Abstract
The antennal lobe (AL) is the primary olfactory center in insect brains. It receives sensory input from the olfactory sensory neurons (OSNs) and sends, through its projection neurons (PNs), reformatted output to secondary olfactory centers, including the mushroom body (MB) calyx and the lateral horn (LH) in the protocerebrum. By injecting dye into the AL of wild-type Drosophila, we identified previously unknown direct pathways between the AL and the ventrolateral, superior medial, and posterior lateral protocerebra. We found that most of these areas in the protocerebrum are connected with the AL through multiple tracts, suggesting that these areas are sites of convergence for olfactory information. Furthermore, areas such as the superior medial protocerebrum now appear to receive olfactory output both directly from the AL and indirectly from lobes of the MB and the LH, suggesting a degree of functional interaction among these areas. We also analyzed the length and number of fibers in each tract. We compare our results obtained from wild-type flies with recent results from transgenic strains and discuss how information about odorants is distributed to multiple protocerebral areas.
INDEXING TERMS: olfactory circuit, olfaction, mushroom body, lateral horn, antennal lobe
In insects and vertebrates, olfactory information is distributed not only by serial, direct routes from one brain area to another but also by multiple, parallel tracts that interconnect numerous brain areas (Strausfeld, 1976; Shepherd and Greer, 1998; Haberly, 1998; Chiang et al., 2011). Information about odorants begins moving through these tracts when volatile chemicals are detected by olfactory sensory neurons (OSNs) housed in peripheral olfactory sensory organs. Each OSN projects an axon to the primary olfactory center, the antennal lobe (AL) in insects or the olfactory bulb in vertebrates. There, OSNs synapse within spherical neuropils called glomeruli, which number in different species from tens to thousands, each of which receives input from a specific type of OSN defined by its receptor. Within the glomeruli, input from OSNs is transmitted to local interneurons (LNs) and projection neurons (PNs; for review see Bargmann, 2006). Olfactory information is then processed and reformatted in several ways by these neural circuits (Kay and Stopfer, 2006; Raman et al., 2010) and is then transferred to secondary olfactory areas, including the mushroom body (MB) calyx and lateral horn (LH) in insects or the piriform cortex, amygdala, and entorhinal cortex in vertebrates.
In insects, multiple tracts of PN axons exit the AL to transfer olfactory information to secondary olfactory sites. Medial, mediolateral, and lateral tracts have all been identified in hymenoptera, diptera, dictyoptera, and lepidoptera (Strausfeld, 1976; Homberg et al., 1988; Stocker et al., 1990; Malun et al., 1993; Müller et al., 2002; Kirschner et al., 2006; Rø et al., 2007; Zube et al., 2008). Most PNs traversing these tracts converge onto the MB calyx and the LH, but some PNs target other protocerebral areas. In hymenoptera, for example, the lateral network is innervated by mediolateral PNs (Kirschner et al., 2006; Zube et al., 2008). In flies, the posterior lateral and superior medial protocerebra also receive input from a few PNs (Strausfeld, 1976; Marin et al., 2002; Lai et al., 2008).
Recent genetic labeling methods have shown that some PNs target a specific region selectively in the secondary sites in Drosophila (Marin et al., 2002; Wong et al., 2002; Tanaka et al., 2004, 2008; Jefferis et al., 2007; Lin et al., 2007). In these studies, two medial and mediolateral fiber bundles, the inner and middle antenno-cerebral tracts (iACT and mACT), have been investigated most intensively, largely because, fortuitously, PNs within these bundles could be labeled in a genetically manipulated fly (GAL4 driver GH146; Marin et al., 2002; Jefferis et al., 2007; Wong et al., 2002). However, Golgi impregnations have revealed additional tracts (such as an mACT running through the pedunculus to the MB calyx; Stocker et al., 1990) that have not been labeled by GAL4 lines. Thus, additional unidentified pathways from the AL to other brain areas might still exist.
To identify possible additional antennal lobe tracts (ALTs), we used a dye-labeling strategy. We mass-injected dextran compounds into the AL of wild type Drosophila and visualized neuronal tracts between the AL and protocerebrum. In addition to labeling the three major ACTs (inner, middle, and outer) described by Stocker et al. (1990), we identified novel mediolateral tracts and new secondary olfactory sites, including the ventrolateral protocerebrum. We also identified fibers emerging from these major tracts and converging upon the posterior lateral protocerebrum (PLP) and ring neuropil in the superior medial protocerebrum.
Additionally, we compared each tract by measuring the length and the number of fibers. The resulting, more complex view of the olfactory system suggests additional olfactory operations that can now be tested.
MATERIALS AND METHODS
Confocal imaging of antennal lobe tracts
We used female Canton S flies and reared them on Jazz-mix Drosophila food (Fisher Scientific, Fair Lawn, NJ) in a box whose temperature was maintained at 23°C or 25 °C and humidity at more than 50%.
