Abstract
Rationale
Intracellular Ca2+ concentration ([Ca2+]i) is regulated and signals differently in various subcellular microdomains, which greatly enhances its second messenger versatility. In the heart, sarcoplasmic reticulum (SR) Ca2+ release and signaling is controlled by local [Ca2+]i in the junctional cleft ([Ca2+]Cleft), the small space between sarcolemma and junctional SR. However, methods to directly measure [Ca2+]Cleft are needed.
Objective
To construct novel sensors that allow direct measurement of [Ca2+]Cleft.
Methods and Results
We constructed cleft-targeted [Ca2+] sensors by fusing Ca2+-sensor GCaMP2.2 and a new lower Ca2+-affinity variant GCaMP2.2Low to FKBP12.6, which binds with high affinity and selectivity to ryanodine receptors (RyRs). The fluorescence pattern, affinity for RyRs and competition by un-tagged FKBP12.6 demonstrated that FKBP12.6-tagged sensors are positioned to measure local [Ca2+]Cleft in adult rat myocytes. Using GCaMP2.2Low-FKBP12.6, we showed that [Ca2+]Cleft reaches higher levels with faster kinetics than global [Ca2+]i during excitation-contraction coupling. Diastolic SR Ca2+ leak or sarcolemmal Ca2+ entry may raise local [Ca2+]Cleft above bulk cytosolic [Ca2+]i ([Ca2+]Bulk), an effect that may contribute to triggered arrhythmias and even transcriptional regulation. We measured this diastolic standing [Ca2+]Cleft–[Ca2+]Bulk gradient using GCaMP2.2-FKBP12.6 vs. GCaMP2.2, using [Ca2+] measured without gradients as a reference point. This diastolic difference ([Ca2+]Cleft=194 nmol/L vs. [Ca2+]Bulk=100 nmol/L) is dictated mainly by the SR Ca2+ leak, rather than sarcolemmal Ca2+ flux.
Conclusions
We have developed junctional cleft targeted sensors to measure [Ca2+]Cleft vs. [Ca2+]Bulk, and demonstrated dynamic differences during electrical excitation and a standing diastolic [Ca2+]i gradient which could influence local Ca2+-dependent signaling within the junctional cleft.
Keywords: Junctional cleft, local Ca2+ concentration, GCaMP, calcium signaling, cardiac myocyte, sarcoplasmic reticulum, fluorescent imaging
INTRODUCTION
Ca2+ is a universal second messenger involved in activation/regulation of cellular processes as diverse as neurotransmitter release, muscle contraction, metabolism, hypertrophic signaling, and cell death. To fulfill such diverse roles and yet be specific enough to trigger distinct responses, information is encoded in the amplitude, temporal properties, and subcellular localization of Ca2+ signals. It is now largely recognized that Ca2+ is regulated and signals differently in various subcellular microdomains.1-3 For example, GABA inhibition in hippocampal neurons results in spatially confined inhibition of Ca2+ transients shortly after a back-propagating action potential, which may play a key role in regulation of synaptic plasticity.4
In the heart, Ca2+ release from the sarcoplasmic reticulum (SR) is a key event for excitation-contraction coupling (ECC), ventricular arrhythmias, mitochondrial function and nuclear transcription.5 Cardiac SR Ca2+ release is controlled by the local [Ca2+] in the junctional cleft ([Ca2+]Cleft), the small, restricted space between the apposing sarcolemma (mostly T-tubules) and junctional SR membrane. Voltage-gated L-type Ca2+ channels (LTCC) and Ca2+-activated Ca2+ channels (or ryanodine receptors, RyRs) are located at these junctions in the sarcolemma and SR membrane, respectively, and are essential in this local control.6-8 During electrical excitation, [Ca2+]Cleft is expected to rise higher and faster than in the bulk cytosol.9-12 Through Ca2+-dependent inactivation, [Ca2+]Cleft feeds back on LTCC and thus plays an important role in shaping the action potential waveform. Several studies12-17 suggest that the Na+/Ca2+ exchanger (NCX), the main route for Ca2+ extrusion in cardiac myocytes, also has access to the cleft and influences [Ca2+]Cleft during both diastole and ECC. In diastole, NCX may help to keep [Ca2+]Cleft low and limit spontaneous RyR activation. Indeed, during stochastic RyR opening events, Ca2+ removal by NCX may limit local Ca2+ release within a given RyRs cluster, which limits Ca2+ sparks and waves, favoring smaller, non-spark Ca2+ release events.18 During an action potential and rapid Na+ influx, local NCX may boost [Ca2+]Cleft and the efficacy of local SR Ca2+ release, especially in the latent period before LTCC opening.19
[Ca2+]Cleft could exceed that in bulk cytosol ([Ca2+]Bulk) even during diastole. This is because spontaneous SR Ca2+ release (leak) and Ca2+ influx caused by stochastic openings of LTCCs may create a standing [Ca2+] gradient between the cleft (where Ca2+ influx and release happen) and the bulk cytosol (where most of SR Ca2+ uptake occurs). Indeed, there is functional evidence for elevated [Ca2+]Cleft in NCX knockout cardiac myocytes, that causes reduced LTCC availability and higher ability of the remaining LTCC to trigger SR Ca2+ release.20
Despite its importance, methods to directly measure [Ca2+]Cleft are only beginning to be tested.21,22 Indirect estimates based on the dynamic properties of Ca2+-dependent inactivation of LTCC12,23 and NCX current12,24 during electrically-triggered SR Ca2+ release suggest that [Ca2+]Cleft peaks within 10-20 ms and reaches tens or even hundreds of μM. In contrast, the global Ca2+ transient reaches a maximum of approximately 1 μM within ~70-80 ms.6 While strongly supporting the theory of local control of ECC, such measurements are indirect, difficult to calibrate, depend on the specific membrane localization of these transporters, and provide no information on diastolic [Ca2+]Cleft. Our aim was to construct novel Ca2+ sensors that are specifically targeted to the sarcolemma-SR junctions and thus report local [Ca2+]Cleft. We used these sensors to assess the dynamic changes in [Ca2+]Cleft during ECC and to demonstrate the existence of a standing [Ca2+] gradient during diastole between the cleft and bulk cytosol.
METHODS
Detailed methods are included in the Online Supplement
Mutagenesis of GCaMP2-based Ca2+ sensors, cleft-targeting, plasmid construction, and expression in adenoviral vectors
The genetically encoded Ca2+ sensors (GECI) GCaMP2.2 and GCaMP2.2Low were constructed by introducing the T203V and, respectively both T203V and D133E mutations in GCaMP2. For targeting to the junctional cleft, GCaMP2.2 and GCaMP2.2Low were fused to the N-terminus of FKBP12.6 through a peptide linker. The GCaMP2.2 constructs were subcloned into the pshuttleCMV vector using Bgl II and Not I restriction sites. Subsequent adenovirus generations and amplifications were done using the Adeasy system (Agilent).
