Abstract
Purpose
Cyclooxygenase-2 (COX-2) has been reported to be ubiquitously expressed in Wilms tumor, the most common malignant renal tumor in children. However, the regulation mechanism of COX-2 expression remains unexplored.
Materials and Methods
Quantitative real-time PCR and western blot analysis were performed to detect COX-2 mRNA and protein expression in WiT49 cells upon the stimulation by sphingosine-1-phosphate (S1P) as well as S1P2 and COX-2 mRNA expression in 10 fresh frozen Wilms tumor tissues and their matched normal tissues. Overexpression, blockade and downregulation of S1P2 were performed using adenoviral transduction, S1P2 antagonist JTE-013 and siRNA transfection, respectively. The level of prostaglandin E2 (PGE2) in WiT49 cells was determined by gas chromatography/mass spectrometry.
Results
S1P induced COX-2 mRNA and protein expression in WiT49 cells in a concentration-dependent manner. Overexpression of S1P2 in WiT49 cells led to a significant increase in COX-2 mRNA and protein expression as well as subsequent PGE2 synthesis. In addition, pretreatment of those cells overexpressing S1P2 with S1P2 selective antagonist JTE-013 completely blocked S1P-induced COX-2 protein expression. In accordance with these results, silencing of S1P2 in WiT49 cells downregulated S1P-induced COX-2 expression. Further research on 10 Wilms tumor specimens found that S1P2 mRNA was greatly increased in Wilms tumor.
Conclusions
S1P induced COX-2 expression in Wilms tumor, and this effect was mediated by S1P2. This finding extends the biological function of S1P2 and provides the biochemical basis for the development of inhibitors targeting S1P/COX-2 signaling pathway.
Keywords: COX-2, sphingosine 1-phosphate, sphingosine 1-phosphate receptor 2, WiT49, Wilms tumor
Introduction
Cyclooxygenase (COX) is a key, rate-limiting enzyme that converts arachidonic acid into prostaglandins (PGs). Two isoforms of COX enzymes have been characterized: COX-1, which is constitutively expressed in most mammalian tissues,1 and COX-2, which is induced by various factors including mitogens, hormones, serum and cytokines.2 Interestingly, COX-2 has been found to be overexpressed in various human cancers. It plays a crucial role in carcinogenesis through synthesis of PGs which stimulate PGs receptors with subsequent enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis and suppression of immune responses.3 With the successful use of celecoxib, the first approved selective COX-2 inhibitor, to reduce the formation of colorectal polyps in patients with familial adenomatous polyposis and inhibit experimentally-induced tumorigenesis in several animal models,4 COX-2 was proposed to be a potential molecular target for cancer chemoprevention.
Sphingosine-1-phosphate (S1P) is an important bioactive lipid that exerts a wide variety of cellular functions via interaction with five G protein-coupled receptors (named S1P1–5).5 Many studies have shown that S1P and its receptors are involved in diverse processes such as angiogenesis, cardiac development, neuronal survival, immunity and recently carcinogenesis.6, 7 Interestingly, S1P has been shown to induce COX-2 expression in some cell types.8–13 In addition, Lee et al. found that COX-2 was ubiquitously expressed in human primary Wilms tumors and the specific COX-2 inhibitor SC-236 suppressed tumor growth and inhibited tumor angiogenesis in an orthotopic xenograft model.14 These findings drove us to investigate the role of S1P regulation of COX-2 expression in Wilms tumor.
In this study, we aimed to study the regulation of COX-2 by S1P in WiT49 cells, a well-characterized Wilms tumor cell line,15 and to determine which S1P receptor was responsible for this effect. In addition, we studied the expression of COX-2 and its related S1P receptor in 10 Wilms tumor specimens.
Materials and Methods
Cell culture, siRNA transfection and adenoviral transduction
WiT49 cells were cultured as described previously.15 Transfection of small interfering RNA (siRNA) oligonucleotide duplexes to block S1P2 expression was done using Oligofectamine reagent (Invitrogen) according to manufacturer’s instructions. The sequences of siRNA (Dharmacon) were as follows: UACCUUGCUCUCUGGCUCU (S1P2 siRNA); UUCUCCGAACGUGUCACGUUU (NS siRNA). For adenoviral transduction, cells were infected with adenovirus containing GFP, S1P1 or S1P2 for 16–24 h (100 multiplicity of infection) before different assays were done.
