Abstract
Small-cell lung carcinoma (SCLC) has a dismal prognosis in part because of multidrug resistance (MDR). Silibinin is a flavonolignan extracted from milk thistle (Silybum marianum), extracts of which are used in traditional medicine. We tested the effects of silibinin on drug-sensitive (H69) and multi-drug resistant (VPA17) SCLC cells. VPA17 cells did not show resistance to silibinin (IC50 = 60µM for H69 and VPA17). Flow cytometry analysis after incubation in 30 µM silibinin showed no changes in cell cycle phases in VPA17 or H69 cells compared with untreated cells. Silibinin (30 µM) incubation was pro-apoptotic in VPA17 cells after >3 days, as measured by ELISA of BUdR labeled DNA fragments. Apoptosis was also indicated by an increase in caspase-3 specific activity and decrease in survivin in VPA17 MDR cells. VPA17 cells had increased Pgp -mediated efflux of calcein acetoxymethyl ester (calcein AM); however, this was inhibited in cells pre-incubated in silibinin for 5 days. Pre-incubation of VPA17 cells in 30 µM silibinin for 5 days also reversed resistance to etoposide (IC50 = 5.50 uM to 0.65 µM) and doxorubicin (IC50 = 0.620 µM to 0.035 µM). The possible synergistic relationship between silibinin and chemotherapy drugs was determined by exposure of VPA17 cells to 1:1 ratios of their respective IC50 values, with serial dilutions at 0.25–2.0 × IC50 and calculation of the combination index (CI). Silibinin and etoposide showed synergism (CI = 0.46 at ED50), as did silibinin and doxorubicin (CI = 0.24 at ED50). These data indicate that in SCLC, silibinin is pro-apoptotic, reverses MDR and acts synergistically with chemotherapy drugs. Silibinin, a non-toxic natural product may be useful in the treatment of drug-resistant SCLC.
Keywords: Lung carcinoma, silibinin, multidrug resistance, apoptosis, chemotherapy, sensitization
1. Introduction
Small-cell lung carcinoma (SCLC) originates from neuroendocrine cells and accounts for 12–15% of all lung cancer. SCLC has a poor prognosis, with 5-year survival less than 5–10%[1]. Most patients present with disseminated disease, so treatment is by chemotherapy, with combinations involving etoposide, doxorubicin, cisplatin and vincristine[2]. Unfortunately, multi-drug resistance usually occurs and this makes further treatment ineffective[3]. One mechanism of multi-drug resistance is the overexpression of P-glycoprotein (Pgp), the drug transporter that is a product of the MDR1 gene.
Milk thistle (Silybum marianum) is a member of the daisy family, indigenous to the Middle East. Extracts of the single-seeded fruit have been used for centuries in Europe to treat hepatitis and mushroom poisoning[4]. The medicinally active hot water extract called silymarin comprises about 5% (w/w) of the fruit and contains a mixture of polyphenols[5]. Among the most active is the flavonolignan, silibinin[6], which is widely available as a dietary supplement.
Following an initial report that silymarin strongly inhibited skin cancer development in a mouse model[7], there has been interest in silymarin and silibinin for cancer prevention and treatment. Of particular interest have been extensive studies on prostate cancer cells and animal models[8], [9] This led to a clinical trial, which is ongoing[10].
Dietary silibinin has been shown to inhibit the growth and progression of urethane-induced lung adenocarcinomas in a mouse model[11] and non-small cell lung carcinoma tumor growth in athymic mice[12], although it did not have an inhibitory effect on benzo(a)pyrene-induced lung adenoma development[13]. The mechanism of the effect of silibinin on non-small-cell lung cancer cells appears to be via multiple signaling pathways and down-regulation of inducible nitric oxide synthase[14]. In extensive studies on prostate cancer cells (reviewed in[15]), silymarin and silibinin have been shown to cause G1 arrest, and to be pro-apoptotic, anti-angiogenic and anti-metastatic.
In this report, we describe the first studies of of silibinin on SCLC and particularly on drug-resistant cells. We examined cytotoxicity, apoptosis and drug-resistance in silibinin-treated cells. We show that silibinin can reverse Pgp-mediated drug resistance and acts synergistically with established chemotherapeutic drugs.
