Abstract
Objective
Whether prolonged exposure to stimulant medication in childhood for the treatment of attention deficit/hyperactivity disorder (ADHD) increases the risk for developing abnormalities in blood pressure or heart rate is unknown. We examined the association between stimulant medication and blood pressure and heart rate over 10 years.
Method
The Multimodal Treatment Study of Children with ADHD (MTA) randomized 579 children, age 7–9 years, to 14-months of behavior therapy, intensive medication treatment, the combination of the two, or community management. The controlled trial was followed by naturalistic treatment with periodic assessments. Blood pressure and heart rate data were first analyzed with linear regression models according to an intent-to-treat approach, using raw data and blood pressure categories of pre-hypertension and hypertension. Currently medicated subjects were then compared with never or no longer medicated subjects. Associations between cumulative stimulant cumulative exposure (in methylphenidate-equivalents) and blood pressure or heart rate were assessed.
Results
No treatment effect on either systolic or diastolic blood pressure could be detected. Children treated with intensive stimulant medication had higher heart rate (mean 84.2 bpm ±SD 12.4 on medication alone and 84.6 ± 12.2 on medication plus behavior therapy) than those treated with behavior therapy alone (79.1±12.0) or the community comparison group (78.9±12.9) at the end of the 14-month controlled trial (p=.01), but not thereafter. Stimulant medication did not increase the risk for tachycardia, but greater cumulative stimulant exposure was associated with higher heart rate at years 3 and 8.
Conclusions
No evidence could be found that stimulant treatment increased the risk for pre-hypertension or hypertension over the 10-year period of observation. Stimulants, however, had a persistent adrenergic effect on heart rate if continued.
ClinicalTrials.gov NCT: 00000388
Keywords: stimulants, children, safety, blood pressure, pulse
Methylphenidate and amphetamines are commonly used in the treatment of attention deficit/hyperactivity disorder (ADHD). By increasing noradrenergic and dopaminergic transmission, these agents exert sympathomimetic activity that is associated with cardiovascular effects(1). Several placebo-controlled investigations have documented a statistically significant increase in heart rate and blood pressure with therapeutic doses of stimulants, in both children and adults(2–6). In children, average increases of 6 to 8 beats per minute (bpm) in heart rate, 3 to 6 mmHg in systolic blood pressure, and 3–4mmHg in diastolic blood pressure over placebo were reported after methylphenidate or amphetamine administration(2,3,5). Some studies found a positive correlation between stimulant dose and cardiovascular changes(7). But not all reports have been consistent, and some adequately powered studies did not find differences between stimulants and placebo after acute treatment (8).Studies for up to 2 years of stimulant treatment suggest that attenuation of acute effects occurs with chronic treatment, but without development of full tolerance (4, 9,10).
Although the magnitude of the cardiovascular changes during stimulant treatment has been dismissed by some as clinically insignificant (3,4,8,10,11), even modest increases in blood pressure or heart rate, when sustained in time, may have relevance as the risk for cardiovascular disease increases monotonically with rising values of blood pressure in young adults(12). Available reports are limited to a few weeks of controlled stimulant administration and up to two years of uncontrolled treatment. It remains unclear whether stimulant treatment in childhood increases the risk for hypertension or for persistently, though modestly, elevated cardiovascular parameters, in future years(13)
In order to clarify the possible clinical significance of stimulant-induced cardiovascular effects, we analyzed the data from the Multimodal Treatment Study of Children with ADHD (MTA), a 14-month randomized controlled clinical trial in 7–9 year-old-children, which was followed by naturalistic treatment with periodic assessment up to 10 years post-randomization. Specifically, we examined whether exposure to stimulant medication was associated with increased heart rate, systolic or diastolic blood pressure, or blood pressure values in the pre-hypertension or hypertension range, over the 10-year period.
Methods
Study Design and Subjects
The data utilized for these analyses were collected as part of the MTA, a publicly funded, multisite, randomized, controlled clinical trial that compared the effectiveness of different treatment interventions for children with ADHD. Design, methods, and main clinical outcomes have been reported in detail(14–18). A total of 579 children, aged 7–9 years(mean 8.5 years), 80% male, 61% White, 20% African American, and 8% Hispanic, with a DSM-IV diagnosis of combined-type ADHD, were randomly assigned to a 14-month treatment with medication(7 days a week, with no interruptions for summer holidays), behavior therapy, a combination of medication and behavior therapy, or usual community care. To enter the study, children had to be medically healthy, without evidence of cardiovascular disease by history or physical examination. After the 14-month controlled trial, all the children received naturalistic community treatment and were assessed at specified time points (2,3, 6, 8, and 10 years after randomization). Intent-to-treat analyses based on random assignment identified statistically significant differential treatment effects on ADHD symptoms at the end of the controlled study and up to 10-month afterwards (year 2),but not at subsequent assessments(15, 18, 19).
Starting with year 2, a local normative comparison group was added to the follow-up study. This group consisted of 289 children who were randomly selected from the same schools and grades and in the same sex proportion as the MTA subjects, and who met the same entry criteria except for ADHD diagnosis (but ADHD was not a reason for exclusion). Their blood pressure and heart rate were assessed in the same manner as in the MTA subjects.
The data were collected between 1994 and 2006 at the following clinical sites: University of California, Berkley/University of California, San Francisco; Duke University Medical Center; University of California, Irvine; Long Island Jewish Medical Center and New York University; McGill University/Montreal Children’s Hospital; University of Pittsburgh; and Columbia University/New York State Psychiatric Institute and Mount Sinai Medical Center, New York.
Stimulant Medication
A total of 289 children were randomly assigned to receive medication treatment, either alone or in combination with behavior therapy. Immediate release methylphenidate was the first-step treatment. Non-responders were given d-amphetamine and, in case of lack of efficacy, other agents. Methylphenidate accounted for 85% of stimulant use in the first 14 months. Stimulant medication was given in 2–3 daily doses, 7 days a week for 14 months. Of the children assigned to community treatment, 92 (63.07%)received stimulant medication and 5 others received non-stimulant medication for ADHD. A few children (N=32) in the behavior therapy group reported taking stimulant medication through their private pediatrician during the 14-month trial. This non-study use was accounted for in the analyses by controlling for current use. At the end of the 14-months, the mean daily dose of stimulant, in methylphenidate-equivalents, was 22.6 mg in the community group, 31.1 mg in the combined medication and behavioral therapy group, and 38.1 mg in the medication only group(20). After month 14, medication use was naturalistically determined, and gradually decreased over time. Across all treatment groups, the number of children currently taking stimulant medication was 316 at month 14, 280 at year 2, 257 at year 3, 169 at year 6, 91 at year 8, and 18 at year 10for those still taking it. Most (69.0%) of the youths medicated at year 10 had been medicated at month 14, indicating continuity of treatment for medicated subjects. Consistent with body growth and with previous reports (18), the average daily dose increased with time, and was 54.3 mg of methylphenidate equivalent at year 10. About 4% of the MTA sample received, at some point during the 10-year period, other, non-stimulant psychotropic medications, mainly antidepressants and mood stabilizers. For 7 of the 289 subjects in the normative comparison group, there was report of use of some psychotropic medication (antidepressants in 6 cases, and atomoxetine in one case).
Blood Pressure and Heart Rate Measurement
The procedure for assessing pulse and blood pressure was as follows: after the subject had been sitting for 5 minutes, the heart rate was obtained with automatic monitor or manually counted for at least 30 seconds. Immediately afterwards, the blood pressure was measured in the right arm using a cuff of adequate size for the subject’s arm. If any of the measurements were out-of-range (i.e., >100 bpm for pulse, >120 mmHg for systolic blood pressure, and >80 for diastolic blood pressure), the measurement was repeated after the subject had been sitting for an additional 5 minutes, and the lower reading was recorded. There were some site differences in the procedure, but at each site all four randomly assigned groups were assessed in the same way. At one of the sites, three immediately subsequent recordings were obtained from each subject; the first reading was automatically discarded, and the average of the latter two was recorded. At4 sites, blood pressure and heart rate were measured using an automatic blood pressure and heart rate monitor, while at the other 3 sites blood pressure was measured with manual sphingomanometry with ausculatory method, and the heart rate with manual measurement at the radial artery pulse. At each visit, body height and weight were also measured, and the youths and their families were queried about the occurrence of significant medical problems, hospitalizations, and other medical services. Time of the assessment varied during the day, as did the time since the last medication dose. Clinicians collecting these measurements were not blind to treatment assignment.