Quantitative real-time PCR
Total RNA was isolated from WiT49 cells treated with S1P (Biomol) under different conditions or Wilms tumor tissues using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. Then cDNA was generated from 1 μg RNA in the presence of random hexamer primers, deoxynucleoside triphosphates and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Primers were designed using Primer Express™ 2.0 (Applied Biosystems) according to the software guidelines. Sequences were as follows: 5′-GTG CAA CAC TTG AGT GGC TAT-3′ (forward) and 5′-AGC AAT TTG CCT GGT GAA TGA T-3′ (reverse) for the COX-2 gene, 5′-GGC CTA GCC AGT TCT GAA AGC -3′ (forward) and 5′-GCG TTT CCA GCG TCT CCT T-3′ (reverse) for the S1P2 gene, 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse) for the GAPDH gene and 5′-GACAGGATGCAGAAGGAGATTACT-3′ (forward) and 5′-TGATCCACATCTGCTGGAAGGT-3′ (reverse) for the β-Actin gene. Real-time PCR was performed using SYBR Green I DNA binding dye technology on an ABI Prism 7900 HT Sequence Detection System (PE Applied Biosystems). Results were expressed relative to the internal control gene β-Actin or GAPDH.
Western blot analysis
WiT49 cells were treated with S1P with or without JTE-013 (Tocris Bioscience) under different conditions. Then they were washed with ice-cold PBS and homogenized in RIPA buffer. Samples were centrifuged at 14,000 g for 20 min at 4 °C, and protein concentrations of supernatants were determined by BCA protein assay Kit (Pierce). Equal amounts of protein were separated on 8% SDS-PAGE and blotted to nitrocellulose membranes. The membranes were incubated with the indicated primary antibodies (anti-COX-2 and anti-β-Actin, both from Santa Cruz), followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was visualized by exposure to X-ray film using Pierce ECL Western Blotting Substrate, according to the manufacturer’s instructions.
Prostaglandin E2 (PGE2) analysis
The level of PGE2 in cell pellets was quantified by a highly precise and accurate assay employing gas chromatography/mass spectrometry utilizing stable isotope dilution methodology as described.16
Statistical Analysis
All experiments on cell lines were performed at least twice on separate occasions. The data are presented as means ± SD from a representative experiment. The statistical significance of differences between two groups was determined by Student’s t test using Microsoft Excel software.
Results
S1P induced COX-2 expression in WiT49 cells
Previous reports have indicated that S1P signaling induces COX-2 expression. However, little is known about this pathway in Wilms tumor. Therefore, WiT49, a well-characterized Wilms tumor cell line,15 was utilized. After treatment of WiT49 cells with different concentrations of S1P for 2 h, quantitative real-time PCR analysis showed that S1P induced COX-2 mRNA expression in a concentration-dependent manner with the maximal effect observed at 100 nM (fig. 1, A). Western blot analysis further confirmed the increase of COX-2 expression by S1P on the protein level (fig. 1, B). However, using FTY720 phosphate (FTY720-P), an agonist for all S1P receptors except S1P2, we did not see such an induction (data not shown), which suggested that this effect might be mediated by S1P2.
Fig. 1.