2. Materials and Methods
2.1 Cells
NCI-H69 SCLC cells were grown at 37°C in suspension culture in an atmosphere with 5% CO2 in AIM-V serum-free medium (Invitrogen). A multidrug-resistant cell line VPA17 derived from H69 cells selected in etoposide[16] was also grown in AIM-V medium. The VPA17 cells showed resistance to etoposide (9-fold), doxorubicin (15-fold) and vincristine (10-fold)[17], but not to cisplatin. They overexpress Pgp[18] Cells were added to 8 ml medium at 3 × 104 cells/ml and reached logarithmic growth in 3 days, at which time they were used in experiments. Doubling time of both cell lines was approximately 30 h. Medium was changed every 4 days in a ratio of 1:5.
2.2 Cytotoxicity
Silibinin, etoposide, doxorubicin or cisplatin (Sigma, St. Louis, MO) were dissolved in DMSO at a concentration of 10 mM and stored at 4°C for up to 2 mo. Dilutions were made in DMSO such that the concentration of DMSO in experiments did not exceed 2%. Drugs were added as indicated to logarithmically growing cells in 500 µl cultures containing 104 cells/ml. After 4 days of continuous incubation (5 days for silibinin experiments), cells were counted using a Coulter Z-1 Particle Counter. Counts were validated microscopically by hemocytometer after staining in trypan blue. All experiments were done in triplicate and repeated at least three times. IC50 values, defined as the concentration of drug that reduced cell counts by 50% compared to controls incubated in 2% DMSO, were calculated and compared by t-test. Retrospective power analysis showed that the triplicate experiments repeated three times was adequate (T = 0.88) for statistical significance (p > 0.05). In experiments involving preincubation, logarithmically growing cells were incubated in 30 µM silibinin for 72 h, after which the medium was removed by centrifugation and the cells washed once with fresh medium. Then the cells were resuspended at 104 cells/ml fresh medium and tested for cytotoxicity as above.
2.3 Cell cycle analysis
Eight-ml cultures of SCLC cells with 2 × 105 cells/ml were incubated in silibinin for 5 days and washed twice in PBS. Cells were fixed in 75% ethanol for 14 h at 4°C. After one additional wash in PBS, cells were stained with 20 µg/ml propidium iodide, 0.1%bTrition X-100, 200 ug/ml RNase A for 16 h at 4°C. They were then analyzed by flow cytometry.
2.4 DNA Fragmentation ELISA for Cell Death
Apoptosis was measured by detection of DNA fragments (Roche Applied Science). Triplicate 3-ml cultures at 105 cells/ml were incubated in AIM-V medium with 10 µM BUdR for 18 h. After washing and resuspension in fresh medium, duplicate 200 µl cultures were incubated with drug as indicated. The cells were pelleted by centrifugation and 100 µl of the culture medium was removed for ELISA of BUdR containing DNA fragments; this is a measure of cell death by necrosis. The cells were then lysed and DNA fragments in the lysate were measured by ELISA; this is a measure of cell death by apoptosis.
2.4. Caspase 3
Cells (3 × 105/ml) were incubated in etoposide or silibinin at 0.5 × IC50 for the times indicated. Caspase 3 was assayed fluorimetrically (Sigma kit), using Ac-DEVD-AMC as substrate and AMC (7-amido-methylcoumarin) product measured by fluorescence at 360 nm excitation−460 nm emission. Experiments were performed at least four times.
2.5. Survivin Assay
Cells were grown in culture for 96 h in the presence or absence of 30 µM silibinin. The Surveyor IC ELISA (RD Systems) was used to measure survivin concentration. A standard curve was generated using purified human survivin. Protein in cell lysates was measured by dye binding (BioRad). Survivin concentration was expressed as µg/pg total cell protein.
2.6 Drug efflux assay
The Vybrant multi-drug resistance assay (Molecular Probes-Invitrogen) was used to measure drug efflux properties of cells treated with silibinin. Calcein acetoxymethyl ester (calcein AM) is taken up by cells and hydrolyzed by cytoplasmic esterases to fluorescent calcein. However, the MDR1-encoded membrane transporter[19]] removes the calcein AM before it can be hydrolyzed, resulting in reduced cellular fluorescence. Two types of experiments were performed. To measure direct inhibition of Pgp-mediated efflux, cells (2 × 105/ 100 ul medium) were incubated with or without 50 µM silibinin for 45 min. Then 250 nM calcein AM was added and after 15 min, cell suspensions were centrifuged and washed and cell fluorescence was measured at 494 nm and compared to controls not incubated in drug. To measure indirect inhibition of Pgp-mediated efflux, 5-ml cell cultures (2 × 105 cells/ml) were incubated for 96 h with or without 30 µM silibinin. Following washing twice by centrifugation in fresh medium, 3 × 104 cells were incubated in 100 µl fresh medium and calcein AM added at 250 nM. After 15 min, the suspensions were centrifuged and washed, and cell fluorescence measured at 494 nm. Because cyclosporin A is an inhibitor of Pgp, experiments were performed with 2 µg/ml cyclosporin A as a control to show reversal of Pgp-mediated drug efflux. All experiments were performed in triplicate and compared to controls not treated with silibinin.
