SUMMARY
Kainate receptors (KARs) play a key role in the regulation of synaptic networks. Here, we show that zinc, a cation released at a subset of glutamatergic synapses, potentiates glutamate currents mediated by homomeric and heteromeric KARs containing GluK3 at 10–100 μM concentrations, whereas it inhibits other KAR subtypes. Potentiation of GluK3 currents is mainly due to reduced desensitization, as shown by kinetic analysis and desensitization mutants. Crystallographic and mutation analyses revealed that a specific zinc binding site is formed at the base of the ligand binding domain (LBD) dimer interface by a GluK3-specific aspartate (Asp759), together with two conserved residues, His762 and Asp730, the latter located on the partner subunit. In addition, we propose that tetrameric GluK2/GluK3 receptors are likely assembled as pairs of heterodimeric LBDs. Therefore, zinc binding stabilizes the labile GluK3 dimer interface, slows desensitization, and potentiates currents, providing a mechanism for KAR potentiation at glutamatergic synapses.
INTRODUCTION
Glutamate released at excitatory synapses acts on ligand-gated ionotropic receptors, which fall into three classes, named after their preferred or selective agonist: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl D-aspartate (NMDA), and kainate. Like other neurotransmitter receptors, ionotropic glutamate receptors harbor binding sites for small molecules that act as allosteric regulators of receptor function. Allosteric modulators are likely to play a key role in the regulation of synaptic transmission and moreover represent potential pharmacological tools of great interest. Positive allosteric modulators of AMPA, kainate, and NMDA receptors (NMDARs) are thought to be promising therapeutic agents in the treatment of cognitive dysfunctions (Traynelis et al., 2010). It is thus important to increase our understanding of the molecular mechanisms by which physiologically relevant allosteric modulators are engaged in the regulation of synaptic transmission.
Ionotropic glutamate receptors are mainly localized at postsynaptic sites where they are directly involved in the transfer of information across synapses. There is however increasing evidence that ionotropic glutamate receptors are also present at presynaptic sites, where they regulate the release of neurotransmitters and participate in presynaptic forms of plasticity (Pinheiro and Mulle, 2008). In the brain, kainate receptors (KARs) support a variety of functions contributing to the regulation of the activity of synaptic networks (Contractor et al., 2011). KARs are tetramers composed of a combination of the five subunits, GluK1–GluK5, previously GluR5–GluR7, KA1–KA2 (Contractor et al., 2011). KARs share a similar architecture with other ionotropic glutamate receptors; the subunits have a large extracellular domain composed of an amino-terminal domain (ATD) and a ligand binding domain (LBD), a membrane region composed of three membrane α helices and a reentrant loop, and an intracellular carboxy-terminal region (Mayer, 2011). The various roles of KARs at pre- or postsynaptic sites arise in part from the diversity of functional properties of the different KAR subtypes (Perrais et al., 2010). At hippocampal mossy fiber synapses onto CA3 pyramidal cells, KARs are present at both pre- and postsynaptic levels (Contractor et al., 2011). Postsynaptic KARs are composed of the GluK2, GluK4, and GluK5 subunits (Contractor et al., 2003; Fernandes et al., 2009; Mulle et al., 1998), whereas presynaptic KARs are thought to comprise the GluK2 and GluK3 subunits (Contractor et al., 2001; Pinheiro et al., 2007). The functional properties of GluK3 (and GluK2/GluK3) receptors set it apart from the other ionotropic glutamate receptors (Perrais et al., 2009a; Schiffer et al., 1997). In particular, its sensitivity to glutamate is the lowest of all known ionotropic glutamate receptors, due in large part to fast desensitization of receptors with only one or two bound glutamate molecules (Perrais et al., 2009a). The low agonist sensitivity of this receptor raises questions about its relevance for synaptic function (Perrais et al., 2010). Therefore, it is possible that endogenous modulators may potentiate its responsiveness to glutamate.
Among potential endogenous modulators of KAR function, we chose to address the role of zinc, known to be present in large amounts in hippocampal mossy fiber terminals (Paoletti et al., 2009). Zinc is accumulated into synaptic vesicles and thought to be coreleased with glutamate in the extracellular milieu during neuronal activity (Paoletti et al., 2009). The best-characterized synaptic zinc targets are NMDARs (Westbrook and Mayer, 1987). Zinc inhibits NMDAR function with affinities ranging from low nanomolar for GluN1/GluN2A receptors to low micromolar for GluN1/GluN2B subunits (Paoletti et al., 1997). The binding site accounting for the high-affinity binding of zinc to GluN2A and GluN2B has been mapped to the large ATD of GluN2 subunits (Choi and Lipton, 1999; Karakas et al., 2009; Paoletti et al., 2000; Rachline et al., 2005). Zinc binding to the ATD has been suggested to inhibit NMDAR channel gating through destabilization of the dimer interface of the LBD (Erreger et al., 2005; Gielen et al., 2008), by mechanisms that resemble desensitization of AMPA and KARs (Armstrong et al., 2006; Weston et al., 2006). Zinc has also been reported to inhibit native and recombinant KARs. Zinc inhibition of KARs is subunit dependent, with KARs containing GluK4 or GluK5 subunits being more sensitive, IC50 ~1–2 μM, than GluK1-GluK2, IC50 ~70 μM (Mott et al., 2008). Despite the proposed presynaptic function of GluK3-containing KARs at hippocampal mossy fiber synapses, which are highly enriched in vesicular zinc, modulation of these receptors by zinc has not yet been addressed.
In this study, we show that zinc at micromolar concentrations potentiates recombinant GluK3 receptor currents evoked by glutamate. Zinc markedly slows receptor desensitization and increases apparent affinity for glutamate. By analysis of chimeric GluK2/GluK3 KARs and of GluK3 bearing selected point mutations, we mapped the zinc binding domain to the S2 segment of the LBD, in a region forming the interface between two GluK3 subunits in an LBD dimer assembly. Crystallographic studies for GluK3 LBD complexes with both glutamate and kainate revealed that zinc ions bind at multiple sites formed by aspartate, histidine, and glutamate residues, which are present in both the upper and lower lobes of the LBD. Based on these crystal structures, a GluK3 LBD dimer model was generated by superposition of GluK3 monomers on previously solved KAR LBD dimers. This identified D730 as the dimer partner component of the binding site underlying zinc potentiation, together with D759 and H762 from the adjacent subunit. Based on these structure-function studies and on modeling of KAR activity, we show that zinc plays a very distinct role in GluK3-KARs by stabilizing the LBD dimer assembly, thereby reducing desensitization. Given the proposed presynaptic localization of GluK3 close to zinc-containing synaptic vesicles, zinc may be an endogenous allosteric modulator for native GluK3-KARs.
RESULTS
Zinc Reversibly Facilitates Recombinant GluK3 Receptor Currents
We tested the effect of zinc on currents activated by fast application of glutamate on lifted HEK293 cells transfected with GluK3 cDNA. Currents evoked by sustained applications (100 ms) of 10 mM glutamate, a concentration close to the EC50 value for GluK3 (Perrais et al., 2009a; Schiffer et al., 1997), were markedly enhanced with preapplication of 100 μM zinc (Figure 1A; 193% ± 38% of control amplitude, n = 17), and this potentiation was rapidly reversible upon removal of zinc. In contrast to GluK3 potentiation, and as previously reported in Xenopus oocytes (Mott et al., 2008), zinc reversibly inhibited GluK2 currents at all concentrations tested (Figures 1A and 1D), with an IC50 of 102 ± 11 μM and a Hill coefficient (nH) of 1.1 ± 0.1 (n = 4–9). Because a glutamate concentration of 10 mM is saturating for GluK2 (Perrais et al., 2009a), this could mask a potentiating effect of zinc. However, currents evoked by 500 μM glutamate, a concentration below the EC50 for GluK2, were also inhibited by 100 μM zinc (48% ± 10%, n = 10; data not shown). It has been proposed that presynaptic GluK2/GluK3 heteromeric receptors regulate glutamate release at hippocampal mossy fiber synapses (Pinheiro et al., 2007). Therefore, it was important to determine the effect of zinc on heteromeric GluK2/GluK3 receptors. To test the specific effects of zinc on GluK2/GluK3 heteromers in cells cotransfected with GluK2 and GluK3, we reduced the likeliness of activating homomeric GluK2 or GluK3 subunits as described previously (Perrais et al., 2009b). First, the GluK2b(Q) splice variant was used because of its reduced expression at the cell surface as a homomer (Jaskolski et al., 2004). Second, GluK3 homomeric receptors were specifically blocked with 1 μM UBP310 (Perrais et al., 2009b). In cells cotransfected with GluK2b(Q) and GluK3, application of 1 μM UBP310 inhibited glutamate-activated currents by 55% (n = 6; p < 0.05). The fraction of current resistant to UBP310 was enhanced by zinc (100 μM) to a similar extent (157% ± 7%, n = 18) as for homomeric GluK3 receptors (p = 0.65; Figures 1B and 1C). The small fraction of homomeric GluK2 receptors at the cell surface would, if anything, lead to an underestimation of the potentiation of GluK2/GluK3 receptors by zinc. Therefore, these results clearly demonstrate that heteromeric GluK2/GluK3 receptors are, like GluK3 receptors, potentiated by zinc.
