Abstract
OBJECTIVES
Optimal vitamin D status is known to have beneficial health effects and vitamin D supplements are commonly used. It has been suggested that vitamin D supplementation may increase blood lead in children and adults with previous lead exposure. The objective was to determine the safety regarding lead toxicity during 12 weeks of high dose vitamin D3 supplementation in children and young adults with HIV.
METHODS
Subjects with HIV (age 8 to 24 yrs) were randomized to vitamin D3 supplementation of 4000 IU/day or 7000 IU/day and followed at 6 and 12 weeks for changes in 25D and whole blood lead concentration. This was a secondary analysis of a larger study of vitamin D3 supplementation in children and adolescents with HIV.
RESULTS
In 44 subjects (75% African American), the baseline mean ± SD serum 25D was 48.3 ± 18.6 nmol/L. 50% of subjects had baseline serum 25D < 50.0 nmol/L. Serum 25D increased significantly with D3 supplementation over the 12 weeks. No subject had a whole blood lead >5.0 μg/dL at baseline or during subsequent visits. Whole blood lead and 25D were not correlated at baseline, and were negatively correlated after 12 weeks of supplementation (p= 0.014). Whole blood lead did not differ between those receiving 4000 IU versus 7000 IU of vitamin D3.
CONCLUSION
High dose vitamin D3 supplementation and the concomitant increased serum 25D did not result in increased whole blood lead concentration in this sample of children and young adults living in a northeastern urban city.
Keywords: Vitamin D supplementation, blood lead, children, HIV/AIDS, nutrition
Introduction
Vitamin D is needed for optimal bone health and muscle strength and may regulate processes such as inflammation and immunity (1). Vitamin D supplementation resulting in optimal serum concentration may have beneficial health effects for multiple diseases and are under evaluation for therapy in many clinical settings (2,3,4,5). Kemp et al. (6) raised a concern regarding vitamin D supplementation for people with previous lead exposure. They showed that seasonal variations in lead levels were associated with changes in vitamin D in young urban children, with both occurring at higher levels in the summer. However, Kersey et al. (7) found no association between serum vitamin D and blood lead concentration in low income children. Additionally, Jackson et al. (8) demonstrated an inverse association between blood lead levels and vitamin D supplementation, and increased blood lead levels during periods of increased bone turnover.
Given the discordance of these previous findings, the objective of this paper was to determine the safety regarding lead toxicity during 12 weeks of high dose vitamin D3 supplementation in children and young adults with HIV. This study was a registered trial (phase 2, NCT 01092338).
Materials and Methods
Study participants ages 8 to 24 years were recruited from the Special Immunology Family Care Clinic and the Adolescent Health Care Clinic at The Children’s Hospital of Philadelphia (CHOP), and from the Jonathan Lax Center. This is a secondary analysis of a larger study to determine the safety and efficacy of high dose vitamin D3 supplementation in children and young adults with HIV. As it is the first high dose vitamin D3 supplementation study in this population, we chose to focus initially on older children and adolescents to demonstrate safety. This study was approved by the Institutional Review Board at CHOP. Informed consent was obtained from young adult participants ≥18 years of age and from emancipated minors (<18 y). Assent was obtained from the other participants <18 y with consent from their parents/guardians. Participants’ racial and ethnic status was obtained via self report. After enrollment, subjects were randomized to a vitamin D3 (cholecalciferol) dose of either 4000 IU/day (two 2000 IU capsules [NSI, Vitacost, Boca Raton, FL]) or 7000 IU/day (one 2000 IU capsule [NSI, Vitacost, Boca Raton, FL] and one 5000 IU supplement [Now Foods, NOW Health Group, Bloomingdale, IL]) with follow up visits at 6 and 12 weeks. Doses of all supplements were confirmed by an independent laboratory (Tampa Bay Analytical Research, Inc [Largo, FL]). For analysis, subjects were stratified by age (8 to 12.9 y, 13 to 18.9 y and 19 to 24.9 y). At all visits, serum 25D was measured using a liquid chromatography-tandem mass spectrometer, and whole blood lead concentration using an inductively coupled plasma mass spectrometry by CHOP Clinical Laboratory. For the purpose of this study, vitamin D status was categorized as deficient, insufficient and sufficient based upon serum 25D concentrations < 50, 50–79, and ≥ 80 nmol/L (< 20, 20–31, and ≥ 32 ng/ml), respectively. The goal of 80 nmol/L was higher than the usual target for healthy people. The cutoff values were based on the ability of the body to regulate intestinal calcium absorption and represent benchmark values for evaluating the immunological effect of vitamin D supplementation (9,10,11).
