Abstract
Localization of GPx2, the gastrointestinal form of glutathione peroxidases (GPx), in the intestinal crypt epithelium points to a specific but so far unknown function of this particular GPx. Therefore, consequences of a GPx2 knockout were tested in mice fed a selenium-restricted, -adequate or -supplemented diet. An unexpected increase in total GPx activity was found throughout the intestine in selenium-fed GPx2 knockout animals. Immunohistochemistry revealed a strong increase of GPx1 in colon and ileum especially in crypt bases where typically GPx2 is localized. GPx1 mRNA was not enhanced in GPx2 KO, indicating that up-regulation most likely occurs at a translational level. Loss of GPx2 was accompanied by an increase of apoptotic cells at colonic crypt bases, an area essential for the self-renewal of the intestinal epithelium, particularly under selenium-restriction. Additionally mitotic cells increased in the middle parts of the crypts, indicating an extension of the proliferative area. The findings corroborate a role of GPx2 in regulating mucosal homeostasis. In GPx2 KO mice, a rise of GPx1 can only partially compensate GPx2 even under selenium-supplementation, indicating that GPx2 is the major anti-apoptotic GPx in the colon. This data explains why spontaneous ileocolitis becomes only manifested if both, gpx2 and gpx1 are deleted.
Keywords: GPx1, GPx2, GPx2 KO, apoptosis, mitosis, intestine
Introduction
Glutathione peroxidase-2 (GPx2) is highly expressed in the mucosal epithelium of the gastrointestinal tract with increased levels from proximal to distal sections of the mouse small intestine [1]. Due to this specific localization it was first suggested to act as a barrier against the absorption of hydroperoxides. Further analyses of ileum and colon revealed that GPx2 levels are much higher at crypt bases than at the luminal sites [2,3]. Crypts harbour the stem cell moiety of the proliferative zone. The coincidence of GPx2 expression and cell growth has led to the suggestion that GPx2 might be involved in the maintenance of the self-renewal of the intestinal mucosa [2,4]. This view is supported by the regulation of GPx2 expression (i) via the Wnt pathway [5,6], which is especially active in the proliferative zone at the crypt bases, and (ii) via p63 signalling which keeps cells in the undifferentiated and, thus, proliferative state [7]. An active Wnt pathway is required to let cells proliferate before they start to differentiate and to migrate towards the villi, which is accompanied by a decrease in the activity of the Wnt pathway. Three days after terminal differentiation, cells reach the tops where they are either shed into the lumen or eliminated by spontaneous apoptosis [8]. In parallel with the decreasing proliferating capacity of mucosal cells, GPx2 levels decrease in the zones of differentiation and migration and are nearly absent in the apoptotic areas [2]. In accordance, GPx2 inhibited migration in HT29 [9] and apoptosis in MCF7 cancer cells [7].
A role of GPx2 in the regulation of proliferation can also be inferred from its up-regulation in cancer cells [4,10-12] and its ability to facilitate in vivo tumor growth of HT29 cells in nude mice [9]. However, the physiological function of GPx2 as well as its role in tumor cells is unknown. Data on GPx2 KO mice are scarce since the mice do not have an obvious phenotype. Only when exposed to UV radiation they display an increased tumor incidence [13]. In contrast, when both, GPx1 and GPx2 are deleted, the double knockout (DKO) mice develop spontaneous ileocolitis and later intestinal cancer [14]. These phenomena were reversed by a single allele of GPx2, but not of GPx1, pointing to a particular relevance of GPx2 for preventing mucosal damage [15].
To learn more about the physiological function of GPx2 in the intestine and the consequences of its deletion, we studied wild-type and GPx2 single KO mice adjusted to a selenium-restricted, a selenium-adequate or a selenium-supplemented status. It was tested whether deletion of GPx2 influenced the synthesis of other GPxs and whether major processes in mucosal homeostasis, apoptosis and proliferation, were affected by the knockout. Surprisingly, we found a drastic increase of GPx1 in the colon and ileum of GPx2 KO mice, especially in areas where typically GPx2 is expressed. Furthermore, the number of apoptotic and mitotic cells in the colon significantly increased under limited selenium supply, but less upon selenium supplementation, indicating that the impaired protection against apoptosis in GPx2 KO mice can in part be compensated by overexpression of GPx1.
Materials and Methods
Animals
Animals were housed under specific pathogen free (SPF) conditions with a 12 h dark light cycle and free access to food and water. Animal experiments and husbandry were carried out in accordance with guidelines from Federation of European Laboratory Animal Science Association (FELASA). The studies were approved by local authorities under the number MLUV 32-2347/4+68. GPx2 KO mice were generated as C57BL/6J × 129SV/J hybrids and 3 times backcrossed to a C57BL/6J background [16] and a 4th time before the experiment started. Mice were reproduced by in house breeding.
Animals investigated here were part of controls of an ongoing study on inflammation-triggered (AOM/DSS) carcinogenesis.1 Thus, animals (10 per group) have been treated once with 300 μl saline i.p. (for AOM control) and were given 100 μl of phosphate buffer by gavage every second day for 4 weeks (for sulforaphane control). This treatment did not affect any of the measured parameters as routinely spot-checked. Animals were sacrificed 3 weeks after saline injection at an age of 11-12 weeks for measuring GPx activity and protein expression. Apoptosis and mitosis was analyzed at 20 weeks of age.