2.7. Combination studies with silibinin and chemotherapeutic drugs
The presence or absence of synergism between silibinin and etoposide or doxoru bicin was determined using the method of Chou using CalcuSyn software v. 2.1 (Biosoft)[20]. Briefly, drug-resistant VPA17 cells were exposed to 1:1 ratios of the respective IC50 values for silibinin (IC50 = 50 µM) and etoposide (IC50 = 6 µM) or silibinin and doxorubicin (IC50 = 0.6 µM) at 1/4 × IC50, 1/2 × IC50, IC50, 2 × IC50, and 4 × IC50. Cells were incubated first in silibinin for 3 days, then washed and resuspended in medium containing etoposide and incubated for a further 4 days. Cell counts were made as described above. The combination index (CI) was calculated to determine the presence of synergism (CI < 1), an additive effect (CI = 1), or antagonism (CI > 1) between silibinin and etoposide/doxorubicin.
3. Results
3.1 Cytotoxicity of silibinin
Silibinin was tested on drug-sensitive (H69) and drug-resistant (VPA17) SCLC cell lines. Cytotoxicities were similar (p > 0.05, t-test) in both cell lines, with an IC50 of 60µM (Figure 1). A similar IC50 was observed in a second drug-resistant cell line (VPA6) (data not shown).
Figure 1.
Cytotoxicity of silibinin. Logarithmically growing drug sensitive (H69) and drug-resistant (VPA17) SCLC cultures were incubated in silibinin in DMSO and cells were counted after 5 days. Shown are the average (S.D.) cell counts from triplicate counts at each drug concentration relative to the 0-drug control. This is a representative experiment that was repeated 3 times.
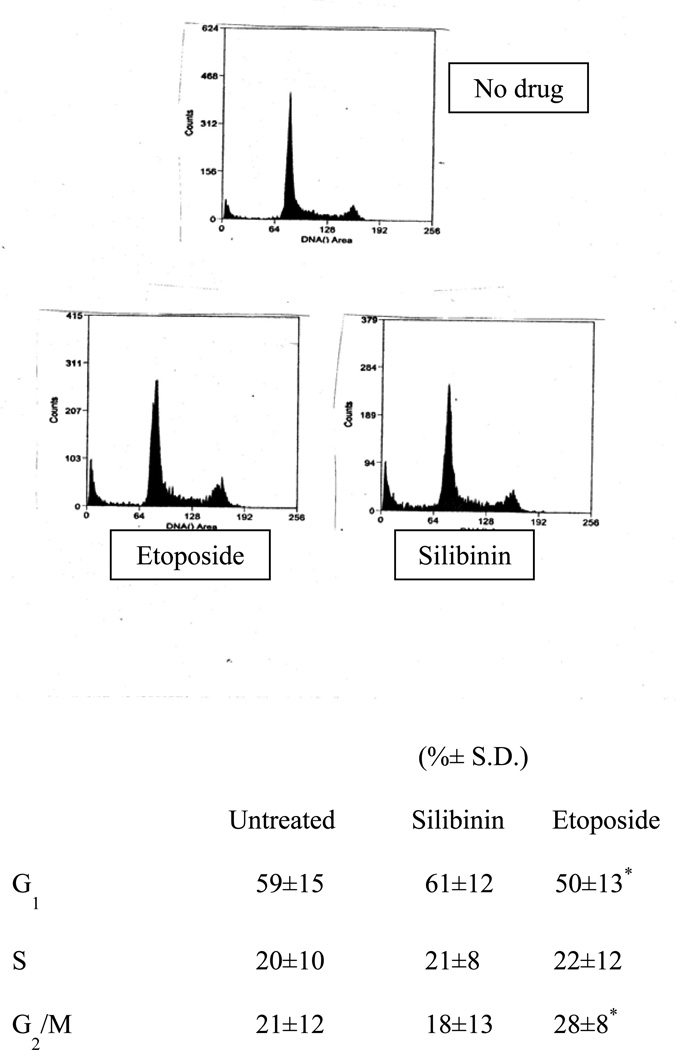
3.2 Cell cycle analysis
SCLC cells incubated in silibinin at 0.5 × IC50 for 5 days showed no difference from controls incubated in solvent in the distribution of cell cycle phases as measured by PI staining and flow cytometry. As a positive control, incubation in 0.5 × IC50 etoposide showed accumulation of cells in G2. Figure 2 shows results with the drug-resistant VPA17 cells. Similar results were obtained for drug-sensitive H69 cells: Untreated: 57% G1, 23% S, 17% G2/M; Silibinin: 58% G1, 20% S, 21 G2/M; Etoposide: 48% G1, 20% S, 30% G2/M).