We anesthetized flies by holding them in plastic vials on ice until they were motionless, for less than 1 minute, and then restrained them in a plastic chamber with wax and epoxy (Tanaka et al., 2009). Drosophila saline (in mM: NaCl 103, KCl 3, TES 5, trehalose 10, glucose 10, sucrose 7, NaHCO3 26, NaH2PO4 1, CaCl2 1.5, MgCl2 4, adjusted to 280 mOsm with sucrose and pH 7.25 with HCl) was applied over the top of the fly body, and a window was opened in the top of the head. After removing fat and air sacs above the brain, we used forceps to gently remove the perineural sheath around the AL. We prepared dextran conjugated with tetramethylrhodamine and biotin (D-7162; Molecular Probes, Eugene, OR) dissolved in water, saline, or goat serum and dried on the tip of a forceps or a tungsten needle sharpened in KNO3. We then jabbed the AL several times with the forceps or needle. Twenty to thirty minutes after this injection, we dissected out the brain within the saline and fixed it in 4% paraformaldehyde/PBS (pH 7.4) for 50 minutes. After washing the brain in 0.2% Triton-X/PBS, we incubated it in Alexa Fluor 568-conjugated streptavidin (S-11226; Molecular Probes; 1 mg/l diluted in 0.2% Triton-X/PBS) in mouse monoclonal nc82 antibody (a gift from Erich Buchner and Alois Hofbauer, diluted at 1:10 in 10% goat serum/0.2% Triton-X/PBS), and in goat anti-mouse IgG conjugated with Alexa Fluor 633 (A21050; Molecular Probes; diluted at 1:200 in 10% goat serum/0.2% Triton-X/PBS), with each step done at 4°C overnight. Finally, we washed the brain in PBS and mounted it in 50% glycerol/PBS. We used the nc82 antibody, which recognizes the ubiquitously expressed active zone protein Bruchpilot (Wagh et al., 2006), to provide visible landmarks within the brain. This commonly used antibody is an IgG produced by a hybridoma clone from a large library generated against Drosophila heads (Hofbauer, 1991) and forms protein bands of 190 and 170 kDa in the Western blots of homogenized Drosophila heads. The immunoreactive signal disappears in the tissue as well as in the Western blots if bruchpilot gene is knocked down, and an additional band is detected specifically if GFP-tagged Bruchpilot is expressed in a pan-neuronal manner (Wagh et al., 2006). We saw no labeling when Alexa Fluor 633-conjugated secondary antibody was applied without the primary antibody.
To label cholinergic neurons, we fixed the brains and soaked them in mouse monoclonal anti-choline acetyltransferase (ChAT) antibody (diluted at 1:500) as described above. The anti-ChAT antibody is an IgG produced by a hybridoma clone made from mice immunized with recombinant dChAT fusion protein (Takagawa and Salvaterra, 1996). This antibody recognizes a protein band positioned at 80 kDa in Western blots of crude fly head samples. ChAT gene mutant shows reduced intensity of labeling of the band compared with the wild type (Takagawa and Salvaterra, 1996). For the secondary antibody, we used goat anti-mouse IgG conjugated with Alexa Fluor 568 (A-11004; Molecular Probes), which does not stain the brain without the primary antibody.
We made confocal serial optical sections of the whole-mount brain samples in 1.0-μm steps with a Zeiss LSM 510 inverted Meta equipped with oil-immersion ×40 Plan-Neofluar (n.a. = 1.3) or dry ×20 Plan-Apochromat (n.a. = 0.75) and reconstructed the image series in three-dimensions (3D) in Volocity 4 (Improvision) or LSM5 image browser (Zeiss). We processed the contrast, size, and resolution of the images in Adobe Photoshop 8.0.
Electron micrography
Brains of female Drosophila were fixed in 1% or 2% glutaraldehyde/PBS for 1 hour. The brains were washed in PBS and fixed in 1% osmium tetroxide in distilled water or 0.2% osmium tetroxide in Sorenson’s buffer for 1 hour. The brains were then rinsed and incubated in 0.5% aqueous uranyl acetate in ice for 40 minutes or in 0.1 M acetate buffer in ice for 20 minutes and dehydrated in ethanol. The brains were incubated in propylene oxide and then in epon. The epon was polymerized for 3 days in an oven set at 60–70°C. Ultrathin sections were cut at 70 or 95 nm thickness on an ultramicrotome (Reichert Ultracut E or Leica EM UC6), mounted on LUXFilm TEM films (12812-CU; Ted Pella, Redding, CA) or copper grids (2010-Cu; VECO), and poststained with 2% uranyl acetate (Polysciences, Warrington, PA) in 50% methanol for 5 minutes and Reynolds lead citrate for 5 minutes. Images were obtained with a Jeol 1010 transmission electron microscope. Thick sections (1 μm) made in series with ultrathin sections were stained with toluidine blue to confirm the orientation and depth of sections and to trace the ALT.
Data analyses
We measured the lengths of neural tracts in confocal stacks in Neurolucida 8 (MicroBrightField, Williston, VT) with z-axis distance multiplied by 0.93 to account for the refractive index of oil immersion and 50% glycerol/PBS mounting solution. The areas of fibers were measured using MetaMorph (Molecular Devices, Sunnyvale, CA).
Nomenclature
We assigned names to each olfactory tract in accordance with nomenclature guidelines recommended by the BrainName working group (Ito et al., in preparation). The inner, middle, and outer antennocerebral tracts are here called medial, mediolateral, and lateral antennal lobe tracts (mALT, mlALT, and lALT), respectively. Tracts that do not belong to the major three tracts, but run through the protocerebrum transversely, are called transverse ALTs (tALTs).