Diets
For adjusting the selenium-status, the selenium-adequate and -supplemented diet was obtained by mixing selenomethionine (Acros, Geel, Belgium) into torula-yeast-based selenium-poor diet (No C1045, with 50% carbohydrates, 17% protein, 5% fat, 4% fibre, and a mixture of micronutrients; Altromin, Lage, Germany) containing 0.086 mg Se/kg [17] to yield a selenium content of 0.15 mg/kg for the selenium-adequate diet corresponding to the dietary reference intake for mice [18] and a selenium content of 0.66 mg/kg for the selenium-supplemented diet. All diets were fed as pellets starting directly after weaning.
Tissue preparation
Animals were anesthetized with isoflurane, killed by cervical dislocation and the intestine was removed. The proximal 4 cm of the ileum and the proximal 2 cm of the colon were taken for measuring GPx activity and frozen at −80°C. The rest of the tissue was used for histology and apoptosis/mitosis counting. It was fixed for 24 h in 4% neutral-buffered formalin and washed under tap water for another 24 h. Then specimens were dehydrated and embedded in paraffin wax. Serial tissue sections (2 μm) from the same area (1 cm proximal to the ileocecal junction and 1.5 cm proximal to the anus) of all 10 animals per group were prepared for histology (hematoxylin staining) and immunohistochemistry.
RNA isolation and quantitative PCR
Ileum and proximal colon were ground under liquid nitrogen. 20–30 mg powder were suspended in 800 μL of cold Trizol (Invitrogen, Karlsruhe, Germany), homogenized with a tissue lyzer (Qiagen, Hilden, Germany), and RNA isolated using the Trizol protocol. RNA from the distal colon was isolated from four 10 μm thick formalin-fixed, paraffin-embedded tissue sections using Rneasy FFPE (Qiagen) according to the manufacturer's instructions. RNA (3 μg or the whole sample in case of the distal colon) was reversely transcribed with 150 fmol oligo(dT)15 primers and 180 U moloney murine leukemia virus reverse transcriptase (Promega, Mannheim, Germany) in a total volume of 45 μL. qPCRs (Mx3005P™ qPCR System, Stratagene, Amsterdam, Netherlands) were performed in triplicates with 1 μL cDNA in 25 μL reaction mixtures using SYBR Green I (Molecular Probes, Eugene, OR, USA) as fluorescent reporter. Primers (Table 1) were designed to be specific for cDNA with PerlPrimer v1.1.14. PCR products were quantified with a standard curve [19]. Rpl13a was used as reference gene.
Table 1. Primer sequences (5′ → 3′).
| Gene | Acc. number | Primer sequence | Product |
|---|---|---|---|
| Ribosomal protein L13a | NM_009438 | fwd GTTCGGCTGAAGCCTACCAG rev TTCCGTAACCTCAAGATCTGCT |
157 bp |
| Gpx1 | NM_008160 | fwd TACACCGAGATGAACGATCTG rev ATTCTTGCCATTCTCCTGGT |
101 bp |
| Gpx3 | NM_001083929 | fwd CCATTTGGCTTGGTCATTCTGGG rev CACCTGGTCGAACATACTTGAGAC |
105 bp |
GPx activity
For GPx activity, 20 mg of tissue powder (frozen tissue ground under liquid nitrogen) were homogenized in a tissue lyzer (Qiagen, Hilden, Germany) for 2 × 2 min at 30 Hz in 500 μl 100 mM Tris/HCl, 300 mM KCl, 0.1% Triton X-100, pH 7.6, containing 4 μl of protease inhibitor cocktail (Calbiochem, Bad Soden, Germany). Cellular debris was removed at 20,000 × g, for 15 min, at 4°C. Protein content was estimated according to Bradford [20] and GPx activity was expressed as mU/mg protein. Absorbance was measured using a 96-well microtitre plate absorbance reader (Synergy 2, Biotek Instruments GmbH, Bad Friedrichshall, Germany). The absorbance reader corrects the different filling levels to a light path length of 1 cm. This way, activity can be calculated according to Lambert-Beer's law. GPx activity was measured in the glutathione reductase-coupled test optimized for tissue samples with a final concentration of 50 μM of H2O2 as substrate [21]. One unit (U) was defined as consumption of 1 μmol NADPH per minute. GPx4 activity was estimated with 50 μM phosphatidylcholine hydroperoxide as substrate, which was prepared as described [22].
Immunohistochemistry
Tissue sections were stained as described [2,23]. Primary antibodies, rabbit anti-human GPx2 antiserum [24] in a 1:12000 dilution and rabbit anti-human-GPx1 (ab 22604, Abcam, Cambridge, UK) in a 1:6000 dilution were applied overnight at 4°C. Dilution buffer was from Dako (antibody diluent S3022). N-Histofine® Simple Stain Mouse Max PO (Rabbit) (Nichirei Biosciences Inc., Tokyo, Japan) and diaminobenzidine (DakoCytomation, Hamburg, Germany) were used for visualization. Specificity of the GPx1 antibody was tested by blocking with the immunizing peptide (ab25301). A concentration of 50 ng/ml was sufficient to block GPx1 staining in epithelial cells as well as in stromal cells.
