Abstract
Cocaine users exhibit a wide range of behavioral impairments accompanied by brain structural, neurochemical and functional abnormalities. Metabolic mapping studies in cocaine users and animal models have shown extensive functional alterations throughout the striatum, limbic system, and cortex. Few studies, however, have evaluated the persistence of these effects following cessation of cocaine availability. The purpose of this study, therefore, was to assess the functional effects of re-exposure to cocaine in nonhuman primates after the discontinuation of cocaine self-administration for 30 or 90 days, using the quantitative autoradiographic 2-[14C]deoxyglucose (2DG) method. Rhesus monkeys self-administered cocaine (fixed interval 3-min schedule, 30 infusions per session, 0.3 mg/kg/infusion) for 100 sessions followed by 30 (n=4) or 90 days (n=3) during which experimental sessions were not conducted. Food-reinforced control animals (n=5) underwent identical schedules of reinforcement. Animals were then re-exposed to cocaine or food for one final session and the 2DG method applied immediately after session completion. Compared to controls, re-exposure to cocaine after 30 or 90 day drug-free periods resulted in lower rates of glucose utilization in ventral and dorsal striatum, prefrontal and temporal cortex, limbic system, thalamus, and midbrain. These data demonstrate that vulnerability to the effects of cocaine persists for as long as 90 days after cessation of drug use. While there was some evidence for recovery (fewer brain areas were affected by cocaine re-exposure at 90 days as compared to 30 days), this was not uniform across regions, thus suggesting that recovery occurs at different rates in different brain systems.
Keywords: cocaine, rhesus monkey, self-administration, functional brain activity, re-exposure
1. Introduction
Chronic cocaine users have significant motivational, emotional, cognitive and sensorimotor impairments. Studies have shown deficits in cognitive performance (Hoff et al. 1996; Rogers and Robbins, 2001) such as decision making (Stout et al. 2004; Bechara et al. 2000; Bolla et al. 2003), complex reasoning (Cunha et al. 2010; Verdejo-Garcia and Perez-Garcia, 2007; Ersche et al. 2008), and attention, as well as abnormal sensitivity to and motivation for both drug and nondrug rewards (Goldstein et al. 2007; Moeller et al. 2009; Coffey et al. 2003; Heil et al. 2006). Furthermore, cocaine users exhibit impaired cognitive control and increased impulsivity (Goldstein and Volkow, 2002; Kjome et al. 2010; Lane et al. 2007), as well as decreased sensorimotor performance (Hanlon et al. 2009, 2010), suggesting a broad range of consequences from long-term cocaine exposure. These impairments are accompanied by alterations in the regulation of transmitter systems (Volkow et al. 2006; Martinez et al. 2004; Mash et al. 2002, 2005), reductions in functional activity in the prefrontal cortex (Volkow et al. 1992), decreases in gray matter volume (Hanlon et al. 2011; Matochik et al. 2003; Fein et al. 2002; Lim et al. 2008), and reductions in the integrity of white matter (Franklin et al. 2002; Lim et al. 2002; Ma et al. 2009; Moeller et al. 2005) among many other deficits when compared to healthy controls.
Studies, particularly those in animal models, have shown that many of these abnormalities result from repeated exposure to cocaine, and are not merely the result of conditions that pre-date drug use. These adaptations likely begin at the time of social experimentation and advance as drug use becomes more compulsive and habitual, and are manifested behaviorally in the form of failure of inhibitory control, preoccupation with drug, craving, and repeated episodes of relapse. Numerous studies have documented many of these neuroadaptations in animal models (Kalivas and O’Brien, 2008; Kreek and Koob, 1998; Weiss et al. 2001; Wolf, 2010), again showing the breadth of changes across multiple systems. Investigations have shown, for example, that early in the course of exposure to cocaine, there are significant alterations in the concentrations of dopamine receptors and transporters largely within the ventral portions of the striatum. But with increasing durations of exposure these adaptations spread to encompass progressively greater territories within the striatum (Letchworth et al. 2001; Nader et al. 2002; Porrino et al. 2004b). These changes in the regulation of dopamine systems are paralleled by a change in the pattern of effects of cocaine on functional brain activity, as reflected by cerebral metabolism measured with the 2-[14C]deoxyglucose (2DG) method. In the initial stages of exposure, cocaine self-administration produces alterations in functional activation in circumscribed portions of the ventral striatum and prefrontal cortex. As cocaine exposure continues, the effects of the drug progress to eventually include the entire extent of the striatum, prefrontal cortex and portions of the temporal lobe (Porrino et al. 2004a; Beveridge et al. 2006). These data support the notion that addiction is a progressive disorder.
One of the key questions in drug abuse research, however, is how the adaptations that develop during chronic cocaine exposure are altered after the cessation of drug use. Specifically, some investigations of affect, craving levels, and the cognitive abilities of addicts have shown that there is some evidence for improvement after drug use has terminated (Coffey et al. 2000; Weddington et al. 1990; Satel et al. 1991), although other studies have provided support for continuing deficits (Herning et al. 1990; Berry et al. 1993). Studies in animal models are consistent with an absence of recovery (Shaham and Hope, 2005; Freeman et al. 2008, 2010; Nestler, 2001; Nader et al. 2006), as well as the development of new adaptations in, for example, the glutamate system (Baker et al. 2003; Conrad et al. 2008). There are, however, also reports of the moderation or amelioration of neurobiological adaptations that have accompanied cocaine exposure (Nader et al. 2006; Beveridge et al. 2009; Freeman et al. 2010). Taken together, these data suggest a complex interaction of numerous new and enduring adaptations in multiple brain systems accompanying discontinuation of drug use. Because the stage that follows drug cessation is considered a period of high vulnerability to relapse that may be triggered by any number of factors, including drug re-experience, acute/chronic stress or exposure to drug-associated cues, the identification of the neurobiological adaptations that occur during this time is an important step in the development of effective treatment strategies to maintain and prolong abstinence in cocaine users.
Many of the above investigations have focused on adaptations in specific transmitter or signaling systems in isolated brain regions or small subset of regions, most frequently in the striatum and/or prefrontal cortex. Few studies, however, have considered the influence of these effects from a more systems-based level. The purpose of the present study, therefore, was to determine the functional consequences of these neuroadaptations that occur following the termination of drug availability in networks and circuits throughout the entire brain using the 2DG method. If there is recovery, or the emergence of new adaptations, the consequences of these changes in brain structure and function should be evident in an altered response to cocaine self-administration itself as compared to previously established patterns of functional activity that accompany self-administration of the drug. To this end, animals with a 100-day history of cocaine self-administration were re-exposed to a single session of cocaine self-administration after discontinuation of cocaine availability for 30 or 90 days, and compared to animals with identical histories of responding for food reinforcement and re-exposure to food reward after 30 or 90 days cessation of operant sessions. Metabolic activity was mapped with the 2DG method immediately following the final session to evaluate the effects of re-exposure to food or cocaine throughout the brain.