RESULTS
Multiple olfactory tracts in the protocerebrum
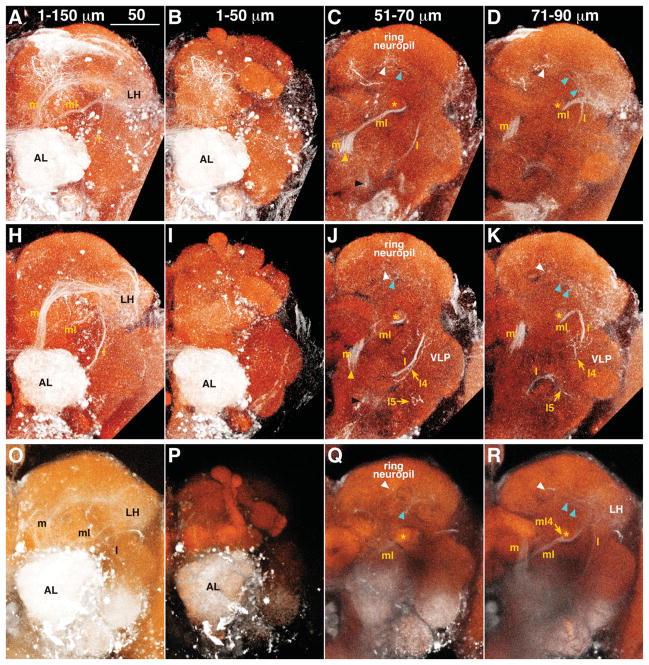
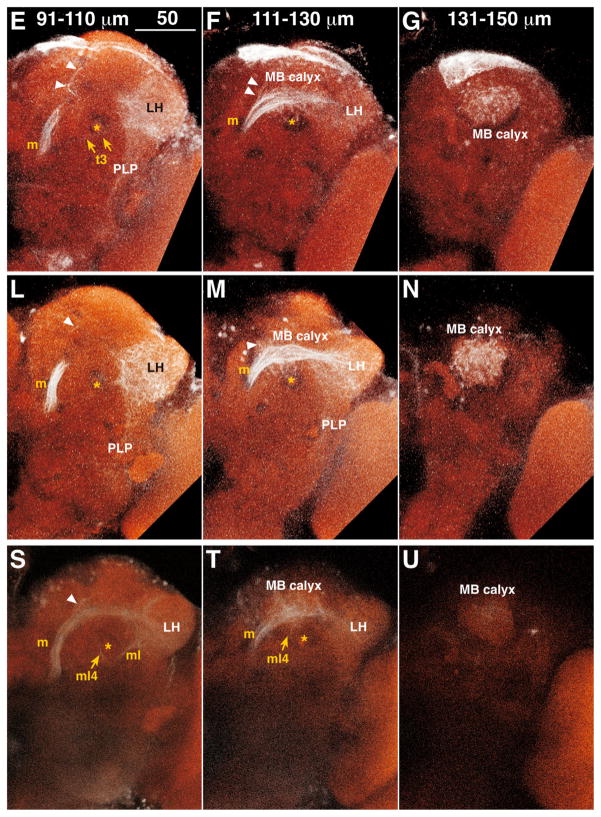
To visualize ALTs, we injected dextran conjugated with tetramethylrhodamine and biotin into the AL. Because dextran can fill neurons in both anterograde and retrograde directions, this method labels both PNs and centrifugal neurons. Our fills revealed not only three major, well-known tracts, the medial, mediolateral, and lateral ALTs (m, ml, and lALTs; original names were inner, middle, and outer antennocerebral tracts, respectively; Figs. 1, 2, Supp. Info. Fig. 1), but also an unknown, transverse ALT (tALT; Figs. 1, 2). Each tract exits from the AL through either the dorsomedial or the ventral root (Fig. 1C,J). In depth, the dorsomedial root was level with the ventral surface of the DP1m glomerulus, 42 μm from the anteriormost surface of the AL (n = 3, vertical position shown by yellow arrowhead in Fig. 1C,J), whereas the ventral root was level with the dorsal surface of the V glomerulus at a depth of 53 μm (n = 3, black arrowhead in Fig. 1C,J). All the m-, ml-, and tALTs shared the dorsomedial root, whereas the lALTs passed through the ventral root.
Figure 1.
A–U: Antennal lobe tracts (ALTs) in the left hemisphere. Results from three animals are shown, one per row. The depths of optical slices are shown in the top panel of each column. White: tetramethylrhodamine signal enhanced with Alexa Fluor 568; red: nc82 antibody staining. White and cyan arrowheads indicate fibers from the medial ALT and LH terminating in the ring neuropil, respectively. Yellow and black arrowheads show the positions of the dorsomedial and ventral root of ALTs, respectively. Asterisk indicates the pedunculus of MB. Dorsal is to the top, lateral to the right. AL, antennal lobe; l, lateral ALT; l4, lateral4 ALT; l5, lateral5 ALT; LH, lateral horn; m, medial ALT; MB, mushroom body; ml, mediolateral ALT; ml4, mediolateral4 ALT; PLP, posterior lateral protocerebrum; t3, transverse3 ALT; VLP, ventrolateral protocerebrum. Scale bars = 50 μm.
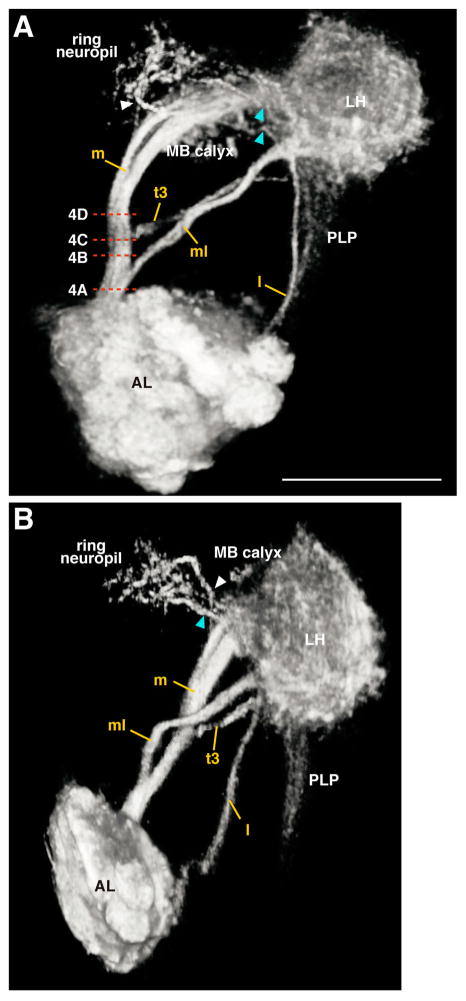
Figure 2.