Weight was measured to the nearest 0.1 kg using a digital scale (Scaltronix, White Plains, NY, USA) and height to the nearest 0.1 cm using a stadiometer (Holtain, Crymych, UK). Age-and gender-specific standard deviation scores (Z-scores) for weight, height, and body mass index were calculated (12). Seasons were defined as Summer (June, July, August), Fall (September, October, November), Winter (December, January, February) and Spring (March, April, May). Adherence to vitamin D3 study supplements was assessed via final pill count and multiple phone calls and questionnaires.
Descriptive statistics were calculated for the total sample at three visits (baseline, 6 weeks, and 12 weeks). Means, standard deviations, and medians and ranges were used to summarize continuous variables, and proportions for categorical variables. Potential trends in whole blood lead, serum 25D, growth and nutritional status over time were assessed using paired Student’s t tests or Wilcoxon rank tests depending upon skewness of the data. Chi squared tests and Fisher exact tests were used to assess differences over time for categorical variables. Pearson correlation coefficients or Spearman rank correlations, as appropriate, were performed to test for significant associations between blood lead and serum 25D status, age, growth and nutritional status at each visit.
Longitudinal mixed effects analysis was used to assess time trends in blood lead and potential differences among the three age groups and between vitamin D3 supplementation dose groups (4000IU vs. 7000IU/day) in these trends (using age group*time or dose group*time interaction terms). Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at CHOP (13). All data were analyzed using STATA 9.0 (STATA Corp., College Station, TX). Statistical significance was defined as P value < 0.05 and data are presented as mean ± SD (unless otherwise indicated).
Results
Forty-four subjects with HIV aged 18.7 ± 4.7 y were enrolled and 42 completed the study. At baseline (Table 1), participants’ characteristics showed altered immune but normal nutritional status. Table 2 presents serum 25D, blood lead and serum alkaline phosphatase over 12 weeks of vitamin D3 supplementation. At baseline, serum 25D was 48.3 ± 18.6 nmol/L with 50% of the subjects under 50nmol/L. A significant increase from baseline in serum 25D was evident at both 6 weeks (114.8 ± 35.6 nmol/L) and 12 weeks (118.0 ± 46.5 nmol/L) and 81% of subjects achieved sufficient 25D concentrations (≥ 80 nmol/L) by 12 weeks. The whole blood lead was low at baseline and no subject had blood lead > 5 μg/dL. Lead values did not change and serum alkaline phosphatase remained stable over 12 weeks. Serum 25 OHD was low at baseline (11.0 to 84.0 nmol/L). Serum 25 OHD increased by 57.1 ± 37.8 nmol/L after 6 weeks and 62.1 ± 46.3 nmol/L after 12 weeks of vitamin D3 supplementation in the 4000 IU/d group. In the 7000 IU/d group, the increase was 77.2 ± 39.1 nmol/L after 6 weeks and 77.4 ± 60.4 nmol/L after 12 weeks of supplementation. No difference was observed in the change in whole blood lead over time between subjects who received 4000 IU versus 7000 IU of vitamin D3.
Table 1.
Baseline characteristics (mean±SD)
| All | 4000 IU/d | 7000 IU/d | |
|---|---|---|---|
| Number | 44 | 22 | 22 |
| Gender, male % | 68 | 68 | 68 |
| Age, yr [Range] | 18.7±4.7 [8.3, 24.7] | 18.4±4.5 [9.4, 23.6] | 19.1±5.0 [8.3, 24.7] |
| Height Z-score | 0.2±1.1 | 0.4±0.9 | −0.1±1.3 |
| Weight Z-score | 0.6±1.3 | 0.8±1.1 | 0.4±1.6 |
| BMI Z-score | 0.5±1.2 | 0.6±1.2 | 0.5±1.3 |
| HIV RNA(log) viral load | 2.5±1.1 | 2.8±1.2 | 2.3±0.9 |
| RNA: % detectable (≥1.6) | 53 | 59 | 48 |
| CD4+, absolute count | 672±374 | 684±390 | 660±365 |
| CD4+, % lymphocytes | 31±10 | 31±11 | 31±10 |
| On HIV medications, % | 82 | 77 | 86 |
| Enrollment season, % | |||
| Summer | 27 | 27 | 27 |
| Fall | 32 | 32 | 32 |
| Winter | 32 | 32 | 32 |
| Spring | 9 | 9 | 9 |
| Race, % | |||
| African American | 75 | 77 | 73 |
| Caucasian | 7 | 5 | 9 |
| Other | 18 | 18 | 18 |
Table 2.