Protein expression and purification
Tagged and untagged GCaMP2.2(Low) sensors were cloned into a pRSET expression vector and amplified in BL21 Star (DE3) pLysS cells, then purified using Profinity IMAC Ni Charged Resins (Bio-Rad) and further subjected to size exclusion chromatography.
Ventricular myocyte isolation, culture and adenoviral infection
All animal protocols were approved by the animal welfare committee at UC Davis and conform to the NIH Publication No. 85-23 (revised in 1996). Rat ventricular myocytes were isolated by digestion with collagenase, cultured in supplemented M199 media and infected with adenoviruses expressing one of the GCaMP2.2 sensors. For some experiments, freshly isolated myocytes were permeabilized with 50 μg/mL saponin for 3 minutes. All experiments were done at room temperature.
Fluorescence measurements
GCaMP2.2s fluorescence was recorded with a Live-5 laser scanning confocal microscope (excitation=488 nm, emission>505 nm). Ca2+ transients were measured in linescan mode (2 ms/line). In other experiments, 2D images were taken 1 sec apart.
Statistical analysis
The statistical differences between groups were determined using the student’s t-test.
RESULTS
Low affinity GCaMP-based genetically-encoded Ca2+ indicator
GCaMPs are GECIs consisting of a circularly permutated, enhanced GFP that is flanked by calmodulin (CaM) at the C-terminus and by the CaM binding peptide myosin light-chain kinase M13 at the N-terminus (Fig. 1A), and have been used to detect [Ca2+] in vivo.25-28 The T203V mutation increases the brightness and dynamic range of GCaMP2 (GCaMP2.2),29 and we used this as a starting point to screen mutations that decrease the Ca2+ binding affinity of GCaMP2.2, while maintaining brightness and dynamic range. EF-hand loop mutational screening identified GCaMP2.2Low, which contains a D133E mutation30 in the EF-hand loop IV of calmodulin (Fig. 1A). GCaMP2.2Low has a similar baseline fluorescence and dynamic range as GCaMP2.2, but its affinity for Ca2+ binding is ~10-fold lower than that of GCaMP2.2 (Kd≈5 μmol/L vs. 450 nmol/L, Fig 1B).
Figure 1. Development and validation of Ca2+ sensors that report local [Ca2+]Cleft.
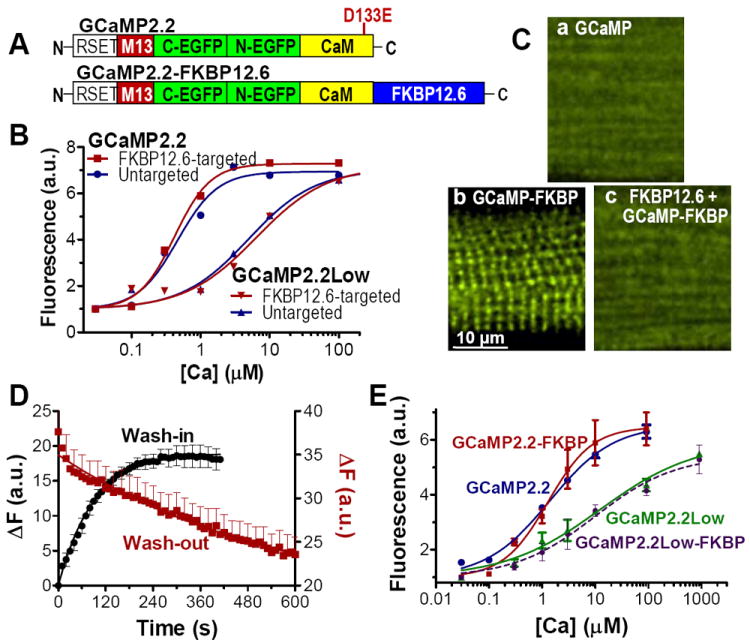
(A) Schematic representation of GCaMP2.2 and GCaMP2.2-FKBP12.6 sensors. The mutation (D133E) indicated in the Top Panel was introduced to obtain GCaMP2.2Low. (B) Ca2+-dependence of purified GCaMP2.2, GCaMP2.2-FKBP12.6, GCaMP2.2Low and GCaMP2.2Low-FKBP12.6 in saline solution. (C) Confocal images of saponin-permeabilized rat ventricular myocytes that were incubated for 10 min with 100 nmol/L purified GCaMP2.2 (a) or GCaMP2.2-FKBP12.6 (b). In c, permeabilized myocytes were first incubated with 10 μmol/L non-fluorescent FKBP12.6 for 5 min, followed by exposure to 100 nmol/L GCaMP2.2-FKBP12.6 in the continued presence of FKBK12.6. The full cell images related to a-c are shown in Online Fig. I. (D) The time course of GCaMP2.2-FKBP12.6 wash-in and wash-out in permeabilized myocytes. We monitored the increase in cell fluorescence upon adding 100 nmol/L GCaMP2.2-FKBP12.6 to the external solution and the decrease in fluorescence following the withdrawal of GCaMP2.2-FKBP12.6. Data are mean ± SEM of 8 separate experiments. These measurements were used to calculate the rate constants of GCaMP2.2-FKBP12.6 binding (kon) and unbinding (koff) to/from RyRs shown in Fig. S2. (E) Ca2+-dependence of the four sensors measured in permeabilized rat ventricular myocytes. To prevent contracture of the cells at elevated [Ca2+], all solutions contained 20 mmol/L BDM. All experiments were done at room temperature.
GCaMP2.2-FKBP12.6 Ca2+ sensors report [Ca2+]Cleft in the space between LTCC and RyR
Our strategy for targeting Ca2+ sensors to a specific subcellular microdomain (the cleft) is to attach GCaMP2.2 and our new GCaMP2.2Low to a molecule that specifically targets to that microdomain. Based on their Ca2+ affinities (Fig 1B), GCaMP2.2 is useful for measuring diastolic [Ca2+]i while GCaMP2.2Low could record the large Ca2+ transients expected in the junctional cleft. To target GCaMP2.2s to the junctions, we fused them to the N-terminus of FKBP12.6 (Fig. 1A). FKBP12.6 is an endogenous protein that binds with high affinity (Kd~1 nmol/L), specificity and 1:1 stoichiometry to RyR2 monomers, but does not greatly influence RyR2 function.31 Our prior work showed that fluorescent-tagged FKBP12.6 binds in a striated pattern along T-tubules at the sites of Ca2+ spark initiation, and that binding is prevented by preincubation with non-fluorescent FKBP12.6.31 Linking FKBP12.6 to the GCaMP sensors does not significantly affect their Ca2+ affinity (Fig. 1B) or Ca2+ binding kinetics (Online Table I). Saponin-permeabilized myocytes exposed to 100 nmol/L purified untagged GCaMP2.2 show uniform cytosolic distribution of the sensor (Fig 1Ca; Online Fig. I shows the entire cell). In contrast, GCaMP2.2-FKBP12.6 has a striated fluorescence pattern (Fig 1Cb) like that seen for purified FKBP12.6 tagged with a small molecule fluorophore,31 which suggests that the sensor is appropriately localized at the z-line where RyRs and clefts are situated. To further test the specificity of GCaMP2.2-FKBP12.6 binding at RyRs, we exposed permeabilized myocytes to GCaMP2.2-FKBP12.6 in the presence of excess non-fluorescent FKBP12.6 to saturate RyRs (Fig 1Cc). In this case, GCaMP2.2-FKBP12.6 shows uniform cytosolic fluorescence. These data suggest that the FKBP12.6-tagged GCaMP2.2 sensors bind with high selectivity to RyRs, which are mainly cleft-localized.