Data Analyses
The database was centrally managed and quality-assured at the National Institute of Mental Health, Bethesda, Md. Statistical analyses were conducted at the University of Illinois Center for Health Statistics, Chicago, Il.
Blood pressure data were analyzed both as absolute values and after classification into the categories of normal, pre-hypertension, hypertension stage 1, and hypertension stage 2, according to age-, sex-, and height-adjusted percentiles from U.S. population norms for children and adolescents through age 17(21). Height percentiles were computed according to the 2002 U.S. Centers for Disease Control and Prevention population norms(22). Blood pressure status was classified as normal if both systolic and diastolic blood pressure were<90th percentile; pre-hypertension if the systolic or/and diastolic blood pressure was ≥90th but <95th percentile; hypertension stage 1 if the systolic or/and diastolic blood pressure was ≥95th but <99th percentile; and hypertension stage 2, if the systolic or/and diastolic blood pressure was ≥99th percentile(21).
For subjects older than 17 years, adult criteria for blood pressure (in mmHg)were used, according to which normal is a systolic blood pressure <120 and a diastolic blood pressure <80; pre-hypertension is a systolic blood pressure =120–139, or a diastolic blood pressure = 80–89; hypertension stage 1 is a systolic blood pressure =140–159, or a diastolic blood pressure=90–99; and hypertension stage 2 is a systolic blood pressure ≥160, or a diastolic blood pressure ≥100 (23).
The heart rate data were analyzed as absolute values and after categorization into normal or tachycardia based on population norms through age 18(24). Tachycardia was defined as a heart rate above the 95 percentile, based on age and sex. For example, at age 16, the cut-off was 100 bpm for girls and 95 bpm for boys.
For the intent-to-treat analyses, linear regression models were applied to the blood pressure and heart rate data, testing for treatment effects from the randomly assigned treatment conditions. In addition, multinomial logistic regression models (under the assumption of proportional odds)were applied to the blood pressure data categorized into normal, pre-hypertension, hypertension stage 1, and hypertension stage 2, and to the heart rate data classified as normal or abnormal. All models included baseline values, site, and race (African American vs. non-African American, given the higher risk for hypertension among African Americans), as covariates, and body mass index and current medication dose as time-varying covariates.
To further account for stimulant use beyond the controlled phase of the study, at each assessment point in the naturalistic follow-up, the sample was classified into the following groups: “never medicated”, “currently medicated”, and “previously medicated” (but not currently on medication), and analyses of blood pressure categories were conducted with these groups.
For analyses testing for possible association between cumulative dose exposure and the blood pressure or heart rate values regardless of random treatment assignment, multinomial logistic regression models were applied. For each subject, the cumulative dose of methylphenidate received up to each point of assessment was computed. Information about the dose was obtained from interviewing the participants and their parents. Amphetamine doses were multiplied by 2 for conversion into methylphenidate-equivalent doses(25).Overall cumulative exposure over the 10-year assessment ranged from 0 to 328,976mg, with 25th percentile being 7,898 mg and 75th percentile 43,460 mg. At each assessment time-point, based on the cumulative dose thus far received, each subject was assigned to one of the following four exposure categories: no medication (0 mg), low (0<cumulative dose≤7,898 mg), medium (7,898 mg<cumulative dose;≤43,460 mg), or high (cumulative dose>43,460 mg). Analyses included baseline values, site, and race (African American vs. non-African American), as covariates, and body mass index and current medication dose as time-varying covariates.
Furthermore, a number of other sensitivity and complementary analyses were conducted: a) a logistic regression model was fitted at each time point using the continuous cumulative stimulant dose variable after log transformation; b) similar multinomial logistic regression models were conducted after combining the four blood pressure categories into three (i.e., normal, pre-hypertension, and hypertension), or two categories (i.e., normal vs. pre-hypertension/hypertension); c) generalized estimating equation methods were used to fit a multinomial logistic regression model simultaneously to all repeated measurements; d) the analyses described above were repeated using the average daily dose of stimulant medication received for at least 15 days during the 30 days prior to the assessment and categorized into no medication (0 mg/day), low (1–24 mg/day), medium(25–40 mg/day), or high (greater than 40 mg/day), in lieu of the cumulative dose percentile method described above; e) the possible effect of actual medication use on the same day of the assessment was examined in a multinomial logistic regression model with cumulative exposure, and also in a longitudinal analysis using generalized estimating equation; f) the effect of medication use on the same day of the assessment was examined in the models with average daily dose, described in d) above; g) stimulant exposure was defined based on percentage of time spent on stimulant medication, as consistent with other analyses of this database that focused on clinical outcomes and physical growth (26). Based on this approach, being medicated was defined as having been treated with a stimulant at least 50% of the days since the previous assessment point, and the sample was divided into the groups of always, sometime, and never/seldom medicated based on the status at each assessment time point. BP and pulse data were reanalyzed using these categories of exposure and the normative comparison group, by fitting mixed-effects models that included site, race, BMI, and stimulant use as a time-varying covariate;
The relationship between cumulative dose, average daily dose, and current medication use, and heart rate was examined based on multiple regression analyses, adjusted for age, race (African American vs. others), study site, and baseline heart rate, and following the same approach described for blood pressure. Logistic regression models were not used to analyze abnormally elevated heart rate due to the fact that very few subjects (i.e., <1.8%) had heart rate above the upper normal range at each time point.
For all the analyses, the statistical significance was set at two-tailed alpha ≤.05, despite multiple tests, in order to prevent Type II error.
Results
Sample Retention
Of the 579 randomized subjects, 506(87.4%)provided BP and HR data at months 14, 505(87.2%) at year 2, 455(78.6%)at year 3, 419 (72.4%)at year 6, 376(64.92)at year 8, and 346(59.8%)at year 10. A comparison of the sample retained through year 10 (N=346) and that was not retained (N=233) showed a lower proportion of males in the retained (76.0%) vs. the non-retained (86.7%), but otherwise no significant differences in age, race, baseline systolic or diastolic blood pressure, heart rate, or distribution among the four randomly assigned treatment groups.
During the controlled trial (first 14 months), no cardiovascular adverse effects leading to drug discontinuation or decrease in drug dosage occurred. During the subsequent naturalistic treatment phase, no cardiovascular event leading to emergency evaluation or hospitalization, or episode of stimulant discontinuation due to cardiovascular adverse events was reported. Three deaths were recorded among the ADHD participants during the 10 years of observation: a suicide at age 14(subject was on methylphenidate), a car accident while being the driver at age 17(subject was on methylphenidate), and a sudden unexplained death of a 17-year-old boy (subject was found dead in bed; no specific cause of death could be determined; he had been previously treated with methylphenidate and had been off medication for more than one year when he died).
Intent-to-treat Analyses of Randomized Treatment Groups
Intent-to-treat analyses of raw systolic and diastolic blood pressure data or of the hypertension categories did not identify any statistically significant treatment related effects on any of these measures, either at month 14 (end of the controlled trial), or afterwards (Tables1 and 2). There was a significant time effect, consistent with physiological increase of blood pressure with age, and significant site effects, due to differences in blood pressure measurement procedures, but no significant site by treatment effects. In particular, at month 14, no site by treatment effect was found for systolic (p=.31)or diastolic blood pressure (p=.75).
Table 1.