Figure 1. Zinc Reversibly Facilitates GluK3-Containing KAR Currents in a Dose-Dependent Manner.
(A) Effect of a sustained application of 100 μM zinc (Zn) on GluK3 (top traces) and GluK2 (bottom traces) currents, evoked by 10 mM glutamate (Glu) for 100 ms and recorded in the whole-cell mode, is shown.
(B) Effect of 100 μM zinc on GluK2b/K3 receptors in presence of 1 μM UBP310 to block homomeric GluK3 is illustrated.
(C) Histogram presents the effect of 100 μM zinc on GluK2, GluK3, and GluK2/GluK3 receptors. ***p < 0.001, **p < 0.01, *p < 0.05, ns, p > 0.05. norm. amplitude, normal amplitude.
(D) Dose-response curves for the effects of zinc on GluK3 (black circles), GluK2 (white circles), and GluK2/GluK3 (black squares) mediated currents elicited by 10 mM glutamate (n = 4–18 for each point) are illustrated. Solid lines show Hill equations (IC50 = 102 μM, nH = 1.1 for GluK2 and EC50 = 477 μM, nH = 0.6 and maximum 286% for GluK2b/K3). The dotted line shows the fit for a combined Hill equation, with an EC50 of 46 μM for potentiation of GluK3, and an IC50 of 100 μM, calculated from inhibition for GluK2 (solid line).
The modulation of GluK3 by zinc showed a dose-dependent biphasic effect: increasing the concentration of zinc up to 100 μM potentiated currents (half-maximal effect around 20 μM), and higher concentrations progressively inhibited currents (Figure 1D). In order to fit the dose-response curve with combined potentiation/inhibition Hill equations, we hypothesized that the inhibition of GluK3 by higher concentrations of zinc was similar to that of GluK2 (a notion supported by the effects of point mutations described in Figure 6). This attempt to separate potentiation and inhibition in the GluK3 dose-response curves yielded an EC50 value of 46 ± 17 μM, nH 1.82 ± 0.95, and a maximal potentiation of 475% ± 47%, although the moderate quality of the combined fit suggests that potentiation and inhibition might not be independent processes. Surprisingly, zinc potentiated currents mediated by GluK2/GluK3 at all concentrations tested (Figure 1D), with an EC50 of 477 ± 1638 μM, nH 0.6 ± 0.4, consistent with a reduced number of binding sites on heteromeric receptors, and a maximal potentiation of 286% ± 195% of control, and by contrast to homomeric GluK3 receptors, there was no inhibition for zinc concentrations up to 1 mM.
Figure 6. D759, H762, and D730 Are Involved in the Facilitatory Effect of Zinc on GluK3 Receptors.
(A) Sequence alignment of a segment of the extracellular S2 region for the five KAR subunits and GluA2 reveals an amino acid that is unique to GluK3, between α helices J and K. Residues known to form zinc binding sites in other proteins are shown in red boxes; Δ represents mutated residues in (B)–(F).
(B–E) Traces illustrate the effects of 100 μM zinc on currents evoked by 10 mM glutamate for GluK3 and GluK2 point mutants.
(F) Histogram presents the effect of 100 μM zinc on the currents evoked by glutamate for GluK2, GluK3, and point mutants recorded in outside-out patches. The asterisks indicate significant difference with control currents without zinc.
(G) Histogram presents the desensitization kinetics of GluK2, GluK3, and point mutants recorded in the same conditions as in (E). The asterisks indicate significant difference with corresponding WT receptors. ***p < 0.001, **p < 0.01, *p < 0.05.
Zinc Changes the Kinetic Properties of GluK3 and Its affinity for Glutamate
Zinc could affect GluK3-mediated currents in several ways: it could increase single-channel conductance, increase open probability, allow activation of “silent” receptors, or slow down receptor desensitization. It was shown previously that the low glutamate sensitivity of GluK3 receptors was due to fast transitions of glutamate bound receptors to desensitized states (Perrais et al., 2009a); hence, a change in desensitization may translate into an increased apparent affinity for glutamate. In order to analyze the mechanisms of zinc potentiation, we characterized key parameters of GluK3 receptor activation using outside-out patches (Figure 2). The current decay (τdes) evoked by 100 ms applications was well fitted with single exponential functions, and zinc increased τdes in a dose-dependent manner (τdes = 5.0 ± 0.2 ms; versus 9.8 ± 0.4 ms, before and after 100 μM zinc, n = 8; p < 0.0001; Figures 2A–2C; see Table S1 available online), whereas it had no effect on GluK2 desensitization (τdes = 3.4 ± 0.1 ms versus 3.7 ± 0.1 ms before and after 100 μM zinc, n = 5; p = 0.20; Figure 2C) despite its strong effect on GluK2 current amplitudes. Therefore, zinc markedly affects GluK3 receptor desensitization, in contrast to its lack of effect on GluK2 kinetics, suggesting a different mechanism of action. Moreover, zinc increased currents evoked by 1 ms pulses of 10 mM glutamate (191% ± 22% of control amplitude, n = 4; Figure 2D) and slowed down their deactivation kinetics (from 1.5 ± 0.05 ms to 2.3 ± 0.1 ms, n = 4; p = 0.002; Figure 2E).
Figure 2. Zinc Changes Desensitization and Sensitivity to Glutamate of GluK3 Currents Recorded in Outside-Out Patches.
(A) Effect of various concentrations of zinc on currents evoked by glutamate (10 mM) on an outside-out patch pulled from a GluK3-expressing cell is presented.
(B) Normalization of traces in (A) reveals the dose-dependent effect of zinc on desensitization kinetics.
(C) Values of τdes for GluK2 and GluK3 mediated currents with increasing zinc concentration (n = 5). A total of 30 and 100 μM zinc induces a significant increase of τdes relative to control (Ctrl). ***p < 0.001.
(D and E) Effect of zinc on currents evoked by 1 ms pulses of glutamate (10 mM) is illustrated. In (E), the two traces of (D) are normalized to maximum amplitude.
(F–H) Effect of zinc on glutamate sensitivity is demonstrated. (F) The amplitude of currents relative to their maximum amplitude (30 mM glutamate) is increased in the presence of 100 μM zinc (red traces). (G) Glutamate dose-response curves in the absence (black circles) or presence (red squares) of 100 μM zinc are shown. The lines are fits with the Hill equation, with EC50 values of 10.1 ± 1.1 and 4.8 ± 1.1 mM, respectively. (H) Relationship between current amplitude and decay time with (red squares) or without (black circles) 100 μM zinc (n = 4–9) is shown.
(I) Paired applications of glutamate (10 mM; 100 ms) separated by intervals ranging from 0.2 to 5 s on an outside-out patch pulled from a cell-expressing GluK3, in the absence (top) or presence (bottom) of 100 μM zinc, are illustrated.
(J) Plot of the amplitude ratio of two successive responses versus time interval for GluK3, with (red squares) or without (black circles) 100 μM zinc, is presented. The lines are fits with an exponential equation, with time constant values of 432 ± 1 and 933 ± 1 ms, respectively (n = 5). fract. amplitude, fraction amplitude.
Next, we measured the EC50 for glutamate in outside-out patches in the absence or presence of zinc (100 μM, Figures 2F and 2G). Zinc increased the sensitivity of GluK3 receptors for glutamate from an EC50 of 10.1 ± 1 mM (nH = 1.6 ± 0.1) in control condition to 4.8 ± 1.1 mM (nH = 1.1 ± 0.2) with 100 μM zinc (n = 4). Consequently, zinc is markedly more potent at low glutamate concentrations (1–3 mM) than at 30 mM. In addition, we found that zinc increases the time constants for desensitization at all glutamate concentrations (Figure 2H). Finally, zinc speeds up recovery from desensitization (time for half-recovery: 902 ± 1.1 ms and 460 ± 1.2 ms in absence and presence of zinc 100 μM, respectively, n = 5; Figures 2I and 2J). Hence, our results suggest that zinc, by affecting the fast desensitization properties of GluK3 receptors, enhances GluK3 currents and increases its sensitivity to glutamate.