We next assessed the affinity of GCaMP2.2-FKBP12.6 binding to RyRs by performing sensor wash-in and wash-out experiments in permeabilized myocytes (Fig 1D). The rate constants for the dissociation (koff) and association (kon) of the sensor from/to RyRs were calculated from exponential fits of the decline in GCaMP2.2-FKBP12.6 fluorescence upon removing the sensor from the solution (kdecline=koff) and the GCaMP2.2-FKBP12.6 fluorescence increase upon addition of the sensor (kincrease=kon•[GCaMP2.2-FKBP12.6]+koff) (Online Fig. II). The dissociation constant of the RyR - GCaMP2.2-FKBP12.6 complex (Kd) was calculated as koff/kon and yielded a value of 15.5±3.3 nmol/L. A similar calculation showed that GCaMP2.2Low-FKBP12.6 binds to RyRs with a Kd of 43.8±6.3 nmol/L. While these affinities are lower than previously reported for FKBP12.6 alone (~1 nmol/L),31 GCaMP2.2-FKBP12.6 and GCaMP2.2Low-FKBP12.6 bind RyR with relatively high affinity.
We then calibrated the [Ca2+]-dependence of the sensors in the myocyte environment (Fig. 1E). The dynamic range of the sensors in myocytes was comparable to that measured in vitro (~6-fold), but the apparent Ca2+ affinity was slightly lower in myocytes for both GCaMP2.2-FKBP12.6 and GCaMP2.2 than in vitro (Kd=1.2 and 1.3 μmol/L, respectively). GCaMP2.2Low and GCaMP2.2Low-FKBP also exhibited roughly a two-fold decrease in apparent affinity in the myocyte environment (Kd~11 μmol/L). While the GCaMP2.2-FKBP12.6 could increase Ca2+ buffering in the cleft, that effect is expected to be small and have limited impact for two reasons. First, there would be at most one GCaMP per RyR, and this is minor compared to the intense >100:1 buffering in the cleft environment.6 Endogenous Ca2+ binding sites include calmodulin and divalent binding sites on RyR, L-type Ca2+ channels and other cleft proteins, membrane sites and ATP. Second, buffering in the cleft will not alter the steady state [Ca2+]cleft at rest, and could only slow achievement of steady state by 1-5 ms during channel opening or closing.6
After characterizing our junctional cleft-targeted Ca2+ sensors, we expressed them in intact rat cardiac myocytes by adenoviral infection. Myocytes were used for experiments 20-24 hours later. Membrane staining with Di-8-ANNEPS indicated that myocytes retain the T-tubules at which most of the junctions reside, under the culture conditions (Online Fig. III). Moreover, a stringent test for normal junctional function utilized transverse line scan images using Fluo-4 (where spatial drop-out or delayed central activation could indicate altered junctional coupling). The results demonstrated that normal transverse synchrony of SR Ca2+ release was well-preserved in myocytes expressing the GCaMP sensors after 24 hr culture (Online Fig. IV). There was no decrement in either the uniformity of local transverse release activation or rate of rise of the Ca2+ transients. Thus, the clefts where the GCaMP2.2-FKBP12.6 sensors are targeted function normally during ECC.
FKBP12.6-tagged sensors express in a striated pattern, with intensity maxima spaced ~2 μm apart (Fig. 2A; full images are shown in Online Fig. V). This strongly supports the conclusion that the FKBP12.6-tagged sensors are targeted to RyR at the z-line, as in permeabilized cells. In contrast, the un-tagged sensors show a rather uniform cytosolic distribution (Fig. 2A). To test the Ca2+ responsiveness of the new sensors when expressed in intact cells, we first depleted myocytes of Ca2+ (using ionomycin and Ca2+-free solution), to determine the minimum sensor fluorescence (Fmin) (Fig. 2Bb vs a). Then, myocytes were Ca2+-overloaded by restoring extracellular Ca2+ in Na+-free solution to assess maximum fluorescence (Fmax) just as hypercontracture begins (Fig. 2Bc). Importantly, GCaMP2.2(Low) and GCaMP2.2(Low)-FKBP12.6 have a similar dynamic range, Fmax/Fmin, in myocytes (5.5±0.6, n=7 for GCaMP2.2 and 5.7±0.5, n=9 for GCaMP2.2-FKBP12.6). These numbers agree well with those in Fig 1E. Thus, our novel FKBP12.6-tagged Ca2+ indicators are highly sensitive to Ca2+ and are positioned to measure local cleft [Ca2+]Cleft vs. un-tagged sensors reporting bulk cytosolic [Ca2+]Bulk in intact myocytes.
Figure 2. Adenovirally-expressed GCaMP2.2(Low)-FKBP12.6 and untagged GCaMP2.2(Low) are positioned to measure local cleft [Ca2+]Cleft and, respectively, bulk cytosolic [Ca2+]Bulk, and are highly sensitive to Ca2+ in intact myocytes.
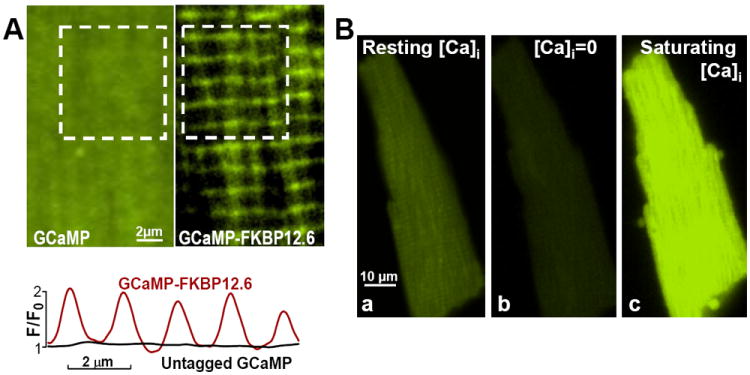
(A) Example of myocytes infected with adenoviruses expressing untagged GCaMP2.2Low (Left) and GCaMP2.2Low-FKBP12.6 (Right). Bottom panel shows the fluorescence profile along the longitudinal axis of the myocyte for the area shown in the white square in the top panels. Right and Left panels show limited areas from each myocyte; entire myocyte images are in Online Fig. V. (B) Ca2+-sensitivity of GCaMP2.2Low-FKBP12.6 in intact cells. A resting myocyte expressing GCaMP2.2Low-FKBP12.6 was imaged first under control conditions (Ba). The cell was then exposed to Ca2+-free external solution in the presence of 10 μmol/L ionomycin (Bb). This resulted in a significant fluorescence drop (Fmin). Then, the cell was Ca2+-overloaded in a 0Na+/1 mmol/L Ca2+ external solution, which favors Ca2+ entry via both ionomycin and reverse mode Na+/Ca2+ exchange, to saturate the sensor and determine Fmax (Bc). The Fmax/Fmin ratio for this cell is 6.2.