Blood Pressure and Heart Rate by Randomly Assigned Treatment Group
| Treatment Group | Time (Month) | Systolic Blood Pressure (mmHg)
|
Diastolic Blood Pressure (mmHg)
|
Heart Rate (bpm)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | mean | SD | n | mean | SD | ||
| Comb | 0 | 143 | 99.5 | 10.3 | 143 | 66.1 | 8.1 | 145 | 84.6 | 10.9 |
| Comb | 14 | 132 | 102.6 | 10.2 | 132 | 66.5 | 10.4 | 135 | 84.6 | 12.2 |
| Comb | 24 | 134 | 104.0 | 11.5 | 134 | 67.4 | 11.0 | 135 | 80.4 | 12.1 |
| Comb | 36 | 118 | 107.3 | 13.4 | 118 | 67.0 | 10.7 | 119 | 80.2 | 12.2 |
| Comb | 72 | 109 | 116.8 | 13.7 | 109 | 67.1 | 9.7 | 113 | 71.3 | 10.6 |
| Comb | 96 | 101 | 120.1 | 15.5 | 101 | 65.4 | 9.0 | 102 | 69.8 | 11.0 |
| Comb | 120 | 93 | 119.6 | 12.8 | 93 | 67.7 | 9.9 | 93 | 69.0 | 12.5 |
| Med | 0 | 142 | 101.0 | 9.8 | 142 | 66.0 | 7.9 | 143 | 83.4 | 11.1 |
| Med | 14 | 125 | 102.4 | 9.7 | 125 | 67.6 | 9.6 | 128 | 84.2 | 12.4 |
| Med | 24 | 115 | 104.4 | 11.6 | 113 | 67.6 | 11.0 | 117 | 81.6 | 12.2 |
| Med | 36 | 106 | 107.8 | 12.3 | 106 | 65.0 | 9.4 | 108 | 76.6 | 12.2 |
| Med | 72 | 96 | 116.4 | 12.7 | 96 | 66.9 | 8.4 | 96 | 72.2 | 11.5 |
| Med | 96 | 89 | 119.8 | 15.6 | 89 | 66.8 | 11.0 | 91 | 71.0 | 12.6 |
| Med | 120 | 77 | 122.2 | 14.8 | 77 | 67.6 | 9.8 | 78 | 69.9 | 11.2 |
| Beh | 0 | 140 | 99.4 | 10.1 | 140 | 65.6 | 9.0 | 142 | 85.3 | 13.3 |
| Beh | 14 | 121 | 103.2 | 10.3 | 121 | 68.9 | 9.1 | 125 | 79.1 | 12.0 |
| Beh | 24 | 128 | 104.4 | 10.9 | 128 | 67.9 | 11.6 | 131 | 79.1 | 12.4 |
| Beh | 36 | 113 | 108.4 | 11.2 | 113 | 66.3 | 11.6 | 116 | 76.3 | 12.7 |
| Beh | 72 | 109 | 114.3 | 12.9 | 109 | 66.3 | 9.0 | 110 | 71.3 | 13.3 |
| Beh | 96 | 97 | 119.1 | 14.0 | 97 | 67.1 | 9.6 | 98 | 70.5 | 11.0 |
| Beh | 120 | 92 | 119.1 | 15.0 | 92 | 68.6 | 11.0 | 92 | 68.6 | 12.4 |
| CC | 0 | 142 | 99.0 | 9.9 | 142 | 64.4 | 8.2 | 143 | 84.5 | 11.4 |
| CC | 14 | 115 | 104.1 | 10.6 | 115 | 67.8 | 8.8 | 118 | 78.9 | 12.9 |
| CC | 24 | 120 | 102.7 | 11.0 | 120 | 65.7 | 10.4 | 122 | 78.8 | 12.1 |
| CC | 36 | 109 | 106.8 | 12.7 | 108 | 64.0 | 10.6 | 112 | 77.8 | 11.6 |
| CC | 72 | 96 | 116.7 | 12.4 | 96 | 64.6 | 8.3 | 100 | 71.3 | 11.7 |
| CC | 96 | 85 | 119.0 | 13.5 | 85 | 66.8 | 8.9 | 85 | 70.6 | 13.7 |
| CC | 120 | 81 | 119.4 | 11.7 | 81 | 68.3 | 8.8 | 83 | 72.4 | 12.1 |
Comb: combined medication management and behavior therapy; Med: medication management; Beh: behavioral therapy; CC: community control.
Linear regression models, with site and race (African American vs. non-African American) as covariates, and current BMI and stimulant medication dose (in mg of methylphenidate) as time-varying covariates:
Systolic Blood Pressure: significant time(p<.0001), site (p<.0001), BMI (p<.0001), and medication dose effects (p=.02); no significant race (p=0.49), treatment (p=.43), or treatment*time effects (p=.85).
Diastolic Blood Pressure: significant time(p<.0001), site (p<.0001), and BMI effects (p<.0001); no significant race (p=0.99), medication dose (p=.08), treatment (p=.15), or treatment*time effects (p=.62).
Heart Rate: significant time (p<.0001), site (p<.0001), BMI (p<.0001), race (p<.01), medication dose (p<.0001) and treatment*time (p=.02) effects; no significant treatment effect (p=.45). A total of 42 pair-wise comparisons were run. Significant pair-wise comparisons: Med>Beth (p=.05), Med>CC (p=.01), Comb>Beh (p=.01), and Comb>CC (p<.01), at month 14; Comb>Med (p=.01)at month 36; and CC>Beh (p<.01) at month 120 (p’s not corrected for multiple comparisons).
Table 2.
Blood Pressure Categories by Randomly Assigned Treatment Group
| Treatment Group | Time (Month) | Normal
|
Pre-hypertension
|
Hypertension Stage 1
|
Hypertension Stage 2
|
Total Subjects (n) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Comb | 0 | 97 | 67.8 | 23 | 16.1 | 22 | 15.4 | 1 | 0.7 | 143 |
| Comb | 14 | 87 | 65.9 | 16 | 12.1 | 28 | 21.2 | 1 | 0.8 | 132 |
| Comb | 24 | 85 | 63.4 | 18 | 13.4 | 28 | 20.9 | 3 | 2.2 | 134 |
| Comb | 36 | 77 | 65.3 | 15 | 12.7 | 21 | 17.8 | 5 | 4.2 | 118 |
| Comb | 72 | 70 | 64.2 | 20 | 18.3 | 11 | 10.1 | 8 | 7.3 | 109 |
| Comb | 96 | 53 | 52.5 | 25 | 24.8 | 17 | 16.8 | 6 | 5.9 | 101 |
| Comb | 120 | 54 | 58.1 | 31 | 33.3 | 8 | 8.6 | 0 | 0.0 | 93 |
| Med | 0 | 97 | 68.3 | 24 | 16.9 | 20 | 14.1 | 1 | 0.7 | 142 |
| Med | 14 | 90 | 72.0 | 14 | 11.2 | 19 | 15.2 | 2 | 1.6 | 125 |
| Med | 24 | 79 | 69.9 | 12 | 10.6 | 17 | 15.0 | 5 | 4.4 | 113 |
| Med | 36 | 74 | 69.8 | 10 | 9.4 | 20 | 18.9 | 2 | 1.9 | 106 |
| Med | 72 | 64 | 66.7 | 11 | 11.5 | 17 | 17.7 | 4 | 4.2 | 96 |
| Med | 96 | 52 | 58.4 | 19 | 21.3 | 14 | 15.7 | 4 | 4.5 | 89 |
| Med | 120 | 35 | 45.5 | 35 | 45.5 | 5 | 6.5 | 2 | 2.6 | 77 |
| Beh | 0 | 107 | 76.4 | 11 | 7.9 | 20 | 14.3 | 2 | 1.4 | 140 |
| Beh | 14 | 74 | 61.2 | 21 | 17.4 | 25 | 20.7 | 1 | 0.8 | 121 |
| Beh | 24 | 85 | 66. | 16 | 12.5 | 25 | 19.5 | 2 | 1.6 | 128 |
| Beh | 36 | 81 | 71.7 | 10 | 8.8 | 19 | 16.8 | 3 | 2.7 | 113 |
| Beh | 72 | 72 | 66.1 | 17 | 15.6 | 17 | 15.6 | 3 | 2.8 | 109 |
| Beh | 96 | 67 | 69.1 | 12 | 12.4 | 14 | 14.4 | 4 | 4.1 | 97 |
| Beh | 120 | 56 | 60.9 | 22 | 23.9 | 12 | 13.0 | 2 | 2.2 | 92 |
| CC | 0 | 110 | 77.5 | 15 | 10.6 | 17 | 12.0 | 0 | 0.0 | 142 |
| CC | 14 | 75 | 65.2 | 19 | 16.5 | 18 | 15.7 | 3 | 2.6 | 115 |
| CC | 24 | 86 | 71.7 | 9 | 7.5 | 24 | 20.0 | 1 | 0.8 | 120 |
| CC | 36 | 79 | 73.1 | 14 | 13.0 | 13 | 12.0 | 2 | 1.9 | 108 |
| CC | 72 | 74 | 77.1 | 3 | 3.1 | 15 | 15.6 | 4 | 4.2 | 96 |
| CC | 96 | 60 | 70.6 | 10 | 11.8 | 12 | 14.1 | 3 | 3.5 | 85 |
| CC | 120 | 47 | 58.0 | 30 | 37.0 | 4 | 4.9 | 0 | 0.0 | 81 |
Based on population derived age-, sex-, and height-adjusted percentiles:
- Normal: <90th percentile for both systolic and diastolic blood pressure
- Pre-hypertension: ≥90th and <95th percentile for either systolic or diastolic blood pressure
- Hypertension Stage 1: ≥95th and <99th percentile for either systolic or diastolic blood pressure
- Hypertension Stage 2: ≥99th percentile for either systolic or diastolic blood pressure
There were 6 records with normal systolic but missing diastolic blood pressure; these records were set to missing for categorical blood pressure. There was one record with abnormal systolic but missing diastolic blood pressure; this record was included as abnormal categorical blood pressure.