2. Materials and Methods
2.1. Subjects
Twelve experimentally-naïve adult male rhesus monkeys (Macaca mulatta) weighing between 9.0–13.0 kg (mean ± SD; 10.6 ± 1.2) at the start of the study served as subjects. Monkeys were individually housed in stainless steel cages with water ad libitum; animals had physical and visual contact with each other. Their body weights were maintained at approximately 95% of free-feeding weights by banana-flavored pellets earned during the experimental sessions and by supplemental feeding of Lab Diet Monkey Chow, provided no sooner than 30 minutes post-session. All efforts were made to minimize animal suffering and to reduce the number of animals used. All procedures were performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
2.2. Behavioral Apparatus
Cocaine self-administration and food-reinforced responding occurred in ventilated and sound-attenuated operant chambers (1.5 × 0.74 × 0.76m, Med Associates, East Fairfield, VT) designed to accommodate a primate chair (Model R001, Primate Products, Redwood City, CA). The chambers contained an intelligence panel (48 × 69 cm), which consisted of two retractable levers (5 cm wide) and three stimulus lights. The levers were positioned within easy reach of the monkey sitting in the primate chair. One-gram food pellets were delivered from a feeder located on the top of the chamber. A peristaltic infusion pump (7531-10, Cole-Parmer Co., Chicago, IL) was used to deliver drug injections at a rate of approximately 1.5 ml/10 sec to those animals self-administering cocaine.
2.3. Surgical Procedures
All monkeys, including controls, were surgically prepared, under sterile conditions, with indwelling intravenous femoral catheters and vascular access ports (Model GPV, Access Technologies, Skokie, IL), as described in detail elsewhere (Nader et al., 2002). Monkeys were given 24–48 hours to recover from surgery prior to returning to the experiment. Approximately five days before the terminal procedure, each monkey was implanted with a chronic indwelling catheter into the adjacent femoral artery for collection of timed arterial blood samples during the 2DG procedure. The surgical procedure was identical to that described for the venous catheter. This procedure took place on a day the animals did not respond in operant sessions. They resumed food or cocaine self-administration the next day, with no obvious differences in rates, pattern or intake.
2.4. Self-administration procedures
Monkeys were initially trained to respond on one of two levers by reinforcing each response on the correct lever with a 1g banana-flavored pellet. Over a period of approximately three weeks the interval between availability of food pellets was gradually increased until a three-minute interval was achieved (i.e., fixed-interval 3-minute schedule; FI 3-min). Under the final schedule conditions, the first response on the lever after three minutes resulted in the delivery of a food pellet; sessions ended after 30 food presentations. In order to habituate animals to the timing of the 2DG procedure, at the end of each session, the response levers were retracted, houselights and stimulus lights were extinguished, and animals remained in the darkened chamber for approximately 30 minutes before they were returned to their home cages. All monkeys responded under the FI 3-min schedule of food presentation for at least 20 sessions and until stable performance was obtained (± 20% of the mean for three consecutive sessions, with no trends in response rates). When food-maintained responding was stable, the feeder was unplugged and the effects of extinction on responding were examined for five consecutive sessions, after which responding was re-established and maintained by food presentation. After baseline performance had been re-established, monkeys were randomly assigned to one of three groups; monkeys in the cocaine self-administration groups were surgically prepared with venous catheters, as described above. Food-maintained performance was allowed to stabilize after surgery (approximately 4–6 days) before cocaine self-administration sessions were initiated.
One group of monkeys served as controls (N=5) and continued to respond under the FI 3-min schedule of food presentation for a total of 100 sessions followed by either 30 (N=3) or 90 (N=2) days during which monkeys remained in their home cage with no operant sessions conducted. The remaining seven monkeys were assigned to two cocaine self-administration groups. Prior to each experimental session, the back of the animal was cleaned with 95% ethanol and betadine scrub and a 22-gauge Huber Point Needle (Model PG20-125) was inserted into the vascular access port leading to the venous catheter, connecting an infusion pump containing the cocaine solution to the catheter. Prior to the start of the session, the pump was operated for approximately three seconds, filling the port with the dose of cocaine that was available during the experimental session. At the end of each session, the port was filled with heparinized saline (100 Units/ml) to help prevent clotting. Chronic exposure to cocaine self-administration consisted of 100 sessions in which responding was maintained by 0.3 mg/kg/injection cocaine (30 injections per session, for a total of 9.0 mg/kg/session), which was then followed by cessation of operant sessions for 30 (N=4) or 90 (N=3) days. Because 0.3 mg/kg cocaine per injection was considered a high dose for previously cocaine-naïve monkeys, this dose was achieved within 2–4 sessions by first allowing the monkeys to self-administer 0.1 mg/kg/injection cocaine.
In addition, in an effort to model drug seeking, probe sessions were conducted every 2 weeks over the course of the experiment. A probe session consisted of a 1, 2 or 3 day period during which operant sessions were suspended, followed by a 2-hr experimental session in which the discriminative stimulus was illuminated but responding was not reinforced over the 2 hr session. Each probe session duration (1, 2, or 3 days) was evaluated twice throughout the course of the experiment, with the order of testing occurring in a semi-random fashion.
Following the cessation of operant sessions, animals remained in their home cages for 30 or 90 days. No self-administration sessions were conducted during this time, however three times per week, all monkeys were placed in their restraint chairs, catheters were flushed with heparinized saline (100 Units/ml) and then animals were returned to their home cages.
A final cocaine self-administration or food-reinforcement session took place 30 or 90 days after the last self-administration session. On that day, a terminal 2DG experiment was conducted. The monkey was placed in the operant chamber and levers were presented, together with cue lights, signaling the availability of cocaine or food reinforcement; the session ended when the animal received the full number of reinforcers available (30). Immediately after the last infusion or food reinforcement, the 2DG procedure was conducted.
2.5. Measurement of local cerebral glucose utilization
A venous and an arterial catheter exited through an opening in the rear of the chamber allowing all infusions and sampling to be accomplished remotely with minimal disruption to the animal. The 2DG procedure was initiated at the end of the final session, two minutes into the timeout, by the infusion of an intravenous pulse of 2.76 MBq/kg 2-deoxy-D-[14C]glucose (DuPont NEN, Boston, MA; specific activity 1850–2035 MBq/mmol) followed by a flush of heparinized saline. For the purposes of the 2DG procedure, this time out was extended to 45 minutes to allow for tracer incorporation and clearance. Timed arterial blood samples (0.25, 0.5, 0.75, 1, 2, 5, 7.5, 10, 15, 25, 35 and 45 min) were drawn thereafter at a schedule sufficient to define the time course of the arterial 2DG and glucose concentrations. Arterial blood samples were centrifuged immediately. Plasma 14C concentrations were determined by liquid scintillation spectrophotometry (Beckman Instruments, Fullerton, CA), and plasma glucose concentrations were assessed using a glucose analyzer (Beckman Instruments, Fullerton CA). Approximately 45 minutes after tracer injection, the animals were euthanized by an intravenous dose of sodium pentobarbital (100 mg/kg). Brains were removed rapidly, blocked in three parts, frozen in isopentane (−45 °C), and stored at −80 °C until they were processed for autoradiograph y. Coronal sections (20 μm) were cut in a cryostat maintained at −22 °C. Four of every 20 sections were thaw -mounted on glass coverslips, dried on a hot plate at 60 °C, and apposed to Kodak MR-1 film (Rochester, NY) for 15–30 days, along with a set of [14C]methylmethacrylate standards (Amersham, Arlington Heights, IL) previously calibrated for their equivalent [14C] concentration in 20 μm brain sections. Autoradiograms were developed in Kodak GBX developer, indicator stop bath, and rapid fix at 68 °C.
