Abstract
Tissue-based determination of Ki-67, a marker of cellular proliferation, has shown prognostic value in solid tumors and hematological malignancies. We developed and validated an electrochemiluminescence-based method for sensitive measurement of circulating Ki-67 in plasma (cKi-67). This assay demonstrated significantly higher levels of cKi-67 in patients with newly diagnosed acute lymphoblastic leukemia (ALL) (n=27; median, 762; range, 0-4574 U/100 μL) than in healthy control subjects (n=114; median, 399; range, 36-2830 U/100 μL). Moreover, elevated plasma cKi-67 was associated with significantly shorter survival in ALL patients (P=0.05). These findings suggest that Ki-67 can be detected in circulation and has potential for use as a biomarker for predicting clinical behavior in ALL.
Keywords: Acute lymphoblast leukemia, circulating KI-67, Plasma
1. Introduction
The expression of Ki-67 protein is associated with cell proliferation [1]. Ki-67 protein is present during all active phases of the cell cycle, G1, S, G2, and mitosis, but is absent from resting cells (G0). This property makes it an excellent marker for determining the growth fraction of a cell population [2]. Ki-67 protein is well characterized on the molecular level and extensively used as a proliferation marker. Although its functional significance remains unclear [3, 4], it has been reported to be a good prognostic factor in various cancers [5-10].
Acute lymphoblastic leukemia (ALL) is a common malignancy in childhood and has a cure rate of around 80% with current treatments [11, 12]. In adults, ALL incidence peaks around the age of 50, and long-term survival remains poor. Identification of biomarkers that better predict clinical behavior of ALL represents an important clinical need that could provide information for devising new therapeutic approaches.
Uncontrolled proliferation is a common feature of ALL and correlates with the expression of the nuclear protein Ki-67. Although Ki-67 expression has prognostic value in solid tumors and hematological malignancies, current Ki-67 assays require tumor tissue samples normally obtained from biopsies, an expensive and invasive procedure. In this paper we show for the first time that Ki-67 circulates in plasma and can be measured using a newly developed electrochemiluminescence-based enzyme immunoassay. We used this plasma-based approach to explore the prognostic relevance of circulating Ki-67 (cKi-67) in hematologic disease, using ALL as a model.
2. Materials and methods
2.1. Cell lines
Cell lines were obtained from ATCC (Manassas, VA) and were maintained in RPMI 1640 supplemented with 10% FCS (Hyclone, Tulare, CA), 1 mmol/L L-glutamine, and antibiotics (streptomycin/penicillin). Cells were cultured at 37 °C in a humid atmosphere with 5% CO2.
2.2. Patients and Samples
Plasma samples were collected from patients with newly diagnosed ALL (n = 27 patients) at The University of Texas MD Anderson Cancer Center (Houston, TX) according to an IRB-approved protocol, after informed consent was obtained according to institutional guidelines. Blood samples were collected 1 or 2 days prior to commencement of chemotherapy. After separation, plasma was stored at −70 °C.
2.3. Reagents
Ninety-six-well small-spot coated anti-mouse plates, Tris wash buffer, Tris lysis buffer, blocker A, Read Buffer T, and antibody diluent buffer were obtained from Meso-Scale Discovery (MSD; Gaithersburg, MD). HL60 lysate was obtained from Novus. Protease inhibitor cocktail set III was from EMD Biosciences (San Diego, CA). Antibodies were obtained from various sources: anti mouse Ki-67 antibody clone 7B11 from Invitrogen (Carlsbad, CA); anti-rabbit Ki-67 antibody clone H300 from Santa Cruz (Santa Cruz, CA); anti PCNA (Cell signalling, Danvers, MA) goat anti-chicken antibody from Rockland (Gilbertsville, PA); anti-rabbit SULFOTAG from MSD; and anti-mouse and rabbit HRP-conjugated antibodies from Bio-Rad Laboratories (Hercules, CA).