Proportional odds models, with site and race (African American vs. non-African American) as covariates, and current BMI and stimulant medication dose as time-varying covariates: Significant time (p<.0001), site (p<.0001), and BMI effects (p<.0001); no race (p=.61), medication dose (p=.09); no significant treatment (p=.08), or treatment by time effects (p=.90).
At 14 months, there was a significant treatment by time effect (p=.02) on heart rate, with children randomized to medication having higher heart rate(mean±SD: 84.2±12.4 in the medication only group, and 84.6±12.0 in the combined medication plus behavior therapy group) than those randomized to behavior therapy alone (79.1±12.0) or community treatment (78.9±12.0). The incidence of tachycardia did not differ by treatment group: 1/128 (0.8%) in the medication only, 3/135(2.2%)in the combined treatment, 1/125(0.8%),in the behavior therapy only, and 3/119(2.5%)in the community group.
In further years, no significant treatment effect on heart rate was found with pair-wise comparisons, except for a greater heart rate at year 3 in the medication plus behavior therapy group as compared with the medication alone group(p=.01), and a greater heart rate at year 10 in the community treatment group than in the behavior therapy alone group (p<.01; p’s were not corrected for multiple comparisons; a total of 29 pair-wise comparisons were conducted).
Stimulant Exposure and Blood Pressure and Heart Rate Over 10 Years
No association was found between current or previous stimulant use, or cumulative methylphenidate equivalent dose and risk for having blood pressure in the pre-hypertensive or hypertensive range (Tables 3 and 4). At year 10, youths with the highest cumulative exposure had rates of abnormal blood pressure (defined as having blood pressure measurement in the prehypertension or hypertension range at both year 8 and 10) that were not statistically different from those of youths with lower exposure or in the normal comparison group(Tab. 5).
Table 3.
Blood Pressure Categories by Past and Current Stimulant Use and in the Comparison Group
| Time (Month) | Stimulant Use1 | Normal
|
Pre-hypertension
|
Hypertension Stage 1
|
Hypertension Stage 2
|
Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| 24 | Never | 67 | 70.5 | 11 | 11.6 | 16 | 16.8 | 1 | 1.1 | 95 |
| 24 | Currently | 184 | 67.2 | 31 | 11.3 | 52 | 19.0 | 7 | 2.6 | 274 |
| 24 | Previously | 84 | 66.7 | 13 | 10.3 | 26 | 20.6 | 3 | 2.4 | 126 |
| 24 | LNCG | 197 | 69.4 | 42 | 14.8 | 43 | 15.1 | 2 | 0.7 | 284 |
| 36 | Never | 45 | 63.4 | 10 | 14.1 | 13 | 18.3 | 3 | 4.2 | 71 |
| 36 | Currently | 184 | 73.3 | 25 | 10.0 | 39 | 15.5 | 3 | 1.2 | 251 |
| 36 | Previously | 82 | 66.7 | 14 | 11.4 | 21 | 17.1 | 6 | 4.9 | 123 |
| 36 | LNCG | 195 | 75.6 | 24 | 9.3 | 36 | 14.0 | 3 | 1.2 | 258 |
| 72 | Never | 40 | 71.4 | 5 | 8.9 | 9 | 16.1 | 2 | 3.6 | 56 |
| 72 | Currently | 108 | 65.5 | 21 | 12.7 | 28 | 17.0 | 8 | 4.8 | 165 |
| 72 | Previously | 132 | 69.8 | 25 | 13.2 | 23 | 12.2 | 9 | 4.8 | 189 |
| 72 | LNCG | 171 | 72.2 | 28 | 11.8 | 30 | 12.7 | 8 | 3.4 | 237 |
| 96 | Never | 32 | 65.3 | 6 | 12.2 | 8 | 16.3 | 3 | 6.1 | 49 |
| 96 | Currently | 50 | 56.2 | 19 | 21.3 | 16 | 18.0 | 4 | 4.5 | 89 |
| 96 | Previously | 150 | 64.1 | 41 | 17.5 | 33 | 14.1 | 10 | 4.3 | 234 |
| 96 | LNCG | 160 | 69.0 | 44 | 19.0 | 25 | 10.8 | 3 | 1.3 | 232 |
| 120 | Never | 30 | 60.0 | 14 | 28.0 | 6 | 12.0 | 0 | 0.0 | 50 |
| 120 | Currently | 12 | 66.7 | 4 | 22.2 | 2 | 11.1 | 0 | 0.0 | 18 |
| 120 | Previously | 150 | 54.5 | 100 | 36.4 | 21 | 7.6 | 4 | 1.5 | 275 |
| 120 | LNCG | 119 | 56.4 | 72 | 34.1 | 19 | 9.0 | 1 | 0.5 | 211 |
Never: never treated with stimulant medication. Currently: taking stimulant medication for the 30 days prior to the assessment. Previously: past treatment with stimulant medication, but no use for at least 30 days prior to the assessment. LNCG: local normative comparison group(not selected for ADHD and not medicated).
No significant differences in blood pressure categories between stimulant use groups (χ2 at each time point).
Table 4.