Quantitative densitometry of autoradiograms was accomplished with a computer-assisted image-processing system (MCID, Interfocus Imaging Ltd. Linton, UK). Optical density measurements for each structure were made in a minimum of eight brain sections. Measurements were made bilaterally and averaged across hemispheres. Tissue [14C] concentrations were determined from the optical densities and a calibration curve obtained by densitometric analysis of the autoradiograms of the calibrated standards. Glucose utilization was then calculated using the operational equation of the method (Sokoloff et al. 1977), local-tissue [14C] concentrations, the time course of the plasma 2-[14C]deoxyglucose and glucose concentrations, and the appropriate kinetic constants (Kennedy et al. 1978). Because of differences in the baseline levels of glycemia in some animals, the lumped constant was adjusted appropriate to the glucose levels according to procedures based on previous work (Kennedy et al. 1978; Schuier et al. 1990; Suda et al. 1990; Porrino et al. 2004b). Identification of structures was made using adjacent thionin-stained sections. Nomenclature was determined using the atlas of Paxinos et al. (2000) and reports by Amaral et al. (1992), Carmichael and Price (1996) and Ongur et al. (1998). The striatum was divided into three distinct anterior-posterior levels (Porrino et al., 2002, 2004b). The anterior level was defined as the rostral portion of the striatum where the caudate nucleus, putamen and nucleus accumbens were present, but anterior to where the core and shell divisions of the nucleus accumbens could be identified (Bregma 4.05 to 3.15 mm; Paxinos et al. 2000). The middle level was defined as the striatal level at which the nucleus accumbens could most clearly be differentiated into core and shell divisions (Bregma 0.45 to – 0.45 mm; Paxinos et al. 2000). The posterior level was defined as that region of the striatum posterior to the crossing of the anterior commissure where the nucleus accumbens is not present (Bregma −4.50 to −9.90 mm; Paxinos et al. 2000).
2.6. Statistical analysis
Operant Behavior: The primary dependent variables were response rates (total session responses/total session time), session time to earn 30 reinforcers, and quarter-life (QL) values which represent the pattern of responding under FI schedules of reinforcement (see Nader et al., 2002). Baseline data between groups was compared using separate Student’s unpaired t-tests. For the re-exposure study, a computer problem precluded the collection of data (but not the conditions of the experiment) for two food-controls and one 90-day cocaine self-administration monkey, weakening statistical comparisons of group performance. The primary dependent variable for probe sessions was total responses during the 2-hr session. Data were analyzed using a two-way repeated measures ANOVA with Group (cocaine vs. food) and Days of abstinence (1, 2, 3) as the factors.
Rates of local cerebral glucose utilization: Standard statistics software (SPSS for Windows, Chicago, IL) was used for statistical analysis. Rates of glucose utilization were measured in 61 discrete brain regions (adjusted for glycemia levels as described above). Global rates of cerebral metabolism (mol/100g/min) for each animal were estimated as the mean (weighted by region size) of all measured brain regions. Global rates were analyzed by means of a one-way analysis of variance. Values of rates of local cerebral glucose utilization obtained for individual brain structures were analyzed in four functional groups (basal ganglia, cortex, thalamus, and limbic areas) by means of a two-way analysis of variance (treatment group X brain region, with brain region considered a repeated measure). This was followed by planned Bonferroni’s post-hoc test for multiple comparisons. Because data obtained from food-reinforced animals 30 and 90 days after their last operant sessions were not significantly different from one another, data from the control groups were combined. Pearson product-moment correlations were used to correlate rates of glucose utilization with the behavioral measures, rates of responding during the final session and final session length, as corrected for multiple tests. Correlations were conducted in food controls and combined cocaine groups.
3. Results
3.1. Self-administration behavior
Under baseline conditions (sessions 97–100) and prior to cessation of operant responding, food-maintained responding by control monkeys was significantly different from cocaine-maintained responding with significantly higher response rates (p< 0.001), shorter session time (p <0.005) and higher QL values (p < 0.0001; Table 1). Although not statistically significant because of the small sample sizes, on the day of the 2DG procedure, differences were apparent in operant performance of animals in which food or cocaine self-administration had been discontinued for 30 or 90 days prior to the final session (Table 1). Response rates were reduced by 60% of baseline in food-reinforced monkeys and by 17% of baseline in monkeys re-exposed to cocaine self-administration conditions. Consistent with the disruption in food-maintained responding, patterns of responding under the FI schedule as assessed with QL values showed reductions (i.e., less appropriate FI responding) going from QL values of 0.69 to 0.46 in food-maintained animals, but were enhanced in monkeys self-administering cocaine (Table 1).
Table 1.
Mean fixed-interval 3-min responding at baseline and following re-exposure to food or cocaine after discontinuation of operant sessions
| Group | Tot Resps | Session Time (min) | Resp Rate (resp/min) | QL |
|---|---|---|---|---|
|
| ||||
| Food SA + 30 or 90 Days Discontinuation | ||||
| Day 100 of SA1 | 1547.40 ± 416.3 | 92.81 ± 1.96 | 16.32 ± 4.45 | 0.69 ± 0.04 |
| Re-exposure2 | 650.00 ± 633.3 | 90.86 ± 26.35 | 6.45 ± 6.30 | 0.46 ± 0.12 |
|
| ||||
| Cocaine SA + 30 or 90 Days Discontinuation | ||||
| Day 100 of SA3 | 176.57 ± 64.27 | 275.05 ± 37.56 | 0.95 ± 0.55 | 0.45 ± 0.02 |
| Re-exposure4 | 168.33 ± 68.06 | 243.75 ± 33.40 | 0.79 ± 0.37 | 0.59 ± 0.09 |
Data are expressed as means ± S.E.M.