Reducing Receptor Desensitization Blocks Potentiation by Zinc
Indeed, previous experimental and kinetic modeling data (Perrais et al., 2009a) have shown that fast desensitization of partially liganded GluK3 receptors limits their activation. Therefore, the potentiating effect of zinc should be reduced in GluK3 mutants in which desensitization is slowed or abolished. We directly tested this prediction with two GluK3 mutant receptors that show reduced desensitization (Figure 3). We constructed a mutant GluK3 receptor by analogy with a mutant GluK2 receptor with four substitutions (K525E, K696R, I780L, and Q784K) that greatly slowed down desensitization, termed GluK2(ERLK) (Chaudhry et al., 2009; Zhang et al., 2006). Indeed, GluK3(ERLK) desensitization (15.3 ± 1.9 ms, n = 7; p < 0.0001; Figures 3A and 3D) was about 3-fold slower than that of wild-type (WT) GluK3, albeit the changes were not as dramatic as with GluK2(ERLK), for which a 50-fold increase is observed (Chaudhry et al., 2009; Zhang et al., 2006). As predicted, GluK3(ERLK)-mediated currents were inhibited rather than potentiated by zinc (Figures 3A and 3C) with no change in desensitization rate (Figure 3D). Another mutant, GluK3(H492C, L753C), for which desensitization is almost entirely suppressed (Perrais et al., 2009a; Weston et al., 2006), was also inhibited by zinc (100 μM) to a similar extent (Figures 3B and 3C). Overall, the potentiating effect of zinc on GluK3 is absent in two variants where GluK3 desensitization is reduced.
Figure 3. Reducing Desensitization Abolishes Potentiation by Zinc.
(A and B) Inhibition by 100 μM zinc of GluK3 mutants attenuating desensitization is shown. GluK3(ERLK) (left traces) and GluK3(CC) (right traces) currents, evoked by 10 mM glutamate for 100 ms, are illustrated.
(C) Histogram represents the effect of 100 μM zinc on GluK3(ERLK) and GluK3(CC) currents, evoked by sustained (100 ms) applications of 10 mM glutamate on outside-out patches.
(D) Histogram presents the desensitization kinetics of GluK2, GluK3, and GluK3(ERLK), recorded in the same conditions as in (C). ***p < 0.001, *p < 0.05, ns, p > 0.05.
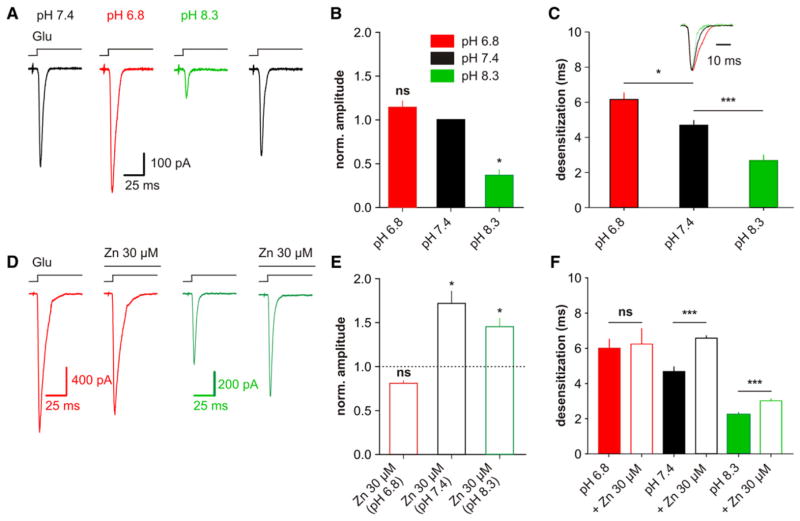
Effect of pH on GluK3 Function and Modulation by Zinc
An interaction between zinc modulation and pH has been documented for many zinc binding sites, in particular for NMDA (Choi and Lipton, 1999; Low et al., 2000) and KARs (Mott et al., 2008). This could reflect the protonation of the zinc binding site or other allosteric mechanisms. Studying the interaction between pH and zinc may provide information on the nature of the site involved in GluK3 potentiation. We have observed a strong effect of pH on GluK3 function: the current amplitude was much smaller at pH 8.3 and slightly higher at pH 6.8 than at pH 7.4. At pH 6.8, in the absence of zinc, there was a slight decrease in rate of desensitization of GluK3 currents (τdes 4.7 ± 0.3 ms, n = 11 at pH 7.4, to 6.0 ± 0.5 ms, n = 8 at pH 6.8; p = 0.014). Interestingly, at pH 8.3, we observed a much lower current amplitude and accelerated desensitization (τdes 2.7 ± 0.3 ms, n = 9; p < 0.0001; Figures 4A–4C). Application of zinc (100 μM) inhibited currents at pH 6.8 but potentiated currents at pH 8.3 (Figures 4D–4F). This suggests that amino acid protonation at pH 6.8, most likely a histidine, might be responsible for the loss of potentiation at low pH.
Figure 4. Effect of pH on GluK3 Function and Modulation by Zinc.
(A) Whole-cell GluK3 currents evoked by glutamate (10 mM) at the indicated pH are demonstrated.
(B) Current amplitude at different pHs, relative to value at pH 7.4 (n = 5), is presented.
(C) Values of τdes at the three pHs tested are illustrated. Inset is of normalized currents shown in (A), with τdes of 5.7, 4.3, and 3.9 ms at pH 6.8, 7.4, and 8.3, respectively.
(D) Effect of zinc (100 μM) on GluK3 at different pHs is presented: at pH 8.3, currents are potentiated, but not at pH 6.8.
(E and F) Effect of zinc on current amplitude and τdes at pH 6.8 (n = 6), 7.4 (n = 11), and 8.3 (n = 8) is shown. Open bars represent conditions with zinc. ***p < 0.001, *p < 0.05, ns, p > 0.05.
Facilitatory Effect of Zinc on GluK3 Receptors Is Mediated by the S2 Extracellular Segment of the LBD
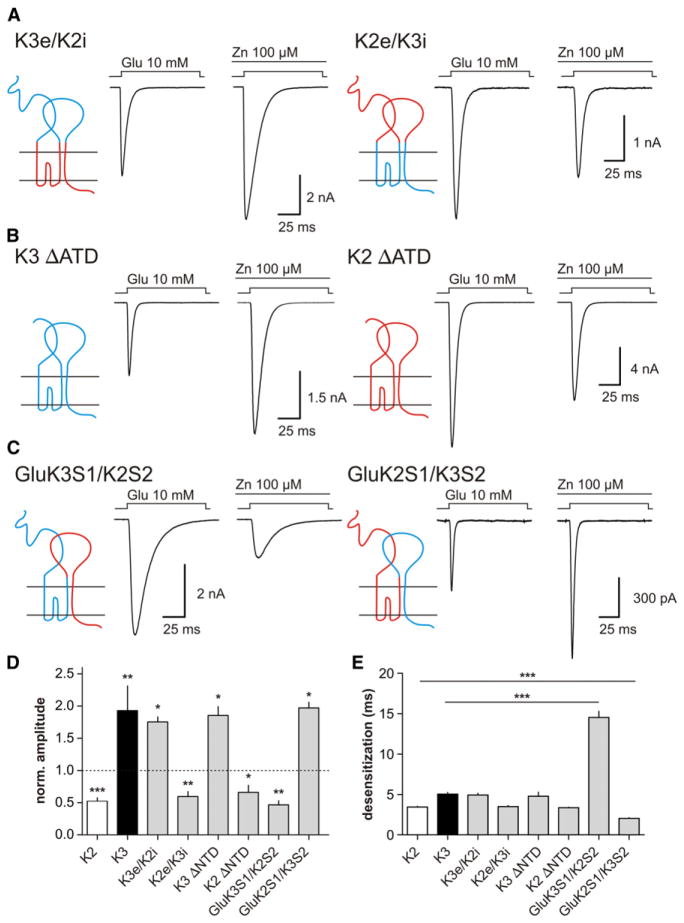
In AMPA receptors (AMPARs) and KARs, several studies have shown that residues lining the interface between the LBDs of two adjacent subunits are a key component of dimer stability and regulate desensitization kinetics (Armstrong et al., 2006; Chaudhry et al., 2009; Horning and Mayer, 2004; Nayeem et al., 2009; Sun et al., 2002; Weston et al., 2006). To identify the zinc binding sites responsible for the facilitatory effect on GluK3 currents, we constructed chimeric receptors of GluK2 and GluK3. Receptors composed of the extracellular domain of GluK3 and the transmembrane and intracellular segments of GluK2 were potentiated by zinc to similar levels as GluK3 (175% ± 9% of control amplitude with 100 μM zinc, n = 5; Figure 5A, left, and Figure 5D). By contrast, zinc inhibited currents mediated by chimeric receptors that contained the transmembrane and intracellular segments of GluK3 and the extracellular domain of GluK2 (40% ± 8%, n = 4; p = 0.0077; Figure 5A, right, and Figure 5D). In the GluN2A and GluN2B subunits of NMDARs, the ATD harbors a discrete zinc binding site (Choi and Lipton, 1999; Karakas et al., 2009; Paoletti et al., 2000; Rachline et al., 2005). GluK3 subunits deleted of their ATD form functional receptors, which fully preserve potentiation by zinc (186% ± 13%, n = 5; p = 0.023; Figures 5B, left and 5D). Conversely, inhibition is conserved in GluK2 receptors where the ATD was excised (44% ± 11%, n = 4; p = 0.035; Figures 5B, right, and 5D). Thus, this first set of experiments seems to rule out a role of the intracellular region, ion channel, and the ATD of GluK3 and points to the LBD as a potential zinc binding domain responsible for the facilitatory effect of zinc. The LBD is formed by two extracellular segments referred to as S1 and S2 (Stern-Bach et al., 1994), which form a clamshell-like structure where S1 forms most of the upper half of the clamshell, and S2 forms most of the lower half. We next tested which of these segments is involved in the facilitatory effect of zinc on GluK3 receptors by using chimeric GluK2 and GluK3 receptors where S2 of one is replaced by the other and vice versa. Interestingly, currents mediated by GluK3/GluK2 chimeric receptors that contain S2 and the intracellular part of the GluK2 subunit (GluK3/K2S2) were inhibited (54% ± 6%; n = 5; p = 0.002; Figure 5C, left, and Figure 5D), whereas currents mediated by GluK3/GluK2 chimeric receptors containing S2 and the intracellular part of the GluK3 subunit (GluK2/K3S2) were facilitated by 100 μM zinc (197% ± 9%, n = 5; p = 0.034; Figure 5C, right, and Figure 5D). Moreover, whereas desensitization kinetics in control conditions was not affected for most of the constructs tested (Figure 5E), the kinetics of GluK3/K2S2 and GluK2/K3S2 was considerably changed (from 5.0 ± 0.2 ms, n = 8 for WT GluK3 to 14.5 ± 0.8 ms, n = 4, p < 0.0001 for GluK3/K2S2; and from 3.4 ± 0.1 ms, n = 5 for WT GluK2 to 2 ± 0.1 ms for GluK2/K3S2, n = 4, p < 0.0001). Slowed desensitization kinetics could explain why GluK3 with the S2 segment substituted for GluK2 is functional as assessed by slow glutamate application on Xenopus oocytes (Strutz et al., 2001). These experiments clearly point to the S2 segment of GluK3 as a target for zinc binding.