Quantification of apoptosis
Apoptotic cells were characterized by morphological changes, including cell shrinkage, nuclear condensation and perinuclear clearing according to the reference standard [25]. Apoptotic cells were counted in 200-300 longitudinally sectioned colonic crypts per animal and numbers indicated per 100 crypts. Crypt sections were divided into quarters 1 to 4 with quarter 4 at the crypt base. Samples were blinded for counting.
Quantification of mitosis
Mitotic cells were characterized by morphological changes, including chromatin condensation, chromosome formation and characteristic phases of mitosis (pro-, meta-, ana- and telophase). Mitotic cells in all animals were counted in the same 200-300 longitudinally sectioned colonic crypts where apoptotic cells were counted and numbers expressed per 100 crypts. As for apoptosis, crypt sections were divided into 4 quarters and samples blinded for counting.
Statistics
Significance (GraphPad Prism® version 5.0, San Diego, CA, USA) was tested by two way ANOVA with Bonferroni's post tests when comparing more than two groups or as indicated in the figure legends. A p-value < 0.05 was considered significant.
Results
Total GPx activity is increased in GPx2 KO mice resulting from an up-regulation of GPx1
To check whether a GPx2 knockout is reflected by an altered total GPx activity it was tested in homogenates of ileum and colon of wild-type and GPx2 KO mice. Surprisingly, total GPx activity was not decreased in GPx2 KO mice as was expected, but compared to wild-type significantly increased in the intestine. Results are shown for ileum and colon (Fig. 1A and B). A slightly higher activity was even observed in the Se-deficient colon (Fig. 1B), yet increased with increased selenium content in the diet. Activity of GPx4, the only GPx whose activity can be measured with a specific substrate, was not influenced by GPx2 KO neither in the ileum nor in the colon.
Fig.1.
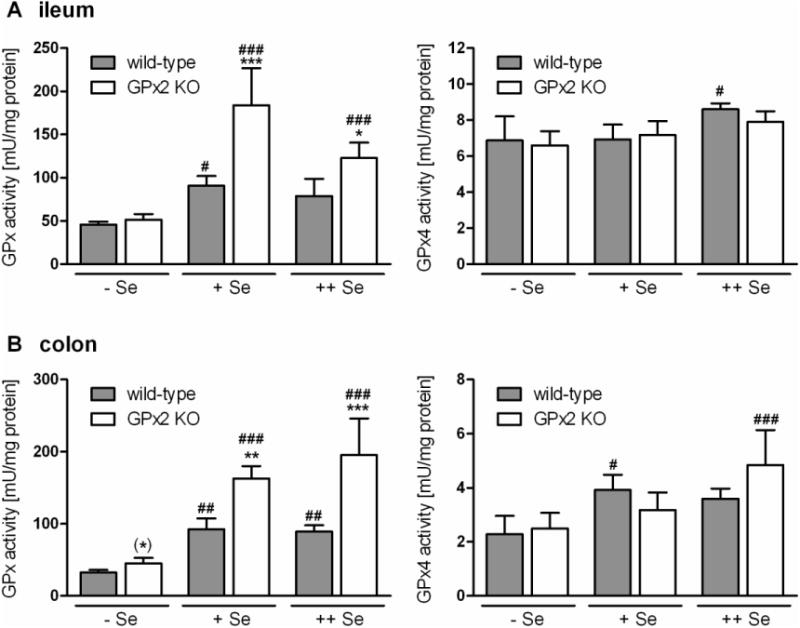
Total GPx and GPx4 activity in the intestine of wild-type and GPx2 KO mice at different selenium status.
Total GPx and GPx4 activity in Se-restricted (-Se), Se-adequate (+Se) and Se-supplemented (++Se) wild-type (WT) and GPx2-KO mice (KO) was measured in homogenates of the ileum (A) and colon (B). Values are means ± SD (n = 4). Significance was estimated by two way ANOVA with Bonferroni's post tests. *p<0.05, **p<0.01, ***p<0.001 vs. respective WT; #p< 0.05, ##p< 0.01, ###p< 0.001 vs. respective groups of Se-restricted mice; (*)p<0.05 vs. the respective WT when Se-restricted groups were analyzed separately by Student's t-test. Further details see ‘Methods’.
To identify the GPx which might be up-regulated by GPx2 deletion, tissues were analyzed for GPx1 and GPx2 expression by immunohistochemistry. Results obtained in the colon are shown in Fig. 2. As observed many times before, in wild-type mice GPx2 was preferentially expressed in the crypt bases (Fig. 2A) and according to its high ranking in the hierarchy of selenoproteins was not completely abolished in selenium-poor animals (Fig. 2C). Fig. 2B confirms the complete absence of GPx2 in the GPx2 KO colon.
Fig. 2.