N=5;
N=3;
N=7;
N=6
Probe sessions, in which responding was not reinforced during 2-hr sessions, were conducted weekly after 1–3 days of abstinence. There was no significant effect of days of abstinence, but there were group differences in total responses [F(1. 9) = 5.75, p < 0.05], with responding by the food group being higher than the cocaine group (data not shown). However, when analyzed as a percentage of daily responses, the cocaine self-administration group had significantly larger increases in responding compared to food controls (p < 0.0001). Post-hoc analyses revealed significant group differences after 1 and 3 days abstinence (73% and 96% of baseline responding vs. 533% and 645% of baseline, for food and cocaine self-administration groups respectively)
3.2. Effects of re-exposure to cocaine self-administration on local rates of cerebral glucose utilization
Plasma glucose levels measured just prior to the initiation of the 2DG procedure did not differ significantly between or within groups: food controls, 0.88 ± 0.07 mg/ml (mean ± SEM); re-exposure 30 days after cessation of cocaine self-administration, 0.88 ± 0.13 mg/ml; re-exposure 90 days after cessation of cocaine self-administration, 0.83 ± 0.14 mg/ml. Rates of local cerebral glucose metabolism were measured in 61 brain regions and the data are shown in Tables 2–4. Global rates of cerebral metabolism were significantly lower (F(2,9) = 15.50, p < 0.001) in animals re-exposed to cocaine 30 (mean ± S.E.M.; 36.7 ± 1.5 μmol/100 g/min) and 90 days (36.9 ± 1.2 μmol/100 g/min) after cessation of drug availability, as compared to those of food controls (47.9 ± 1.6 μmol/100 g/min). There were no differences in global rates of cerebral metabolism between the two cocaine self-administration groups. Data from specific brain regions are described in detail below.
Table 2.
Effects of cocaine re-exposure on rates of local cerebral glucose utilization in striatum and basal ganglia 30 or 90 days after cessation of self-administration†
| Brain Region | Food SA + 30 or 90 Day Cessation (N=5) | Cocaine SA + 30 Day Cessation (N=4) | Cocaine SA + 90 Day Cessation (N=3) |
|---|---|---|---|
|
| |||
| Anterior Striatum | |||
| Caudate | 58 ± 1.9 | 44 ± 2.5 ** | 44 ± 3.3 ** |
| Putamen | 62 ± 2.3 | 45± 1.8 ** | 45 ± 1.5 ** |
| Nucleus accumbens | 43 ± 0.8 | 34 ± 2.4* | 38 ± 2.1 |
| Middle Striatum | |||
| Caudate | 59 ± 3.0 | 44 ± 2.7 ** | 46 ± 1.7 ** |
| Putamen | 63 ± 2.8 | 46 ± 2.0 ** | 46 ± 2.3 ** |
| Nucleus accumbens core | 46 ± 0.2 | 34 ± 0.7 ** | 36 ± 0.8 * |
| Nucleus accumbens shell | 41 ± 0.4 | 30 ± 0.7 ** | 32 ± 1.2 ** |
| Posterior Striatum | |||
| Caudate | 62 ± 2.1 | 43 ± 1.7 ** | 47 ± 3.0 ** |
| Putamen | 68 ± 2.4 | 46 ± 1.7 ** | 50 ± 1.3 * |
| Basal ganglia | |||
| Ventral tegmental area | 32 ± 7.4 | 16 ± 0.8 ** | 21 ± 2.7 |
| Globus pallidus | 32 ± 2.5 | 37 ± 2.3 | 23 ± 6.2 |
| Subthalamus | 72 ± 5.3 | 64 ± 9.8 | 62 ± 5.9 |
| Substantia nigra pars compacta | 52 ± 9.2 | 32 ± 4.0 * | 38 ± 2.7 |
| Substantia nigra pars reticulata | 41 ± 2.7 | 36 ± 2.9 * | 38 ± 2.1 |
| Rostromedial tegmental nucleus | 18 ± 1.7 | 15 ± 1.4 | 15 ± 1.1 |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01 different from food control, two-way ANOVA followed by Bonferroni t-tests comparing self-administration groups to food controls.
Table 4.
Effects of cocaine re-exposure on rates of local cerebral glucose utilization in limbic and related subcortical areas 30 or 90 days after cessation of self-administration†
| Brain Region | Food SA + 30 or 90 Day Cessation (N=5) | Cocaine SA + 30 Day Cessation (N=4) | Cocaine SA + 90 Day Cessation (N=3) |
|---|---|---|---|
|
| |||
| Amygdala | |||
| Lateral | 38 ± 2.9 | 27 ± 1.4 ** | 30 ± 2.3 |
| Central | 24 ± 2.2 | 16 ± 0.7 * | 20 ± 1.5 |
| Medial | 30 ± 1.2 | 19 ± 1.0 ** | 24 ± 2.0 |
| Basolateral (parvicellular) | 34 ± 2.0 | 22 ± 1.6 ** | 25 ± 1.1 * |
| Basolateral (magnocellular) | 45 ± 2.6 | 33 ± 1.8 ** | 37 ± 2.8 * |
| Accessory basal | 34 ± 1.8 | 22 ± 1.5 ** | 26 ± 1.2 * |
| Hypothalamus | |||
| Preoptic area | 34 ± 1.2 | 23 ± 1.0 ** | 26 ± 1.5 * |
| Lateral | 32 ± 2.5 | 21 ± 1.6 ** | 26 ± 1.1 |
| Dorsomedial | 35 ± 2.3 | 24 ± 2.0 ** | 29 ± 2.0 |
| Ventromedial | 32 ± 2.2 | 21 ± 1.3 ** | 25 ± 1.5 |
| Paraventricular | 32 ± 2.2 | 23 ± 2.1 ** | 25 ± 1.5 |
| Posterior | 38 ± 1.5 | 23 ± 2.5 ** | 30 ± 2.7* |
| Limbic-Associated | |||
| Bed nucleus of stria terminalis | 25 ± 2.1 | 20 ± 1.6 | 22 ± 2.3 |
| Lateral septum | 35 ± 2.2 | 25 ± 1.2 * | 27 ± 0.8 * |
| Extended amygdala | 31 ± 2.2 | 23 ± 1.3* | 25 ± 0.8 |
| Hippocampal Formation | |||
| CA1 | 37 ± 4.8 | 27 ± 2.4 ** | 31 ± 1.6 |
| CA3 | 35 ± 4.4 | 24 ± 2.4 ** | 29 ± 1.4 |
| Dentate gyrus | 41 ± 4.3 | 30 ± 2.3 ** | 34 ± 2.6 |
| Subiculum | 43 ± 4.5 | 32 ± 1.6 ** | 37 ± 2.7 |
| Entorhinal cortex | 46 ± 3.4 | 34 ± 3.3 ** | 32 ± 2.2 ** |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01 different from food control, one-way ANOVA followed by Bonferroni t-tests comparing self-administration groups to food controls.