3D reconstruction of major ALTs. Frontal (A) and anterior lateral (B) views of ALTs of the top row in Figure 1 are shown. White and cyan arrowheads indicate fibers from the medial ALT and LH terminating in the ring neuropil, respectively. Abbreviations are as in Figure 1. Dashed lines in A represents the horizontal level of the electron micrographs in Figure 4. Scale bar = 50 μm.
The mALT linked the AL to the LH (m in Fig. 1; Stocker et al., 1990), with two branching points along the way. The first branching point has not been previously described. Containing several branches, it began at a depth of 122.1 μm (n = 3, white arrowhead in Fig. 1F,M,S) near the medial surface of the MB calyx, projected toward the anterior, and terminated in the ring neuropil, which is the area surrounding the MB vertical lobe (white arrowheads in Fig. 1C,J,Q). The second branching point has been described previously; it began at a depth of 127.8 μm (n = 3) in front of the MB calyx and projected into the calyx (Stocker et al., 1990).
The mlALT shared the same root with the mALT and branched off from the mALT near the ventrolateral surface of the fan-shaped body at a depth of 64.8 μm (n = 3) and ran below the MB pedunculus to the LH (ml in Fig. 1C,D; Stocker et al., 1990). The mlALT bifurcated at the MB pedunculus at a depth of 38 μm (n = 1). The branched fiber (named here the mediolateral4 [ml4]-ALT) ran posteriorly through the pedunculus to the MB calyx (ml4 in Fig. 1R,S,T; see also Fig. 13c in Stocker et al., 1990). This tract is different from one previously identified as imACT (Tanaka et al., 2008), which also innervates the MB pedunculus; the imACT does not run through the mlALT to reach the pedunculus.
One transverse tract, named the transverse3 ALT (see Fig. 5C in Tanaka et al., 2012), was also stained. It branched off the mALT at a depth of 104.3 μm (n = 3, t3 in Fig. 1E), turned laterally and then ventrally below the LH, and terminated in the posteriorlateral protocerebrum (PLP).
Three lALTs were labeled (Fig. 1J,K). The major lALT previously reported as oACT projected dorsolaterally to the medial periphery of the LH, where the lALT and mlALT crossed to enter the LH (Fig. 1K, see also Stocker et al., 1990; Tanaka et al., 2008). Two lALTs not previously described were labeled as well, both branching from the major lALT at a depth of 81.4 μm (n = 3) where the lALT intersected the commissure of the antennomechanosensory and motor center and the ventrolateral protocerebrum (VLP). One lALT, named lateral4 ALT, turned dorsolaterally, ran parallel to the lALT, and then terminated in the central and posterior dorsomedial VLP (l4 in Figs. 1J,K, 3, Supp. Info. Fig. 1, Fig. 6D in Tanaka et al., 2012). The other lateral ALT, named lateral5 ALT, turned ventrolaterally and then arborized in the posterior ventral part of the VLP (l5 in Figs. 1J,K, 3, Supp. Info. Fig. 1).
Figure 3.
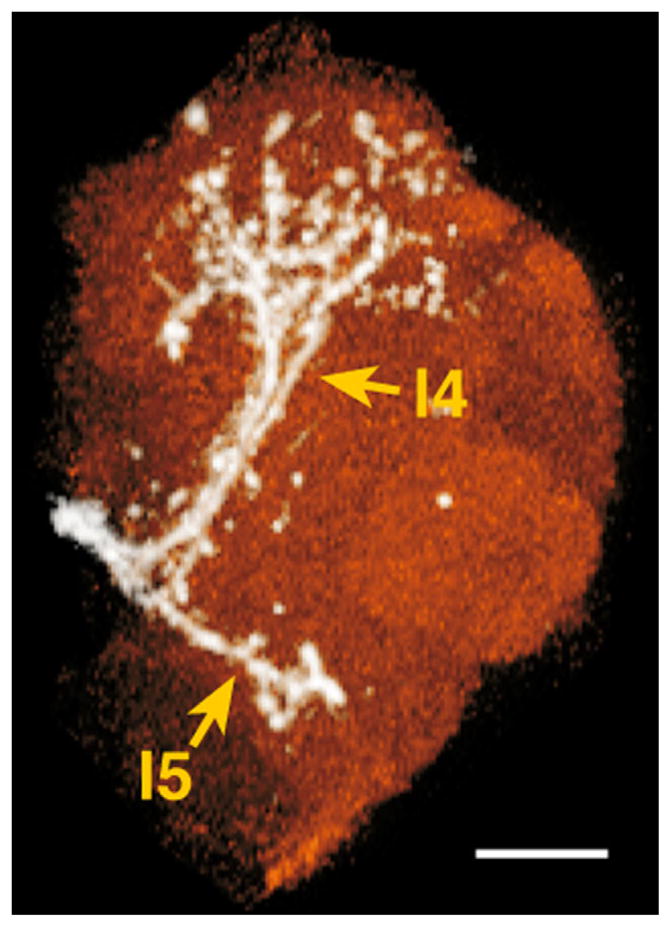
Minor lateral ALT neurons terminating in the ventrolateral protocerebrum. White, lateral ALT neurons; red, ventrolateral protocerebrum. l4, lateral4 ALT; l5, lateral5 ALT. Dorsal is to the top, lateral to the right. Scale bar = 20 μm.