Blood Pressure Category by Cumulative Stimulant Use Over Time
| Time (months) | Stimulant Use | Normal
|
Pre-hypertension
|
Hypertension Stage 1
|
Hypertension Stage 2
|
Total | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| 24 | No Medication | 67 | 70.5 | 11 | 11.6 | 16 | 16.8 | 1 | 1.1 | 95 |
| CUM ≤ 7,898 mg | 74 | 68.5 | 8 | 7.4 | 24 | 22.2 | 2 | 1.9 | 108 | |
| 7,898 mg < CUM ≤43,460 mg | 193 | 66.3 | 36 | 12.4 | 54 | 18.6 | 8 | 2.7 | 291 | |
| CUM > 43,460 mg | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | |
| LNCG | 197 | 69.4 | 42 | 14.8 | 43 | 15.1 | 2 | 0.7 | 284 | |
| 36 | No Medication | 45 | 63.4 | 10 | 14.1 | 13 | 18.3 | 3 | 4.2 | 71 |
| CUM ≤ 7,898 | 41 | 67.2 | 8 | 13.1 | 9 | 14.8 | 3 | 4.9 | 61 | |
| 7,898 mg < CUM ≤ 43,460 mg | 191 | 73.2 | 26 | 10.0 | 39 | 14.9 | 5 | 1.9 | 261 | |
| CUM > 43,460 mg | 34 | 65.4 | 5 | 9.6 | 12 | 23.1 | 1 | 1.9 | 52 | |
| LNCG | 195 | 75.6 | 24 | 9.3 | 36 | 14.0 | 3 | 1.2 | 258 | |
| 72 | No Medication | 40 | 71.4 | 5 | 8.9 | 9 | 16.1 | 2 | 3.6 | 56 |
| CUM ≤ 7,898 | 28 | 75.7 | 3 | 8.1 | 5 | 13.5 | 1 | 2.7 | 37 | |
| 7,898 mg < CUM ≤ 43,460 mg | 104 | 69.3 | 20 | 13.3 | 21 | 14.0 | 5 | 3.3 | 150 | |
| CUM > 43,460 mg | 108 | 64.7 | 23 | 13.8 | 25 | 15.0 | 11 | 6.6 | 167 | |
| LNCG | 171 | 72.2 | 28 | 11.8 | 30 | 12.7 | 8 | 3.4 | 237 | |
| 96 | No Medication | 32 | 65.3 | 6 | 12.2 | 8 | 16.3 | 3 | 6.1 | 49 |
| CUM ≤ 7,898 mg | 23 | 74.2 | 4 | 12.9 | 4 | 12.9 | 0 | 0.0 | 31 | |
| 7,898 mg < CUM ≤ 43,460 mg | 73 | 64.0 | 20 | 17.5 | 15 | 13.2 | 6 | 5.3 | 114 | |
| CUM > 43,460 mg | 104 | 58.4 | 36 | 20.2 | 30 | 16.9 | 8 | 4.5 | 178 | |
| LNCG | 160 | 69.0 | 44 | 19.0 | 25 | 10.8 | 3 | 1.3 | 232 | |
| 120 | No Medication | 30 | 60.0 | 14 | 28.0 | 6 | 12.0 | 0 | 0.0 | 50 |
| CUM ≤ 7,898 | 16 | 61.5 | 6 | 23.1 | 3 | 11.5 | 1 | 3.8 | 26 | |
| 7,898 mg< CUM ≤43,460 mg | 54 | 54.0 | 39 | 39.0 | 6 | 6.0 | 1 | 1.0 | 100 | |
| CUM > 43,460 mg | 92 | 55.1 | 59 | 35.3 | 14 | 8.4 | 2 | 1.2 | 167 | |
| LNCG | 119 | 56.4 | 72 | 34.1 | 19 | 9.0 | 1 | 0.5 | 211 | |
CUM: cumulative stimulant dose in methylphenidate equivalent mg.
LNCG: local normative comparison group (not selected for ADHD and not medicated).
Proportional odds models with 4(normal, prehypertension, hypertension stage 1, and hypertension stage 2), 3(normal, prehypertension, and hypertension), or 2 (normal vs. prehypertension/hypertension) blood pressure categories: no significant differences in any of these models.
No significant treatment effects on hypertension categories emerged in any of the sensitivity analyses, including dichotomizing the cumulative stimulant dose into no-medication vs. medication; log transformation of the cumulative dose; combining the blood pressure categories into 3 (normal vs. pre-hypertension vs. hypertension) or 2 (normal vs. pre-hypertension and hypertension combined) categories; longitudinal generalized estimating equation models; using average daily dose instead of cumulative dose; and accounting for being currently in treatment with stimulant and having taken the medication the same day of the assessment.
No significant effect of stimulant exposure, defined as “always”, “sometimes”, or “never” based on percentage of days in the past year at each assessment point was found for blood pressure or heart rate, using mixed effects with stimulant use as a time-varying covariate.
Significant effects of stimulant exposure on HR were detected at year 3 (p=.019) and 8 (p<.001), but not at year 10(p=.122)(Table 6). When controlling for current medication use, the effect remained significant at year 8, but not at year 3.
Table 6.
Heart Rate by Cumulative Stimulant Dose
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (months) | Stimulant Category | Current Medication Use | Number of Subjects | Mean | Standard Deviation | Controlled for current medication use | |
| No | Yes | ||||||
|
|
|||||||
| 36 | No Medication | NoMed | 57 | 77.8 | 10.6 | ||
| CUM ≤ 7,898 | NoMed | 39 | 77.6 | 13.8 | |||
| Med | 9 | 80.7 | 12.4 | ||||
| 7,898 < CUM ≤43,460 | NoMed | 75 | 77.1 | 11.7 | |||
| Med | 133 | 78.9 | 11.9 | ||||
| CUM > 43,460 | NoMed | 7 | 78.7 | 12.1 | |||
| Med | 32 | 84.4 | 10.7 | ||||
| LNCG | 199 | 76.1 | 11.8 | ||||
| p=.019 | p=.084 | ||||||
| 96 | No Medication | NoMed | 50 | 66.4 | 8.7 | ||
| CUM ≤7,898 | NoMed | 32 | 69.6 | 13.4 | |||
| Med | 1 | 74.0 | |||||
| 7,898 < CUM ≤43,460 | NoMed | 106 | 69.3 | 12.5 | |||
| Med | 9 | 77.1 | 10.8 | ||||
| CUM > 43,460 | NoMed | 128 | 70.4 | 11.7 | |||
| Med | 51 | 76.2 | 11.6 | ||||
| LNCG | 233 | 67.9 | 10.4 | ||||
| P<.001 | p<.001 | ||||||
| 120 | No Medication | NoMed | 50 | 68.9 | 11.0 | ||
| CUM ≤ 7,898 | NoMed | 26 | 70.2 | 14.7 | |||
| Med | 0 | ||||||
| 7,898 < CUM ≤43,460 | NoMed | 98 | 68.1 | 11.3 | |||
| Med | 2 | 82.0 | 5.7 | ||||
| CUM > 43,460 | NoMed | 145 | 70.7 | 12.7 | |||
| Med | 24 | 73.7 | 11.1 | ||||
| LNCG | 212 | 67.7 | 10.4 | ||||
|
|
|||||||
| p=.122 | p=.144 | ||||||
CUM: cumulative stimulant dose in methylphenidate equivalent mg.
LNCG: local normative comparison group (not selected for ADHD and not medicated).
Discussion
These analyses, conducted on the database of a 14-month controlled clinical trial that was followed by naturalistic treatment for a cumulative 10-year period of evaluation, extend the findings from previous studies using much shorter periods of observation. Although this clinical trial was not specifically designed to evaluate cardiovascular function, it provides an opportunity to assess blood pressure and heart rate abnormalities as they are likely to emerge in clinical settings. Despite extensive analyses, taking different approaches to the data, no evidence could be found that intensive, sustained, and continuous treatment with stimulant medication starting at age 7–9 years increased the risk for pre-hypertension or hypertension over a period of 10years of observation. This conclusion was supported by a comprehensive series of sensitivity analyses that were conducted to account for overall, recent, and current exposure.
However, stimulant treatment was found to increase heart rate at several time points, as shown by intent-to-treat analyses at month 14 and significant association with actual stimulant exposure at year 3 and 8. The effect on heart rate after 8 years of treatment indicates that complete tolerance to the adrenergic activity of stimulant medication does not develop. As shown in Table 6, the never medicated group had consistently lower mean heart rate than the intensively medicated groups, although the difference was no longer statistically significant at year 10, possibly due to the smaller number of subjects still on medication at this time-point. The effect on heart rate was in large part driven by current use of medication, although at one time point (8 years) there was a significant effect of cumulative exposure regardless of current use.