Basal ganglia
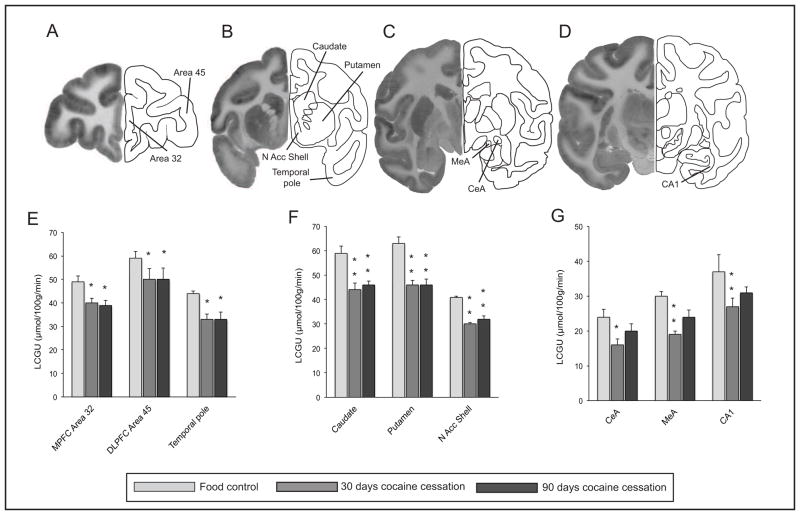
Rates of cerebral glucose utilization for regions of the basal ganglia are shown in Table 2. Statistical analysis revealed a main effect of group (F(2,9) = 15.64, p < 0.001) and of brain region (F(14,9) = 40.366, p < 0.001), as well as a significant interaction of group with brain region (F (14,9) = 1.728, p < 0.02). Further analysis using pre-planned multiple comparisons (Bonferroni t-statistics) in specific basal ganglia regions showed that within the striatum, re-exposure to cocaine self-administration 30 days after cessation of cocaine self-administration decreased glucose utilization throughout all segments: caudate nucleus, putamen, and ventral striatum (Table 2). These differences were observed throughout all three distinct anterior-posterior levels of the striatum. Compared to food controls, significantly lower rates of glucose utilization were present in the pre-commissural caudate and putamen in response to re-exposure to cocaine 30 days after cessation of drug availability at both the anterior (caudate nucleus, −24%; putamen, −27%) and middle level of the striatum (caudate −25%; putamen, −27%; Figures 1 and 2). In addition significant decreases were observed in all portions of the ventral striatum including the rostral accumbens (−21%), nucleus accumbens core (−26%) and nucleus accumbens shell, (−27%; Figures 1 and 2). Within the posterior (post-commissural) striatum, rates of glucose metabolism were also significantly lower in response to cocaine re-exposure in both the caudate nucleus (−31%) and putamen (−32%), compared to food controls (Table 2). Finally, significantly lower rates of metabolism were present in the ventral tegmental area (−50%), substantia nigra pars compacta (−38 %), and pars reticulata (−12%).
Figure 1.
Effect of re-exposure to cocaine self-administration 30 or 90 days after cessation of drug availability, compared to food-reinforced controls. The left hemisphere in Panels A–D displays the distribution of [14C]-deoxyglucose, while the right hemisphere indicates the anatomical localization of brain areas shown in the histograms below (Panels E–G). Rates of local cerebral glucose utilization (LCGU, μmol/100g/min) in response to food or cocaine self-administration are shown in Panels E–G. Asterisks represent significant differences from food controls, * P<0.05, ** P<0.01
Figure 2.
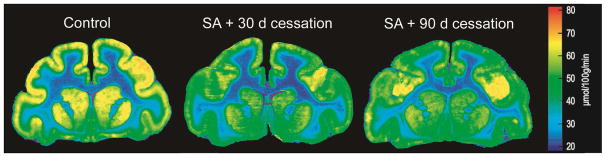
Effect of re-exposure to cocaine self-administration 30 or 90 days after cessation of drug availability on rates of local cerebral glucose utilization in nonhuman primates, as compared to rates of local cerebral glucose utilization of food-reinforced control animals. The 2[14C]DG method was applied immediately after the end of the behavioral session. Shown are color-coded transformations of autoradiograms of coronal sections of nonhuman primate brain at the level of the striatum. Each color represents a range of rates of local cerebral glucose utilization (μmol/100 g per min) according to the calibration scale to the right of the autoradiograms.
Re-exposure to cocaine self-administration 90 days after cessation of drug availability produced a very similar pattern of alterations in cerebral metabolism, with lower rates, as compared to food controls, in the anterior (caudate, −24% and putamen, −27%) and middle (caudate, −22% and putamen, −27%; Figures 1 and 2) striatum, as well as the posterior (post-commissural) striatum (caudate −24% and putamen, −26%). Similar decreases to those observed after 30 days of discontinuation were also observed in the ventral striatum (nucleus accumbens core, −22%; nucleus accumbens shell, −22%; Figures 1 and 2). However, there were no significant differences in rates of glucose utilization in any ventral midbrain structures after re-exposure to cocaine self-administration 90 days after cessation of cocaine availability as compared to controls.
Although there were no statistically significant differences between rates of glucose utilization in groups re-exposed to cocaine 30 or 90 days after cessation of drug availability in striatal and basal ganglia regions, the number of brain regions in which significant differences were observed was much smaller 90 days after cessation than 30 days (Table 5).
Table 5.
Proportion of brain regions in which rates of glucose utilization were significantly different from rates of controls following initial (5 days; Porrino et al, 2002), chronic (100 days; Beveridge et al, 2006) exposure to cocaine self-administration and after re-exposure to cocaine 30 or 90 days after cessation of self-administration. The individual brain regions within each system are listed in Tables 2–4.
| System | 5 days Cocaine SA§ | 100 days Cocaine SA# | 100 days Cocaine SA + 30 days Cessation | 100 days Cocaine SA + 90 days Cessation |
|---|---|---|---|---|
|
| ||||
| Striatum | 4/9 | 9/9 | 9/9 | 8/9 |
| Limbic | 1/20 | 12/20 | 19/20 | 6/20 |
| Prefrontal/motor cortex | 6/13 | 10/13 | 11/13 | 11/13 |
| Temporal cortex | 0/5 | 4/5 | 5/5 | 5/5 |
| Thalamus | 3/8 | 4/8 | 7/8 | 6/8 |
| Basal ganglia | 4/6 | 1/6 | 3/6 | 1/6 |
Data taken from Porrino et al., 2002
Data taken from Beveridge et al. 2006
Frontal and temporal cortex
Rates of glucose utilization for cortical areas are shown in Table 3. Statistical analysis revealed a significant main effect of group (F(2,9) = 9.774, p < 0.01) and of brain region (F(10,9) = 15.986, p < 0.0001). Further analysis using pre-planned multiple comparisons within divisions of frontal and temporal cortex showed that re-exposure to cocaine self-administration 30 days after cessation of drug availability resulted in lower rates of glucose utilization, as compared to rates in food controls, throughout large portions of the cortex analyzed in the current study (Table 3; Figure 1). Within the prefrontal cortex these regions included Areas 10 (−21%), 12 (−15%), 13 (−18%), 14 (−19%), 25 (−18%), and 32 (−18%) along the medial and ventral aspects, as well as areas 45 (−15%) and 46 (−13%) within the lateral prefrontal cortex. Within the temporal lobe, significant reductions included the medial and lateral aspects of the temporal pole (−25%), the dorsal (−29%) and ventral (−22%) insula, superior temporal gyrus (−25%) and area TE (−19%). Finally, rates of glucose metabolism were lower, as compared to rates in food controls, in portions of the motor (−19%) and premotor (−20%) cortex.
Table 3.