Figure 2.
Cell cycle analysis. VPA17 SCLC cells were incubated for 118 h in medium (control, top left), with 30 µM silibinin (top right) or with 3 µM etoposide (bottom) and then fixed and stained with propidium iodide prior to analysis by flow cytometry. The table shows the average (±S.D.) percentage of cells in each cell cycle phase from three independent experiments. (*Values for etoposide different from controls, p<0.05)
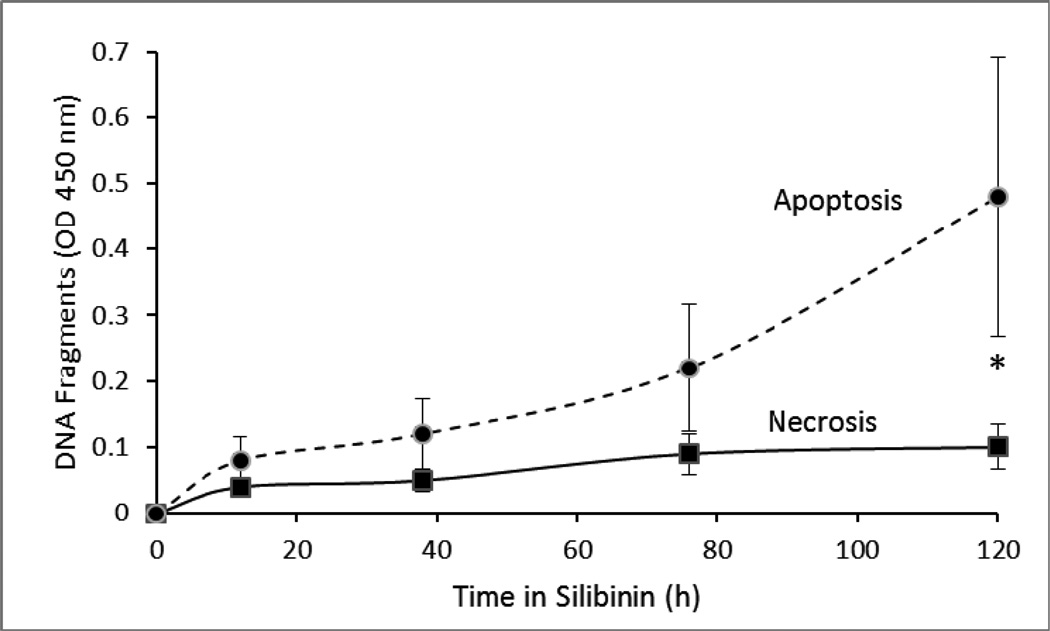
3.3 DNA fragmentation
To determine the mechanism of cytotoxicity, we treated VPA17 cells with silibinin at 0.5 × IC50 and used an ELISA test to measure DNA fragmentation. As incubation time in silibinin increased, there was an increase in DNA fragments in cell lysates (apoptosis), as opposed to released by intact cells into the medium (necrosis) (Figure 3). Similar results were obtained with drug-sensitive H69 cells (e.g., OD450 nm at 120h: 0.7 cell lysate, 0.13 culture medium).
Figure 3.