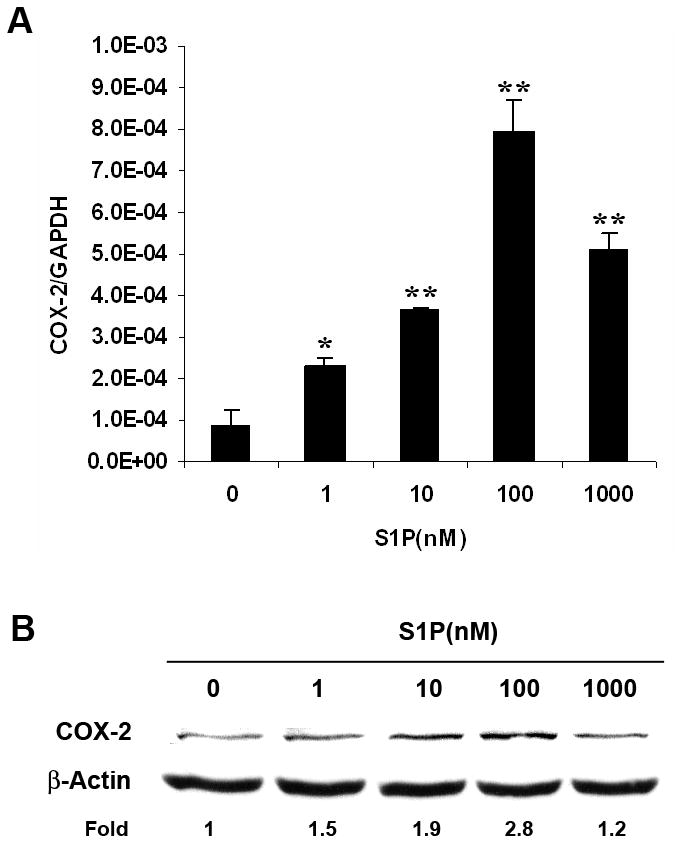
S1P induced COX-2 expression in WiT49 cells. WiT49 cells were serum starved for 24 h and then treated with different concentrations of S1P for 2 h before quantitative real-time PCR (A) and western blot analysis (B) were done. The mRNA expression of COX-2 was normalized to that of GAPDH and presented as means ± SD. *, P<0.05, **, P<0.01 versus without S1P.
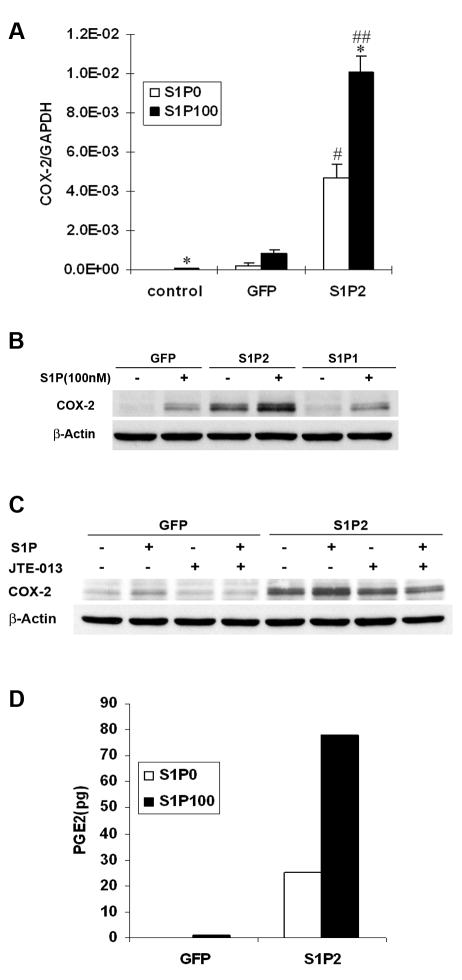
Overexpression of S1P2 increased S1P-induced COX-2 expression and PGE2 synthesis in WiT49 cells
To prove this notion, we overexpressed S1P2 in WiT49 cells by adenoviral transduction. Consistent with our hypothesis, overexpression of S1P2 into WiT49 cells dramatically increased the expression level of COX-2 mRNA and a further increase was seen with S1P stimulation by quantitative real-time PCR analysis (fig. 2, A). Western blot analysis further confirmed this result on COX-2 protein level while overexpression of S1P1 had no such effect compared to that of GFP control cells (fig. 2, B). In addition, pretreatment of WiT49 cells overexpressing S1P2 with S1P2 selective antagonist JTE-013 17 completely blocked S1P-induced COX-2 expression (fig. 2, C).
Fig. 2.
Overexpression of S1P2 increased COX-2 and PGE2 synthesis in WiT49 cells. WiT49 cells were infected with adenovirus containing GFP, S1P2 or S1P1 with MOI 100. After 16–24 h, cells were serum starved for 24 h and then stimulated with 100 nM S1P for 2 h before quantitative real-time PCR (A) and western blot analysis (B) were done. *, P<0.05 versus without S1P; #, P<0.05, ##, P<0.01 versus corresponding GFP control. C, WiT49 cells overexpressing S1P2 or GFP were serum starved and pretreated with 1 μM JTE-013 for 30 min before stimulation with 100 nM S1P for another 2 h. Then western blot analysis was done. D, WiT49 cells were seeded at the cell density of 1x106 cells per dish (60 mm). After attachment, they were infected with adenovirus containing S1P2 or GFP with MOI 100 for 16 h followed by serum starvation for another 24 h. Then the cells were treated with 100 nM S1P for 2 h and collected for PGE2 analysis. Data shown are representative of two independent experiments.