Larger and faster local Ca2+ transients in the junctional space vs. bulk cytosol
We expressed the low-affinity untagged and FKBP12.6-tagged sensors to measure Ca2+ transients in the bulk cytosol and junctional cleft, respectively (Fig. 3). Myocytes were field-stimulated at 0.5 Hz and Ca2+ transients were measured in linescan mode using a laser scanning confocal microscope. Fig. 3A shows representative Ca2+ transients reported by GCaMP2.2Low and GCaMP2.2Low-FKBP12.6. Junctional cleft transients recorded by GCaMP2.2Low-FKBP12.6 are ~2-fold larger (Fig. 3B) and have a much faster upstroke (time-to-peak=46±5 vs. 90±7 ms; Fig. 3C) and decay (decay τ=254±19 vs. 421±42 ms; Fig. 3D) compared to global Ca2+ transients measured by the un-targeted GCaMP2.2Low. This is encouraging (see Discussion for inferred [Ca2+] values), but based on theoretical models11 and indirect cleft Ca2+ transient assessments12,24 one would expect a much larger difference in both amplitude and kinetics between the targeted and untargeted sensor. An obvious explanation is that the kinetics of local [Ca2+] change in the cleft are likely to be very fast, and GCaMP sensors lack the temporal resolution to detect such rapid kinetics. The dissociation rate constant for GCaMP2.2Low (~6 s-1: Online Table I) is consistent with this notion. While this new low-affinity Ca2+-sensor is not fast enough to accurately report [Ca2+]Cleft dynamics during ECC, it may still be useful in determining relative changes in [Ca2+]cleft with acute treatments (e.g. slower or larger). Targeted GCaMP2.2Low may also provide more accurate values of local [Ca2+]i in microdomains with local Ca2+ flux rates that are lower than in striated muscle junctions, where local Ca2+ flux rates may be among the highest in nature.
Figure 3. Electrically stimulated Ca2+ transients measured with GCaMP2.2Low and GCaMP2.2Low-FKBP12.6.
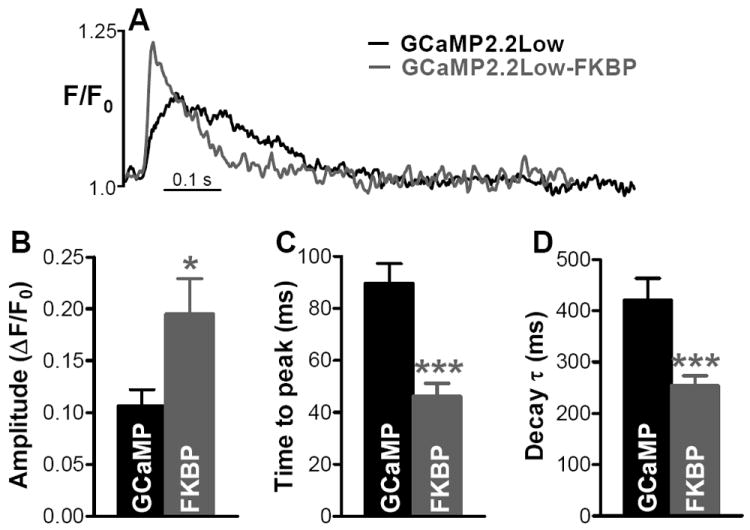
(A) Representative recordings in myocytes expressing GCaMP2.2Low and GCaMP2.2Low-FKBP12.6. (B-D) Amplitude (B), time-to-peak (C) and decay time (D) of Ca2+ transients reported by GCaMP2.2Low (GCaMP) and GCaMP2.2Low-FKBP12.6 (FKBP). Data are the mean±SEM of 10 (GCaMP2.2Low) and 13 (GCaMP2.2Low-FKBP12.6) separate experiments.
Upon β-adrenergic activation with isoproterenol (ISO; Online Fig VI), untargeted GCaMP2.2Low readily detects the expected large increase in Ca2+ transient amplitude and two-fold acceleration of [Ca2+]i decline due to enhanced SR Ca2+ uptake. The cleft-targeted sensor Ca2+ transient decays much faster than the untargeted sensor under control conditions, but fails to accelerate with ISO, despite a similar doubling of amplitude. Since [Ca2+]cleft is expected to sense released Ca2+ and diffusion from the cleft to cytosol, rather than SR Ca2+ uptake rate (which is distributed throughout the cytosol), this is exactly what one would expect. Thus, the cleft-targeted GCaMP2.2Low is more sensitive to Ca2+ release at the cleft than to transport by SERCA, and differs qualitatively from bulk [Ca2+]i sensors. This relates to Ca2+ sparks which decay primarily via diffusion from the source, with minor influence by SERCA function,32 despite much of the indicator signal coming from outside the cleft. In contrast, global [Ca2+]i decline is dictated almost entirely by Ca2+ transport out of the cytosol.6
Standing diastolic [Ca2+] gradient between the junctional space and bulk cytosol
During diastole, Ca2+ leak from the SR or Ca2+ influx across sarcolemma may raise local [Ca2+]Cleft above [Ca2+]Bulk (distant from Ca2+ sources). Moreover, the very high density of both L-type Ca2+ channels and RyR2 at the cleft, compared to the broader distribution of the transporters that remove Ca2+ from the cytosol (SR Ca2+-ATPase and Na+/Ca2+ exchange) could allow even modest Ca2+ leak through these channels to produce a standing [Ca2+] gradient (Fig 4A, left). Increases in SR Ca2+ leak have been implicated in pathological states, where [Ca2+]Cleft could be preferentially elevated. We used our targeted GCaMP2.2 to directly test for such diastolic standing [Ca2+] gradients between the cleft and the cytosol.
Figure 4. Measurement of standing diastolic Ca2+ gradient between cleft and bulk cytosol.