DNA fragmentation. VPA17 cells were prelabeled with BUdR and then incubated for the indicated periods in 30 µM silibinin. DNA fragments released into the medium (open squares) or cell lysate (solid squares) were determined by ELISA. Error bars indicate SD. Controls treated with solvent only gave absorbances of 0.03 (medium) and 0.06 (lysate). (*, p < 0.05 by t-test comparing medium and cell lysate at each time indicated)).
3.4 Caspase-3 activity and survivin concentration
Incubation of drug-resistant VPA17 cultures in 0.5 × IC50 silibinin resulted in an increase in the specific activity of caspase-3 compared to untreated controls (Figure 4). As a positive control, etoposide also resulted in an increase in caspase-3 activity. Similar results were obtained with drug-sensitive H69 cells (e.g., Caspase specific actrivity at 120 h: 17.3 etoposide, 19.1 silibinin, 6.3 control).
Figure 4.
Caspase activity. VPA17 cells were grown in 30 µM silibinin (circles), 3 µM etoposide (triangles), or medium without drug (squares) for the times indicated. Equivalent number of cells were assayed for caspase 3 activity using a fluorimetric assay. Results of four experiments are expressed as mean ± SD. (*, p < 0.05 by -t-test comparing control to either etoposide or silibinin at each time indicated).
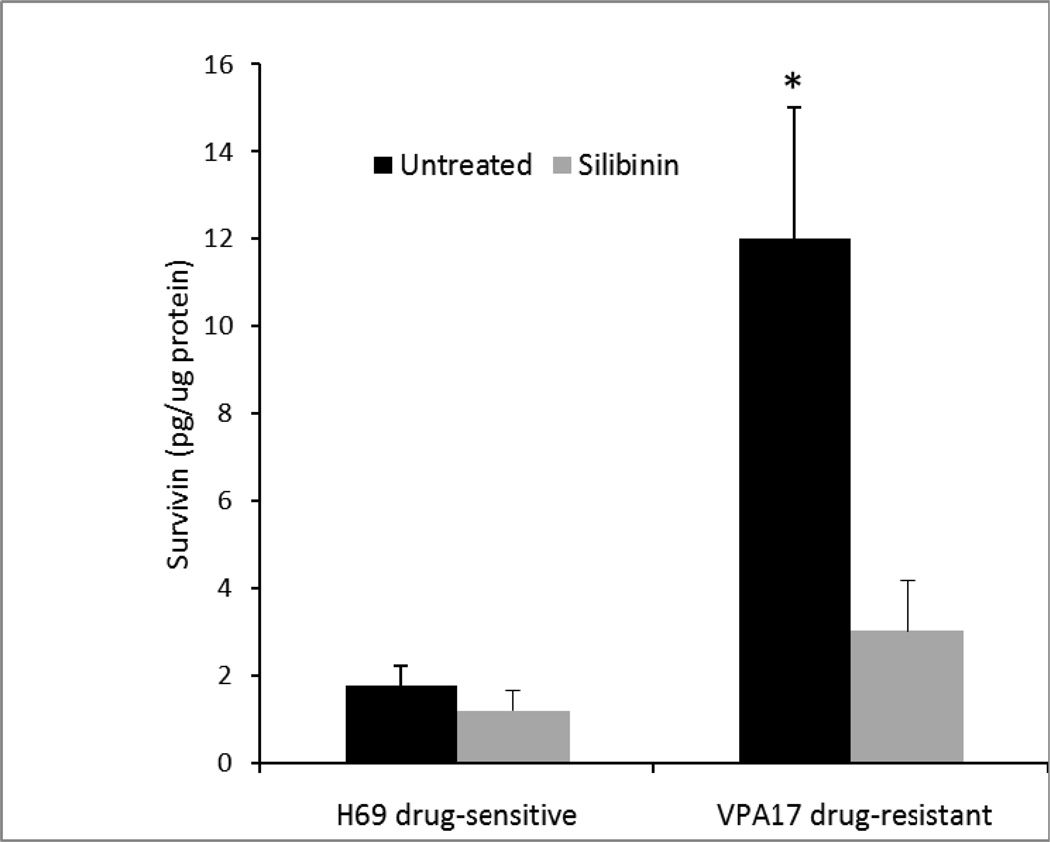
The baseline concentration of survivin on a total protein basis was significantly higher in drug-resistant VPA17 cells than in drug-sensitive H69 cells (Figure 5, dark bars). Incubation of H69 cells in 0.5 × IC50 silibinin did not significantly alter the survivin level; however, survivin was significantly reduced in drug-resistant VPA17 cells (Figure 5, light bars).