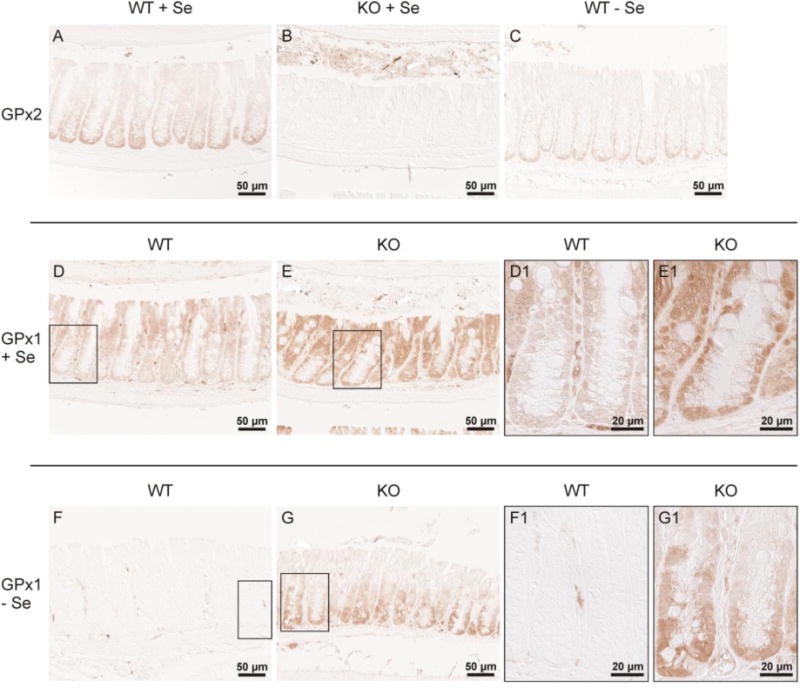
GPx1 and GPx2 in the colon of wild-type and GPx2 KO mice.
Glutathione peroxidases were immunohistochemically stained in slices of the colon of wild-type (WT) and GPx2 KO mice. (A-C) shows GPx2 staining, where (A) and (C) indicate WT and (B) the KO animal, both under the selenium states indicated. (D-G) shows staining for GPx1. (D) and (E) represent the Se-adequate and (F) and (G) the selenium-poor status of the indicated genotypes. (D1, E1, F1, G1) indicate the higher magnification of the area marked in the respective overview. The figure confirms the gradual increase of GPx2 expression from the top of the crypt towards the crypt base (A), the absence of GPx2 in the KO mice (B), and the only weak decline in selenium-restriction (C). Under both, selenium-adequate (D,E) and -poor (F,G) conditions, GPx1 is increased in GPx2 KO mice (E,G), especially at the crypt base. Shown are representative slices of each group stained simultaneously. Further details see text and ‘Methods’.
In wild-type mice, the localization of GPx1 is opposite to that of GPx2. In selenium-adequate wild-type mice GPx1 is mainly seen at the luminal surface and only weakly at crypt base (Fig. 2D and D1). In GPx2 KO mice, GPx1 increases dramatically throughout the crypt, notably also at the base, when selenium supply is sufficient (Fig. 2E and E1). No GPx1 is detectable in epithelial cells of selenium-restricted crypts; the minor staining is located in stromal cells (Fig. 2F and F1).The increase of GPx1 at the crypt base in GPx2 KO mice, however, remains under selenium-restriction, whereas it disappears at the luminal side (Fig. 2G and G1). Thus, GPx1 is up-regulated in areas where usually GPx2 is located (Fig. 2A and C).
A similar situation was observed in the ileum (Fig. 3). Expression of GPx2 is more pronounced in crypts than in villi in selenium-adequacy (Fig. 3A) and under selenium-restriction (Fig. 3C). In the GPx2 KO ileum GPx2 is not expressed (Fig. 3B). GPx1 staining is weak even in Se-adequate wild-type mice (D), but visible throughout crypts and villi with a slightly higher level towards the bottoms (Fig. 3D). More distinct signals are detectable in the surrounding stroma cells which is maintained in the selenium-restricted tissue where GPx1 is almost absent in the epithelial cells (Fig. 3F and F1). As in the colon, GPx1 is dramatically increased throughout crypts and villi in selenium-adequate ileum of GPx2 KO mice (compare Fig. 3E with D and E1 with D1). Interestingly, up-regulated GPx1 was also localized in crypts, where preferentially GPx2 is expressed (Fig. 3A). As expected, GPx1 levels were much lower under selenium-restriction but compared to wild-type its increase in GPx2 KO crypts GPx1 was still visible (compare Fig. 3G with F and G1 with F1).
Fig. 3.