Effects of cocaine re-exposure on rates of local cerebral glucose utilization in cortico-thalamic areas 30 or 90 days after cessation of self-administration†
| Brain Region | Food SA + 30 or 90 Day Cessation (N=5) | Cocaine SA + 30 Day Cessation (N=4) | Cocaine SA + 90 Day Cessation (N=3) |
|---|---|---|---|
|
| |||
| Prefrontal Cortex | |||
| Area 10 | 53 ± 1.4 | 42 ± 1.8 * | 44 ± 2.6 * |
| Area 14 | 47 ± 2.4 | 38 ± 2.2 * | 37 ± 0.6 * |
| Area 32 | 49 ± 2.4 | 40 ± 1.9 * | 39 ± 2.1 * |
| Area 24 | 54 ± 1.9 | 47 ± 2.5 | 43 ± 1.3 ** |
| Area 25 | 45 ± 1.2 | 37 ± 3.0 * | 34 ± 1.7 * |
| Area 12 | 59 ± 2.3 | 50 ± 3.0 * | 50 ± 3.7 * |
| Area 13 | 62 ± 2.7 | 51 ± 3.1 * | 47 ± 2.7 ** |
| Area 9 | 54 ± 3.5 | 48 ± 2.4 | 49 ± 2.1 |
| Area 45 | 59 ± 3.0 | 50 ± 4.5 * | 50 ± 4.8 * |
| Area 46 | 67 ± 3.4 | 58 ± 3.7 * | 52 ± 4.9 ** |
| Motor Cortex | |||
| Area 6 | 59 ± 2.1 | 47 ± 2.3 * | 48 ± 1.0* |
| Area 4 | 58 ± 1.5 | 47 ± 2.7 ** | 47 ± 1.3 ** |
| Area 8 | 65 ± 3.1 | 55 ± 2.9 | 47 ± 1.6 |
| Temporal Cortex | |||
| Temporal pole | 44 ± 1.0 | 33 ± 2.2 * | 33 ± 3.2 * |
| Dorsal anterior insula | 62 ± 2.4 | 44 ± 2.9 ** | 39 ± 2.5 ** |
| Ventral anterior insula | 54 ± 3.1 | 42 ± 2.9 ** | 37 ± 1.5 ** |
| Superior temporal gyrus | 64 ± 3.9 | 48 ± 3.3 ** | 42 ± 2.8 ** |
| Area TE | 52 ± 3.6 | 42 ± 1.0 * | 40 ± 2.1 * |
| Thalamus | |||
| Anterior | 67 ± 2.9 | 54 ± 8.8 * | 41 ± 2.6 * |
| Mediodorsal | 57 ± 1.3 | 45 ± 4.5 * | 45 ± 4.3 * |
| Ventral anterior | 56 ± 4.6 | 43 ± 3.1 * | 37 ± 3.2 ** |
| Centromedian | 55 ± 3.8 | 40 ± 3.0 * | 42 ± 1.0 * |
| Parafascicular | 50 ± 5.9 | 37 ± 2.3 * | 39 ± 2.0 |
| Ventrolateral | 52 ± 1.6 | 43 ± 5.1 * | 35 ± 7.1 ** |
| Medial habenula | 36 ± 2.2 | 27 ± 4.0 | 29 ± 4.0 |
| Lateral habenula | 49 ± 3.6 | 23 ± 2.8** | 25 ± 1.0** |
Data represent rates of local cerebral glucose utilization (μmol/100g/min) expressed as means ± S.E.M.
P < 0.05,
P < 0.01 different from food control, two-way ANOVA followed by Bonferroni t-tests comparing self-administration groups to food controls.
Re-exposure to cocaine 90 days after cessation of drug availability also resulted in similar widespread decreases in glucose utilization throughout prefrontal, frontal and temporal cortical areas (Table 3; Figure 1). These areas included medial (areas 10, −17%; 14, −21%; 24, −20%; 25, −24%), orbital (area 13, −24%) and motor regions (areas 6, −19%; 4, −19%). Within the temporal lobe significant decreases were present in the temporal pole (−25%), dorsal (−37%) and ventral (−31%) anterior insula, superior temporal gyrus (−34%) and area TE (−23%). There were no significant differences between groups re-exposed to cocaine 30 or 90 days after cessation of cocaine availability.
Thalamus
Rates of glucose utilization for nuclei within the thalamus are shown in Table 3. Statistical analysis revealed a significant main effect of group (F(2,9) = 8.755, p < 0.01) and of brain region (F(10,9) = 15.443, p < 0.001). Further analysis using pre-planned multiple comparisons in specific nuclei showed that re-exposure to cocaine 30 days after cessation of cocaine self-administration resulted in decreased rates of glucose utilization, as compared to rates in food controls, throughout most nuclei of the thalamus that were analyzed in the current study (Table 3). Significantly lower rates were observed in anterior, (−19%); mediodorsal, (−21%); ventral anterior, (−23%); centromedian, (−27%); parafascicular, (−27%); and ventrolateral, (−17%) nuclei. In addition, rates of glucose utilization were significantly lower in the lateral habenula (−53%).
Re-exposure to cocaine 90 days after discontinuation of operant sessions also resulted in lower thalamic rates of glucose utilization as compared to rates in food controls in a very similar pattern to that following the 30 day time period. Significant decreases were observed in anterior (−39%), mediodorsal (−22%), ventral anterior (−35%), centromedian (−23%), and ventrolateral (−33%) nuclei, as well as the lateral habenula (−49%). In general, the magnitude of these reductions in glucose utilization were as great, if not greater, than those following re-exposure 30 days after cessation of self-administration, although differences between rates at the 30 and 90 day time points did not reach statistical significance.
Limbic system
Rates of glucose utilization for limbic-related brain regions including the amygdala, hypothalamus, and hippocampus are shown in Table 4. Statistical analysis revealed a significant main effect of group (F(2,9) = 17.893, p < 0.002) and of brain region (F(18,9) = 35.218, p < 0.0001). Further analysis using pre-planned multiple comparisons in specific nuclei showed that re-exposure to cocaine self-administration 30 days after the cessation of drug availability resulted in widespread decreases in rates of glucose utilization, as compared to rates in food controls. Within the amygdala (Figure 1) significant decreases, as compared to food controls, were observed in lateral (−29%), central (−33%), medial (−37%), basolateral (parvicellular, −35%; magnocellular, −27%) and accessory basal (−35%) nuclei (Table 4). Within those regions of the hypothalamus in which measurements were made, significant decreases were noted in the preoptic area (−32%), as well as lateral (−34%), dorsomedial (−31%), ventromedial (−34%), paraventricular (−28%) and posterior (−39%) regions. Finally, reduced rates of glucose utilization were seen in the CA1 (−27%; Figure 2) and CA3 fields (−26%), dentate gyrus (−27%) and subiculum (−26%) of the hippocampus, the lateral septum (−29%) and extended amygdala (−26%).
Re-exposure to cocaine 90 days after cessation of self-administration sessions also decreased rates of glucose utilization, as compared to rates in food controls, but in the majority of limbic brain regions the decreases were of much smaller magnitude. Significant reductions were restricted to the accessory basal (−24%) and both parvicellular (−26%) and magnocellular (−17%) basolateral nuclei of the amygdala (−26%), lateral septum (−23%) and the preoptic region of the hypothalamus (−24%). Although there were no statistically significant differences between rates of glucose utilization in groups re-exposed to cocaine 30 or 90 days after cessation of drug availability, the number of brain regions in which significant differences were observed was much smaller 90 days after cessation than 30 days (Table 5).