Figure 5.
Survivin concentration. VPA16 cells were grown in culture for 96 h in the presence or absence of 30 µM silibinin. Concentration of survivin was measured by ELISA. Protein in cell lysates was measured by dye binding. Results are expressed as mean of three experiments ± SD. (*, p <0.05 by t-test, for the comparison between VPA17 in the presence and absence of silibinin)
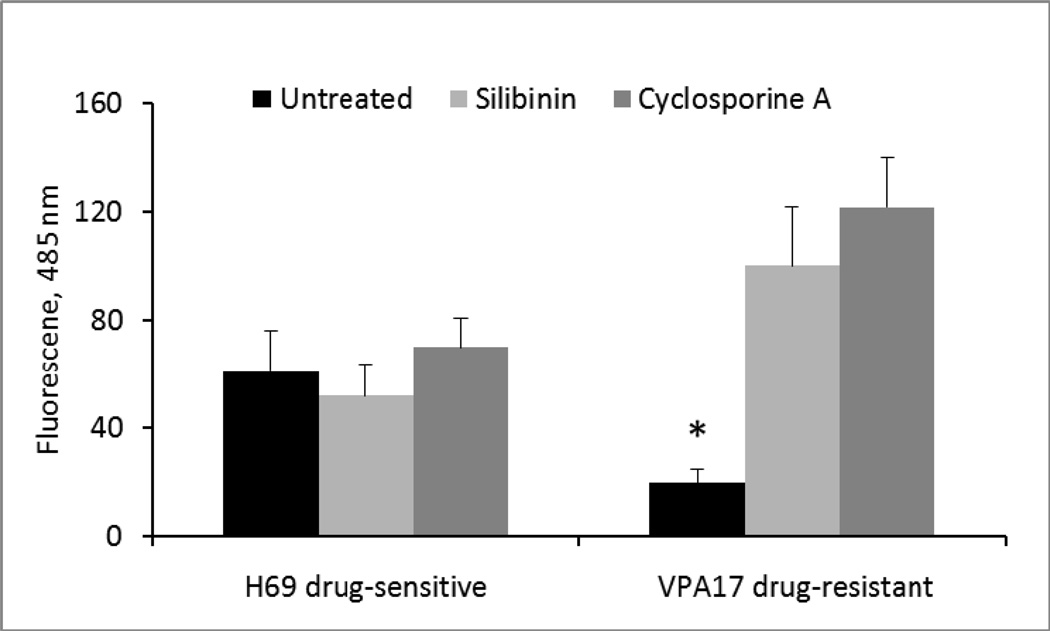
3.5 Inhibition of drug efflux and reversal of multi-drug resistance
Drug efflux via Pgp was measured by a fluorescence assay. Compared to drug-sensitive H69 cells, there was significantly greater efflux in drug-resistant VPA17 cells (Figure 6, black bars). Pre-incubation of the cells in 0.5 × IC50 silibinin for 4 days did not alter the low level of efflux in H69 cells, but it did significantly reduce efflux in VPA17 cells (Figure 6, light bars). As a control, cyclosporine A, which blocks Pgp-mediated efflux, also did so in the VPA17 cells (Figure 6, medium bars). When untreated VPA17 cells were incubated in silibinin for 45 min and then immediately tested for drug efflux, there was no inhibition of the efflux (data not shown).
Figure 6.
Calcein AM efflux from silibinin-treated and control cells. Triplicate cultures were incubated for 96 h with or without 30 µM silibinin. Following washing twice, cells were incubated for 15 min in calcein AM and cell fluorescence measured (RD Systems). Experiments were also performed with 2 µg/ml cyclosporin A as a control. Results are expressed as mean ± SD. (*, p < 0.05 by ANOVA for the comparison between all treatments and paired t-test between silibinin-treated and untreated cells, and cyclosporine-treated and untreated cells).
To test for reversal of the overall drug resistance phenotype, SCLC cells were pre-incubated in 0.5 × IC50 silibinin for 5 days and then tested for cytotoxicity with etoposide or doxorubicin (Table 1). Compared to cells not treated with silibinin, the IC50’s for etoposide and doxorubicin were significantly reduced in silibinin-treated VPA17 cells. There was no effect of silibinin on drug-sensitive H69 cells, nor was there an effect on the cytotoxicity of cisplatin, which is not part of the multi-drug resistance phenotype in these cells.