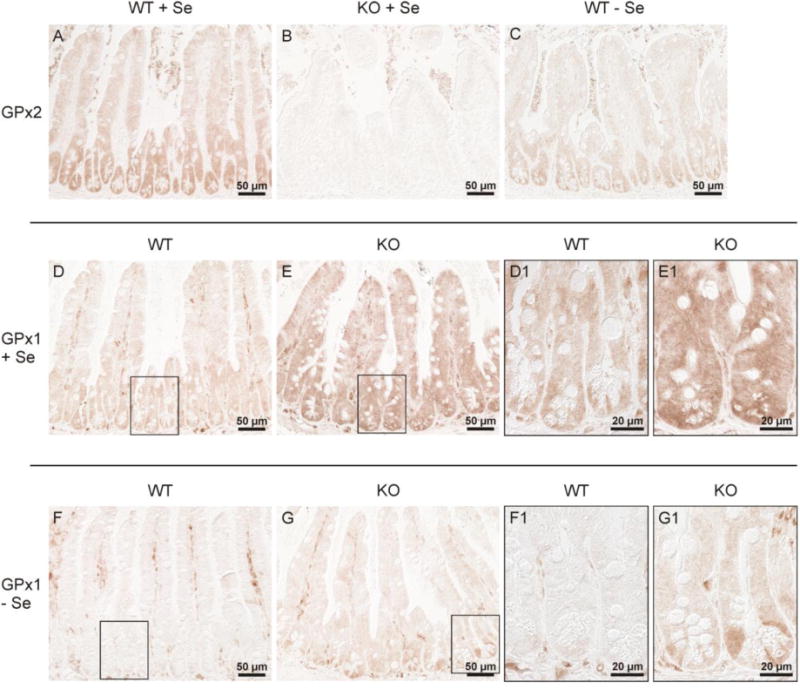
GPx1 and GPx2 in the ileum of wild-type and GPx2 KO mice.
Immunohistochemical GPx2 staining in the ileum of Se-adequate wild-type (A) and GPx2 KO (B) mice shows a similar distribution of GPx2 in the ileum as in the colon: increasing levels from villi towards crypts (A), confirmation of the absence of GPx2 in GPx2 KO mice (B), and a still visible expression under selenium-restriction (C). Staining for GPx1 reveals a drastic increase in GPx2 KO (E, E1) compared to wild-type (D, D1) selenium-adequate mice. GPx1 is almost completely decreased in selenium-poor wild-type (F, F1), but remains slightly up-regulated in selenium-poor GPx2 KO (G, G1) mice, especially in the crypts. The higher magnification of the area marked in the respective overview is labelled by 1. Shown are representative slices of each group stained simultaneously.
Selenium-supplementation did not strikingly increase GPx1 staining, neither in the colon nor in the ileum (not shown). Total GPx activity was also increased in the distal and proximal jejunum, but not in the duodenum and liver of GPx2 KO mice (not shown), whereas the activity of thioredoxin reductase, another selenoprotein, was not significantly affected by GPx2 deletion (not shown).
Up-regulation of GPx1 is not based on an increase in mRNA synthesis
Up-regulation of GPx1 in GPx2 KO mice raised two questions. First, is the up-regulation based on a transcriptional or a translational level, and second, are also other glutathione peroxidases affected. To test this, RNA from ileum and proximal colon was isolated from the same samples from which activity was tested, i.e. whole tissue homogenates, and quantified for GPx1 and 3 (Fig. 4A, B). According to their hierarchical ranking, mRNA of both GPxs was unstable under selenium-restriction and decreased to about 60%. RNA from the distal colon was taken from the same sections in which the histological data were obtained (Fig. 2 and 3). For this, RNA was isolated from the paraffin-embedded tissue. Also here GPx1 RNA significantly decreased with decreasing selenium state, whereas levels of GPx3 mRNA were more scattered and not significantly influenced by the selenium state (Fig. 4C). This corresponds to the observations made in microarray studies with mice fed identically [17]. The RNA level of neither GPx1 nor GPx3 was influenced by GPx2 deletion which clearly indicated that up-regulation of GPx1 must have occurred at the translational level. Since GPx4 activity was not changed (Fig. 1) a change in mRNA was not expected and, therefore, not tested.
Fig. 4.
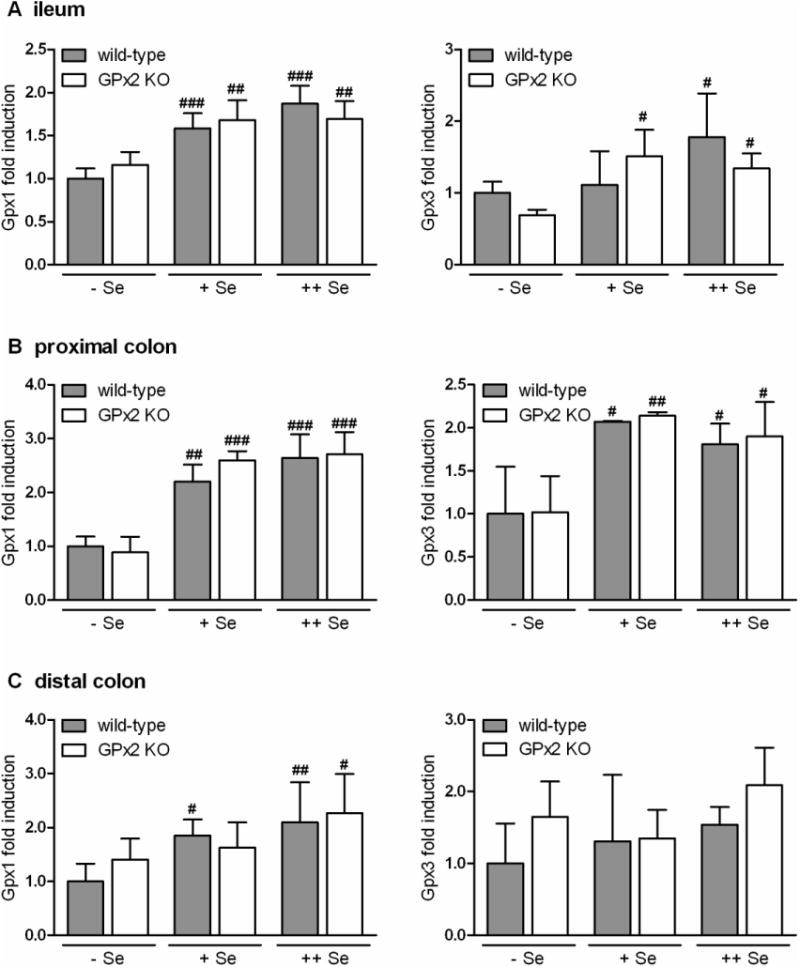
mRNA levels of Gpx1 and Gpx3 in the ileum (A), proximal (B) and distal (C) colon of wild-type and GPx2 KO mice at different selenium status.