2.4. Protein extracts
Whole-cell protein extracts were prepared by lysing 1–2 × 107 cells in RIPA buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1% NP40, 0.1% SDS, 1% deoxycholic acid, 1 mM Na-orthovanadate, 1 mM NaF, 100 μg/ml phenylmethylsulfonyl fluoride/PMSF/, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) for 30 min on ice. Lysates were centrifuged at 12,000 rpm for 15 min and the supernatant was collected. Protein concentration was assessed by means of the Bio-Rad assay method.
2.5. Immunoblot analysis
Cell lysates were prepared in RIPA buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton × 100, 0.5% deoxycholate, 0.1% SDS, 5 mM EDTA) containing protease inhibitors (Complete Protease Inhibitor Cocktail Tablets; Roche Applied Science, Palo Alto, CA). Lysates were normalized for total protein (50 μg) and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 4% to 20% gradient gels; PIERCE) and immunoblot analysis. Primary antibodies included anti-Ki-67, albumin (Cell Signaling, Danvers, MA), and β-actin (Sigma, St. Louis, MO). Immunodetection was accomplished with the use of HRP-conjugated secondary antibodies and an enhanced chemiluminescence (ECL) method (PIERCE) involving exposure to x-ray film (XAR; Kodak, Sigma).
2.6. Immunoprecipitation
500 μl of plasma were incubated with 2 μg of anti Ki-67 clone H300 or rabbit IgG in 1 ml lysis buffer for 2 h at 4 °C; followed by incubation with 20 μl of protein A/G Sepharose beads (Sigma) for 1 h at room temperature. The supernatants were removed and stored for further Western blot analysis. The beads were then washed 4 times with lysis buffer and complexes were resuspended in loading buffer (100 mM Tris HCl [pH 6.8], 12.5% glycerol, 8% β-mercaptoethanol, 0.1% bromophenol blue, and 3% SDS). Immunocomplexes were harvested and subjected to Western blot analysis with appropriate antibodies.
2.7. Circulating Ki-67 ECL assay
This assay employs an electrochemiluminescence (ECL)-based technology from MSD, an adaptation of enzyme-linked immunosorbent assays (ELISAs). Nonspecific binding of the MSD anti-species plate was first blocked by overnight incubation at 4 °C with blocking buffer (MSD). A capture antibody specific for Ki-67 (mouse) had been coated onto the wells of the MSD anti-species (antibody-coated 96-well small spot Anti Mouse) plates for 2 h. The Ki-67 mAb was prepared in the antibody diluent described above. Samples including standards of known Ki-67 concentrations (HL60 lysates), specimens, and controls were diluted 1/5 (volume/volume) with sample diluent, pipetted into these wells, and incubated for 2 h. After the unbound material was washed away with MSD washing buffer, blocking antibody (goat anti-chicken antibody) was added to the plates and incubated for 1 h. Plates were then washed 3 times and the detection antibody anti Ki-67 (rabbit) was added. After incubation for 1 h and washing to remove the unbound enzyme, SULFO-TAG anti-rabbit was added to the wells. The reading was achieved by adding a Read Buffer (MSD) solution containing tripropylamine (TPA) to the plate. Each sample was measured in duplicate, and standard curves for the estimation of cKi-67 concentration were generated by using serial dilutions of HL60 lysate. The plate was read using an MSD Sector Imager 2400 instrument for electrochemiluminescence RLU (relative light unit) signal. This RLU signal was analyzed and compared with RLU signals of standards to determine the concentration of each sample; each sample had a value calculated in units Ki-67/100 μl. The unit is arbitrarily defined as equivalent Ki-67 molecules found in HL60 lysate.
2.8. Statistics
The Kruskal-Wallis test was used for categorical data, and Student's t test was used for continuous data. Correlations in plasma and peripheral blood cell samples were determined using Spearman's correlation coefficients. The sign test was used to compare results obtained by 2 different methodologies. The log-rank test was used to compare Kaplan-Meier survival curves between ALL patients with different plasma levels of cKi-67. Clinical and biological findings were analyzed for correlation with survival using Cox proportional hazard models.