Figure 5. Facilitatory Effect of Zinc on GluK3 Receptors Is Mediated by the S2 Segment of the GluK3 LBD.
(A–C) Chimeric GluK2/GluK3 receptors were designed to isolate the region involved in the facilitatory effect of zinc. Currents are evoked by glutamate (10 mM, 100 ms) with or without 100 μM zinc. (A) Left view shows chimeric receptors composed of the extracellular domains of the GluK3 subunit (in blue) with the transmembrane and intracellular domains of the GluK2 subunit (in red). Right view illustrates chimeric receptors composed of the extracellular domains of the GluK2 subunit (in red) with the transmembrane and intracellular domains of the GluK3 subunit (in blue). (B) Left view presents GluK3 receptors with ATD deletion. Right view demonstrates GluK2 receptors with ATD deletion. (C) Left view shows chimeric GluK3 receptors that contain the S2 segment, the last transmembrane α helix, and the C-terminal domain of the GluK2 subunit. Right view illustrates chimeric GluK2 receptors that contain the S2 segment, the last transmembrane α helix, and the C-terminal domain of the GluK3 subunit.
(D) Histogram presents the effect of 100 μM zinc on GluK2, GluK3, and chimeric receptor current amplitude in whole cells. The asterisks indicate significant difference with control currents without zinc.
(E) Histogram presents the desensitization kinetics of GluK2, GluK3, and chimeric receptor currents recorded in the same conditions as in (D). The asterisks indicate a significant difference with GluK3 or GluK2 currents.
***p < 0.001, **p < 0.01, *p < 0.05.
Identification of Key Residues at the Dimer Interface Mediating the Facilitatory Effect of Zinc
To further characterize the zinc binding site, we hypothesized that it might stabilize the interface between LBDs by binding to a unique site generated by amino acids found only in GluK3. Among residues that usually bind zinc (histidine, cysteine, aspartate, and glutamate), a single residue in S2 differs between GluK3 and the other KARs: An aspartate in GluK3 (D759) is replaced by a glycine in GluK1 and GluK2 and by an asparagine in GluK4 and GluK5 (Figure 6A). We tested the effects of zinc (100 μM) on the reciprocal mutants GluK3(D759G) and GluK2(G758D). Glutamate-activated currents were potentiated by zinc in GluK2(G758D) receptors to the same extent as GluK3 (177% ± 7% of control amplitude, n = 6; p = 0.008; Figures 6B and 6F). Conversely, GluK3(D759G) currents were inhibited by zinc (32% ± 9%, n = 5; Figures 6C and 6F). These results clearly indicate that the replacement of G758 in GluK2 by an aspartate is sufficient to confer zinc potentiation in GluK2. Moreover, desensitization was markedly slower in GluK3 (D759G) (τdes = 18.4 ± 1.8 ms; n = 8; p < 0.0001) and greatly accelerated in GluK2(G758D) (τdes = 1.3 ± 0.1 ms; n = 6; p < 0.0001; Figure 6G) compared to WT GluK3 and GluK2, respectively. We observed the same effect when the whole S2 segment was substituted in GluK3/K2S2 and GluK2/K3S2 chimeric receptors (Figure 5E). Hence, in addition to its role in the zinc action on GluK3, D759 in GluK3 appears as a key residue to explain the specific desensitization properties of GluK3 as compared to GluK1 and GluK2.
We searched for additional residues involved in the binding of zinc in the vicinity of D759. This residue is localized in the turn between helices J and K of the GluK3 LBD (Venskutonyté et al., 2011), three residues downstream of a conserved histidine near the N terminus of helix K. This conserved histidine, together with D759, is a candidate for residues forming part of the zinc binding site for GluK3. To test this hypothesis, GluK3 H762 was replaced by an alanine. As expected, the facilitatory effect of zinc was turned to an inhibitory effect in GluK3(H762A) (peak amplitude 45% ± 6%, n = 5; p = 0.014; Figures 6D and 6F), strongly suggesting that H762 participates in the zinc binding site of GluK3 receptors. In addition, the desensitization kinetics of GluK3(H762A) was faster (τdes = 3.9 ± 0.1 ms, n = 5; p = 0.0009; Figure 6G) than for WT GluK3, ruling out an indirect effect of reduced desensitization on the effect of zinc in this mutant.
Structure-Based Analysis of the Zinc Binding Site in GluK3
To obtain further insight into the zinc binding site on GluK3, three independent crystal structures were solved for the GluK3 LBD in the presence of zinc: two in a complex with glutamate, and one in a complex with kainate (Table S2). The structure for the six GluK3 protomers in the glutamate complexes was similar to that reported recently by Venskutonyté et al. (2011) but with small variations in the extent of domain closure, from 25.3° to 23.4°, and larger cavity volumes (299 ± 6 Å3, mean ± SD, n = 6) than the value of 274 ± 4 Å3 reported previously, indicating that the LBD of GluK3 is more similar to GluK1 than GluK2, with cavity volumes of 305 and 255 Å3, respectively (Mayer, 2005). For the two protomers in the GluK3 kainate complex (Figure S1), domain closure was 90% and 70% of that induced by glutamate, indicating that the GluK3 LBD can adopt both more closed, and also more open, conformations than observed previously for the GluK3 kainate complex for which a value of 81% was reported by Venskutonyté et al. (2012). In the crystal forms reported here, the GluK3 protomers assemble as two different dimers, both of which diverge from the canonical arrangement found in full-length GluA2 (Sobolevsky et al., 2009). In the P2221 glutamate and P2221 kainate complexes, two GluK3 protomers are arranged head to tail such that helix D of subunit A packs against the N terminus of helix K in subunit B (Figure 7A). Crystallographic symmetry operations generate a second dimer, arranged in a head-to-head assembly but with a >20 Å lateral displacement of the two protomers such that helix J is packed against helix J of its symmetry mate (Figure S1C). In the P21212 glutamate complex, which has four protomers in the asymmetric unit, both dimer forms are generated by noncrystallographic symmetry.
Figure 7. Multiple Zinc Binding Sites in GluK3 LBD Crystal Structures.
(A) Ribbon diagram shows two protomers in the asymmetric unit of the GluK3 complex with kainate. Zinc ions are drawn as gray spheres; anomalous difference Fourier maps contoured at 4 σ are shown for eight zinc ions labeled as Zn1–Zn8; the side chains that coordinate zinc ions and the kainate ligand are drawn as sticks.
(B) Enlarged view of the Zn1 binding site created by the side chains of D759 and H762 in helix K of protomer A, together with H492 and E495 in helix D of protomer B, is illustrated; dashed lines indicate intermolecular contacts for the zinc ion with distances shown in angstroms.