Serum 25D and blood lead (mean±SD) over 12 weeks of vitamin D3 supplementation
| Baseline
|
Week 6
|
Week 12
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All | 4000 IU/d | 7000IU/d | All | 4000 IU/d | 7000 IU/d | All | 4000 IU/d | 7000 IU/d | |
| Number | 44 | 22 | 22 | 43 | 22 | 21 | 42 | 21 | 21 |
| Serum 25D, nmol/L | 48.3±18.6 | 44.9±21.2 | 51.8±15.3 | 114.8±35.6* | 102.0±32.9* | 128.3±33.9* | 118.0±46.5* | 107.5±36.6* | 128.5±53.4* |
| <50, % | 50 | 59 | 41 | 2 | 5 | 0 | 2 | 5 | 0 |
| 50–79, % | 45 | 36 | 54 | 12 | 18 | 5 | 17 | 19 | 14 |
| ≥ 80, % | 5 | 5 | 5 | 86* | 77* | 95* | 81* | 76* | 86* |
| Blood Lead, ug/dL [Range] | 1.5±0.9 [0.5, 4.9] | 1.4±1.0 [0.05, 4.9] | 1.5±0.9 [0.6, 3.7] | 1.6±1.1 [0.5, 5.0] | 1.5±0.9 [0.5, 4.0] | 1.7±1.2 [0.5, 5.0] | 1.5±1.0 [0.2, 4.7] | 1.5±1.1 [0.6, 4.3] | 1.5±1.0 [0.2, 4.7] |
| Serum Alkaline Phosphatase, U/L | 149±113 | 159±145 | 138±68 | 147±114 | 156±145 | 138±70 | 145±113 | 157±150 | 133±60 |
Significant difference from baseline at p <0.01
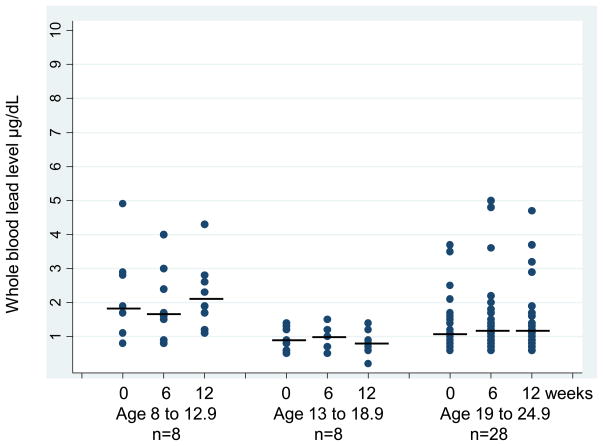
Figure 1 presents the whole blood lead levels over time by age group where subjects were grouped by age (8 to 12.9y, n=8/13 to 18.9y, n=8 and 19 to 24.9y, n=28). Although the whole blood lead remained under 5μg/dL, a significantly higher level at baseline and 12 weeks was found in the youngest age group when compared to the older subjects (p ≤ 0.02). Whole blood lead was not correlated with 25D at baseline, however, blood lead was significantly negatively correlated (r = −0.38, p = 0.014) with 25D after 12 weeks of supplementation evaluating all subjects together. Subjects enrolled in Winter and Spring had significantly lower serum 25D at baseline than those enrolled in Summer and Fall (38.5 ± 17.7 vs 55.1 ± 16.2 nmol/L, p = 0.003) but both groups had comparable whole blood lead levels at baseline. Increase in serum 25D after 12 weeks of supplementation was significantly greater in the subjects enrolled during Winter and Spring compared to the others enrolled during Summer and Fall (+100.7 ± 51.6 vs +48.7 ± 44.9 nmol/L, p = 0.001). This increase in 25D was accompanied by no change in whole blood lead level in the group of all subjects. For participants enrolled in Winter and Spring who had robust increases in 25D over the subsequent 12 weeks (into Spring and Summer) whole blood lead decreased (− 0.12 ± 0.32 μg/dL, p = 0.04).
Figure 1.
Whole blood lead level is presented over time (0 = baseline, 6 = 6 weeks, 12 = 12 weeks) separately by age group: age 8 to 12.9, age 13 to 18.9 and age 19 to 24.9. The horizontal lines represent the median values in blood lead level. The Figure shows a higher whole blood lead throughout the study in the youngest age group compared to both older age groups (p ≤ 0.02).