3. Results
3.1 Development of cKi-67 measurement assay
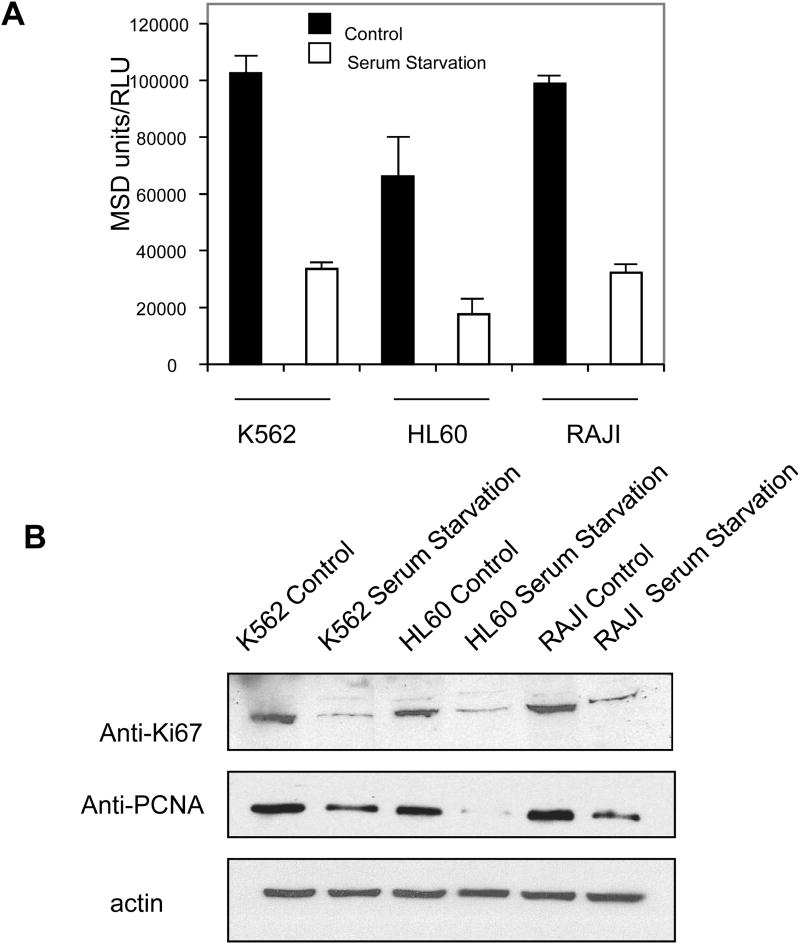
This sandwich immunoassay using ECL technology was developed with a Ki-67 monoclonal antibody as the capture reagent and polyclonal rabbit anti-Ki-67 for detection. Ki-67 protein is expressed during all phases of the cell cycle except G0, and serum starvation can be used to induce growth arrest and synchronize (or enrich) cultured cells in G0 [13]. We therefore tested the reliability of our ECL assay on leukemic cell lines (K562, HL60, and Raji, Fig1) deprived of serum for 72 h, with simultaneous monitoring of Ki-67 levels in cell lysates by Western blot (Fig 1 A). Results from the ECL assay showed that the level of Ki-67 was downmodulated during growth arrest in all leukemic cell lines tested (Fig 1 A). Western blot experiments confirmed that serum starvation decreased Ki-67 levels in these cell lines but had no effect on actin (loading control; Fig. 1 B). PCNA is a prolifereation marker and its expression increases from the late G1 phase through the S-phase of the cell cycle [14]. PCNA antibody was used as a control and show that PCNA protein expression following serum starvation confirmed that serum starvation decreased Ki-67 levels in these cell lines (Fig. 1 B).
Figure 1.
Serum starvation induces down-regulation of Ki-67 protein. A) K562, HL60, and Raji cells were cultured in media containing 10% FBS (■; Control) or 0.2% FBS (□; Serum Starvation). Lysates were prepared from the cells indicated, and Ki-67 levels were evaluated using the Meso Scale Discovery (MSD)-based enzyme immunoassay. Data represent the mean (SD) of at least 3 experiments. B) Lysates (100 μg) from cells cultured in media containing 10% (Control) or 0.2% (Serum Starvation) FBS were analyzed by SDS-PAGE/immunoblotting using anti-Ki-67, anti-PCNA or anti-actin antibodies. Antibody detection was accomplished by electrochemiluminescence with exposure to x-ray film. All experiments were repeated at least 3 times.