Several fiber bundles from the LH targeted two protocerebral areas: the ring neuropil and the PLP. Fibers linking the LH and the ring neuropil emerged from the LH near the intersection of the mlALT and lALT and extended anterior-medially toward the MB vertical lobe, terminating around the shaft of the MB vertical lobe (cyan arrowheads in Fig. 1C,D,J,K,Q,R). Two distinct bundles targeted the ring neuropil. One appeared to be the mlALT reported by Marin et al. (2002); the other appeared to originate from the lateral ALT (see Fig. 6B in Tanaka et al., 2012). Another group of fibers emerged from the LH at a depth of 108 μm and formed a wide bundle (n = 3, Fig. 1E,L) that terminated in the PLP. This bundle included the mALT fibers and lALT (Lai et al., 2008; see Figs. 3C,D, 6B in Tanaka et al., 2012).
Lengths of major AL tracts
The MB calyx and LH are innervated by three major ALTs, the medial, mediolateral, and lateral tracts (Stocker et al., 1990; Tanaka et al., 2008). Here we dye labeled all three and compared the length of each tract from the root of the AL to its entry sites at the MB calyx and the LH (Table 1).
TABLE 1.
Distance (micrometers) Between the AL and the MB Calyx or LH Through Major ALTs (mean ± SD)
| mALT
|
mlALT
|
lALT
|
|||
|---|---|---|---|---|---|
| MB calyx | LH | MB calyx | LH | MB calyx | LH |
| 148.9 ± 6.4 | 192.6 ± 4.2 | 157.8 ± 6.7 | 144.1 ± 1.6 | 232.5 ± 7.2 | 163.4 ± 7.5 |
The lengths of the three ALTs from the AL to the LH were all significantly different from each other (ANOVA, F = 70.99, P < 0.0001): the mlALT was the shortest, averaging 144.1 ± 1.6 (mean ± SD, n = 3) μm; the lALT averaged 163.4 ± 7.5 μm; and the mALT averaged 192.6 ± 4.2 μm. For the distance between the AL and MB calyx, the medial and mediolateral4 ALTs were about the same length (148.9 ± 6.4 and 157.8 ± 6.7 μm, respectively), but the lALT (232.5 ± 7.2 μm) was significantly longer than the other two ALTs (ANOVA, F = 138.1, P < 0.05).
Numbers of fibers in ALTs
Previous work has shown that the iACT (mALT), near its exit from the AL, contains ~200 medium-sized to thick axons, with another ~50 thin axons (Fig. 6a in Stocker et al., 1990). At the depth of the basal central complex, fibers in this tract numbered 467, of which 126 were anti-ChAT antibody immunopositive (Yasuyama et al., 2003). To investigate further the number of fibers of different tracts arising from the same root, we made horizontal ultrathin sections at the levels of the inter-AL commissure and inferior and middle parts of the fan-shaped body (Fig. 4). We traced the mALTs by using toluidine blue stains of thick sections cut in sequence with the ultrathin sections that we observed with the light microscope (EM). We were not able to trace the lALT in thick sections. Horizontal sections shown here are almost perpendicular to the ALTs between the levels of the inter-AL commissure and the top of the fan-shaped body, which also helped us to identify the ALTs both in light microscopic (LM) and in EM images. At the level of the inter-AL commissure, we could identify m/ml/tALTs running together (Fig. 4A), where the total number of fibers was 435 ± 8 (mean ± SD, n = 3), which is compatible with findings of Yasuyama et al. (2003). Medial to the m/ml/tALTs at this level, about 60 fibers ran parallel to the ALTs. Because glial processes appeared to separate these fibers partially from the ALTs, we concluded that these fibers are not part of these tracts. At the level of the bottom of the fan-shaped body, the mlALT ran apart from m/tALTs. Above this, at the level of the inferior part of the fan-shaped body, the m/tALTs bundle contained 366 fibers (n = 1; Fig. 4B). Nearly the same number of fibers was present at the point the m/tALTs adjoined an additional bundle running posterior to the inferior part of protocerebral bridge (348 ± 8, n = 3; Fig. 4C). The tALT then ran parallel to this additional bundle, separating from the mALT as the tALT and the additional bundle coursed together to the posterior. These results suggest that the mlALT contains 80–100 fibers at maximum. About 8 μm above the previous point measured, where mALT ran aparts from the tALT, 288 fibers were contained in the mALT (n = 1, Fig. 4D). These results suggest the tALT contains about 60 fibers at maximum.
Figure 4.
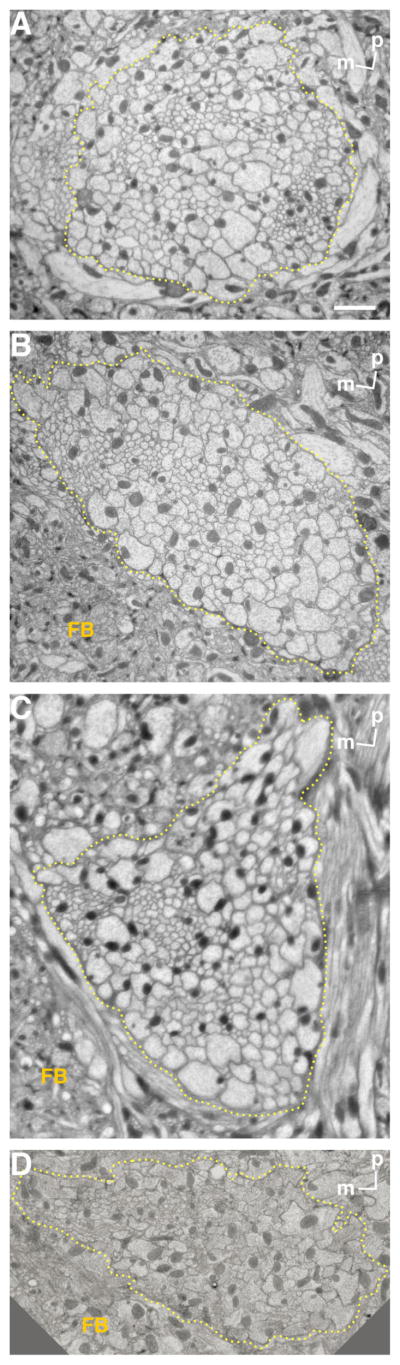
Electron micrographs of ALTs. Horizontal ultrathin sections at a depth of inter-antennal lobe tract (A) and inferior (B,C) and middle (D) parts of the fan-shaped body. These levels are shown in Figure 2. Note that the area surrounded by dashed lines contains m/ml/tALTs in A, m/tALTs in B,C, and only mALT in D. FB, fan-shaped body; m, medial; p, posterior. Scale bar = 2 μm.