Prostaglandin E2 (PGE2) is the major prostaglandin product of COX-2 enzyme in many human tumors.3 Functional assay measuring PGE2 synthesis showed that cells overexpressing S1P2 contained relatively abundant PGE2 compared to GFP control cells in which the basal level of PGE2 is undetectable in our assay system. Consistent with the data on COX-2 mRNA and protein, stimulation with S1P further increased PGE2 synthesis in S1P2-overexpressing cells. Moreover, it resulted in the amount of PGE2 in GFP control cells becoming detectable (fig. 2, D).
Downregulation of S1P2 reduced S1P-induced COX-2 expression in WiT49 cells
To further confirm the role of S1P/S1P2 signaling on COX-2 expression, we used siRNA technology to downregulate S1P2 expression in WiT49 cells. Quantitative real-time PCR analysis proved that the siRNA against S1P2 was significantly effective in reducing the mRNA level of S1P2 (fig. 3, A). Treatment of WiT49 cells with this S1P2 siRNA potently inhibited S1P-induced COX-2 mRNA and protein expression (fig. 3, B and C). Taken together, all the above data clearly demonstrated that S1P-induced COX-2 expression was mediated by S1P2.
Fig. 3.
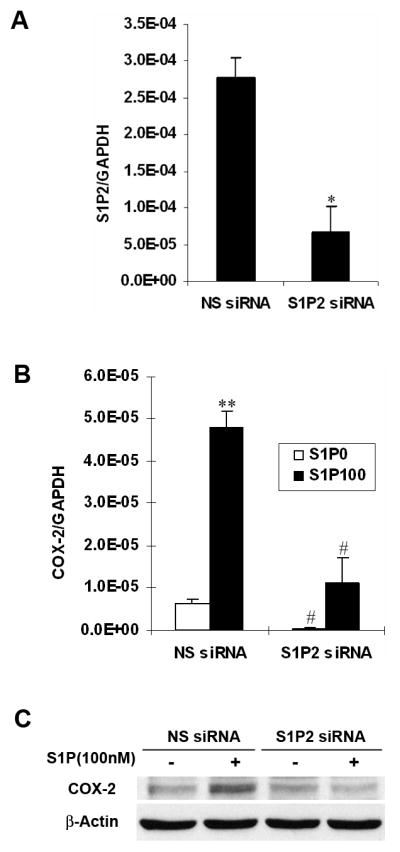
Downregulation of S1P2 reduced S1P-induced COX-2 expression in WiT49 cells. A, WiT49 cells were transfected with 100 nM S1P2 siRNA or NS siRNA, harvested 48 h later and assayed for the level of S1P2 mRNA by quantitative real-time PCR. *, P<0.05 versus NS siRNA. B and C, After transfection with S1P2 siRNA for 24 h, WiT49 cells were serum starved for 24 h and then stimulated with 100 nM S1P for another 2 h before quantitative real-time PCR (B) and western blot analysis (C) were done. **, P<0.01 versus without S1P; #, P<0.05, ##, P<0.01 versus corresponding NS siRNA control.
S1P2 mRNA was increased in Wilms tumor
Having shown the role of S1P2 in S1P-induced COX-2 expression in vitro and based on the finding that COX-2 was extensively expressed in Wilms tumor,14, 18 we were interested in knowing whether this pathway also exists in vivo. Therefore, quantitative real-time PCR analysis was performed to detect the expression level of COX-2 and S1P2 mRNA in 10 Wilms tumor specimens. Compared to their matched normal tissues, COX-2 mRNA was slightly increased in Wilms tumor specimens while S1P2 mRNA was greatly increased (P<0.01; fig. 4), which indicated that S1P/S1P2/COX-2 pathway may also exist in vivo.