The reproducibility of the assay was demonstrated by evaluating the intra-assay and inter-assay variation (supplemental Table 1 and 2). The mean coefficients of variation (CVs) for this assay were below 10%, indicating acceptable variability. Human EDTA-plasma retained acceptable levels of Ki67 activity at 4 °C for at least 5 days (supplemental Table 3) and at room temperature for 3 days (supplemental Table 4) acceptable Ki-67 activity was also maintained after 3 additional freeze/thaw cycles from the initial freeze/thaw ((supplemental Table 5).
3. 2. Detection of cKi-67 in plasma samples by Western blot
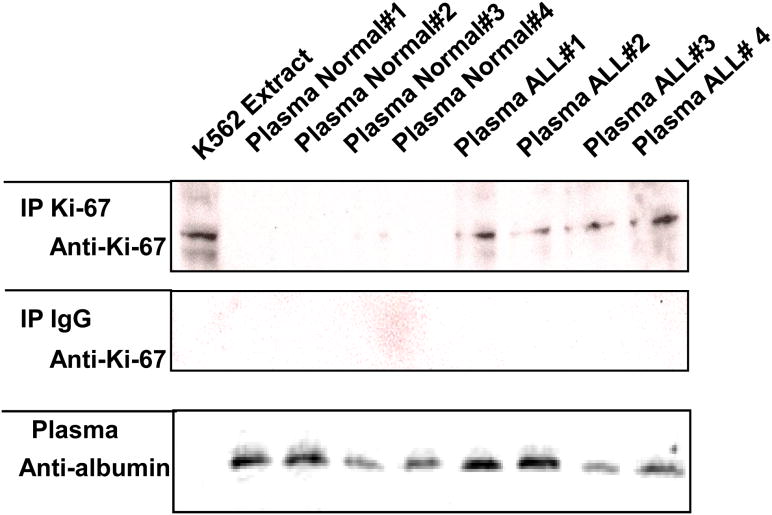
To determine whether Ki-67 is present in plasma, we immunoprecipitated cKi-67 from clinical plasma samples diluted 1:1 in lysis buffer and then analyzed the immunoprecipitated proteins by immunoblotting with anti-Ki-67 antibody. Ki-67 was detected by immunoblot in ALL patient samples but was not present at detectable levels in normal plasma samples (Fig 2). K562 immunoprecipitated extracts served as a control (lane 1). The variation in the quantity of Ki-67 was not due to variations in the amount of protein loaded, as shown with the anti-albumin antibody control. Immunoprecipitation with anti-IgG antibody served as a control for this experiment. Taken together, these data demonstrate that cKi-67 protein is present and measurable in the plasma of patients with ALL.
Figure 2.
Detection of circulating Ki-67 protein in the plasma of patients with acute lymphoblastic leukemia (ALL), but not control subjects, by immunoblot assay. K562 extracts (100 μg, lane 1) and diluted plasma from 4 normal control subjects and 4 ALL patients (1:5 in lysis buffer, lanes 2-9) were analyzed by SDS-PAGE/immunoblotting using anti-albumin (bottom) antibodies. Alternatively, lysate and plasma were prepared and subjected to immunoprecipitation (IP) with anti-Ki-67 antibody or anti-IgG antibody (top and middle). The resulting immune complexes were analyzed by Western blotting using Ki-67 antibody, as indicated. Antibody detection was accomplished by enhanced chemiluminescence with exposure to x-ray film.
3.3. Measurement of cKi-67 in plasma by ECL-based assay
Having established that cKi-67 can be detected in plasma of patients with ALL, we used the ECL-based assay to measure plasma levels of cKi-67 in samples from normal control subjects and patients with ALL. Patients with newly diagnosed ALL (n = 27) had significantly higher levels of cKi-67 (median, 762; range, 0-4574 U/100 μL) than did normal control subjects (n = 113; median, 399; range, 36-2830 U/100 μL) (Fig 3).
Figure 3.