RNA was isolated and GPx1 and 3 mRNA quantified in triplicate by PCR and normalized to the reference gene Rpl13a (see Methods). Selenium state and genotype is indicated in the figure. Levels are expressed relative to the level of selenium-restricted wild-type mice, which was set to 1. Values are means ± SD (n = 4). Significance was estimated by two way ANOVA with Bonferroni's post tests. #p<0.05, ##p<0.01, ###p<0.001 vs. respective groups of Se-restricted (-Se) mice. For further details see ‘Methods’.
Deletion of GPx2 elevates apoptotic cells in the colon
Silencing of GPx2 has been shown to increase apoptosis in MCF7 cells [7] and in the intestine of GPx1/GPx2 double knockout (DKO) mice [14]. It was, therefore, tested whether apoptosis was also increased in the single GPx2 knockout. A characteristic overview of the colon with apoptotic and mitotic cells marked is shown in Fig. 5. For quantification, apoptotic cells were counted in 200-300 longitudinally sectioned colonic crypts of wild-type and GPx2 KO mice (10 animals each) adjusted to the different selenium states at the age of 20 weeks. Numbers of apoptotic cells in the 4th colonic crypt quarter (crypt base) were generally higher in GPx2 KO mice than in wild-type and increased even more under Se-restriction where also the 3rd crypt quarter was affected (Fig. 6A). Selenium-containing diet was only partially able to prevent the increase in apoptosis. The number of apoptotic cells in the 4th crypt quarter was still enhanced under the selenium-supplemented diet.
Fig. 5.
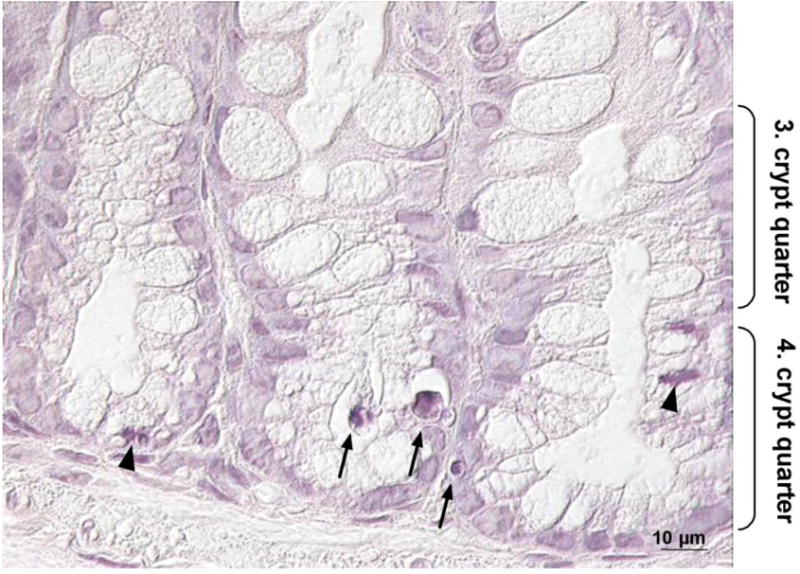
Apoptotic and mitotic cells in the colon of Se-adequate GPx2 KO mice. Slices of colon tissues from 20 weeks old animals were stained with hematoxylin to identify apoptotic and mitotic cells. A representative photograph is shown with apoptotic cells marked by arrows and mitotic cells by arrowheads.
Fig. 6.