(C) Sequence alignment of a section of the extracellular S1 segment for GluK1, GluK2, GluK3, and GluA2 reveals an amino acid that is unique to GluK3 in α helix D. Residues known to be involved in the binding of zinc are shown in red boxes; Δ indicates the mutated residue in α helix D.
(D) Traces illustrate the effects of 100 μM zinc on currents evoked by 10 mM glutamate for GluK3(H492Y).
(E) Traces illustrate the effects of 100 μM zinc on currents evoked by 10 mM glutamate for GluK2(Y490H).
(F) Histogram presents the effect of 100 μM zinc on the currents evoked by glutamate for GluK2, GluK3, and point mutants. The asterisks indicate significant difference with control currents without zinc.
(G) Histogram presents the desensitization kinetics of GluK2, GluK3, and point mutants recorded in the same conditions as in (E). The asterisks indicate a significant difference with corresponding WT GluK3 or GluK2 receptors. ***p < 0.001, **p < 0.01, *p < 0.05.
See also Figure S1.
Calculation of anomalous difference Fourier electron density maps revealed the location of zinc ions that form numerous intermolecular contacts between protomers in both crystal forms (Figures 7A and S1C). It is likely that these zinc-mediated contacts play a key role in assembly of the two dimer forms observed in the crystal structures, which could explain why GluK3 failed to pack in the canonical arrangement found in many crystal structures for other iGluR LBDs. However, GluK3 LBD glutamate and kainate complexes, which were crystallized in the absence of zinc ions (Venskutonyté et al., 2011, 2012), were also packed in a noncanonical dimer configuration in the P41 space group, highlighting the tendency of KAR LBDs to pack in a variety of nonbiological assemblies, as observed previously for GluK1 and GluK2 (Mayer, 2005; Naur et al., 2005). Relevant to the zinc potentiation of GluK3, zinc ions were bound at a site labeled Zn1, which was created by D759 in all three crystal forms (Figure 7B); the zinc ion at this site was also coordinated by H762, mutation of which abolished potentiation by zinc, and by H492 and E495 located in helix D of the dimer partner subunit. Additional zinc binding sites, which stabilize the alternative dimer assembly, were created by E757 at the base of helix J together with E757 of its symmetry mate (Zn2), by H444 at the N terminus of helix B (Zn3), by E713 in helix I together with H762 and E766 from a symmetry related molecule (Zn4), by H479 at the C terminus of helix C (Zn5), by the main-chain carbonyl oxygen of Glu495 and the side-chain carboxylate of D499 just after the C terminus of helix D (Zn6), by H479 with its symmetry mate (Zn7), and by E441 with H444 of a symmetry-related molecule (Zn8). For most of these sites, the binding of zinc was characterized by short bond lengths, on the order of 1.9–2.0 Å (Figure 7A). Although the GluK3 LBD dimer arrangements observed here differ from the canonical arrangement of full-length GluA2 receptors (Sobolevsky et al., 2009), we tested functionally the involvement of H492, which participates in the Zn1 site (Figure 7B), but which is absent in other iGluR subunits. We mutated the histidine into a tyrosine, the equivalent residue in GluK2, and also into an alanine. For both GluK3(H492Y) and GluK3(H492A), zinc still potentiated currents (Figures 7D and 7F). Conversely, GluK2(Y490H) was, like WT GluK2, inhibited by zinc (Figures 7E and 7F). Therefore, H492 does not participate in the functional zinc binding site for GluK3 potentiation. This result is consistent with the fact that substitution of the S1 region of GluK2 to GluK3 does not abolish zinc potentiation (Figures 5C and 5D).
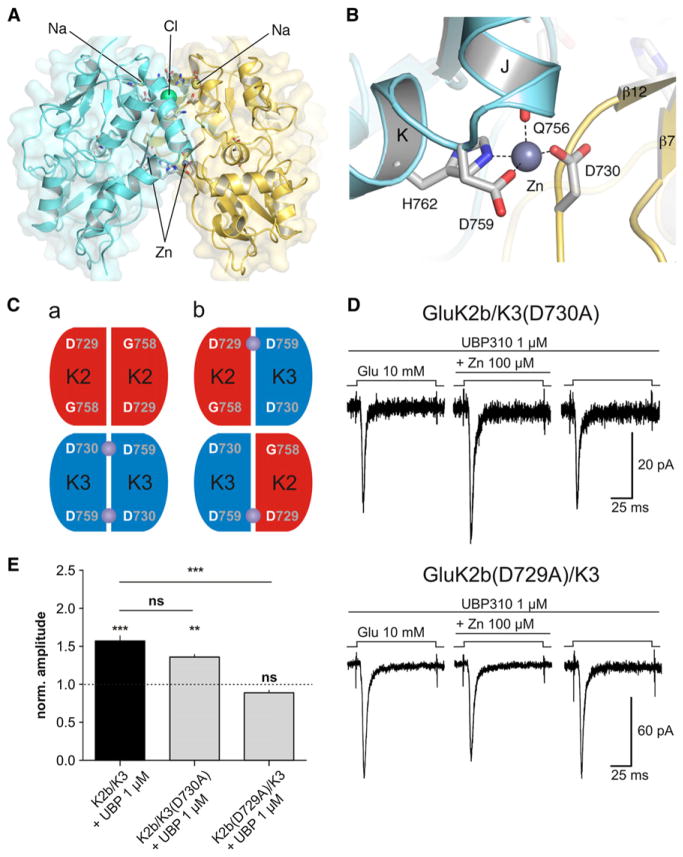
The essentially identical conformation of GluK2 and GluK3 LBD glutamate complexes in crystal forms obtained both in the presence and absence of zinc (Mayer, 2005; Venskutonyté et al., 2011), combined with 87% amino acid identity, and 94% amino acid similarity of the GluK2 and GluK3 LBDs, provides a basis for modeling a biological dimer assembly for GluK3, based on GluK2 LBD dimer crystal structures. This approach is further validated by the similar LBD dimer assemblies found in the full-length GluA2 structure (Sobolevsky et al., 2009). The rmsd for superposition of a protomer from the GluK3 P2221 glutamate complex on each of the two subunits in a GluK2 LDB dimer assembly (Protein Data Bank ID Code [PDB] 3G3F) was 0.42 and 0.40 Å for 242 Cα atoms, indicating that the structures of the GluK2 and GluK3 LBDs are nearly identical. Following this superposition, inspection of the GluK3 dimer model revealed that selection of new rotamers for D730, D759, and H762 would allow formation of intersubunit contacts with appropriate bonding distances for zinc coordination; likewise, binding sites for Na+ and Cl− like those found in GluK1 and GluK2 LBD dimers (Plested et al., 2008; Chaudhry et al., 2009) could be created by adjusting side-chain torsion angles for E495 and R745. The resulting GluK3 dimer model shows the location and stoichiometry of three discrete binding sites for allosteric ions: with a single Cl− ion on the 2-fold axis of dimer symmetry, two Na+ ions binding near the upper surface of domain 1, and two zinc ions binding at the base of domain 1 (Figure 8A). This model identified D730 as the residue that completes the coordination shell for zinc, together with the main-chain carbonyl oxygen atom of Q756 and the side chains of D759 and H762 from the adjacent subunit, together with one or two water molecules that were not included in the model (Figure 8B). The resulting structure reveals two key features. First, zinc acts as an intermolecular bridge between the pair of subunits in an LBD dimer assembly. Second, in the absence of zinc, the side chains of D730 and D759, which are separated by only 2.9–3.8 Å, would likely repel each other, destabilizing the dimer assembly and accelerating desensitization. In support of this, neutralizing these charges by mutating D759 into a glycine strongly reduces desensitization. Conversely, introducing a negatively charged aspartate at the equivalent position in GluK2(G758D) markedly accelerates desensitization (Figures 6B, 6C, and 6G). We suggest that the bound zinc ions act as a countercharge that reduces this repulsive interaction. We tested the prediction that D730 participates in the zinc binding site by constructing the GluK3(D730A) mutant. This receptor was no longer potentiated but rather inhibited by zinc (33% ± 2% of control amplitude, n = 6; p = 0.02; Figures 6E–6G), whereas the GluK3(D730N) mutant retained zinc potentiation (Figure 6F). Therefore, the GluK3 zinc binding site is formed by residues located on two adjacent LBDs.
Figure 8. A Model for GluK3 Zinc Binding Sites Based on GluK2 LBD Crystal Structures.
(A) Ribbon diagram and molecular surface of a GluK3 model dimer in which the pair of subunits was arranged by least-squares superposition on the crystal structure of a GluK2 LBD dimer assembly, illustrating the location of the binding sites for two Zn2+ (D730, Q756, D759, and H762), two Na+ (E495, I498, and D499), and one Cl− (K502, R745, T749).