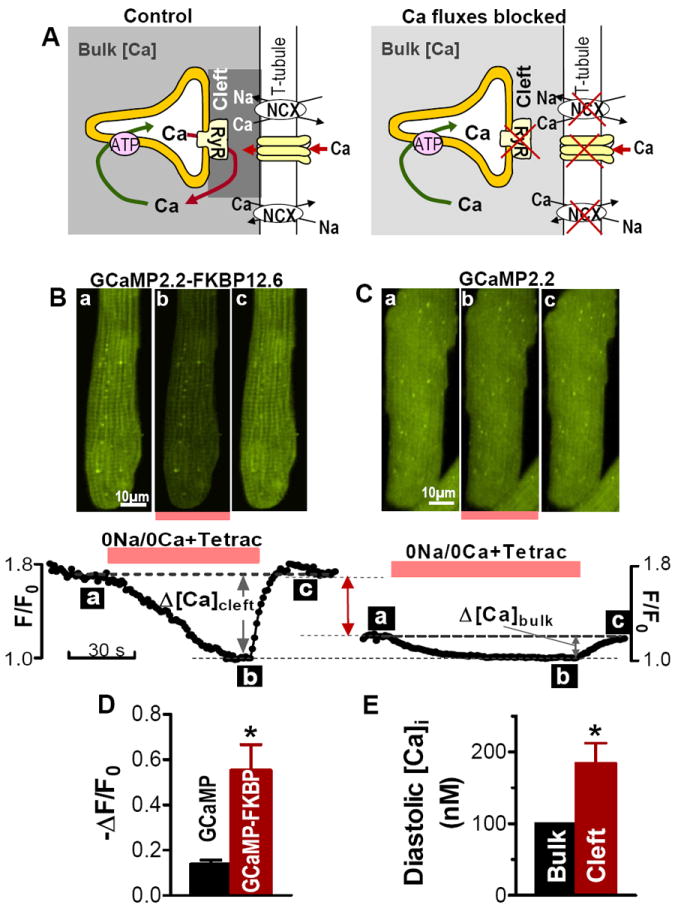
(A) Cartoon illustrating the expected effect of blocking Ca2+ fluxes through RyRs, NCX and L-type Ca2+ channels on [Ca2+]Cleft and [Ca2+]Bulk. We hypothesize that [Ca2+]Cleft > [Ca2+]Bulk during diastole (left). Upon inhibition of both SR Ca2+ leak and sarcolemmal Ca2+ fluxes, [Ca2+] decreases in both compartments and at steady-state [Ca2+]Cleft = [Ca2+]Bulk (right). (B-C) Representative examples of the change in the fluorescence of GCaMP2.2-FKBP12.6 (B) and GCaMP2.2 (C) during application and washout of 1 mmol/L tetracaine and 0Na+/0Ca2+ external solution. Myocytes were first field-stimulated (at 0.5 Hz) to reach steady-state, then stimulation was stopped and we added tetracaine and 0Na+/0Ca2+ solution. Top panels show the fluorescence 2D images of myocytes expressing GCaMP2.2-FKBP12.6 (B) and GCaMP2.2 (C) at the time points (a-c) indicated in the lower panels. Lower panels show time-dependence of average fluorescence. Fluorescence of both sensors decreased rapidly in the presence of tetracaine and 0Na+/0Ca2+ solution and the decrease was larger for GCaMP2.2-FKBP12.6. (D) Mean decrease in fluorescence intensity upon application of tetracaine and 0Na+/0Ca2+ external solution for myocytes expressing GCaMP2.2-FKBP12.6 and untagged GCaMP2.2 (n=6 cells for each). (E) Conversion of data in panel (D) to [Ca2+].
Upon blocking Ca2+ fluxes into the cleft, [Ca2+] should decline in both compartments, and importantly, any [Ca2+]Cleft vs. [Ca2+]Bulk gradient should dissipate (Fig. 4A, right). This maneuver is valuable so that we can define a point (F0) where both the cytosolic and targeted GCaMP2.2 sense the same [Ca2+]i. Figure 4B-C shows how [Ca2+]Cleft and [Ca2+]Bulk decline when we abruptly block RyRs with 1 mM tetracaine and both LTCC and NCX by rapidly switching the external solution to a 0Na+/0Ca2+ solution. Both [Ca2+]Cleft and [Ca2+]Bulk dropped rapidly after the application of tetracaine and 0Na+/0Ca2+ solution, reaching the F0 steady-state (where [Ca2+]Cleft =[Ca2+]Bulk) in about 1 min (Figs. 4B-C, transition from time a to b). Note that [Ca2+] decline was much larger for GCaMP2.2-FKBP12.6. These results suggest that the initial diastolic [Ca2+]Cleft was higher (F/F0 =1.8) than [Ca2+]Bulk (F/F0 =1.2). In both cases, fluorescence was restored on return to the normal bath solution (time point c in Figs. 4B-C).
Mean data confirm that inhibition of SR and sarcolemmal Ca2+ fluxes in the cleft produces a significantly larger decline in the fluorescence of GCaMP2.2-FKBP12.6, which reports local [Ca2+]Cleft, vs. the untagged GCaMP2.2, which reports [Ca2+]Bulk (-ΔF/F0=0.55±0.10 for GCaMP2.2-FKBP12.6 vs. 0.13±0.02 for GCaMP2.2; Fig. 4D). This indicates that there is indeed a standing [Ca2+]i gradient during diastole between the junctional cleft and bulk cytosol. Assuming that diastolic [Ca2+]Bulk =100 nmol/L (prior to tetracaine, 0Na+/0Ca2+) and using data in Fig. 1E, we calculated that diastolic [Ca2+]Cleft is 194±28 nmol/L.
Another way of reducing diastolic SR Ca2+ leak is to block SERCA with thapsigargin (Fig 5A). SERCA inhibition slowly unloads the SR, which is paralleled by a slow decrease in SR Ca2+ leak.33 As expected, with 10 μM thapsigargin and 0Na+/0Ca2+ solution, the fluorescence signal in myocytes expressing either GCaMP2.2 or GCaMP2.2-FKBP12.6 declined more slowly, reaching a new steady-state in ~10 min (Fig. 5B). A similar time course was previously demonstrated for thapsigargin-induced cessation of SR Ca2+ leak, measured as the rate of decline in SR [Ca2+].33,34 Similar to RyR blockade with tetracaine, the fluorescence decline was significantly larger for GCaMP2.2-FKBP12.6 vs. untagged GCaMP2.2 (-ΔF/F0=0.77±0.11 vs. 0.41±0.06; Fig. 5C). The overall decline in [Ca2+]i was larger for thapsigargin vs. tetracaine (presumably due to longer time in Ca2+-free solution) but the difference between cleft and bulk was similar for both methods (-ΔF/F0=0.36 vs. 0.42).
Figure 5. The standing diastolic [Ca2+]i gradient is caused mainly by the SR Ca2+ leak.