Table 1.
Cytotoxicity of drugs after preincubation with silibinin
| Condition | H69 IC50 (µM) | VPA17 IC50 (µM) |
|---|---|---|
| Etoposide, no preincubation | 0.45 ± 0.24 | 5.50 ± 2.35a |
| Etoposide, preinc. SB | 0.70 ± 0.40 | 0.65 ± 0.45b |
| Doxorubicin, no preincubation | 0.040 ± 0.025 | 0.620 ± 0.255a |
| Doxorubicin, preinc. SB | 0.045 ± 0.030 | 0.035 ± 0.025b |
| Cisplatin, no preincubation | 2.0 ± 0.8 | 2.8 ± 1.3 |
| Cisplatin, preincubation SB | 4.0 ± 2.8 | 2.6 ± 1.6 |
Values significantly different (p<0.05) by t-test comparing H69 and VPA17 cells under stated condition.
Values significantly different (p<0.05) by t-test comparing VPA17 cells with and without preincubation in silibinin.
3.6 Combinations of silibinin and chemotherapeutic drugs
Drug-resistant VPA17 cells were first exposed to silibinin and then to either doxorubicin or etoposide at a constant 1:1 (IC50:IC50) ratio of silibinin:drug at varying dilutions (0.25–2 × IC50) to determine possible interactions. The combination index calculations (Table 2) showed synergism (combination index <1) between silibinin and etoposide and between silibinin and doxorubicin.
Table 2.
Combination Index (CI) for Silibinin and Chemotherapy Drugs
| Condition | CI at ED50 | CI at ED95 |
|---|---|---|
| Etoposide and silibinin | 0.46 | 0.95 |
| Doxorubicin and silibinin | 0.24 | 0.96 |
4. Discussion
Lung cancer is a leading cause of cancer deaths worldwide, and most people die with drug-resistant disease[1]. Small-cell lung carcinoma (SCLC) is a particularly lethal form of this disease. Milk thistle (Silybum marianum) and its extract silymarin are widely used herbs to treat various ailments[4]. This is the first report to examine the effects of silibinin, a flavonolignan extracted from milk thistle, on drug-resistant SCLC cells.
Silibinin was cytotoxic to SCLC cells, and was not part of drug resistance (Figure 1). In contrast to its effect on other tumor cell types, where silibinin causes inhibition at G1[15], it did not affect cell cycle parameters in SCLC cells (Figure 2).
Several lines of evidence indicate that silibinin is pro-apoptotic in SCLC cells. First, release of DNA fragments into the extracellular medium is characteristic of necrotic cells, with disrupted cell membranes. Instead, we observed a time-dependent accumulation of DNA fragments within silibinin-treated cells that could be released upon cell lysis with detergent (Figure 3). This is characteristic of apoptosis. Second, the apoptosis effector caspase-3[21] activity was increased in silibinin-treated cells (Figure 4). Third, the apoptosis inhibitor survivin[22] was reduced in silibinin-treated drug-resistant SCLC cells (Figure 5).
Since silibinin was cytotoxic to drug-resistant SCLC cells, we examined its effect on the phenotype of drug resistance in two ways. First, we tested the effects of silibinin on drug efflux mediated by the Pgp membrane transporter. Our results (Figure 6) showed that preincubation for 4 days in silibinin was effective in blocking the drug efflux phenotype in drug-resistant SCLC cells (Figure 6).
Silibinin did not block efflux when presented to cells for 45 min, indicating that its effect is probably not a result of directly inhibiting Pgp-mediated drug efflux. There might be effects on Pgp expression over the 4 days of silibinin pretreatment, which would indirectly affect drug efflux activity and drug resistance. Second, with a view to clinical use, we performed experiments in which drug-resistant SCLC cells incubated in silibinin were tested for resistance to etoposide and doxorubicin, two drugs commonly used in SCLC chemotherapy[2]. Our data (Table 1) showed that silibinin effectively reversed the drug resistance phenotype. From our quantitative analysis of drug combination experiments, silibinin was synergistic with both etoposide and doxorubicin (Table 2). This indicates that with the addition of silibinin, a dose reduction of etoposide and/or doxorubicin may be possible with clinical benefit in drug-resistant SCLC.