(B) Enlarged view of one of the pair of zinc binding sites, showing coordination of the zinc ion by the main chain of Q756, and the side chains of D730, D759, and H762.
(C) Possible arrangements of heteromeric LBD dimers on a KAR composed of two GluK2 and two GluK3 subunits are demonstrated: homodimers in a, and heterodimers in b. Purple spheres show the predicted location of zinc binding sites.
(D) Effect of zinc on currents evoked by glutamate (10 mM) in a cell expressing GluK2b and GluK3(D730A) (top) and in a cell expressing GluK2b(D729A) and GluK3 (bottom). In the former, the potentiation by zinc is preserved, whereas in the latter, the potentiation is lost.
(E) Summary graph of the effect of zinc on heteromeric mutant receptors is presented. ***p < 0.001, **p < 0.01, ns, p > 0.05.
The contribution of two LBD subunits to the zinc binding site explains the potentiation of heteromeric GluK2/GluK3 receptors by zinc (Figures 1B–1D). In principle, for receptors composed of two GluK2 and two GluK3 subunits, two arrangements are possible: (1) pairs of LBD homodimers, one composed of GluK3 containing two zinc binding sites, with no zinc binding sites in the GluK2 homodimer; and (2) pairs of LBD heterodimers, each containing one zinc binding site formed by residues Q756, D759, and H762 in GluK3 and D729 in GluK2 (Figure 8C). To distinguish between these two possibilities, we measured the effect of zinc on receptors composed of GluK2b and GluK3(D730A) in the presence of 1 μM UBP310 to record primarily the activity of heteromeric receptors. Application of zinc (100 μM) led to potentiation of currents (Figures 8D and 8E), similar to WT receptors, whereas for GluK3(D730A) mutant homomeric dimers, zinc potentiation was abolished (Figure 6E). This result is consistent with hypothesis (2). Moreover, in cells transfected with GluK2b(D729A) and GluK3, zinc did not potentiate currents (Figures 8D and 8E), strongly suggesting that the zinc binding site is lost in these heteromeric receptors. Again, this is consistent with hypothesis (2), namely that heteromeric GluK2/GluK3 contains at least an LBD heterodimer and, if composed of two GluK2 and two GluK3 subunits, is arranged as a pair of heterodimers at the level of the LBDs.
DISCUSSION
Our results identify zinc as a positive allosteric modulator of KARs containing the GluK3 subunit and provide a molecular and mechanistic basis for this allosteric modulation. We identify critical amino acids at the interface between the LBD of two partner subunits that form a pocket for zinc binding. Zinc stabilizes the interface by cross-bridging the two partner LBDs in the dimer. By its action as a counter ion that reduces repulsion between opposed aspartate side chains, hence strongly reducing desensitization, zinc binding translates into potentiation of the GluK3 response. Our data also provide a mechanistic and structural explanation for the specific properties of the GluK3 subunit of KARs and reveal important information about KAR architecture. In particular, our study provides a structural explanation for the functional differences between the two closely related KAR subunits GluK2 and GluK3 and about the probable arrangement of subunits in a heteromeric GluK2/GluK3 receptor, the only native GluK3-containing receptor identified so far (Pinheiro et al., 2007).
Zinc Acts as a Positive Allosteric Modulator of KARs Containing GluK3
The positive allosteric modulation of KARs by zinc appears as a specific feature of GluK3. Homomeric GluK1 and GluK2, as well as GluK2/GluK4 and GluK2/GluK5, are inhibited by zinc in the concentration range that potentiates GluK3 (this study and Mott et al., 2008). The properties of GluK3, especially the fast desensitization and low agonist sensitivity, set it apart from the other KARs (Perrais et al., 2010; Schiffer et al., 1997). We previously showed that the properties of GluK3 are dominant over those of GluK2 when expressed in heteromeric combinations (Perrais et al., 2009a; Pinheiro et al., 2007). Interestingly, zinc potentiates GluK2/GluK3 receptors as well as GluK3 receptors, indicating that the GluK3 subunit also imposes its allosteric properties on the heteromer. Zinc potentiation appears to act by reducing the desensitization rate of GluK3. To confirm this link between reduced desensitization and potentiation of the GluK3 response, we showed that the effects of zinc on GluK3 are abrogated in GluK3 variants with reduced desensitization rates. In addition to its potentiating effect, zinc at concentrations above 300 μM for WT GluK3 receptors and for many mutant GluK3 receptors (Table S1) clearly inhibits currents. Similar to GluK2, this inhibition is not accompanied by a change in desensitization kinetics, suggesting that these two effects of zinc rely on different mechanisms and, hence, different binding sites. Interestingly, we did not see inhibition of heteromeric GluK2/GluK3 receptors; moreover, when potentiation is abolished in the GluK2(D729A)/K3 heteromeric receptor, zinc also does not induce any inhibition. This suggests that inhibitory zinc binding sites are screened or absent in heteromeric receptors.
There is a growing number of positive allosteric modulators related to aniracetam and cyclothiazide that bind to the LBD dimer assembly of structurally related AMPARs and that potentiate activity through modification of the deactivation and/or desensitization time course (Traynelis et al., 2010). The situation is quite different for KARs, for which only concanavalin A (ConA) and a few other plant lectins have been identified as positive allosteric modulators, although not of GluK3 (Perrais et al., 2009a; Schiffer et al., 1997). ConA appears to reduce desensitization of KARs and increase the apparent agonist affinity (Partin et al., 1993; Bowie et al., 2003). There are however clear differences with the mode of action of zinc, including the fact that lectins stabilize different KAR open states (Bowie et al., 2003) and bind to carbohydrate chains in the ATD (Everts et al., 1999). Moreover, although monovalent ions, such as Na+ and Cl−, also regulate the gating of KARs, but not AMPA or NMDARs (Bowie, 2010), they act as necessary cofactors for KAR function (Figure 8A; Plested and Mayer, 2007; Plested et al., 2008). Finally, protons typically inhibit the function of KARs (Mott et al., 2003; 2008). Here, we show that for GluK3 receptors, desensitization is instead increased at pH 8.3, suggesting that protonation of dimer interface residues stabilizes the LBD dimer assembly. In addition, zinc potentiation is lost at pH 6.8, which suggests that the zinc binding site itself can be protonated, which is likely the case for the key histidine H762.
Zinc Binds GluK3 at the Dimer Interface and Stabilizes the Interaction
We took advantage of the opposite modulation of GluK2 and GluK3 by zinc to narrow down the region responsible for zinc binding. By studying chimeric GluK2/GluK3 receptors and point mutants of the two subunits, we identified key residues in the S2 segment of GluK3 that are responsible for the potentiating effects of zinc. Strikingly, the single mutation D759G in GluK3 reverts zinc potentiation into an inhibition, and the converse mutation in GluK2 imparts potentiation by zinc. In addition to D759, which is unique to GluK3, the binding site for zinc is composed of a carbonyl oxygen from the main chain and two conserved residues: H762 in the same subunit as D759, and D730 in the dimer partner. Prior analysis of the effects of mutations at the LBD dimer interface has confirmed that there is a common mechanism for desensitization in AMPA/KARs, dependent on the stability of the LBD dimer interface (Weston et al., 2006). We propose that D759 facing D730 induces a destabilization of the dimer interface by electrostatic repulsion (Figure S1D), generating fast desensitization properties. The binding of zinc to the dimer interface cancels this repulsion and stabilizes the LBD dimer. Consistent with this, GluK3(D759G) desensitizes much more slowly, whereas the converse mutant GluK2(G758D) desensitizes very rapidly. However, mutation of the other aspartate in the zinc binding site, D730, did not yield receptors with reduced desensitization: for GluK3(D730A), desensitization is similar to WT, and for GluK3(D730N), it is even faster than WT (Table S1). This unexpected effect could be due, for example, to His762, which would attract Asp730, stabilizing the interaction between LBDs. The presence of Asp759 in the D730A mutant would cancel this effect. Alternatively, structural changes in the mutant receptors could complicate the interpretation. Similar results have been reported for some GluK2 LBD dimer interface mutants, for which the GluK2(E757Q) mutant, which swaps a GluA2 for GluK2 residue, increases desensitization (Chaudhry et al., 2009), most likely by subtly perturbing the structure of helix J.