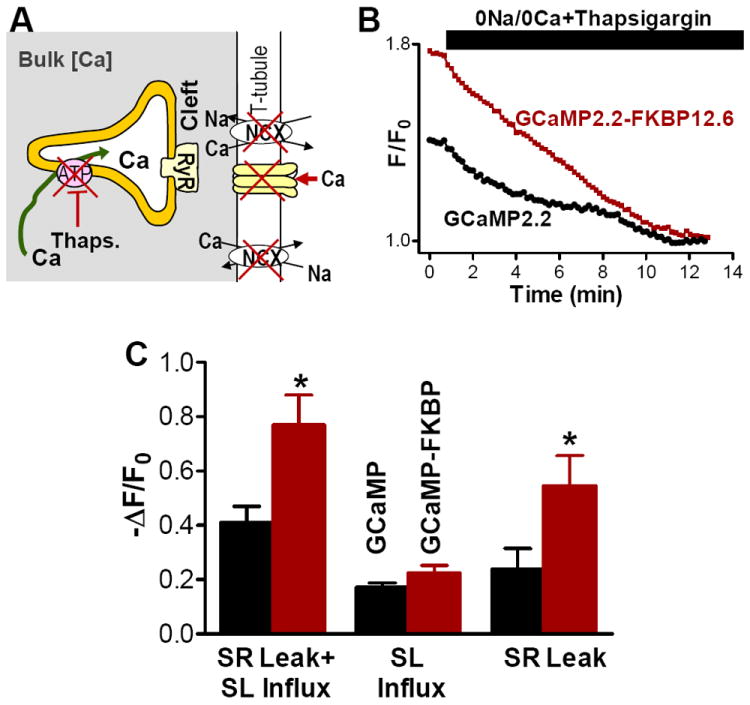
(A) Cartoon illustrating a second way of progressive reduction of diastolic SR Ca2+ leak by blocking SERCA with 10 μmol/L thapsigargin. (B) Time course of GCaMP2.2-FKBP12.6 and GCaMP2.2 fluorescence during application of thapsigargin and 0Na+/0Ca2+ external solution (mean of 8 separate experiments). (C) Mean decrease in fluorescence intensity in myocytes expressing GCaMP2.2-FKBP12.6 and untagged GCaMP2.2 upon inhibition of both SR and sarcolemmal Ca2+ fluxes with thapsigargin and 0Na+/0Ca2+ external solution (SR Leak+SL Influx), blockade of sarcolemmal Ca2+ transport in 0Na+/0Ca2+ solution (SL Influx) and inhibition of SR Ca2+ leak by emptying the SR with thapsigargin (SR Leak).
To assess the relative contribution of the SR and sarcolemmal Ca2+ fluxes to Ca2+ entry into the cleft, we did separate experiments where only sarcolemmal Ca2+ fluxes are blocked (0Na+/0Ca2+ solution). In this case, the decrease in the sensor fluorescence was similar for the FKBP12.6-tagged and the untagged sensor (-ΔF/F0=0.22±0.03 for GCaMP2.2-FKBP12.6 vs. 0.17±0.02 for GCaMP2.2; Fig. 5C). In contrast, inhibition of SR Ca2+ leak alone resulted in a significantly larger drop in the GCaMP2.2-FKBP12.6 fluorescence vs. the untagged sensor (-ΔF/F0=0.55±0.11 vs. 0.24±0.07; Fig. 5C). These results indicate that most of the Ca2+ entering the junctional cleft during diastole comes from the SR and SR Ca2+ leak is the main factor in setting a diastolic [Ca2+]i gradient between the cleft and bulk cytosol.
DISCUSSION
In many cell types, [Ca2+]i is regulated and signals differently in various subcellular microdomains, which greatly enhances its versatility as a secondary messenger. Recognition of such subcellular regulation stimulated us to develop methods for measuring local [Ca2+]i in microdomains. Here, we attached the genetically encoded Ca2+ sensor GCaMP2.2 and a newly developed lower Ca2+ affinity variant GCaMP2.2Low to the N-terminus of FKBP12.6 to construct novel Ca2+ sensors that bind specifically to RyRs. Our data indicate that GCaMP2.2Low-FKBP12.6 has a high affinity and selectivity for RyRs, although slightly reduced compared to untagged FKBP12.6. In cardiac myocytes, RyRs are localized predominantly in the junctional SR membrane, where the SR comes into close proximity of the external sarcolemma (the dyads). Due to this specific targeting, the FBP12.6-tagged sensors report local [Ca2+]i in the small dyadic cleft, [Ca2+]Cleft. This local [Ca2+]Cleft controls and is influenced by both RyRs and L-type Ca2+ channels. Therefore, [Ca2+]Cleft critically affects myocyte function at rest (during diastole) and during electrical excitation. Despite its importance, methods to directly measure [Ca2+]Cleft are only now becoming available.21,22 This key parameter has typically been inferred from the dynamics of NCX12,24 and L-type Ca2+ currents12,23 in electrically stimulated myocytes, but no such estimates could be made for diastolic [Ca2+]Cleft. Our novel Ca2+ sensors allow direct measurement of [Ca2+]Cleft, both during ECC (the low affinity sensor GCaMP2.2Low-FKBP12.6) and at rest (GCaMP2.2-FKBP12.6). However, the extremely rapid changes of [Ca2+]Cleft expected during ECC are too fast to be accurately captured by even the 10 μM Kd sensor, although they are likely to be useful to assess relative changes in [Ca2+]cleft during perturbations that might alter the amplitude or kinetics of [Ca]cleft changes during ECC (as we have seen for ISO stimulation).
We use FKBP12.6 to target the sensors because of the very high affinity and selectivity for RyR2 in cardiac myocytes.31 A limitation with this approach is that FKBP12.6 can also influence RyR channel gating, stabilizing the closed state.35 FKBP12.6 overexpression in myocytes can reduce spontaneous Ca2+ spark frequency and either increase or decrease Ca2+ transient amplitude.36-37 Thus, using FKBP12.6 to target these Ca2+ sensors may reduce diastolic RyR2 opening. On the other hand, in studies measuring simultaneously FKBP12.6 binding to RyR2 and Ca2+ sparks, saturating RyR2 with FKBP12.6 only reduced Ca2+ spark frequency by 18%.31 This effect would mean that we are probably slightly underestimating diastolic [Ca2+]Cleft.