Fig. 4.
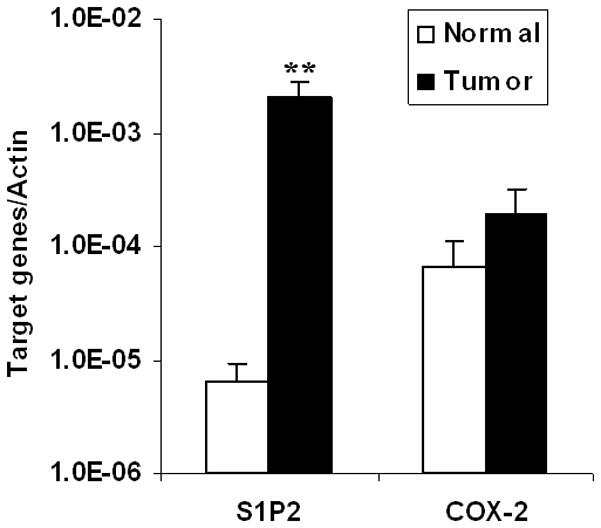
Relative mRNA expression of S1P2 and COX-2 in Wilms tumor and their matched normal tissues. Quantitative real-time PCR was performed in 10 Wilms tumor tissues and their matched normal tissues. The mRNA expression of S1P2 and COX-2 was normalized to that of the housekeeping gene β-Actin and presented as means ± SE from 10 samples..**, P<0.01 versus that of their matched normal tissues.
Discussion
Wilms tumor is the most common malignant renal tumor in children. Although it has a relatively high cure rate, which is achieved by surgery, chemotherapy and radiotherapy, some children with tumors that harbor adverse biologic features still succumb to their disease.19 Moreover, current therapies are usually associated with significant late sequelae. To date, our knowledge of the mechanisms leading to Wilms tumor progression and metastasis is limited. Therefore, a better understanding of the stimuli and signaling pathways involved in Wilms tumor progression is needed in order to develop future therapeutic strategy.
Recently, S1P signaling has been reported to induce COX-2 expression in different cell types.8–13 However, it is unknown whether this effect also exists in human cancers, such as Wilms tumor. We detected the effect of S1P on COX-2 expression in WiT49 cells and found that S1P induced COX-2 mRNA and protein expression concentration-dependently (fig. 1). S1P displays diverse cellular functions by interaction with its five specific receptors S1P1–5, in which S1P1 mainly couples Gi protein while S1P2 couples G12/13 protein.5 In rheumatoid arthritis synoviocytes, Kitano et al. found that S1P-induced COX-2 expression was sensitive to pertussis toxin, an inhibitor of the Gi protein,11 in accordance with Kim et al.’s findings in human amnion-derived WISH cells.10 However, in mouse embryonic fibroblast cells, S1P-induced COX-2 expression was specifically regulated by Gα12.9 These findings indicated that S1P-induced COX-2 expression might be cell type-specific.
To delineate which S1P receptor was responsible for S1P-induced COX-2 expression in Wilms tumor, we used different approaches. FTY720-P, an S1P analogue that binds all S1P receptors except S1P2, could not induce COX-2 expression suggesting that this effect might be mediated by S1P2 signaling. Further, overexpression or downregulation of S1P2 expression either increased or decreased COX-2 mRNA and protein expression confirming that S1P/S1P2 signaling was required for COX-2 induction (figs. 2 and 3). In addition, the specific S1P2 antagonist JTE-013 completely blocked S1P-induced COX-2 expression in cells overexpressing S1P2 (fig. 2, C), further confirming the requirement of S1P2 in COX-2 induction by S1P. PGE2 is the principle metabolite of COX-2 enzyme. As expected, the cells overexpressing S1P2 produced much more PGE2 than GFP control cells. And stimulation with S1P led to a further increase (fig. 2, D). Taken together, these data clearly demonstrate that S1P2 was responsible for S1P-induced COX-2 expression and its downstream molecule PGE2 synthesis in vitro.
