa H. Coronary vasoconstrictive effects of neuropeptide Y and their modulation by the ATP-sensitive potassium channel in anesthetized dogs. J Am Coll Cardiol. 1997;29(6):1380–1389. doi: 10.1016/s0735-1097(97)82759-3. [DOI] [PubMed] [Google Scholar]
- 60.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92(26):12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutafova-Yambolieva VN, Keef KD. Adenosine-induced hyperpolarization in guinea pig coronary artery involves A2b receptors and KATP channels. Am J Physiol. 1997;273(6 Pt 2):H2687–H2695. doi: 10.1152/ajpheart.1997.273.6.H2687. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778(1):88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478(2):136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6. 1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem. 2008;283(5):2488–2494. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension. 2008;52(3):499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- 66.Haba M, Hatakeyama N, Kinoshita H, Teramae H, Azma T, Hatano Y, Matsuda N. The modulation of vascular ATP-sensitive K+ channel function via the phosphatidylinositol 3-kinase-Akt pathway activated by phenylephrine. J Pharmacol Exp Ther. 2010;334(2):673–678. doi: 10.1124/jpet.110.167775. [DOI] [PubMed] [Google Scholar]
- 67.Kinoshita H, Matsuda N, Kaba H, Hatakeyama N, Azma T, Nakahata K, Kuroda Y, Tange K, Iranami H, Hatano Y. Roles of phosphatidylinositol 3-kinase-Akt and NADPH oxidase in adenosine 5′-triphosphate-sensitive K+ channel function impaired by high glucose in the human artery. Hypertension. 2008;52(3):507–513. doi: 10.1161/HYPERTENSIONAHA.108.118216. [DOI] [PubMed] [Google Scholar]
- 68.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94(10):1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 69.Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1205–R1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Purves GI, Kamishima T, Davies LM, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J Physiol. 2009;587(Pt 14):3639–3650. doi: 10.1113/jphysiol.2009.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orie NN, Thomas AM, Perrino BA, Tinker A, Clapp LH. Ca2+/calcineurin regulation of cloned vascular KATP channels: crosstalk with the protein kinase A pathway. Br J Pharmacol. 2009;157(4):554–564. doi: 10.1111/j.1476-5381.2009.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 73.Ross J, Armstead WM. Differential role of PTK and ERK MAPK in superoxide impairment of KATP and KCa channel cerebrovasodilation. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R149–R154. doi: 10.1152/ajpregu.00003.2003. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular KATP channels by S-glutathionylation. J Biol Chem. 2010;285(49):38641–38648. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erdos B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004;35(4):964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- 76.Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92(2):151–158. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 77.Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. Molecular basis and structural insight of vascular KATP channel gating by S-glutathionylation. J Biol Chem. 2011;286(11):9298–9307. doi: 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren Y, Xu X, Wang X. Altered mRNA expression of ATP-sensitive and inward rectifier potassium channel subunits in streptozotocin-induced diabetic rat heart and aorta. J Pharmacol Sci. 2003;93(4):478–483. doi: 10.1254/jphs.93.478. [DOI] [PubMed] [Google Scholar]
- 79.Fan LH, Tian HY, Wang J, Huo JH, Hu Z, Ma AQ, Cao YX. Downregulation of Kir6. 1/SUR2B channels in the obese rat aorta. Nutrition. 2009;25(3):359–363. doi: 10.1016/j.nut.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol. 2003;285(4):C959–C967. doi: 10.1152/ajpcell.00511.2002. [DOI] [PubMed] [Google Scholar]
- 81.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74(5):1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 82.Xiao D, Longo LD, Zhang L. Role of KATP and L-type Ca2+ channel activities in regulation of ovine uterine vascular contractility: effect of pregnancy and chronic hypoxia. Am J Obstet Gynecol. 2010;203(6):596e6–596e12. doi: 10.1016/j.ajog.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raeis V, Philip-Couderc P, Roatti A, Habre W, Sierra J, Kalangos A, Beghetti M, Baertschi AJ. Central venous hypoxemia is a determinant of human atrial ATP-sensitive potassium channel expression: evidence for a novel hypoxia-inducible factor 1α-Forkhead box class O signaling pathway. Hypertension. 2010;55(5):1186–1192. doi: 10.1161/HYPERTENSIONAHA.109.148767. [DOI] [PubMed] [Google Scholar]
- 84.Vanelli G, Hussain SN, Aguggini G. Glibenclamide, a blocker of ATP-sensitive potassium channels, reverses endotoxin-induced hypotension in pig. Exp Physiol. 1995;80(1):167–170. doi: 10.1113/expphysiol.1995.sp003832. [DOI] [PubMed] [Google Scholar]
- 85.Landry DW, Oliver JA. The ATP-sensitive K+ channel mediates hypotension in endotoxemia and hypoxic lactic acidosis in dog. J Clin Invest. 1992;89(6):2071–2074. doi: 10.1172/JCI115820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovalev H, Quayle JM, Kamishima T, Lodwick D. Molecular analysis of the subtype-selective inhibition of cloned KATP channels by PNU-37883A. Br J Pharmacol. 2004;141(5):867–873. doi: 10.1038/sj.bjp.0705670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Brien AJ, Thakur G, Buckley JF, Singer M, Clapp LH. The pore-forming subunit of the KATP channel is an important molecular target for LPS-induced vascular hyporeactivity in vitro. Br J Pharmacol. 2005;144(3):367–375. doi: 10.1038/sj.bjp.0706065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Czaika G, Gingras Y, Zhu E, Comtois AS. Induction of the ATP-sensitive potassium (uKATP-1) channel by endotoxemia. Muscle Nerve. 2000;23(6):967–969. doi: 10.1002/(sici)1097-4598(200006)23:6<967::aid-mus19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 89.Jin X, Malykhina AP, Lupu F, Akbarali HI. Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G274–G285. doi: 10.1152/ajpgi.00472.2003. [DOI] [PubMed] [Google Scholar]
- 90.Shi W, Cui N, Wu Z, Yang Y, Zhang S, Gai H, Zhu D, Jiang C. Lipopolysaccharides up-regulate Kir6. 1/SUR2B channel expression and enhance vascular KATP channel activity via NF-κB-dependent signaling. J Biol Chem. 2010;285(5):3021–3029. doi: 10.1074/jbc.M109.058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collin S, Sennoun N, Dron AG, de la Bourdonnaye M, Montemont C, Asfar P, Lacolley P, Meziani F, Levy B. Vascular ATP-sensitive potassium channels are over-expressed and partially regulated by nitric oxide in experimental septic shock. Intensive Care Med. 2011;37(5):861–869. doi: 10.1007/s00134-011-2169-5. [DOI] [PubMed] [Google Scholar]
- 92.Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular KATP channels and coronary vasodilation produced by isoproterenol. Br J Pharmacol. 2011;164(8):2064–2072. doi: 10.1111/j.1476-5381.2011.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aziz Q, Thomas A, Khambra T, Tinker A. Phenformin has a direct inhibitory effect on the ATP-sensitive potassium channel. Eur J Pharmacol. 2010;634(1–3):26–32. doi: 10.1016/j.ejphar.2010.02.023. [DOI] [PubMed] [Google Scholar]