The clinical implication of persistent adrenergic stimulation, especially for individuals with underlying heart abnormalities are unclear, and cannot be elucidated from these data, but a graded relationship, independent of systolic blood pressure, between increasing values of resting heart rate and mortality is well documented epidemiologically in adults(27–30). Thus, the adrenergic effect of stimulants cannot be dismissed and should be reason for concern and further evaluation of the long-term safety of these medications. To this end, the recent launching of the publicly funded study ADHD Drugs Use Chronic Effects (ADDUCE) in Europe seems especially timely(31).
No symptomatic cardiovascular events leading to medical attention were reported during the period of observation, and no stimulant treatment discontinuation consequent to cardiovascular adverse effects occurred during the 10-year period. This study sample might have been too small to detect the association between stimulant use and increased risk of cardiac emergency room visits for cardiac symptoms that has been reported in large epidemiological databases (32). Moreover, participants underwent a selection process to enter the study that excluded children with significant medical conditions. An issue of great concern has been a possible link between therapeutic use of stimulants and increased risk for sudden cardiac death in youth(33,34). While the rarity of this event prevents testing for causality through randomized, prospective investigations, it is currently recommended that stimulants generally should not be used in individuals with underlying cardiac abnormalities that may place them at higher vulnerability to the sympathomimetic effects of these medications(35). Whether or not stimulants can increase the risk for sudden death among children with no detectable structural heart abnormality is open to speculation. The MTA sample was selected for absence of history or physical signs of cardiovascular problems. Even though no cardiovascular adverse events were recorded during the 10-year period of observation, the sample size was too small to contribute information about an event for which the annual incidence is estimated to be between 0.6 and 6.2 per 100,000 young people(36). Our data, however, indicate that therapeutic use of stimulants can be accompanied by detectable adrenergic stimulation even after years of ongoing treatment. As a number of cardiac disorders, such as hypertrophic cardiomyopathy, long QT syndrome, or catecholaminergic polymorphic ventricular tachycardia, often entail adrenergic stimulation for arrhythmia induction, stimulant-induced sympathomimetic activity might have clinical implications for some individuals with underlying heart abnormalities(37,38).
A number of limitations must be taken into account in considering these findings. The MTA study was designed to evaluate treatment effects on behavioral outcomes and not specifically focused on assessing cardiovascular parameters. The blood pressure and heart rate measurements were not conducted under double-blind conditions and the methods varied across the clinical sites, with most sites using a manual method, while others used an automatic monitor. This variability does not vitiate the comparison of the randomized treatment groups, which were all measured the same way within a given site. Between-site differences were accounted for by including site as a covariate in the data analyses. It should be noted that at none of the six sites was there a treatment effect on blood pressure at the end of the controlled trial (14 months), thus suggesting that variability in methods between sites did not undermine the results. The time during the day when measurements were made was variable according to when individual patients reported to the clinic for their visits. Moreover, the time since stimulant dosing on the day of the assessments could vary. This lack of standardization is likely to have introduced variability that contributed to experimental error, thus possibly obscuring effects that might have been detected with better standardization.
Another important limitation is that abnormal blood pressure values were not systematically confirmed over three separate assessments as required for a diagnosis of pre-hypertension or hypertension(21). In fact, blood pressure decreases with repeated measurements. In an epidemiological study of school-aged children, abnormally elevated BP was found in 19.4% of the children after the first screening, but in 9.5% after the second, and in only 4.5% after the third assessment (39).Thus, the rates of elevated blood pressure that we report cannot be taken as evidence of clinically defined pre-hypertension or hypertension, but only as an indication of increased risk for these clinical conditions. As a reference, the National Health and Nutrition Examination Survey estimated that an age-adjusted 28.7% of the U.S. adult population has hypertension(40),and there are indications that there is a historical trend for blood pressure to increase over the years(41).
With the stated limitations, these data obtained from a large sample over an extended period of many years suggest that intensive and chronic stimulant treatment does not increase the risk for developing blood pressure in the prehypertension or hypertension range. Stimulant administration, however, continues to have a detectable adrenergic effect even after years of treatment. This effect may have clinical implications, especially for individual patients with underlying heart abnormalities, and deserves further investigation.
Figure 1.
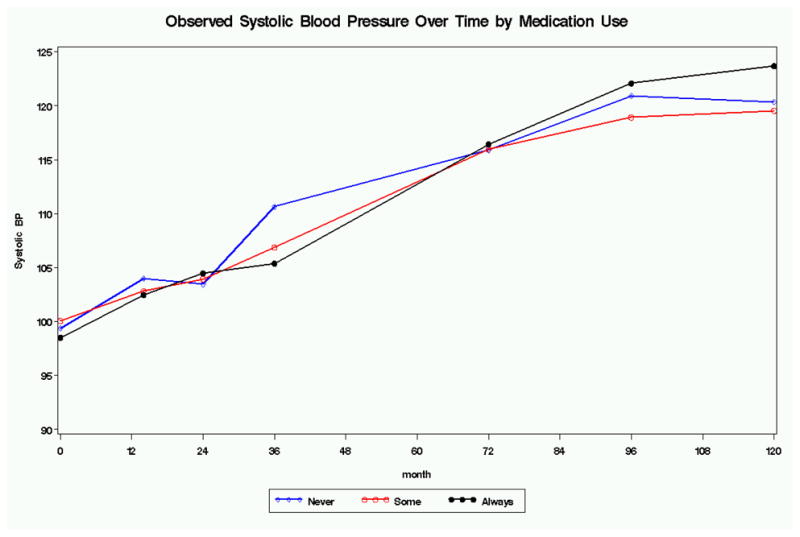

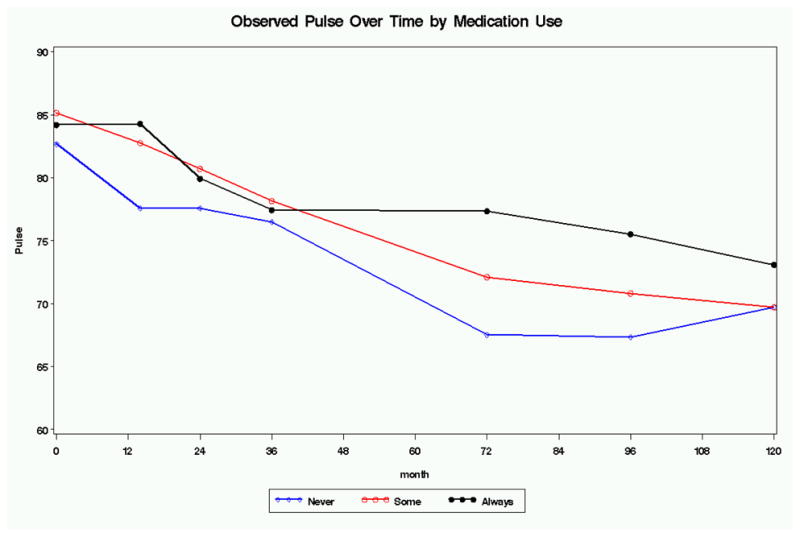
Figure 2.