Electron micrographs showed that thinner fibers are grouped together in the anterior lateral area, separately from thicker fibers in the sections containing all the m-, ml-, and tALTs (Fig. 4A). The thin fibers numbered 84 ± 4 (n = 3), and their mean area was 0.054 μm2 (n = 1). This area is less than one-fifth that of the other fibers (0.29 μm2 on average). We labeled the m/mlALTs with green fluorescent protein (GFP) expressed by GAL4 enhancer trap NP5288 (Tanaka et al., 2004) costained with anti-choline acetyltransferase (ChAT) antibody. Interestingly, fibers that were not labeled with anti-ChAT antibody correspond to the positions of thin fiber groups (Fig. 5). The lateral fibers lacking the anti-ChAT antibody signals form the mlALT, which is known to be GABAergic (Okada et al., 2009). The number of fibers in the anterior lateral thin cluster approximately matches that of the mlALT fibers, suggesting that the thin fibers form the mlALT.
Figure 5.
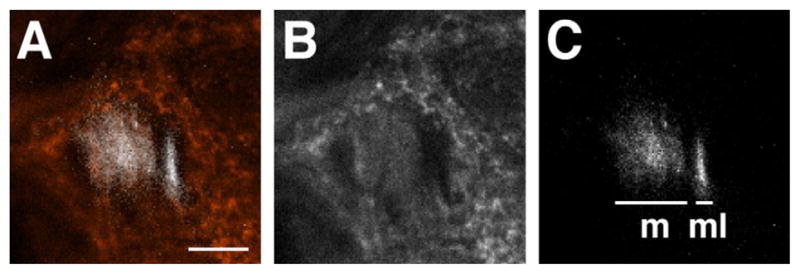
Localization of mALT and mlALT. A vertical confocal section behind the AL. The mALT (m in C) is colocalized with anti-choline acetyltransferase signal (red in A and white in B), whereas the mlALT (ml in C) is not. Note that the mlALT is running at the lateral side of mALT. Medial is to the left, dorsal to the top. Scale bar = 10 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Genetically controlled neural labels have often been used to delineate olfactory pathways in the adult Drosophila brain (Wong et al., 2002; Marin et al., 2002; Tanaka et al., 2004, 2008, 2012; Jefferis et al., 2007; Lai et al., 2008; Chiang et al., 2011). Single-cell morphology of various olfactory neurons can also be seen at http://www.flycircuit.tw/ (Chiang et al., 2011). However, genetic manipulations may affect neural circuits (Aso et al., 2009) and may fail to detect pathways for which genetic labels have not yet been identified. Here we report results obtained through mass dye injection in wild-type flies. Our work adds to the number of known olfactory pathways and reveals new patterns of connectivity among the AL and protocerebrum (Table 2). More detailed analyses of the projection patterns of individual or specific neurons in wild-type flies will require intracellular dye injections or Golgi staining (Stocker et al., 1990; Strausfeld et al., 2003).
TABLE 2.
Connections Between the AL and Protocerebral Areas Via Multiple ALTs1
| mALT
|
mlALT
|
tALT
|
lALT
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB calyx | LH | RN | PLP | LH | MB calyx | RN | MB calyx | LH | RN | PLP | MB calyx | LH | RN | ASLP | PLP | VLP | |
| This study | + | + | + | ? | + | + | ? | − | − | − | + | ? | + | ? | − | ? | + |
| Golgi stains | + | + | − | − | + | + | − | − | − | − | − | − | + | − | − | − | − |
| GAL4 lines | + | + | − | + | + | − | + | + | + | + | + | + | + | + | + | + | + |
Connections in Drosophila identified in this study and in previous work based on analyses of Golgi staining (Stocker et al., 1990) and GAL4 lines (Marin et al., 2002; Wong et al., 2002; Lai et al., 2008; Tanaka et al., 2012; see also Fig. 31 in Tanaka et al., 2012). We assigned a question mark for some MB calyx, PLP, and RN columns when we could not trace fibers of each tract from the LH to these areas in this study although we observed fibers from the LH terminating in these areas. ASLP, anterior superiorlateral protocerebrum; RN, ring neuropil.
We counted, in total, about 435 fibers passing through the dorsomedial root of the AL. Currently, the projection patterns of only about 100 AL PNs have been analyzed at the cellular level using GAL4 enhancer-trap strains (Wong et al., 2002; Marin et al., 2002; Tanaka et al., 2004, 2008; Jefferis et al., 2007). Some of the additional fibers that we identified may originate in previously uncounted PNs. However, our labeling method does not allow us to distinguish AL-associated PNs from centrifugal neurons, because dextran can fill neurons in both anterograde and retrograde directions. Further studies will be needed to describe fully the connectivity between the AL and the protocerebrum.