Correlations
Neither rate of responding or session length was significantly related to any measures of glucose utilization in the food control group. Rates of responding were also not related to any measures of glucose utilization in the cocaine self-administration group. However, rates of glucose utilization in the anterior caudate (+. 65) and the rostral (+.87) and shell (+.82) of the nucleus accumbens were positively correlated with session length. In addition, session length was negatively correlated with rates of glucose utilization in Area 13 of the prefrontal cortex (−.67). Correlations were significant at the p< 0.05 level corrected for multiple correlations.
4. Discussion
The findings of the present study in a nonhuman primate model of cocaine self-administration demonstrate that re-exposure to cocaine up to 3 months after cessation of cocaine availability continued to produce significant and substantial changes in brain functional activity, as reflected by local rates of glucose utilization measured with the 2DG method. In the majority of brain regions including prefrontal cortex, temporal cortex, striatum, thalamus and hippocampus, the functional response to cocaine was similar regardless of the duration (30 or 90 days) of the absence of drug availability. The intensity and pattern of these alterations in glucose utilization in response to cocaine re-exposure were very similar to those measured during chronic exposure without any interposed periods of drug unavailability (Porrino et al. 2004b; Beveridge et al. 2006). In fact, there was some suggestion of even greater responses to cocaine in areas such as the temporal cortex and thalamus, although none reached statistical significance. In contrast, there was evidence for amelioration of the effects of cocaine in the ventral striatum, limbic regions, and ventral midbrain where the degree of change appeared less intense or more restricted anatomically following longer periods after the cessation of self-administration. These data suggest that recovery from the effects of chronic cocaine use is not uniform across the brain and that the rate of recovery may be very different across systems with effects in some areas persisting well-beyond those in other systems. This may be particularly important when considering either pharmacological or behavioral interventions for cocaine dependence where different strategies at different durations of abstinence may improve outcomes.
The 2DG method measures functional activity as reflected by rates of glucose utilization which reflect the maintenance of cellular gradients, along with other energy-requiring processes such as neurotransmitter synthesis and processing, maintenance of ionic gradients, intracellular signaling cascades, and protein synthesis and trafficking. In the current study, this includes the sum total of both the immediate or acute effects of cocaine in combination with the accumulated consequences of previous drug experience and subsequent drug cessation. These effects cannot be attributed simply to the acute effects of cocaine since the significant effects of cocaine in the initial stage of self-administration (Porrino et al., 2002), as illustrated in Table 5 are of smaller magnitude and more restricted topographically than those in the current study. Thus, the profound functional response of the brain to cocaine up to 90 days after drug discontinuation underscores the persistence of the underlying neuroadaptations that result from chronic cocaine experience.
Neurobiological adaptations that may underlie the continued responsiveness to cocaine following discontinuation of use have been identified in rodent models of cocaine self-administration and relapse (for reviews see Loweth et al., 2014; Pickens et al., 2011; Wolf et al., 2010). These include alterations in BDNF levels in the ventral tegmental area, nucleus accumbens and amygdala (Ghitza et al. 2010; Grimm et al. 2003; Li and Wolf, 2011), enhanced ERK signaling in the central amygdala (Lu et al. 2005) and ventral prefrontal cortex (Koya et al. 2009), increases in cell surface AMPA receptors in the nucleus accumbens (Boudreau and Wolf, 2005; Conrad et al. 2008; McCutcheon et al. 2011), and decreases in glutamate concentrations induced by dysregulation of cystine-glutamate exchange (Baker et al. 2002, 2003), among many others. Similar adaptations on the molecular and cellular level may underlie the persistent metabolic alterations in response to cocaine seen on the systems level in the present study.
Though less is known about the nature of cocaine-induced neuroadaptations in nonhuman primates, significant alterations in the dopamine system have been reported (Beveridge et al. 2009; Bradberry, 2000; Farfel et al. 1992; Henry et al. 2009; Nader et al. 2002), and more recently changes in the glutamate system have been substantiated (Beveridge et al. 2011). Although these studies have only begun to explore the changes in primate species, they parallel the findings reported from rodent studies. While the majority of these studies have focused on the striatum and prefrontal cortex, the present study demonstrates that other systems are also affected – brain regions that have received less attention. Further work is needed to explore the structural and functional neuroadaptations that accompany the discontinuation of chronic drug use.
During cocaine self-administration, sessions ended only after monkeys received 30 injections of 0.3 mg/kg/injection cocaine, so session length was a dependent variable. For control monkeys, session length was approximately 90 min, while monkeys self-administering cocaine required 3 times as long to receive 30 reinforcers (Table 1). There are several hypotheses to account for this long session length – the monkeys may be titrating their cocaine dose or the increased session length is a result of the rate-decreasing effects of cocaine or both. It is important to note that tolerance did not develop to these effects over the 100 sessions of cocaine self-administration. After operant sessions were discontinued for 30 or 90 days, all animals were once again given access to 30 cocaine infusions. Session length did not significantly differ between the final three sessions before cessation and the re-exposure session. This suggests that compensatory adaptations, including sensitization to the behavioral effects of cocaine, did not develop in the absence of continued drug exposure. Interestingly, the pattern of responding under the FI schedule did appear sensitive to the re-exposure conditions, although QL values were still representative of schedule-controlled responding. Unfortunately, the experimental design did not allow for the assessment of whether tolerance developed to this effect (and the QL value increased over time).
Session length during the re-exposure session was significantly correlated with rates of glucose utilization in the nucleus accumbens and anterior caudate; the shorter the session length, the lower the rates of glucose utilization. The more rapid intake of cocaine is likely to lead to higher blood levels which in turn result in greater reductions in rates of glucose utilization. This would suggest that the effects on glucose utilization in the ventral and medial striatum are due to the direct pharmacological actions of cocaine. In contrast, session length was negatively correlated with rates of metabolism in the medial prefrontal cortex; the longer the session length, the lower the rates of metabolism. When high doses of cocaine are available, one could hypothesize that shorter session lengths represent less disruptive effects of cocaine and an “addictive phenotype”. Whether the relationship between behavior and cortical function (positive in the nucleus accumbens and negative in the PFC) represents a biomarker for vulnerability will require additional research.