Quantitation of circulating Ki-67 (cKi-67) in plasma among normal control subjects (N) and patients with acute lymphoblastic leukemia (ALL), measured using an electrochemiluminescence (ECL)-based method with a Meso Scale Discovery format. cKi-67 levels were significantly higher in ALL patients than in healthy control subjects (P <0.001). Median and 75 percentile are shown in boxes. Asterisks indicate outliers; open circles indicate extreme values
3.4. Clinical correlations
In patients with ALL, cKi-67 levels did not correlate significantly with WBC, platelet, or blast counts, or with levels of hemoglobin, lactate dehydrogenase, blood urea nitrogen, or creatinine. However, 26% of older ALL patients (age >70 years) had higher cKi-67 levels than did younger patients (P = 0.05). High levels of cKi-67 were found in 2 patients with Burkitt lymphoma (999 and 1623 U/100 μL, respectively), consistent with the known high Ki-67 staining demonstrated by IHC in these patients.
3.5. Correlation of plasma cKi-67 levels with survival in ALL
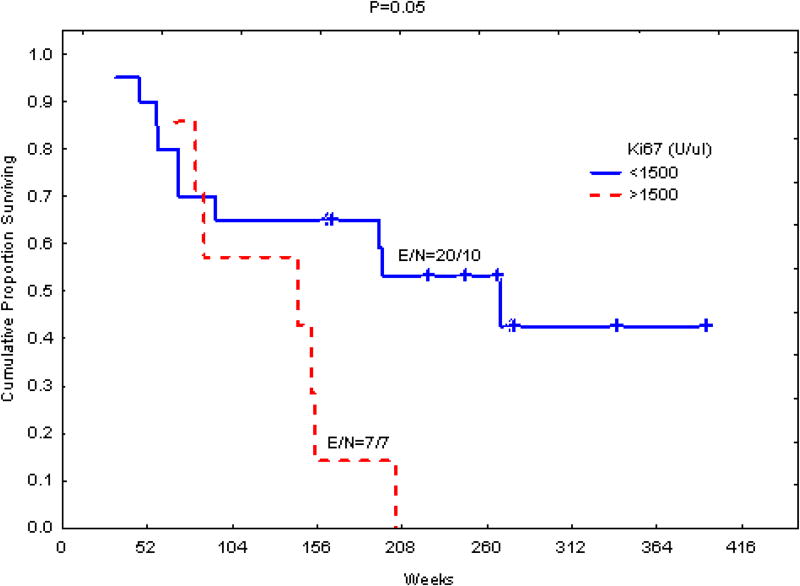
ALL patients were divided into 2 groups on the basis of their plasma levels of cKi-67 (Fig 4). The cut-off level was set at 1500 units Ki-67/100 μL plasma, a value corresponding to the upper quartile level detected in ALL patients. Patients with high plasma levels of cKi-67 had shorter survival than those with lower levels (P = 0.05, log-rank test; Fig. 4).
Figure 4.
Association of circulating Ki-67 (cKi-67) levels in plasma with survival in patients with acute lymphoblastic leukemia (ALL; n = 27). Patients with higher levels of plasma cKi-67 had shorter survival. The cut-off ((1500 U/100 μL) corresponds to the upper quartile detected in the control group. E refers to events and N refers to total number.
4. Discussion
Proliferation is a key feature of the progression of tumors and is now widely measured in a subjective, semi-quantitative fashion by means of immunohistochemical staining for the nuclear antigen Ki-67. Quantitation of Ki-67 for diagnosis and disease monitoring is becoming the standard of care for cancer patients. Ki-67 has been reported to be particularly useful in studies of the prognostic value of cell growth in clinical samples of human neoplasms, most notably prostate and breast carcinomas. For these types of tumors, the prognostic value for survival and tumor recurrence has repeatedly been demonstrated in univariate and multivariate analysis. Ki-67 labeling may serve as a prognostic factor of prostatic cancer [15]. In breast cancer high Ki-67 expression is a sign of poor prognosis associated with a good clinical response to chemotherapy [16-19]. In addition, high levels of proliferation and high levels of staining have been reported in patients with ALL, and particularly in patients with Burkitt lymphoma [20].