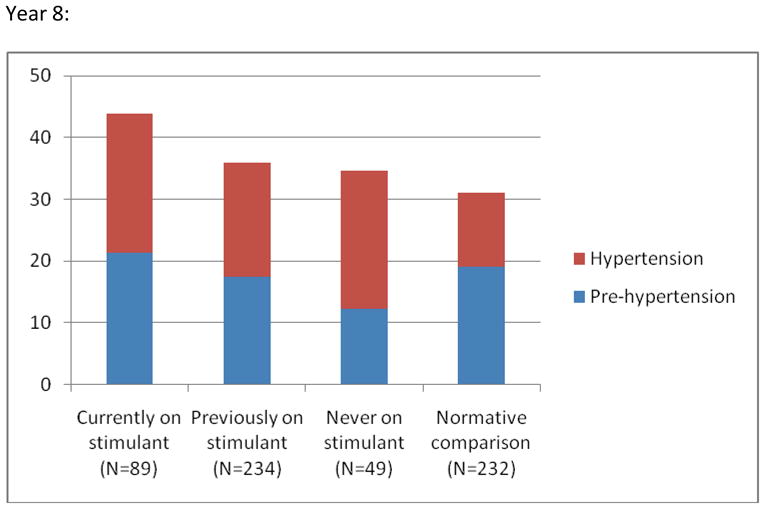
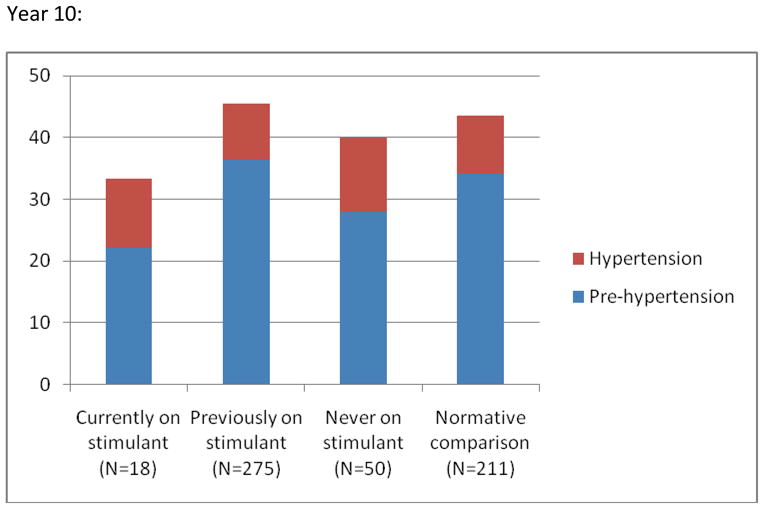
Prevalence (%) of Blood Pressure Reading in the Prehypertension and Hypertension Range by Stimulant Use at Year 8 and 10 Respectivelya
Note: aPre-hypertension is defined as a systolic and/or diastolic reading ≥90th but <95th percentile for age, gender, and height. Hypertension is defined as a systolic and/or diastolic reading ≥95th percentile for age, gender, and height. Based on one reading only and as such not necessarily evidence of hypertension. No statistically significant differences between the groups (see text and table 3 for details).
Table 5.
Rate of Sustained Increase in Blood Pressure by Cumulative Exposure to Methylphenidatea
| Cumulative 10-year methylphenidate dosage (mg)b | N | % with BP ≥90th percentile at years 8 and 10 | ||
|---|---|---|---|---|
| ADHD sample: | Lower 95% C.I. | Upper 95% C.I. | ||
| 0 | 50 | 18.0 | 6.2 | 29.8 |
| 1–7,898 | 26 | 19.2 | 2.4 | 36.1 |
| 7,899–43,460 | 100 | 23.0 | 13.6 | 32.4 |
| >43,460 | 169 | 21.3 | 14.3 | 28.3 |
| Normative comparison group: | ||||
| 0 | 212 | 17.9 | 12.2 | 23.6 |
sBP or/and dBP ≥90th percentile for age, gender, and height. Based on one BP measurement at each year (see text for discussion).
Cumulative stimulant medication exposure, in methylphendiate-equivalent mg. Groups were defined based on cumulative exposure at year 10.
Acknowledgments
Funded by cooperative agreement grants and contracts from the National Institute of Mental Health to the University of California, Berkeley (U01MH50461, N01MH12009, and HHSN271200800005-C), Duke University (U01MH50477, N01MH12012, and HHSN271200800009-C), University of California, Irvine (U01MH50440, N01 MH12011, and HHSN271200800006-C),Research Foundation for Mental Hygiene-New York State Psychiatric Institute/Columbia University (U01MH50467, N01 MH12007, and HHSN271200800007-C),Long Island-Jewish Medical Center (U01MH50453), New York University (N01 MH12004, and HHSN271200800004-C),University of Pittsburgh (U01MH50467, N01 MH12010, and HHSN271200800008-C),and McGill University (N01 MH12008,and HHSN271200800003-C). Statistical analyses were funded by a professional contract to the University of Illinois Center for Health Statistics, Chicago, Il.
Footnotes
The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the National Institute of Mental Health.
Dr. Vitiello had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Dr. Elliott has received research funding from Cephalon, McNeil, Shire, Sigma Tau, and Novartis; has consulted to Cephalon and McNeil; and has been on the speakers’ bureaus of Janssen, Eli Lilly, and McNeil. Dr Swanson has received research support from Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalone, Watson, CIBA, Janssen, and Mcneil; has been on the advisory boards of Alza, Richwood, Shire, Celgene, Novartis, Celltech, UCB, Gliatech, Cepahlon, McNeil, and Eli Lilly; has been on the speakers’ bureaus of Alza, Shire, Novartis, Celltech, UCB, Cephalon, CIBA, Janssen, and McNeil; and has consulted to Alza, Richwood, Shire, Celgene, Novartis, Celltech, UCB, Gliatech, Cephalon, Watson,, CIBA, Janssen, McNeil, and Eli Lilly. Dr. Arnold has received research funding from Celgene, Curemark, Shire, Noven, Eli Lilly, Targacepts, Sigma Tau, Novartis, and Neuropharm; has consulted to Shire, Noven, Sigma Tau, Ross, Organon, Targacept, and Neuropharm; and has been speaker for Abbott, Shire, McNeil, Targacept, and Novartis. Dr. Hechtman has received research funding from the National Institute of Mental Health, Eli Lilly, GlaxoSmithKline, Janssen Ortho, Purdue Pharma, and Shire; has been on the speakers’ bureaus of Eli Lilly, Janssen-Ortho, Purdue Pharma, and Shire; and has been on the advisory board of Eli Lilly, Janssen-Ortho, Purdue Pharma, and Shire. Dr. Abikoff has received research funding from McNeil, Shire, Eli Lilly, and Bristol-Myers-Squibb; has consulted to McNeil, Shire, Eli Lilly, Pfizer, Celltech, Cephalon, and Novartis; and has been on the speakers’ bureaus of McNeil, Shire, and Celltech. Dr. Wigal has received research funding from Eli Lilly, Shire, Novartis, and McNeil; and has been on the spearkers’ bureaus of McNeil and Shire. Dr. Jensen has received research funding from McNeil and unrestricted grants from Pfizer; has consulted to Best Practice, Shire, Janssen, Novartis, Otsuka, and UCB; and has participated in speakers’ bureaus for Janssen,-Ortho, Alza, McNeil, UCB, CMED, CME Outfitters, and the Neuroscience Education Institute. Greenhill has received research funding from or has been a consultant to the National institute of Mental Health, National Institute on Drug Abuse, American Academy of Child and Adolescent Psychiatry, Johnson & Johnson, Otsuka, and Rhodes Pharmaceuticals. Dr. Gibbons has consulted to the US Department of Justice, Wyeth, and Pfizer. Ms. Odbert, Ms. Severe, and Drs. Hur, Kaltman, Wells, Molina, and Vitiello report no relevant financial relationships.