A fraction (~15-20%) of RyRs are located outside the sarcolemma-SR junctions in rat myocytes.38 Thus, the GCaMP2.2-FKBP12.6 signal is not generated entirely by [Ca2+]Cleft, but is slightly contaminated by [Ca2+] at such non-junctional RyRs. Of note, local [Ca2+] at non-junctional RyRs may also be relevant, even if less influential on sarcolemmal currents. The adenoviral expression of GCaMP sensors in cardiac myocytes could risk alteration of dyad structure or function. While cleft dimensions were not directly measured (e.g. via electron microscopy), the FKBP12.6-targeted sensors are highly localized along T-tubules (of which ~50% are in dyads in rat myocytes)6 and normal synchronous transverse activation during ECC was maintained (Online Fig. IV). Therefore, we assume that the ultrastructure of the junctional cleft was not appreciably altered by the experimental conditions. FKBP12.6-GCaMP2.2 also has to be studied in a practical range of protein expression. If one waits too long or transfects too aggressively, one can express enough FKBP12.6-targeted protein to more than saturate the ~1 μM of RyR binding sites. In that case, the excess sensor will not be effectively targeted. Any amount of untargeted GCaMP2.2-FKBP12.6 (as above) would cause underestimation of the difference between [Ca2+]Cleft and [Ca2+]Bulk. This means that our [Ca2+]Cleft measurements and the diastolic [Ca2+] gradient are lower limit estimates. True [Ca2+]Cleft would be larger. On balance, with awareness of these challenges with respect to interpretation, we think the advantages of targeting sensors to the cleft this way outweigh the limitations, and do not affect any of our conclusions. Potential enhancements in live cell imaging resolution that selectively visualize such local domains would complement approaches like ours with locally target sensors.22
[Ca2+]Cleft and [Ca2+]Bulk in cardiac myocytes
Our novel cleft-targeted sensor GCaMP2.2Low-FKBP12.6 reported a larger and faster rise in [Ca2+]Cleft vs. [Ca2+]Bulk during electrical excitation of myocytes, in good agreement with previous estimates from indirect measurements.9-12 Weber et al.24 inferred the submembrane [Ca2+] near NCX during a normal action potential as peaking at >3.2 μM within <32 ms (vs. a global Ca2+ transient that peaks at 1.1 μM in 81 ms). However [Ca2+]Cleft ought to be much higher than submembrane [Ca2+]i. Acsai et al.12 estimated the Ca2+ transient near Ca2+ release sites from the kinetics of L-type Ca2+ current and from a fraction of NCX current. They calculated that this local Ca2+ transient has 10-15 μM in amplitude, peaks at about 10 ms after depolarization and recovers within ~50 ms. In contrast, the global Ca2+ transient reached a maximum of ~1 μM within ~70-100 ms. Estimates of [Ca2+]Cleft based on geometry, diffusion and Ca2+ flux measurements suggest even higher (100 μM) and faster [Ca2+]Cleft changes during electrical excitation.11 Based on the measured diastolic [Ca2+]cleft and [Ca2+]bulk (194 nmol/L and 100 nmol/L, respectively, Fig. 4E), the characteristics of GCaMP2.2Low and GCaMP2.2Low-FKBP response to Ca2+ in myocytes (Fig. 1E), and the Ca2+ transient amplitude (Fig. 3C), we calculated that peak [Ca2+]bulk=560±73 nmol/L while peak [Ca2+]cleft is ~2-fold larger (1272±199 nmol/L). This peak [Ca2+]cleft is much smaller than previous estimates. The time-to-peak of [Ca2+]Cleft was 46±5 ms (vs. 90±7 ms for [Ca2+]Bulk), slightly longer than the estimates based on NCX and L-type Ca2+ current. We believe that these differences in the amplitude and dynamics of cleft Ca2+ transients vs. previous estimates are mostly due to the relatively slow kinetics of Ca2+ binding and unbinding of the GCaMP2.2 sensors (see Online Table I). Thus, while GCaMP2.2Low detects larger and faster cleft Ca2+ transients vs. the global sensor and can detect changes in amplitude and kinetics (e.g. with ISO), much faster indicators will be required for accurate [Ca2+]Cleft measurement during the dynamics of active SR Ca2+ release in heart.
Using GCaMP2.2-FKBP12.6 we could directly assess diastolic [Ca2+]Cleft, a parameter not previously measured. We demonstrated that during diastole, [Ca2+]Cleft is >90 nmol/L higher than [Ca2+]Bulk. This standing diastolic [Ca2+] gradient indicates that Ca2+ extrusion from the cleft (via junctionally-located NCX and diffusion into bulk cytosol) cannot keep up with Ca2+ entry into the cleft. Our data show that diastolic cleft Ca2+ influx is caused mainly by the SR Ca2+ leak (vs. entry through LTCC). At the same time, elevated diastolic [Ca2+]Cleft can reduce LTCC availability (via Ca2+ -dependent inactivation) and alter RyR gating to increase their activity by direct RyR activation, and also indirectly via activation of CaMKII followed by CaMKII-dependent RyR phosphorylation.39 Increased RyR opening leads to a larger SR Ca2+ leak and a higher risk for generation of propagating Ca2+ waves, which are a known risk for arrhythmias. The balance of Ca2+ fluxes in the cleft is altered under pathological conditions, e.g. in heart failure SR Ca2+ leak and Na+/Ca2+ exchange function are increased.40
The high diastolic [Ca2+]Cleft may also have important effects on local signaling cascades beyond the sphere of E-C coupling. Indeed, there is much indirect evidence that local [Ca2+]i in these environments may be important in the activation of calcineurin or CaMKII that can signal to the nucleus and trigger alterations in gene expression.41 In pathological conditions where SR Ca2+ leak is known to be enhanced, the higher local level of diastolic [Ca2+]Cleft may have an important impact on these (and other) broader signaling pathways.
In summary, we have developed sensors for measuring [Ca2+]Cleft vs. [Ca2+]Bulk and demonstrated dynamic differences during excitation-contraction coupling. We have also provided measures of a normal standing [Ca2+]i gradient during diastole between the junctional cleft and the bulk cytosol and showed that this gradient is set mainly by the SR Ca2+ leak.
Supplementary Material
Novelty and Significance.
What Is Known?
Ca2+ is regulated and signals differently in various subcellular microdomains, which greatly enhances its second messenger versatility.
In the heart, sarcoplasmic reticulum (SR) Ca2+ release is controlled by local [Ca2+]i in the junctional cleft, the small space between sarcolemma and junctional SR.
This local cleft [Ca2+]i is thought to be important in both the regulation of excitation-contraction coupling, but also to downstream Ca2+-dependent signaling (e.g. via calmodulin, calcineurin and CaMKII).
What New Information Does This Article Contribute?
We constructed novel Ca2+-sensors that are targeted to microdomains rich in ryanodine receptors.
In cardiac myocytes, the novel Ca2+-sensors are positioned to measure local Ca2+ in the junctional cleft.
There is a standing Ca2+ gradient during diastole between the junctional cleft and bulk cytosol in cardiac myocytes, which could influence local Ca2+-dependent signaling within the cleft.
Ca2+ is a universal second messenger involved in activation/regulation of very diverse cellular processes. To allow such versatility, Ca2+ is regulated and signals differently in various subcellular microdomains, which generated a large interest in developing methods for measuring local rather than bulk Ca2+. Here we targeted novel Ca2+-sensors to microdomains rich in ryanodine receptors. This was accomplished by fusing the genetically encoded Ca2+-sensor GCaMP2.2 and a lower Ca2+-affinity variant GCaMP2.2Low to FKBP12.6, a protein that binds with high affinity and selectivity to ryanodine receptors. In cardiac myocytes, these sensors report local [Ca2+] in the small junctional cleft between the plasmalemma and junctional sarcoplasmic reticulum ([Ca2+]Cleft), a parameter that critically affects heart function and dysfunction. Using these sensors, we demonstrate that [Ca]cleft reaches higher levels with faster kinetics than global [Ca2+]i during excitation-contraction coupling. We also show that there is a substantial standing diastolic [Ca2+] gradient between [Ca2+]Cleft and bulk [Ca2+]i, due mainly to ryanodine receptor-dependent Ca2+ leak. Such [Ca2+]Cleft characteristics may have important impact on local Ca2+-dependent signaling.
Acknowledgments
Authors thank Dr. M. Khafaga, K. Dao and L. Lee for help in preliminary experiments and Dr. KS. Ginsburg for technical support.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health [R01-HL109501 to S.D., R01-HL081526, R37-30077 and R01-HL092097 to D.M.B., and 5-P01-HL095488 to M.K.].