Recent work has identified ~100 ipsilaterally projecting and ~100 bilaterally projecting local neurons in each AL in Drosophila (Chou et al., 2010). Compared with the number of local neurons, the number of afferent and efferent neurons is much larger in Drosophila than that in hymenoptera such as honeybee, in which there are ~4,000 local neurons but only ~800 PNs (for review see Galizia and Rössler, 2010). One could speculate that the larger population of LNs in hymenoptera might allow more complex odor processing than in the fly.
Our dye fills revealed many ALTs, some of which have not previously been described. However, we did not fill two small groups of fibers that were identified previously with GAL4 enhancer-trap strains (Sachse et al., 2007; Tanaka et al., 2008, 2012): the transverse1 ALT, which projects from the V glomerulus and runs above the MB pedunculus to the LH (Fig. 5A in Tanaka et al., 2012), and the im (transverse2; Fig. 5B in Tanaka et al., 2012) ALT, which, like the mediolateral4 ALT, runs through the pedunculus but branches off the mALT more dorsoposteriorly (Stocker et al., 1990; Tanaka et al., 2008). These two ALTs might be composed of too few fibers to be detected by our labeling technique.
Secondary olfactory sites in the Drosophila protocerebrum
We labeled fibers directly linking the AL to five protocerebral areas: the MB calyx, LH, ring neuropil, PLP, and VLP (Fig. 2). The olfactory pathway that includes the MB has been shown to be necessary for olfactory learning and memory (Heisenberg, 2003). Because flies lacking the MB show normal olfactory responses in situations that do not require learning, another olfactory pathway, that extending through the LH, has been proposed to be important for experience-independent olfactory behavior (Heimbeck et al., 2001). By analogy, we speculate that olfactory processing that occurs in other secondary sites might also contribute primarily to functions that do not require associative learning. The ring neuropil and VLP appear to receive input from relatively few neurons, because the bundles projecting to these areas are much thinner than those projecting to the other areas (Fig. 1). This leads us to speculate that these areas, unlike the MB, might not integrate the output of large numbers of uniglomerular projection neurons but rather process more specific forms of olfactory information, such as that provided by pheromones or sources of food or oscillatory timing (Tanaka et al., 2009, 2012).
We labeled fibers running through both the mALT and the LH that contact the ring neuropil (Fig. 2). Although we could not be certain of the origins of all the bundles associated with the LH, at least one is likely to contain the mlALT neurons (identified by Marin et al., 2002). The other is likely to contain the lALT neurons, because a GAL4 line visualizing the lALT also targets the ring neuropil with a pathway similar to that seen here (Fig. 6B in Tanaka et al., 2012). Single-cell images of AL neurons might also help to reveal the connective patterns of these neurons (Chiang et al., 2011; http://flycircuit.tw/). In hymenoptera, the ring neuropil is also innervated by PNs but, unlike the case in Drosophila, only by the neurons of the transverse tracts (Kirschner et al., 2006; Zube et al., 2008). Despite such differences, the ring neuropil is a secondary olfactory site in both hymenoptera and diptera.
Protocerebral areas connecting the MB and LH have previously been described for Drosophila. The ring neuropil region is linked to the calyx and lobes of the MB and to the LH by extrinsic neurons of the MB and by LH neurons (Tanaka et al., 2004, 2008). Thus, the ring neuropil may receive direct olfactory output from the AL, and indirect olfactory output from other secondary centers, possibly forming a circuit in which direct output from the AL interacts within the ring neuropil with information processed in the MB and LH. Another possibility is that output from the AL to the ring neuropils, via the ALT, may modulate olfactory processing in the MB and LH.
The PLP, which is known to receive extensive input from the optic lobe (Strausfeld, 1976; Otsuna and Ito, 2006), has previously been reported to be a target of the mALT PNs (Lai et al., 2008). Here, we describe a previously unknown transverse tract of fibers that also targets the PLP (see also Fig 5C in Tanaka et al., 2012). Because these fibers broadly fan out within their target (Fig. 2), they appear to carry the output of many neurons. A comparable link between the PLP and the AL has not been reported for moth or hymenoptera (Homberg et al., 1988; Kirschner et al., 2006; Zube et al., 2008).
The VLP receives input from the optic lobe and has been described as an optic focus (Strausfeld, 1976). Here, we describe lALT fibers that follow separate paths to two different areas in the VLP, terminating in the ventralmost and dorsomedial areas (Fig. 3). Areas of the VLP receiving lALT fiber bundles do not appear to overlap with those receiving input from optic lobe neurons (Shinomiya, unpublished findings). The ventralmost VLP is also innervated by sensory neurons of Johnston’s organ (Kamikouchi et al., 2006). Our results add to a view of VLP as a site of multimodal integration and indicate that some integration of olfactory information with other modalities may occur in the protocerebrum outside of the central complex, MB, and LH. However, the organization of this area remains largely unknown. A further exploration of the projection patterns of neurons bearing other sensory modalities in these areas will be necessary for understanding olfactory processing in a multimodal context.
Transmission of olfactory information to secondary sites
It has been reported that the MB calyx and the LH are both innervated by multiple tracts of PNs in the brain of the fly (Strausfeld, 1976; Stocker et al., 1990; Tanaka et al., 2008). We show here that the ring neuropil and PLP are also innervated by multiple tracts of PNs (see also Fig. 31 in Tanaka et al., 2012).
The PNs bundled into different ALTs appear to use different neurotransmitters: PNs of the m- and lALTs are cholinergic and thus excitatory, whereas PNs of the mlALT express glutamate decarboxylase (which synthesizes GABA), suggesting that mlALT PNs are inhibitory (Okada et al., 2009; Tanaka et al., 2012). Both excitatory and inhibitory ALTs converge upon common secondary sites, including the LH and the ring neuropil. Because the neurotransmitters prevalent in the other tracts of neurons have not yet been identified, it is not known whether the MB calyx and PLP also receive input from both excitatory and inhibitory PNs.