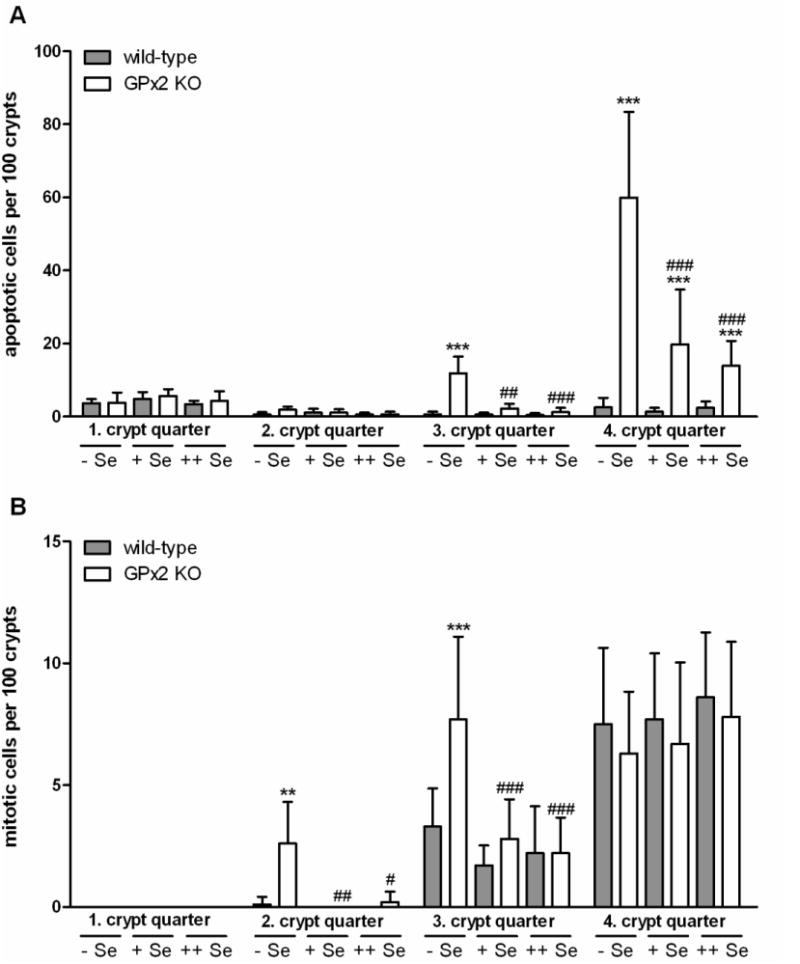
Increase of apoptotic (A) and mitotic (B) cells in the colon of GPx2 KO mice. Colon sections of 20 weeks old animals grown on the different selenium diets from weaning were stained with hematoxylin and apoptotic and mitotic cells (see Fig. 5) were counted in quarters 1 to 4 (where 4 is the base) of 200-300 intact crypts in each animal. Numbers of respective cells are indicated per 100 crypts. Values are means ± SD (n = 10). There were no mitotic cells in the first and 2nd quarters under selenium-adequacy. Significance was estimated by two way ANOVA with Bonferroni's post tests. **p<0.01, ***p<0.001 vs. respective samples of WT mice in the same crypt quarter (GPx2 KO effect). #p<0.05, ##p<0.01; ###p<0.001 vs. Se-restricted KO mice in the same crypt quarter (selenium effect).
Deletion of GPx2 shifts mitosis of colonic epithelial cells to luminal regions when selenium supply is limited
It has been suggested several times that GPx2 might support cell proliferation [4,9,12]. Therefore, also mitotic cells were counted in the same 200-300 longitudinally sectioned crypts of wild-type and GPx2 KO mice used for counting of apoptotic cells. The number of mitotic cells was not changed by GPx2 deletion in Se-adequacy or Se-supplementation neither in the 3rd nor the 4th crypt quarter, where usually GPx2 expression is highest (Fig. 6B). As expected, there were no mitotic cells detectable in the 1st and 2nd crypt quarter. In contrast, under Se-restriction mitotic cells significantly increased in GPx2 KO mice in the 3rd crypt quarter to a level usually present at the very bottom (4th quarter) and showed up also in the 2nd quarter. In contrast to apoptosis, selenium supply was able to prevent the increase in mitosis already when applied in the adequate amount.
Discussion
Up-regulation of GPx1 in GPx2-deficiency
The most surprising result during the present study was the distinct up-regulation of GPx1 in the intestine in GPx2 KO mice especially in areas where otherwise GPx2 is expressed. Reports on the localization of GPx1 and GPx2 are controversal. Based on an activity approach a rather homogeneous expression of GPx1 and GPx2 throughout the mouse intestine and along the crypt-to-villus axis was suggested [1], GPx1 protein was not observed in the colonic epithelium when compared to epithelium environment like lamina propria or sub-mucosa [26]. When epithelial fractions were separated from the rest of intestinal tissues, mRNA of GPx1 was only found in the remnants, GPx2 in the epithelial fractions [1,27] with preference at the crypt base [26]. We also observed a low GPx1 expression in ileal epithelial cells (Fig. 3D). Substantial expression of GPx1 in epithelial cells was only detected at the apical surface of colon crypts (Fig. 2D). Regarding GPx2, we consistently find an increasing gradient of GPx2 protein from the top to the base of the crypt in selenium adequate and even selenium-poor colon of wild-type mice ([2]; Fig. 2A and C). What is common to all observations is a preference for GPx2 in the intestinal epithelium and an apparent mutual exclusion of GPx1 and GPx2, indicating that the expression of GPx2 might be regulated differently from GPx1 but in an interrelated manner. Regulation of both GPxs can occur at the transcriptional or the translational level.