Because D730 is conserved between GluK3 and GluK2, it provides an explanation why zinc potentiates heteromeric GluK2/GluK3 receptors, with the zinc binding site partitioning between the two subunits in the dimer. Our structural model suggests that there is only one zinc binding site in a heteromeric LBD dimer (see Figure 8C). Consistent with this, GluK2/GluK3 receptors have a higher EC50 and lower nH for zinc than homomeric receptors. Moreover, the analysis of mutant heteromeric receptors shows that zinc binding requires Asp729 on the GluK2 subunit. Consequently, the zinc binding site is most probably shared by GluK2 and GluK3 in LBD heterodimers (model b in Figure 8C). Asp729 is conserved for all GluK subunits, and therefore, we propose that other combinations of heteromeric receptors containing GluK3 could all comprise a zinc binding site leading to potentiation. Moreover, the GluK3 specificity of potentiation by zinc provides structural insights into the specific gating and desensitization properties of GluK3. Notably, in the LBD of 14 out of 18 iGluR subunits, with the exception of GluK3, GluK4, GluK5, and GluN2D, the turn between helices J and K contains a highly conserved glycine residue. In GluK3, this glycine residue is replaced by D759, producing unique rapid desensitization, whereas in GluK4 and GluK5, the asparagine substitution at this position would likely not destabilize the LBD dimer interface, imparting different gating properties to these receptors.
Functional Consequences
Zinc can modulate excitatory synaptic transmission through multiple mechanisms, which are not all well described (Paoletti et al., 2009). Although the inhibitory regulation of postsynaptic glutamate receptors, principally NMDARs, appears as a primary function of synaptic zinc, other potential roles in the regulation of synaptic transmission have also been proposed by Paoletti et al. (2009). Technical limitations have yet precluded a direct measurement of zinc in the synaptic cleft. However, the peak concentration was initially estimated to be in the order of 100 μM (Vogt et al., 2000). This value is well within the range of efficacy for the allosteric potentiation of GluK3 by zinc but may be overestimated and may depend on experimental conditions (Paoletti et al., 2009). Moreover, simultaneous application of zinc and glutamate does not potentiate GluK3-mediated currents (data not shown), which likely excludes an effect of zinc during low-frequency stimulation. However, high-frequency trains of synaptic stimulation are thought to trigger a substantial increase in extracellular zinc, and the accumulated zinc could potentiate presynaptic GluK2/GluK3 receptors present at hippocampal mossy fiber synapses (Pinheiro et al., 2007). Interestingly, it has been shown that vesicular zinc is required for presynaptic LTP at hippocampal mossy fiber synapses by a yet undisclosed mechanism (Pan et al., 2011). This result can be correlated with the fact that mossy fiber LTP is absent in GluK3−/− mice (Pinheiro et al., 2007). The positive allosteric modulation of these presynaptic GluK2/GluK3 receptors may impart increased sensitivity to glutamate and prolonged channel opening, inducing a possible increase in presynaptic Ca2+ influx. Hence, allosteric modulation of presynaptic GluK3 receptors may be one of the mechanisms by which zinc promotes presynaptic long-term potentiation.
In conclusion, we have identified zinc as a positive allosteric modulator of presynaptic KARs, with a potential role in synaptic plasticity. Our structure-function analyses lend further support to the notion that the stability of the LBD dimer interface is essential for dictating the desensitization properties of KARs. Our data help explain the fast desensitization properties of GluK3 as compared to GluK2 and pinpoint a single amino acid residue in the upper lobe of the clamshell of the GluK3 LBD, D759, as responsible for the specific properties of GluK3. Finally, the structural data about the site for positive allosteric modulation will be instrumental for the discovery of ligands that may promote synaptic plasticity and cognitive processes by acting on the regulatory KARs.
EXPERIMENTAL PROCEDURES
Receptor Mutagenesis
Clones corresponding to rat GluK2a(Q), GluK2b(Q), and GluK3a were described previously (Jaskolski et al., 2004, 2005; Pinheiro et al., 2007). Clones corresponding to GluK2e/3i and GluK3e/2i were described in Perrais et al. (2009a). Site-directed mutagenesis was performed using QuikChange XL Site-Directed Mutagenesis Kit (Stratagene) with specific oligonucleotides corresponding to each mutant (oligonucleotide forward sequences, primed on their respective WT subunits: GluK3(D759G), 5′-GCTGCAGGAGGAGGGCAA GCTTCACATCATGAAGGAG-3′; GluK3(H762A), 5′-GCTGCAGGAGGAGGAC AAGCTGGCCATCATGAAGGAGAAGTGGTGGC-3′; GluK3(D730A), 5′-CGG CGGCCTCATCGCTTCCAAGGGCTACGG-3′; GluK3(D730N), 5′-AACTGCAATCTCACCCAGATCGGCGGCCTCATCaATTCCAAGGGCTACGGCATCGGCA CGCCCATGG-3′; GluK3(H492Y), 5′-GGCTCCCCTGACCATCACATATGTCC GAGAGAAGGCC-3′; GluK3(H492A), 5′-CCCCTGACCATCACCGCGGTCCGA GAGAAGGCC-3′; GluK2(Y490H), 5′-GCTCCACTGGCTATAACCCACGTGCG TGAGAAGGTCATCG-3′; GluK2(G758D), 5′-CAGCTGCAGGAGGAAGACAA GCTTCAC ATGATGAAGGAG-3′; GluK2b(D729A), 5′-ATTGGCGGCCTTATA GCATCCAAAGGCTATGGC-3′).
N-terminal deletion versions of GluK2a(Q) and GluK3a(Q) were generated using restriction site addition by PCR before the amino acid sequence GRI at positions 344 and 347, respectively: GluK2 ΔATD: forward 5′-GAATTCCGG CAGAATAACATTT AACAAAACCAATGG-3′, reverse 5′-CACCAAATGC CTC CCACTATC-3′ primed on GluK2a ; GluK3 ΔATD: forward: 5′-GAATTCAG GACGGATTGTTTTCAACAAAACCAGTGGC-3′, reverse 5′-GGCTTAGAGAA GTCAATGGCCTCCTCTCGGACATGGG-3′ primed on GluK3a.
The GluK2/3S2 and GluK3/2S2 chimeras were constructed by exchange of the C-terminal domain of one GluK subunit to the other one using the EcoRV restriction site already present on GluK2 in position 532 on the first transmembrane domain and one created by mutagenesis into the same amino acid sequence in position 534 on GluK3 with a forward primer of 5′-CCCCC TGTCCCCAGATATCTGGATGTACGTGC-3′. All DNAs were verified by restriction analysis and sequencing.
Cell Culture and Transfection
HEK293 cells (European Collection of Cell Cultures) were grown and transfected as previously described by Coussen et al. (2005). Cells were cotransfected using FuGENE 6 (Roche) with green fluorescent protein (GFP) and GluK2a(Q), GluK3a, or mutated forms of these receptors at a cDNA ratio of 1:3. For experiments on heteromeric GluK2/GluK3 receptors, cells were transfected with GFP, GluK2b(Q), and GluK3a at a DNA ratio of 1:1.5:1.5. Cells were used 1–3 days after transfection and replated the day before recording for lifted cells, or 1–2 days before recording for outside-out patches.
Electrophysiological Recordings
Brightly fluorescent, isolated cells were selected for recording. Cells were bathed in a solution containing the following: 145 mM NaCl, 2 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM HEPES, adjusted to 320 mOsm/l and pH 7.4 with NaOH, at room temperature. Recording pipettes (resistance 3–5 MΩ) were filled with a solution containing the following: 130 mM CsCH3SO3, 2 mM NaCl, 2 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 4 mM Na2ATP, and 0.1 mM spermine, adjusted to 310 mOsm/l, and pH 7.2 with CsOH. Cells were recorded at room temperature (22°C–25°C), in whole-cell or outside-out patch mode, held at −80mV to −40mV, and placed under the flow of a theta tube pulled to a final opening of ~100 μm mounted on a piezoelectric translator (P-245.50 and E-470 amplifier; Polytec PI). Currents were evoked by long (100 ms) or short (1 ms) applications of glutamate every 20 s and were filtered at 2.9 kHz and recorded at a sampling frequency of 20 kHz by an EPC10 amplifier (HEKA). Up to four different glutamate concentrations, or three zinc concentrations, were applied to a cell or an outside-out patch with a manual valve. Zinc (ZnCl2) was added in both control and glutamate solutions. The exchange between two different concentrations was completed within 2 min. All chemicals were from Sigma-Aldrich. UBP310 was from Tocris.
Data Analysis
All electrophysiological recordings were analyzed with IGOR Pro 5 (WaveMetrics). Current amplitudes were measured with built-in tools, and τdes was measured with exponential fit using a least-squares algorithm. For each condition, we averaged five sweeps and corrected amplitude changes for run down. Statistical analysis was performed using GraphPad (Prism). In the text and figures, data are presented as mean ± SEM; Student’s t test was used for assessment of difference, with the following coding: ***p < 0.001, **p < 0.01, *p < 0.05, ns p > 0.05. The actual p value is indicated for critical tests.