The GABAergic mlALT is shorter than the cholinergic m- and lALTs, raising the possibility that inhibitory output from the AL might reach its targets before excitatory output. However, because fibers in the mlALT appear to be thinner than those of the mALT, the transmission through the mlALT may be slower. Transmission time among olfactory areas, which can be tested electrophysiologically, could have important consequences for understanding the processing of olfactory information in the Drosophila brain.
Acknowledgments
We thank Chi-Hon Lee for providing Canton S; Erich Buchner and Alois Hofbauer for nc82 antibody; the Hybridoma Bank at the University of Iowa for anti-choline acetyltransferase antibody, Chris McBain for the use of Neurolucida; Joby Joseph for technical suggestions; Kazunori Shinomiya, Kei Ito, Christina Zube, and Wolfgang Rössler for helpful discussions; Kei Ito for updated terminology; and the NICHD Microscopy and Imaging Core facility for the use of Volocity, MetaMorph, and electron and confocal microscopes.
Grant sponsor: JSPS; Grant number: 22770068; Grant sponsor: JST PREST Program (to N.K.T.); Grant sponsor: NIG Cooperative Research Program; Grant number: 2010-A83; Grant number: 2011-A60 (to N.K.T., E.S., A.E.); Grant sponsor: National Institute of Child Health and Human Development (to M.S.).
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Aso Y, Grübel K, Busch S, Friedrich AB, Siwanowicz I, Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- Chiang AS, Lin CY, Chuang CC, Chang HM, Hsieh CH, Yeh CW, Shih CT, Wu JJ, Wang GT, Chen YC, Wu CC, Chen GY, Ching YT, Lee PC, Lin CY, Lin HH, Wu CC, Hsu HW, Huang YA, Chen JY, Chiang HJ, Lu CF, Ni RF, Yeh CY, Hwang JK. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr Biol. 2011;21:1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JCS, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–451. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia CG, Rössler W. Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol. 2010;55:399–420. doi: 10.1146/annurev-ento-112408-085442. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford: Oxford University Press; 1998. pp. 377–416. [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hofbauer A. Habilitation thesis. University of Würzburg; Würzburg, Germany: 1991. Eine Bibliothek monoklonaler Antikörper gegen das Gehirn von Drosophila melanogaster. [Google Scholar]
- Homberg U, Montague RA, Hildebrand JG. Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res. 1988;254:255–281. doi: 10.1007/BF00225800. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi A, Shimada T, Ito K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J Comp Neurol. 2006;499:317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- Kay LM, Stopfer M. Information processing in the olfactory systems of insects and vertebrates. Semin Cell Dev Biol. 2006;17:433–442. doi: 10.1016/j.semcdb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Kirschner S, Kleineidam CJ, Zube C, Rybak J, Grünewald B, Rössler W. Dual olfactory pathway in the honeybee, Apis mellifera. J Comp Neurol. 2006;499:933–952. doi: 10.1002/cne.21158. [DOI] [PubMed] [Google Scholar]
- Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Malun D, Waldow U, Kraus D, Boeckh J. Connections between the deutocerebrum and the protocerebrum, and neuroanatomy of several classes of deutocerebral projection neurons in the brain of male Periplaneta americana. J Comp Neurol. 1993;329:143–162. doi: 10.1002/cne.903290202. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Müller D, Abel R, Brandt R, Zöckler M, Menzel R. Differential parallel processing of olfactory information in the honeybee, Apis mellifera L. J Comp Physiol A. 2002;188:359–370. doi: 10.1007/s00359-002-0310-1. [DOI] [PubMed] [Google Scholar]
- Okada R, Awasaki T, Ito K. Gamma-aminobuyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514:74–91. doi: 10.1002/cne.21971. [DOI] [PubMed] [Google Scholar]
- Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–958. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- Raman B, Joseph J, Tang J, Stopfer M. Temporally diverse firing patterns in olfactory receptor neurons underlie spatio-temporal neural codes for odors. J Neurosci. 2010;30:1994–2006. doi: 10.1523/JNEUROSCI.5639-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rø H, Müller D, Mustaparta H. Anatomical organization of antennal lobe projection neurons in the moth Heliothis virescens. J Comp Neurol. 2007;500:658–675. doi: 10.1002/cne.21194. [DOI] [PubMed] [Google Scholar]
- Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford: Oxford University Press; 1998. pp. 159–203. [Google Scholar]
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Atlas of an insect brain. Heidelberg: Springer; 1976. [Google Scholar]
- Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Techniq. 2003;62:151–169. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
- Takagawa K, Salvaterra P. Analysis of choline acetyltransferase protein in temperature sensitive mutant flies using newly generated monoclonal antibody. Neurosci Res. 1996;24:237–243. doi: 10.1016/0168-0102(95)00999-x. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila sencond-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci. 2009;29:8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Endo K, Ito K. The organization of antennal lobe-associated neurons in the adult Drosophila melanogaster brain. J Comp Neurol. 2012;520:4067–4130. doi: 10.1002/cne.23142. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schürmann FW. Synaptic connections of cholinergic antennal lobe relay neurons innervating the lateral horn neuropile in the brain of Drosophila melanogaster. J Comp Neurol. 2003;466:299–315. doi: 10.1002/cne.10867. [DOI] [PubMed] [Google Scholar]
- Zube C, Kleineidam CJ, Kirschner S, Neef J, Rössler W. Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J Comp Neurol. 2008;506:425–441. doi: 10.1002/cne.21548. [DOI] [PubMed] [Google Scholar]