Nonstandard Abbreviations and Acronyms
- [Ca2+]i
intracellular Ca2+ concentration
- [Ca2+]Cleft
local [Ca2+] in the junctional cleft
- [Ca2+]Bulk
[Ca2+] in the bulk cytosol
- CaM
calmodulin
- ECC
excitation – contraction coupling
- GECI
genetically-encoded Ca2+ indicator
- LTCC
L-type Ca2+ channels
- NCX
Na+/Ca2+ exchanger
- RyR
ryanodine receptors
- SR
sarcoplasmic reticulum
Footnotes
DISCLOSURES
None
References
- 1.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol. 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.McCarron JG, Chalmers S, Bradley KN, MacMillan D, Muir TC. Ca2+ microdomains in smooth muscle. Cell Calcium. 2006;40:461–493. doi: 10.1016/j.ceca.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Kanemoto Y, Matsuzaki M, Morita S, Hayama T, Noguchi J, Senda N, Momotake A, Arai T, Kasai H. Spatial distributions of GABA receptors and local inhibition of Ca2+ transients studied with GABA uncaging in the dendrites of CA1 pyramidal neurons. PLoS One. 2011;6:e22652. doi: 10.1371/journal.pone.0022652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM. Calcium cycling and signaling in cardiac myocytes. Ann Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2 Kluwer Academic Publishers; 2001. [Google Scholar]
- 7.Guatimosim S, Dilly K, Santana LF, Saleet Jafri M, Sobie EA, Lederer WJ. Local Ca2+ signaling and EC coupling in heart: Ca2+ sparks and the regulation of the [Ca2+]i transient. J Mol Cell Cardiol. 2002;34:941–950. doi: 10.1006/jmcc.2002.2032. [DOI] [PubMed] [Google Scholar]
- 8.Cannell MB, Kong CH. Local control in cardiac E-C coupling. J Mol Cell Cardiol. 2012;52:298–303. doi: 10.1016/j.yjmcc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of ICa during calcium release from the sarcoplasmic reticulum in guinea-pig ventricular myocytes. Circ Res. 1995;76:102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- 10.Soeller C, Cannell MB. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J. 1997;73:97–111. doi: 10.1016/S0006-3495(97)78051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acsai K, Antoons G, Livshitz L, Rudy Y, Sipido KR. Microdomain [Ca2+] near ryanodine receptors as reported by L-type Ca2+ and Na+/Ca2+ exchange currents. J Physiol. 2011;589:2569–2583. doi: 10.1113/jphysiol.2010.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserstrom JA, Vites AM. The role of Na+-Ca2+ exchange in activation of excitation-contraction coupling in rat ventricular myocytes. J Physiol. 1996;493:529–542. doi: 10.1113/jphysiol.1996.sp021401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sipido KR, Maes M, Van de Werf F. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na+-Ca2+ exchange. Circ Res. 1997;81:1034–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- 15.Litwin SE, Li J, Bridge JH. Na-Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neco P, Rose B, Huynh N, Zhang R, bridge JH, Phlipson KD, Goldhaber JI. Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J. 2010;99:755–764. doi: 10.1016/j.bpj.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayasinghe ID, Cannell MB, Soeller C. Organization of ryanodine receptors, transverse tubules, and sodium-calcium exchanger in rat myocytes. Biophys J. 2009;97:2664–2673. doi: 10.1016/j.bpj.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato D, Despa S, Bers DM. Can the sodium-calcium exchanger initiate or suppress calcium sparks in cardiac myocytes? Biophys J. 2012;102:L31–L33. doi: 10.1016/j.bpj.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH. Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol. 2010;588:4249–4260. doi: 10.1113/jphysiol.2010.194688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pott C, Yip M, Goldhaber JI, Philipson KD. Regulation of cardiac L-type Ca2+ current in Na+-Ca2+ exchanger knockout mice: functional coupling of the Ca2+ channel and the Na+-Ca2+ exchanger. Biophys J. 2007;92:1431–1437. doi: 10.1529/biophysj.106.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Despa S, Bossuyt J, Shui B, Kotlikoff M, Bers DM. Junctional cleft [Ca]i measurements using novel cleft-targeted Ca sensors. Biophys J. 2012;102:408a. doi: 10.1161/CIRCRESAHA.115.303582. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang W, Lu F, Sun T, Xu J, Li LL, Wang Y, Wang G, Chen L, Wang X, Cannell MB, Wang SQ, Cheng H. Imaging Ca2+ nanosparks in heart with a new targeted biosensor. Circ Res. 2014;114:412–420. doi: 10.1161/CIRCRESAHA.114.302938. [DOI] [PubMed] [Google Scholar]
- 23.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional SR Ca release by total and free intra-SR Ca concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber CR, Piacentino V, III, Ginsburg KS, Houser SR, Bers DM. Na/Ca exchange current and submembrane [Ca] during the cardiac action potential. Circ Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 25.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothellial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40 BAC GCaMP2 transgenic mice. Circ Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 26.Roell W, Lewalter T, Sasse P, Tallini YN, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 27.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci. 2010;13:1433–1440. doi: 10.1038/nn.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallini YN, Ohkura M, Choi BR, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Reid RE. Conservative D133E mutation of calmodulin site IV drastically alters calcium binding and phosphodiesterase regulation. Biochemistry. 1997;36:3608–3616. doi: 10.1021/bi962149m. [DOI] [PubMed] [Google Scholar]
- 31.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010;106:1743–1752. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gómez A, Cheng H, Lederer WJ, Bers DM. Ca diffusion and SR transport both contribute to the [Ca]i decline during Ca sparks in rat ventricular myocytes. J Physiol. 1996;496:575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zima A, Bovo E, Bers DM, Blatter LA. Properties of sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588(Pt 23):4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bovo E, Mazurek SR, Blatter LA, Zima AV. Regulation of sarcoplasmic reticulum Ca2+ leak by cytosolic Ca2+ in rabbit ventricular myocytes. J Physiol. 2011;589:6039–6050. doi: 10.1113/jphysiol.2011.214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 36.Gomez AM, Schuster I, Fauconnier J, Prestle J, Hasenfuss G, Richard S. FKBP12.6 overexpression decreases Ca2+ spark amplitude but enhances [Ca2+]i transient in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1987–H1993. doi: 10.1152/ajpheart.00409.2004. [DOI] [PubMed] [Google Scholar]
- 37.Gellen B, Fernández-Velasco M, Briec F, et al. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation. 2008;117:1778–1786. doi: 10.1161/CIRCULATIONAHA.107.731893. [DOI] [PubMed] [Google Scholar]
- 38.Scriven DR, Asghari P, Schulson MN, Moore ED. Analysis of Cav1.2 and ryanodine receptor clusters in rat ventricular myocytes. Biophys J. 2010;99:3923–3929. doi: 10.1016/j.bpj.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo T, Zhang T, Mestril R, Bers DM. Ca/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 40.Bers D. Cardiac sarcoplasmic reticulum Ca leak: Basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 41.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: contractile versus signaling Ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