Crystallography
The GluK3 LBD construct was created by PCR from the full-length cDNA and contained residues N402-K515 in S1 connected via a GT linker to P638-E776 in S2, with an N-terminal His8 affinity tag and LVPRGS thrombin site. The GluK3 LBD was expressed as a soluble protein in Origami B(DE3) E. coli and purified as described previously for other KAR LBD constructs (Mayer, 2005). Crystals were grown using hanging drops in the presence of either 5 mM glutamate or 4 mM kainate, together with 2–10 mM zinc acetate added to the protein solution at 5–10 mg/ml in a buffer containing 200 mM NaCl, 10 mM HEPES (pH 7), and 1 mM EDTA. The reservoir solution for the glutamate complexes contained 6%–14% PEG 3350, 100 mM Bis-Tris propane (pH 8.4–8.5), and 0.2 M Na2SO4. For the kainate complexes, the reservoir contained 4%–6% PEG 8K and 0.1 M Tris (pH 7.8). Crystals were cryoprotected by serial transfers to artificial mother liquor containing 18%–22% glycerol. X-ray diffraction data were collected from single crystals at 100 K at APS 22-ID. Data sets were indexed, scaled, and merged using DENZO and SCALEPACK from the HKL2000 suite (Otwinowski and Minor, 1997). None of the data sets showed twinning as analyzed by PHENIX xtriage (Adams et al., 2010). For the glutamate complex, two different crystal forms were obtained; the kainate complex was isomorphous with one of the glutamate complexes (Table S2). The structures were solved by molecular replacement using Phaser (McCoy et al., 2007) with a GluR6 LBD monomer (PDB 1S50) as a search probe. The solution found two protomers with high rotation and translation Z scores for the glutamate P2221 (RFZ1 = 15.5, TFZ1 = 17.4; RFZ2 = 17.4 and TFZ2 = 52.4) and kainate P2221 (RFZ1 = 12.1, TFZ1 = 20.5; RFZ2 = 14.8, TFZ2 = 40.8) complexes. For the second crystal form of the glutamate complex in the P21212 space group, the molecular replacement solution located four protomers, also with high Z scores (RFZ1 = 13.8, TFZ1 = 17.6; RFZ2 = 18.6 and TFZ2 = 31.4; RFZ3 = 13.0, TFZ3 = 61.8; RFZ4 = 13.0 and TFZ4 = 67.1). The models were initially built using ARP/wARP (Morris et al., 2003) and then refined by alternate cycles of crystallographic refinement with PHENIX (Adams et al., 2010) coupled with rebuilding and real-space refinement with Coot (Emsley and Cowtan, 2004) using TLS groups determined by motion determination analysis (Painter and Merritt, 2006). The final models (Table S2) were validated with MolProbity (Davis et al., 2004). Figures were prepared using PyMOL (Schrödinger).
Supplementary Material
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, the Fondation pour la Recherche Medicale, the Conseil Régional d’Aquitaine, the Agence Nationale de la Recherche (contract SynapticZinc), and the intramural research program of NICHD, NIH. Synchrotron diffraction data were collected at SER-CAT beamline 22 ID. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. We thank Remi Sterling for cell culture maintenance, and Françoise Coussen, Séverine Desforges, and Carla Glasser for help with molecular biology. Pierre Paoletti provided insightful suggestions along the course of this study. We are also grateful for members of the C.M. laboratory for helpful discussions.
Footnotes
ACCESSION NUMBERS
The coordinates and structure factors the GluK3 kainate, GluK3 glutamate P2221, and GluK3 glutamate P21212 complexes have been deposited into the Protein Data Bank under ID codes 3U92, 3U93, and 3U94, respectively.
Supplemental Information includes one figure and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2012.08.027.
References
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Bowie D. Ion-dependent gating of kainate receptors. J Physiol. 2010;588:67–81. doi: 10.1113/jphysiol.2009.178863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J Physiol. 2003;547:373–385. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009;28:1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Lipton SA. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 1999;23:171–180. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J Neurosci. 2003;23:422–429. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, Marin P, Mulle C. Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron. 2005;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Davis IW, Murray LW, Richardson JS, Richardson DC. MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res. 2004;32(Web Server issue):W615–W619. doi: 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Petroski R, Kizelsztein P, Teichberg VI, Heinemann SF, Hollmann M. Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci. 1999;19:916–927. doi: 10.1523/JNEUROSCI.19-03-00916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Catches JS, Petralia RS, Copits BA, Xu J, Russell TA, Swanson GT, Contractor A. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron. 2009;63:818–829. doi: 10.1016/j.neuron.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P. Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron. 2008;57:80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Coussen F, Nagarajan N, Normand E, Rosenmund C, Mulle C. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J Neurosci. 2004;24:2506–2515. doi: 10.1523/JNEUROSCI.5116-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskolski F, Normand E, Mulle C, Coussen F. Differential trafficking of GluR7 kainate receptor subunit splice variants. J Biol Chem. 2005;280:22968–22976. doi: 10.1074/jbc.M413166200. [DOI] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low CM, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors. Proc Natl Acad Sci USA. 2000;97:11062–11067. doi: 10.1073/pnas.180307497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Structure and mechanism of glutamate receptor ion channel assembly, activation and modulation. Curr Opin Neurobiol. 2011;21:283–290. doi: 10.1016/j.conb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Perrakis A, Lamzin VS. ARP/wARP and automatic interpretation of protein electron density maps. Methods Enzymol. 2003;374:229–244. doi: 10.1016/S0076-6879(03)74011-7. [DOI] [PubMed] [Google Scholar]
- Mott DD, Washburn MS, Zhang S, Dingledine RJ. Subunit-dependent modulation of kainate receptors by extracellular protons and polyamines. J Neurosci. 2003;23:1179–1188. doi: 10.1523/JNEUROSCI.23-04-01179.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott DD, Benveniste M, Dingledine RJ. pH-dependent inhibition of kainate receptors by zinc. J Neurosci. 2008;28:1659–1671. doi: 10.1523/JNEUROSCI.3567-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Naur P, Vestergaard B, Skov LK, Egebjerg J, Gajhede M, Kastrup JS. Crystal structure of the kainate receptor GluR5 ligand-binding core in complex with (S)-glutamate. FEBS Lett. 2005;579:1154–1160. doi: 10.1016/j.febslet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Nayeem N, Zhang Y, Schweppe DK, Madden DR, Green T. A nondesensitizing kainate receptor point mutant. Mol Pharmacol. 2009;76:534–542. doi: 10.1124/mol.109.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. [20] Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Painter J, Merritt EA. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr D Biol Crystallogr. 2006;62:439–450. doi: 10.1107/S0907444906005270. [DOI] [PubMed] [Google Scholar]
- Pan E, Zhang XA, Huang Z, Krezel A, Zhao M, Tinberg CE, Lippard SJ, McNamara JO. Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron. 2011;71:1116–1126. doi: 10.1016/j.neuron.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Perrais D, Coussen F, Mulle C. Atypical functional properties of GluK3-containing kainate receptors. J Neurosci. 2009a;29:15499–15510. doi: 10.1523/JNEUROSCI.2724-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Pinheiro PS, Jane DE, Mulle C. Antagonism of recombinant and native GluK3-containing kainate receptors. Neuropharmacology. 2009b;56:131–140. doi: 10.1016/j.neuropharm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Perrais D, Veran J, Mulle C. Gating and permeation of kainate receptors: differences unveiled. Trends Pharmacol Sci. 2010;31:516–522. doi: 10.1016/j.tips.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2007;104:12181–12186. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron. 2007;53:829–841. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Plested AJ, Vijayan R, Biggin PC, Mayer ML. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58:720–735. doi: 10.1016/j.neuron.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O’Hara PJ, Heinemann SF. Agonist selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid-binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Strutz N, Villmann C, Thalhammer A, Kizelsztein P, Eisenstein M, Teichberg VI, Hollmann M. Identification of domains and amino acids involved in GLuR7 ion channel function. J Neurosci. 2001;21:401–411. doi: 10.1523/JNEUROSCI.21-02-00401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venskutonyté R, Frydenvang K, Gajhede M, Bunch L, Pickering DS, Kastrup JS. Binding site and interlobe interactions of the ionotropic glutamate receptor GluK3 ligand binding domain revealed by high resolution crystal structure in complex with (S)-glutamate. J Struct Biol. 2011;176:307–314. doi: 10.1016/j.jsb.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Venskutonyté R, Frydenvang K, Hald H, Rabassa AC, Gajhede M, Ahring PK, Kastrup JS. Kainate induces various domain closures in AMPA and kainate receptors. Neurochem Int. 2012;61:536–545. doi: 10.1016/j.neuint.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. Conformational restriction blocks glutamate receptor desensitization. Nat Struct Mol Biol. 2006;13:1120–1127. doi: 10.1038/nsmb1178. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nayeem N, Nanao MH, Green T. Interface interactions modulating desensitization of the kainate-selective ionotropic glutamate receptor subunit GluR6. J Neurosci. 2006;26:10033–10042. doi: 10.1523/JNEUROSCI.2750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.