Taken together, our data indicate that in SCLC, silibinin is pro-apoptotic, reverses drug resistance and acts synergistically with chemotherapeutic drugs. As a non-toxic natural product, silibinin may be useful in treatment of SCLC.
Acknowledgements
We thank Ms. L. Brown for assistance with flow cytometry, Ms. C. Donohue for assistance with synergism experiments, Drs. R. Agarwal, L. Gu, and W. Tiemann for discussions and the Alpern Foundation and NIH-NCI (CA137914) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Govindan R, Page N, Morgensztern D. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Pass HI, Carbone DP, Johnson DH, Minna JD, Scagliotti G, Turrisi, editors. Principles and Practice of Lung Cancer. 4th ed. Philadelphia: Lippincott Williams Wilkins; 2010. pp. 847–865. [Google Scholar]
- 3.Nishio W, Nakamura T, Koh Y, Suzuki T, Fukumoto H, Saijo N. Drug resistance in lung cancer. Curr. Opin. Oncol. 1999;11:109–115. doi: 10.1097/00001622-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ladas E, Kroll DJ, Kelly KA. Milk Thistle. In: Coates PM, Blackman MR, Cragg GM, Levine M, White JD, editors. Encyclopedia of Dietary Supplements. New York: Marcel Dekker; 2005. [Google Scholar]
- 5.Wagner H, Diesel P, Seitz M. Arzneimittelforschung. 1974;24:466–471. [PubMed] [Google Scholar]
- 6.Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME. Complete isolation and characterization of silibins and isosilybins from milk thistle. Org. Biomol. Chem. 2003;1:1684–1689. doi: 10.1039/b300099k. [DOI] [PubMed] [Google Scholar]
- 7.Lahari-Chaterjee M, Katijar SK, Mohan RR, Agarwal RA. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59:622–632. [PubMed] [Google Scholar]
- 8.Singh R, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol. Carcinogenesis. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 9.Singh R, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse. Clin. Cancer Res. 2008;14:7773–3380. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaig TW, Gustafson DL, Su L-J, Zirolli J, Curghton F, Harrison G, Pierson A, Agarwal R, Glode LM. A phase I and pharmacokinetic study of silybinphytosome in prostate cancer patients. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 11.Singh R, Deep G, Chittezehath M, Kaur M, Dwyer-Nield A, Malkinson L, Agarwal R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J. Natl. Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Mallikarjuna G, Sharma G, Tyagi A, Chan D, Agarwal C, Agarwal R. Oral silibinin inhibits lung tumor growth in athymic nude mice. Clin. Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Wang Y, Tan Q, Lubet R, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene induced lung tumorigenesis in A/J mice. Neoplasia. 2005;7 doi: 10.1593/neo.05532. 1-53-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittezhath M, Deep G, Singh R, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down regulates iNOS in human lung carcinoma A549 cells. Mol. Cancer Ther. 2008;7:1817–1826. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramasamy K, Agarwal R. Multi-targeted therapy of cancer by slilymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minato K, Kanzawa F, Nishio K, Nakagawa K, Fujiwara Y, Saijo N. Characterization of an etoposide-resistant human small-cell lung carcinoma line. Cancer Chemother. Pharmacol. 1990;26:313–327. doi: 10.1007/BF02897284. [DOI] [PubMed] [Google Scholar]
- 17.Sadava D, Ahn J, Zhan M, Pang M-L, Ding J, Kane SE. Effects of four Chinese herbal extracts on drug-sensitive and multidrug-resistant small-cell lung carcinoma cells. Cancer Chemother. Pharmacol. 2002;49:261–266. doi: 10.1007/s00280-002-0427-5. [DOI] [PubMed] [Google Scholar]
- 18.Sadava D, Hamman W. RNA interference against MDR1 but not MRP1 reverses drug resistance in small-cell lung carncer cells. J. Cancer Mol. 2007;3:91–94. [Google Scholar]
- 19.Homolya L, Hollo Z, Germann UA, Pastan I, Gottesman MM, Sarkadi H. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 20.Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 21.Earnshaw W, Martins S, Kaufmann S. Mammalian caspases: structure, activation, substrates and functions during apoptosis. Ann. Rev. Biochem. 1999;69:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 22.Zaffaroni N, Daidone M. Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug. Resist. Update. 2002;5:65–72. doi: 10.1016/s1368-7646(02)00049-3. [DOI] [PubMed] [Google Scholar]