We and others previously reported that plasma from patients with leukemia is enriched with leukemia-specific nucleic acid [21] and cellular proteins, such as CD20 and CD52, which can be readily detected in laboratory assays. This plasma enrichment presumably derives from the more rapid turnover of leukemic (relative to normal) cells and the resulting release of cellular proteins and nucleic acids into circulation [22-25]. We took advantage of this concept and measured cKi-67 in plasma. Based on ECL detection, this method offers a unique combination of sensitivity, dynamic range, and convenience. This new cKi-67 assay exhibited high reproducibility (CV<10%) and was sufficiently sensitive to detect the low levels of cKi-67 present in normal individuals (Figures 1A and 3).
In this study we demonstrated that Ki-67 protein is present in the plasma of normal control subjects and patients with ALL. Our data correlate with a previous report on the expression of functional markers in ALL, which showed that the cellular Ki-67 (+) fraction was increased in ALL [26]. Taken together, these findings indicate that Ki-67 can be measured as circulating protein, and that cKi-67 levels in plasma can be used as a maker as demonstrated in ALL.
In addition, cKi-67 testing may hold potential as a pharmacodynamic marker for monitoring the efficacy of therapy. Studies analyzing tissue sections have shown that sulindac sulfide inhibits the proliferation of colon cancer cells by reducing expression of the proliferation markers PCNA and Ki-67 [27]. A quantitaive cKi-67 assay could be very useful to monitor drug efficacy in clinical trials, without the need for biopsy. Ki-67 is also a potential drug target in oncology, and Zheng et al have shown inhibition of renal cancer cell growth in vitro and in vivo with oncolytic adenovirus armed short hairpin RNA targeting Ki-67 encoding mRNA [28]. Plasma cKi-67 may have the potential to be used to monitor such therapy as well.
In summary, our findings provide a strong rationale for further studies on the biologic function, prognostic significance, and therapeutic significance of cKi-67 in patients with hematologic malignancies.
Supplementary Material
Acknowledgments
We thank Jeff Radcliff for editorial contributions.
Footnotes
Conflicts of interest: We attest that the authors have no financial conflicts of interest.
Authors Contributions: MA, JMB. Conception and design of the study.
HK, ZE, WM, FA, AA, DT, SB acquisition of data, or analysis and interpretation of data.
JMB, CY, MA, ZZ. Drafting the article or revising it critically for important intellectual content.
JMB, MA. Final approval of the version to be submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 2.Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 3.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res. 2000;257:231–7. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 5.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–20. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 6.Khatami A, Hugosson J, Wang W, Damber JE. Ki-67 in screen-detected, low-grade, low-stage prostate cancer, relation to prostate-specific antigen doubling time, Gleason score and prostate-specific antigen relapse after radical prostatectomy. Scand J Urol Nephrol. 2009;43:12–8. doi: 10.1080/00365590802469543. [DOI] [PubMed] [Google Scholar]
- 7.Oshima CT, Iriya K, Forones NM. Ki-67 as a prognostic marker in colorectal cancer but not in gastric cancer. Neoplasma. 2005;52:420–4. [PubMed] [Google Scholar]
- 8.Ferrara C, Tessari G, Poletti A, et al. Ki-67 and c-jun expression in pancreatic cancer: a prognostic marker? Oncol Rep. 1999;6:1117–22. doi: 10.3892/or.6.5.1117. [DOI] [PubMed] [Google Scholar]
- 9.Sulik M, Guzinska-Ustymowicz K. Expression of Ki-67 and PCNA as proliferating markers in prostate cancer. Rocz Akad Med Bialymst. 2002;47:262–9. [PubMed] [Google Scholar]