Previous reports have shown that COX-2 was ubiquitously expressed in human Wilms tumor.14, 18 Consistent with these findings, our study on 10 Wilms tumor specimens also showed that COX-2 mRNA was extensively expressed in Wilms tumor specimens. Interestingly, we also found that among the four S1P receptors (S1P1–3,5) that Wilms tumor expressed (data not shown), S1P2 was significantly upregulated in Wilms tumor, which indicate that S1P/S1P2/COX-2 pathway may also have physiopathological relevance in vivo.
In summary, our study for the first time clearly demonstrated that S1P induced COX-2 expression and subsequent PGE2 synthesis in WiT49 cells, and this effect was mediated by S1P2, which extends the biological function of S1P2 in human cancers and provides the biochemical basis for the development of inhibitors targeting S1P/COX-2 signaling pathway. In previous studies S1P2 was usually regarded as a negative modulator in tumor progression.20 Here for the first time we suggest that S1P2 might act as a positive tumor modulator and thus promote tumor progression. Further work in other human cancers will allow us to better understand the role of S1P2 in tumor progression.
Conclusions
This study for the first time clearly demonstrated that S1P induced COX-2 expression and subsequent PGE2 synthesis in WiT49 cells, and this effect was mediated by S1P2, which extends the biological function of S1P2 in human cancers and provides the biochemical basis for the development of inhibitors targeting S1P/COX-2 signaling pathway.
Acknowledgments
This work was supported by the Seraph Foundation (to F.F.), NIH grants k08DK070468A (to F.F.) and GM15431, DK48831, ES13125 (to J.D.M.). We thank Dr. Herman Yeger for the WiT49 cell line, Novartis Pharma for FTY720-P and the Children’s Oncology Group Biopathology Center for the Wilms tumor specimens.
Abbreviations
- COX
cyclooxygenase
- PGs
prostaglandins
- S1P
sphingosine-1-phosphate
- FTY720-P
FTY720-phosphate
- siRNA
small interfering RNA
- NS
non-specific
- PGE2
prostaglandin E2
References
- 1.O’Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:156. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JA, Belvisi MG, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall J, et al. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol. 1994;113:1008. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telliez A, Furman C, Pommery N, Hénichart JP. Mechanisms leading to COX-2 expression and COX-2 induced tumorigenesis: topical therapeutic strategies targeting COX-2 expression and activity. Anticancer Agents Med Chem. 2006;6:187. doi: 10.2174/187152006776930891. [DOI] [PubMed] [Google Scholar]
- 4.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 6.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 7.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol. 2006;207:757. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- 9.Ki SH, Choi MJ, Lee CH, Kim SG. Galpha12 specifically regulates COX-2 induction by sphingosine 1-phosphate. Role for JNK-dependent ubiquitination and degradation of IkappaBalpha. J Biol Chem. 2007;282:1938. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- 10.Kim JI, Jo EJ, Lee HY, Cha MS, Min JK, Choi CH, et al. Sphingosine 1-phosphate in amniotic fluid modulates cyclooxygenase-2 expression in human amnion-derived WISH cells. J Biol Chem. 2003;278:31731. doi: 10.1074/jbc.M300625200. [DOI] [PubMed] [Google Scholar]
- 11.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- 12.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. Faseb J. 2003;17:1411. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 13.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee A, Frischer J, Serur A, Huang J, Bae JO, Kornfield ZN, et al. Inhibition of cyclooxygenase-2 disrupts tumor vascular mural cell recruitment and survival signaling. Cancer Res. 2006;66:4378. doi: 10.1158/0008-5472.CAN-05-3810. [DOI] [PubMed] [Google Scholar]
- 15.Alami J, Williams BR, Yeger H. Derivation and characterization of a Wilms’ tumour cell line, WiT 49. Int J Cancer. 2003;107:365. doi: 10.1002/ijc.11429. [DOI] [PubMed] [Google Scholar]
- 16.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 18.Fridman E, Pinthus JH, Kopolovic J, Ramon J, Mor O, Mor Y. Expression of cyclooxygenase-2 in Wilms tumor: immunohistochemical study using tissue microarray methodology. J Urol. 2006;176:1747. doi: 10.1016/j.juro.2006.03.118. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich PF. Wilms tumor: progress to date and future considerations. Expert Rev Anticancer Ther. 2001;1:555. doi: 10.1586/14737140.1.4.555. [DOI] [PubMed] [Google Scholar]
- 20.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]