Adherence was assessed via patient report in 42 subjects (by phone contact and in-person questionnaires), and via pill count in 31 subjects. Adherence assessed with pill count was 87% for the 4000 IU dose and 93% for the 7000 IU dose. Adherence assessed with phone calls was 94% for both doses. Adherence assessed with questionnaires was 92% for the 4000 IU dose and 90% for the 7000 IU dose. No correlation was found between adherence to supplementation and whole blood lead level.
Discussion
Increased serum 25D concentration did not result in increased whole blood lead in this group of children and young adults with HIV living in the northeast urban United States, 75% of whom were African American. The more robust increase in serum 25D after 12 weeks of vitamin D3 supplementation for participants enrolled during Winter and Spring was accompanied by a decrease in whole blood lead concentration. Whole blood lead did not differ between those receiving 4000 IU versus 7000 IU of vitamin D3. These data are likely generalizable outside HIV care as there is no known impact of HIV on lead metabolism, and limited impact on vitamin D metabolism.
Previous work included Kemp et al. (6) who reported an increase in blood lead during summer which was significantly associated with an increase in serum 25D in urban African-American and Hispanic children aged 4 to 8 years old in Newark, NJ. Our youngest participants were eight years of age, which may be an important consideration since higher blood levels are mainly observed in younger children. Data from the National Report on Human Exposure to Environmental Chemicals 1999–2008 showed that children aged 1 to 5 years had a higher blood lead level compared to other age groups (14). Kemp et al. (6) also reported an increased blood lead during summer in the subgroup of 1 to 3 years old children without a significant increase in serum 25D. Kersey et al. (7) studied healthy toddlers and children under 6 years of age in Minneapolis, MN and found no association between vitamin D and lead levels. We did not find a positive association between 25D and blood lead and there was no increase in blood lead during the summer months in our 8–24 years old sample of subjects. Jackson et al. (8) found that the use of vitamin D supplements (by report) in the past month was associated with significantly lower adjusted mean blood lead levels in postmenopausal women suggesting that one month of vitamin D supplementation per se did not increase blood lead in these women. The association between high dose vitamin D3 supplementation and blood lead in contemporary groups of infants and pre-school children remains to be evaluated.
The relationship of serum vitamin D and whole-blood lead is possibly influenced by growth and/or calcium homeostasis in some children and adults. More than 95% of body lead is located in bone (15). Mobilization of bone lead can be increased during periods of higher bone turnover including childhood growth (16,17). Low dietary intake of vitamin D and calcium are known risk factors for high bone lead levels (18). Vitamin D enhances calcium absorption and calcium competes with lead for gut binding sites (19). Animal studies have shown an inverse relationship between calcium intake and lead levels (20,21). This inverse relationship was also documented in pregnant women (22) and calcium supplementation during pregnancy was associated with reductions in blood lead (23).
The United States has seen dramatic decreases in environmental lead exposure since 1980 (24). Blood lead levels declined in all age groups during the 1999–2008 survey period (25). The Kemp et al. (6) study reporting seasonal blood lead variations was conducted in 2001 and 2002 while the Kersey et al. (7) study and the present study were more recent. The participants of the three studies were from Newark, Minneapolis and Philadelphia. Although all three are urban, northern U.S. cities with socioeconomically similar populations, differences in environmental exposures and in adherence to lead abatement may exist. Of note, there were still 130 children between 7 and 16 years old with confirmed elevated blood lead level (>10 μg/dL) in Pennsylvania in 2010 (year of our study), 66 of whom lived in Philadelphia (26).
These results demonstrate that in this sample of children and young adults aged 8 to 24 with HIV and low baseline blood lead levels, 12 weeks of high dose vitamin D3 supplementation resulted in significantly increased serum 25D with no concomitant change in whole blood lead concentration. These data provide safety information when considering higher dose vitamin D intervention.
Acknowledgments
This project was supported in part by the NIH (R01 AT005531) and the Clinical Translational Research Center (UL1RR024134) and the Nutrition Center at the Children’s Hospital of Philadelphia, Philadelphia, PA.
We thank the subjects, parents, and other care providers for their participation and cooperation in this study, the CHOP Special Immunology Family Care Clinic, the Adolescent Health Care Clinic, the Jonathan Lax Center, the CHOP Clinical and Translational Research Center, and Alia Tanko and Savannah Knell.