References
- 1.Volkow ND, Wang GJ, Fowler JS, Molina PE, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas NR, Zhu W, Swanson JM. Cardiovascular effects of methylphenidate in humans are associated with increases of dopamine in brain and of epinephrine in plasma. Psychopharmacology (Berl) 2003;166(3):264–270. doi: 10.1007/s00213-002-1340-7. [DOI] [PubMed] [Google Scholar]
- 2.Ballard JE, Boileau RA, Sleator EK, Massey BH, Sprague RL. Cardiovascular responses of hyperactive children to methylphenidate. JAMA. 1976;236(25):2870–2874. [PubMed] [Google Scholar]
- 3.Findling RL, Short EJ, Manos MJ. Short-term cardiovascular effects of methylphenidate and Adderall. J Am Acad Child Adolesc Psychiatry. 2001;40(5):525–529. doi: 10.1097/00004583-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Wilens TE, Biederman J, Lerner M the Concerta Study Group. Effects of once-daily osmotic-release methylphenidate on blood pressure and heart rate in children with attention-deficit/hyperactivity disorder: results from a one-year follow-up study. J Clin Psychopharmacol. 2004;24(1):36–41. doi: 10.1097/01.jcp.0000106223.36344.df. [DOI] [PubMed] [Google Scholar]
- 5.Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21(1):92–95. doi: 10.1007/s00467-005-2051-1. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Mick E, Surman C, Doyle R, Hammerness P, Harpold T, Dunkel S, Dougherty M, Aleardi M, Spencer T. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2006;59(9):829–835. doi: 10.1016/j.biopsych.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Stowe CD, Gardner SF, Gist CC, Schulz EG, Wells TG. 24-hour ambulatory blood pressure monitoring in male children receiving stimulant therapy. Ann Pharmacother. 2002;36 (7–8):1142–1149. doi: 10.1345/aph.1A367. [DOI] [PubMed] [Google Scholar]
- 8.Findling RL, Biederman J, Wilens TE, Spencer TJ, McGough JJ, Lopez FA, Tulloch SJ. Short-and long-term cardiovascular effects of mixed amphetamine salts extended release in children. J Pediatr. 2005;147(3):348–354. doi: 10.1016/j.jpeds.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Safer D. Relative cardiovascular safety of psychostimulants used to treat attention-deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 1992;2(4):279–290. doi: 10.1089/cap.1992.2.279. [DOI] [PubMed] [Google Scholar]
- 10.Hammerness P, Wilens T, Mick E, Spencer T, Doyle R, McCreary M, Becker J, Biederman J. Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr. 2009;155(1):84–9. doi: 10.1016/j.jpeds.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Donner R, Michaels MA, Ambrosini PJ. Cardiovascular effects of mixed amphetamine salts extended release in the treatment of school-aged children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):706–712. doi: 10.1016/j.biopsych.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161(12):1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 13.Nissen S. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354(14):1445–1448. doi: 10.1056/NEJMp068049. [DOI] [PubMed] [Google Scholar]
- 14.Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Pelham WE, Richters JE, Schiller E, Severe JB, Swanson JM, Vereen D, Wells KC. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design, challenges and choices. Arch Gen Psychiatry. 1997;54(9):865–870. doi: 10.1001/archpsyc.1997.01830210113015. [DOI] [PubMed] [Google Scholar]
- 15.The MTA Cooperative Group. A 14-Month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder (ADHD) Arch Gen Psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 16.Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, Arnold LE, Abikoff HB, Bukstein OG, Conners CK, Elliott GR, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Severe JB, Wells K, Wigal T. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40(2):180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Hechtman L, Epstein J, Pelham W, Abikoff HB, Newcorn J, Molina B, Hinshaw S, Wells K, Hoza B, Severe JB, Jensen PS, Gibbons R, Hur K, Stehli A, Davies M, March J, Caron M, Volkow ND, Posner MI for the MTA Cooperative Group. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1014–1026. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- 18.Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal TL, Gibbons RD, Hur K, Houck PR and the MTA Cooperative Group. The MTA at 8 years: prospective follow-up of children treated for combined type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113(4):754–761. doi: 10.1542/peds.113.4.754. [DOI] [PubMed] [Google Scholar]
- 20.Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein O, Elliott GR, Hechtman L, Jensen PS, Hinshaw SP, March JS, Newcorn JH, Swanson JM, Cantwell DP. Methylphenidate dosage for children with ADHD over time under controlled conditions: Lessons from the MTA. J Am Acad Child Adolesc Psychiatry. 2001;40(2):188–196. doi: 10.1097/00004583-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 21.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. National Heart, Blood and Lung Institute. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. [access verified November 30, 2010];Pediatrics. 2004 114(2):555–576. Also available at Website: http://www.nhlbi.nih.gov/guidelines/hypertension/hbp_ped.htm. [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein D. The Cardiovascular System. In: Behrman RE, Kliegman RM, Jensen HB, editors. Nelson Textbook of Pediatricts. 16. WB Saunders Co; Philadelphia, PA: 2000. [Google Scholar]
- 25.Arnold LE, Christopher J, Huesti R, Smeltzer DJ. Methylphenidate vs dextroamphetamine vs caffeine in minimal brain dysfunction. Arch Gen Psychiatry. 1978;35(4):463–473. doi: 10.1001/archpsyc.1978.01770280073008. [DOI] [PubMed] [Google Scholar]
- 26.Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, Hechtman L, Epstein J, Pelham W, Abikoff HB, Newcorn J, Molina B, Hinshaw S, Wells K, Hoza B, Severe JB, Jensen PS, Gibbons R, Hur K, Stehli A, Davies M, March J, Caron M, Volkow ND, Posner MI for the MTA Cooperative Group. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1014–1026. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart disease and cardiovascular mortality: the Framingham Study. Am Hear J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 28.Gillum RF, Makuc DM, Feldman JJ. Pulse rate, coronary heart disease, and death: the NHANES I. Epidemiologic Follow-up Study. Am Heart J. 1991;121(1):172–177. doi: 10.1016/0002-8703(91)90970-s. [DOI] [PubMed] [Google Scholar]
- 29.Jouven X, Empana JP, Escolano S, Buyck JF, Tafflet M, Desnos M, Ducimetiere P. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol. 2009;103(2):279–283. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 30.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159(4):612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 31.European Commission. Attention Deficit Hyperactivity Disorder Drugs Use Chronic Effects (ADDUCE) Website: http://cordis.europa.eu/fetch?CALLER=FP7_PROJ_EN&ACTION=D&DOC=1&CAT=PROJ&RCN=96780.
- 32.Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A. Cardiac safety of central nervous system stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2007;120(6):e1494–501. doi: 10.1542/peds.2007-0675. [DOI] [PubMed] [Google Scholar]
- 33.Gould MS, Walsh BT, Munfakh JL, Kleinman M, Duan N, Olfson M, Greenhill L, Cooper T. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166(9):992–1001. doi: 10.1176/appi.ajp.2009.09040472. [DOI] [PubMed] [Google Scholar]
- 34.Vitiello B, Towbin K. Stimulant treatment of ADHD and risk of sudden death in children. Am J Psychiatry. 2009;166(9):955–957. doi: 10.1176/appi.ajp.2009.09050619. [DOI] [PubMed] [Google Scholar]
- 35.McNeil Pharmaceuticals. [access verified May 27, 2010];Concerta Prescribing Information. Revised November 2009. Available at: http://www.concerta.net/assets/Prescribing_Info-short.pdf.
- 36.Berger S, Utech L, Fran Hazinski M. Sudden death in children and adolescents. Pediatr Clin North Am. 2004;51(6):1653–1677. doi: 10.1016/j.pcl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Anderson KP. Sympathetic nervous system activity and ventricular tachyarrhythmias: recent advances. Ann Non-Invasive Electrocardiol. 2003;8(1):75–89. doi: 10.1046/j.1542-474X.2003.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terai H, Shimizu M, Ino H, Yamaguchi M, Hayashi K, Sakata K, Kiyama M, Hayashi T, Inoue M, Taki J, Mabuchi H. Cardiac sympathetic nerve activity in patients with hypertrophic cardiomyopathy with malignant ventricular tachiarrthythmias. J Nucl Cardiol. 2003;10(3):304–310. doi: 10.1016/s1071-3581(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 39.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113(3):475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 40.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 41.Muntner P, Jiang He, Cutler JA, Widman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]