Within the limbic system, alterations in functional activity in response to re-exposure to cocaine after drug cessation appeared shorter-lived than in cortex, dorsal striatum and thalamus. However, cocaine produced somewhat more widespread reductions in glucose utilization in the amygdala, hippocampus and hypothalamus 30 days after self-administration was discontinued when compared to rates measured without any interruption in cocaine availability or 90 days after cessation (Figure 1). These data suggest that these limbic-related areas may become particularly sensitive to the effects of cocaine early in the course of discontinuation of use, which coincides with a time when the rates of relapse in human addicts are especially high (Gawin, 1991; McKay et al. 1999; Sinha, 2001). This exaggerated responsiveness to the re-exposure to cocaine may indicate the neurobiological impact of the dynamic changes that occur within these regions during this time frame. Further, our data suggest that this sensitivity may diminish with longer periods of discontinuation of use, in that there was a trend towards an amelioration of the functional effects of cocaine within the limbic system when measured after a longer period of 90 days. The dynamic nature of the functional response to cocaine after cessation of chronic cocaine use is also consistent with alterations in the dopamine system following cessation of drug exposure in a similar model of cocaine exposure in nonhuman primates. In these studies, concentrations of dopamine transporters and dopamine D1 receptors were shown to be elevated above those seen during ongoing drug exposure, but approached baseline control levels over a similar timeframe of unavailability of cocaine (Beveridge et al. 2009). It is possible that these fluctuations in dopamine regulation may contribute, in part, to the increased responsiveness to cocaine during this early phase of discontinuation of drug use.
The current data, however, are in contrast to a recent study examining the effects of cessation of cocaine self-administration on rates of glucose utilization measured with in vivo positron emission tomography (PET) procedures (Henry et al. 2010). In that study, the non-contingent administration of cocaine to monkeys four weeks after their last self-administration session produced only very limited brain activation in the anterior cingulate, in contrast to the very broad pattern of activation observed prior to cessation of operant sessions. The inconsistencies in the outcomes between this and the current study can be attributed to a number of key differences in procedures including the use of PET vs. autoradiography (with its higher resolution), differences in drug histories, route of administration, and amount of drug administered in the final session. But perhaps most important was the environment in which the mapping occurred. Monkeys in the PET study received a single intramuscular injection of cocaine in their home cages prior to imaging, whereas in the current study tracer uptake occurred in the chambers in which animals had a long history of self-administration and followed completion of the experimental session. The presence of drug-associated cues and the contextual environment may have resulted in the more widespread effects of cocaine and the similarity of this pattern of functional effects to that seen prior to drug cessation.
In summary, the present data utilizing a nonhuman primate model of cocaine self-administration demonstrate that re-exposure to cocaine 30 or 90 days after the cessation of drug access continues to produce significant alterations in brain functional activity. In many regions, including dorsal striatum, prefrontal and temporal cortex, as well as the thalamus, these effects were largely unchanged by the duration of time since prior access to cocaine, suggesting the persistence of neuroadaptations resulting from chronic cocaine use. In contrast, in limbic regions as well as the ventral striatum, the magnitude and/or anatomical extent of cocaine’s effects were lessened with longer periods of time since cessation of drug use. Recovery, then, is likely to progress more rapidly in some systems than others. The relative stability of the functional response to cocaine, particularly in cortical areas, suggests that some of the neuroadaptations that are produced by chronic drug exposure endure well after the cessation of drug use. However, it is apparent that recovery can occur, but this is likely to be a highly dynamic process that emerges over multiple time courses involving numerous systems.
Highlights.
Re-exposure to cocaine after self-administration cessation was studied in NHPs.
Cocaine continued to produce significant changes in functional brain activity.
There was some evidence of recovery 90 days after cessation of drug exposure
The time course was different in limbic as compared to cortico-striatal systems.
Acknowledgments
Funding for this study was provided by NIDA grants DA09085 and DA06634. The authors thank Tonya Calhoun for excellent technical assistance.
Footnotes
Author Contribution
MAN and LJP were responsible for the study concept and design. TJRB and HRS contributed to the acquisition of the data. All authors assisted with the data analysis and interpretation of findings. TJRB and LJP drafted the manuscript and HRS, SHN and MAN provided critical revision of the manuscript for important intellectual contact. All authors critically reviewed content and approved final version for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas J.R. Beveridge, Email: tbeverid@wakehealth.edu.
Hilary R. Smith, Email: hsmith@wakehealth.edu.
Susan H. Nader, Email: snader@wakehealth.edu.
Michael A. Nader, Email: mnader@wakehealth.edu.
References
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. Wiley-Liss; New York: 1992. [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berry J, van Gorp WG, Herzberg DS, Hinkin C, Boone K, Steinman L, Wilkins JN. Neuropsychological deficits in abstinent cocaine abusers: preliminary findings after two weeks of abstinence. Drug Alcohol Depend. 1993;32:231–237. doi: 10.1016/0376-8716(93)90087-7. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobe of nonhuman primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Group II metabotropic glutamate receptors in the striatum of non-human primates: dysregulation following chronic cocaine self-administration. Neurosci Lett. 2011;496:15–19. doi: 10.1016/j.neulet.2011.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Dansky BS, Carrigan MH, Brady KT. Acute and protracted cocaine abstinence in an outpatient population: a prospective study of mood, sleep and withdrawal symptoms. Drug Alcohol Depend. 2000;59:277–286. doi: 10.1016/s0376-8716(99)00126-x. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: Abstract reasoning, motor programming, and cognitive flexibility. Addict Behav. 2010;35:875–881. doi: 10.1016/j.addbeh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992;578:235–243. doi: 10.1016/0006-8993(92)90252-5. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Lull ME, Patel KM, Brucklacher RM, Morgan D, Roberts DC, Vrana KE. Gene expression changes in the medial prefrontal cortex and nucleus accumbens following abstinence from cocaine self-administration. BMC Neurosci. 2010;11:29. doi: 10.1186/1471-2202-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zahi H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Henry PK, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010;4:212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning RI, Glover BJ, Koeppl B, Weddington W, Jaffe JH. Cognitive deficits in abstaining cocaine abusers. NIDA Res Monogr. 1990;101:167–178. [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L. Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol. 1978;4:293–301. doi: 10.1002/ana.410040402. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, Swann AC, Moeller FG. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010;178:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lane SD, Moeller FG, Steinberg JL, Buzby M, Kosten TR. Performance of cocaine dependent individuals and controls on a response inhibition task with varying levels of difficulty. Am J Drug Alcohol Abuse. 2007;33:717–726. doi: 10.1080/00952990701522724. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76:287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Qin Y, Pablo J. Norepinephrine transporter immunoblotting and radioligand binding in cocaine abusers. J Neurosci Methods. 2005;143:79–85. doi: 10.1016/j.jneumeth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, Mulvaney FD, Koppenhaver JM. Predicting proximal factors in cocaine relapse and near miss episodes: clinical and theoretical implications. Drug Alcohol Depend. 1999;56:67–78. doi: 10.1016/s0376-8716(99)00013-7. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2000. [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Smith HR, Nader MA. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004a;27:813–820. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004b;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148:1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- Schuier F, Orzi F, Suda S, Lucignani G, Kennedy C, Sokoloff L. Influence of plasma glucose concentration on lumped constant of the deoxyglucose method: effects of hyperglycemia in the rat. J Cereb Blood Flow Metab. 1990;10:765–773. doi: 10.1038/jcbfm.1990.134. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Suda S, Shinohara M, Miyaoka M, Lucignani G, Kennedy C, Sokoloff L. The lumped constant of the deoxyglucose method in hypoglycemia: effects of moderate hypoglycemia on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1990;10:499–509. doi: 10.1038/jcbfm.1990.92. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology (Berl) 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]