Abbreviations
- 25D
Serum 25-hydroxy vitamin D, nmol/L
- BMI
Body Mass Index kg/m2
- CHOP
Children’s Hospital of Philadelphia
- HIV
Human immunodeficiency virus
- HT
Height, cm
- WT
Weight, kg
Footnotes
The authors declare they have no or potential competing financial interest.
References
- 1.Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dawson-Hughs B, Stocklin E, et al. Oral supplementation with 25(OH)D(3) versus vitamin D(3): effects on 25(OH)D levels, lower extremity function, blood pressure and markers of innate immunity. J Bone Miner Res. 2011 doi: 10.1002/jbmr.551. Online 25 October 2011. [DOI] [PubMed] [Google Scholar]
- 3.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 4.Spector SA. Vitamin D and HIV: letting the sun shine in. Top Antivir Med. 2011;19(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301–23. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 6.Kemp FW, Neti PV, Howell RW, et al. Elevated Blood Lead Concentrations and Vitamin D Deficiency in winter and summer in young urban children. Environ Health Perspect. 2007;115(4):630–5. doi: 10.1289/ehp.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kersey M, Chi M, Cutts DB. Anemia, lead poisoning and vitamin D deficiency in low-income children: do current screening recommendations match the burden of illness? Public Health Nutrition. 2010;14(8):1424–8. doi: 10.1017/S1368980010003617. [DOI] [PubMed] [Google Scholar]
- 8.Jackson LW, Cromer BA, Panneerselvamm A. Association between bone turnover, micronutrient and blood lead levels in pre- and postmenopausal women, NHANES 1999–2002. Environ Health Perspect. 2010;118(11):1590–6. doi: 10.1289/ehp.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaney RP. Vitamin D: criteria for safety and efficacy. Nutr Rev. 2008;66(10 Suppl 2):S178–S181. doi: 10.1111/j.1753-4887.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135(2):317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 11.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC (Center for Disease Control and Prevention) [Accessed Jan 27, 2012];National Report on Human Exposure to Environmental Chemicals 2012. Available at: http://www.cdc.gov/exposurereport/data_tables/LBXBPB_DataTables.html.
- 15.Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101(7):598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Flaherty EJ. Physiologic changes during growth and development. Environ Health Perspect. 1994;102 (Suppl 11):103–6. doi: 10.1289/ehp.94102s11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Willet WC, Schwartz J, et al. Relation of nutrition to bone lead and blood lead levels in middle-aged to elderly men. The Normative Aging Study. Am J Epidemiol. 1998;147(12):1162–74. doi: 10.1093/oxfordjournals.aje.a009415. [DOI] [PubMed] [Google Scholar]
- 19.Fullmer CS. Intestinal interactions of lead and calcium. Neurotoxicology. 1992;13(4):799–807. [PubMed] [Google Scholar]
- 20.Dowd TL, Rosen JF, Gundberg CM, et al. The displacement of calcium from osteocalcin at submicromolar concentrations of free lead. Biochim Biophys Acta. 1994;1226(2):131–7. doi: 10.1016/0925-4439(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 21.Fullmer CS. Lead-calcium interactions: involvement of 1,25-dihydroxyvitamin D. Environ Res. 1997;72(1):45–55. doi: 10.1006/enrs.1996.3689. [DOI] [PubMed] [Google Scholar]
- 22.Zentner LE, Rondo PH, Duran MC, et al. Relationships of blood lead to calcium, iron, and vitamin C intakes in Brazilian pregnant women. Clin Nutr. 2008;27(1):100–4. doi: 10.1016/j.clnu.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect. 2009;117(1):26–31. doi: 10.1289/ehp.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AAP (American Academy of Pediatrics) Committee on Environmental Health. Lead exposure in children: prevention, detection and management. Pediatrics. 2005;116(4):1036–46. doi: 10.1542/peds.2005-1947. [DOI] [PubMed] [Google Scholar]
- 25.EPA (United States Environmental Protection Agency) [Accessed Jan 28, 2012];Report on the Environment, Blood Lead Level. 2009 Available at: http://cfpub.epa.gov/eroe/index.cfm?fuseaction=detail.viewInd&lv=list.listbyalpha&r=224030&subtop=208.
- 26.Pennsylvania Childhood Lead Surveillance Program. [Accesses June 19, 2012];Annual Report. 2010 Available at: http://www.portal.state.pa.us/portal/server.pt?open=514&objID=558053&mode=2.