- 10.Uzoaru I, Rubenstein M, Mirochnik Y, Slobodskoy L, Shaw M, Guinan P. An evaluation of the markers p53 and Ki-67 for their predictive value in prostate cancer. J Surg Oncol. 1998;67:33–7. doi: 10.1002/(sici)1096-9098(199801)67:1<33::aid-jso7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Bassan R, Gatta G, Tondini C, Willemze R. Adult acute lymphoblastic leukaemia. Crit Rev Oncol Hematol. 2004;50:223–61. doi: 10.1016/j.critrevonc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 13.Cooper S. Reappraisal of serum starvation, the restriction point, G0, and G1 phase arrest points. FASEB J. 2003;17:333–40. doi: 10.1096/fj.02-0352rev. [DOI] [PubMed] [Google Scholar]
- 14.Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci U S A. 1985;82:3262–6. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallee MP, van Steenbrugge GJ, ten Kate FJ, Schroeder FH, van der Kwast TH. Determination of the proliferative fraction of a transplantable, hormone-dependent, human prostatic carcinoma (PC-82) by monoclonal antibody Ki-67: potential application for hormone therapy monitoring. J Natl Cancer Inst. 1987;79:1333–40. [PubMed] [Google Scholar]
- 16.Lee J, Im YH, Lee SH, et al. Evaluation of ER and Ki-67 proliferation index as prognostic factors for survival following neoadjuvant chemotherapy with doxorubicin/docetaxel for locally advanced breast cancer. Cancer Chemother Pharmacol. 2008;61:569–77. doi: 10.1007/s00280-007-0506-8. [DOI] [PubMed] [Google Scholar]
- 17.Jones RL, Salter J, A'hern R, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 18.Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjallskog ML, Blomqvist C. Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology. 2007;51:491–8. doi: 10.1111/j.1365-2559.2007.02798.x. [DOI] [PubMed] [Google Scholar]
- 19.Hrushesky WJ, Retsky M, Baum M, Demicheli R. Re: Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:1053–4. doi: 10.1093/jnci/djm019. [DOI] [PubMed] [Google Scholar]
- 20.Nomura Y, Yoshida S, Karube K, et al. Estimation of the relationship between caspase-3 expression and clinical outcome of Burkitt's and Burkitt-like lymphoma. Cancer Sci. 2008;99:1564–9. doi: 10.1111/j.1349-7006.2008.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers A, Joe Y, Manshouri T, et al. Relative increase in leukemia-specific DNA in peripheral blood plasma from patients with acute myeloid leukemia and myelodysplasia. Blood. 2004;103:2799–801. doi: 10.1182/blood-2003-06-1840. [DOI] [PubMed] [Google Scholar]
- 22.Manshouri T, Do KA, Wang X, et al. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood. 2003;101:2507–13. doi: 10.1182/blood-2002-06-1639. [DOI] [PubMed] [Google Scholar]
- 23.Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin's lymphoma or Hodgkin's disease. Br J Haematol. 2003;123:850–7. doi: 10.1046/j.1365-2141.2003.04683.x. [DOI] [PubMed] [Google Scholar]
- 24.Albitar M, Do KA, Johnson MM, et al. Free circulating soluble CD52 as a tumor marker in chronic lymphocytic leukemia and its implication in therapy with anti-CD52 antibodies. Cancer. 2004;101:999–1008. doi: 10.1002/cncr.20477. [DOI] [PubMed] [Google Scholar]
- 25.Giles FJ, Albitar M. Plasma-based testing as a new paradigm for clinical testing in hematologic diseases. Expert Rev Mol Diagn. 2007;7:615–23. doi: 10.1586/14737159.7.5.615. [DOI] [PubMed] [Google Scholar]
- 26.Oh EJ, Kahng J, Kim Y, et al. Expression of functional markers in acute lymphoblastic leukemia. Leuk Res. 2003;27:903–8. doi: 10.1016/s0145-2126(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 27.Qiao L, Shiff SJ, Rigas B. Sulindac sulfide inhibits the proliferation of colon cancer cells: diminished expression of the proliferation markers PCNA and Ki-67. Cancer Lett. 1997;115:229–34. doi: 10.1016/s0304-3835(97)04740-x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng JN, Pei DS, Mao LJ, et al. Inhibition of renal cancer cell growth in vitro and in vivo with oncolytic adenovirus armed short hairpin RNA targeting Ki-67 encoding mRNA. Cancer Gene Ther. 2009;16:20–32. doi: 10.1038/cgt.2008.61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.