Regulation at the transcriptional level
GPx1 was induced by Nrf1 activation in endothelial cells [28], by the haematopoietic-specific Ets family transcription factor PU.1 in neutrophils [29], and its promoter is activated by p53 [30]. Nrf1 was translocated into the nucleus by genistein accompanied by an up-regulation of GPx activity and protection of endothelial cells from oxidative stress. PU.1 is activated by cytokines, growth factors, antigen and cellular stress, the latter pointing to a role of GPx1 in the protection of neutrophils from O2•−/H2O2 produced by NADPH oxidase. The tumor suppressor p53 acts primarily via regulation of cell cycle arrest, survival and DNA repair and by induction of apoptosis. Depending on its concentration, p53 is able to regulate the cellular oxidation state [31]. Under unstressed or physiologically stressed conditions, levels of p53 remain moderate and induce antioxidant genes including GPx1 in a series of cancer and normal cells, thus decreasing the probability of DNA damage [31]. Under severe stress, e.g., increased H2O2 production, p53 becomes stabilized and induces pro-oxidant and, thus, pro-apoptotic genes thereby eliminating irreparably damaged cells [32].
GPx2 is regulated by retinoic acid in some (MCF-7) but not all (HT29) tumor cells [33], by Nrf2 [34], β-catenin/TCF [17] and an isoform of the p63 transcription factor [7]. The control of GPx2 by the Nrf2/Keap1 system complies with its presumed protective role in inflammation and cancer development. The β-catenin/TCF system triggers proliferation via the Wnt pathway [5,8]. The decrease in GPx2 expression by an impaired Wnt pathway [5] supports the assumption of a role of GPx2 in the maintenance of the mucosal homeostasis. The latter point is further supported by the induction of GPx2 by ΔNp63, a transcription factor required for proliferation [7]. Furthermore, GPx2 expression is down-regulated by a mutated Nkx3.1, a homeobox gene responsible for the appropriate prostate epithelial differentiation [35], indicating that GPx2 might also be involved in the suppression of differentiation in the proliferative area.
A link between the regulation of both enzymes, GPx1 and GPx2, might be p53. P53 is stabilized by H2O2 which is prevented by overexpression of GPx2 leading to p53 degradation [7]. Deletion of GPx2 may allow p53 stabilisation and might, thus, increase GPx1 expression. The hypothesis sounds attractive; however, the present RNA data do not support an induction of GPx1 at the transcriptional level. GPx1 RNA is increased with increasing selenium content in the diet reflecting its low ranking in the hierarchy of selenoproteins, which is characterized by a decreased mRNA stability under selenium restriction (see below). GPx3 obviously ranks a bit higher in the hierarchy. Consequently its mRNA is more stable and not significantly affected by the moderate selenium deficiency (Fig. 4), which was also observed in a previous study [17]. In contrast, GPx1 RNA is not increased by the loss of GPx2. Thus, up-regulation must have its reason at a translational level.
Regulation at the translational level
Selenoprotein expression, so far, is best studied at the translational level which depends on selenium availability. Selenoproteins do not respond identically to the selenium status of cells, but are synthesized at limited selenium supply according to a strict hierarchy, a phenomenon that depends on (i) their mRNA stability in selenium-deficiency [36-38], (ii) interactions of their 3′UTRs harbouring the selenocysteine inserting sequence (SECIS) with so far unidentified sequences within the coding sequence deduced from the failure of the 3′UTR of a stable selenoprotein to transfer stability to an otherwise unstable selenoprotein [39], and (iii) the affinity of the selenoprotein-specific translation factor SBP2 (SECIS binding protein-2) to the different 3′UTRs of individual selenoproteins [40]. In this hierarchy, GPx1 and 2 occupy extreme positions, GPx2 ranking by far higher than GPx1 [38]. If GPx2 is deleted more selenium might be available for GPx1 synthesis. This idea is supported by the only moderate decrease of GPx2 staining in selenium-restricted colon (Fig. 2C) and ileum (Fig. 3C) of wild-type mice, whereas GPx1 is almost absent (Figs. 2F and 3F, respectively). In selenium-restricted GPx2 KO mice, however, the low amount of selenium otherwise used for GPx2 synthesis at the crypt base could be shifted into GPx1 synthesis (Figs. 2G and 3G). When selenium supply is adequate or supernutritive, additional available selenium can easily be used for GPx1 biosynthesis. Additional selenium is preferentially shifted to GPx1 since activity of GPx4 was not affected and GPx3 protein was hard to detect in the ileum or colon under all three selenium states.
Finally one should keep in mind that selenium availability in the colon is also influenced by the presence of bacteria [21]. Spontaneous development of colitis and cancer in GPx1/2 DKO mice was only observed in mice colonized with microbiota [14]. Selenoprotein expression was lower in colonized than in germ-free mice [21] suggesting a competition for selenium between bacteria and host. Thus, a compensatory up-regulation of GPx1 would be more efficient in germ-free GPx2 KO animals.
Taken together, the dramatic change of GPx1 protein in the ileum and colon in GPx2 KO mice obviously is not caused by an increased mRNA production or stability but rather by an increase in GPx1 biosynthesis due to the higher availability of selenium by a knock out of GPx2.
Increased apoptosis in GPx2 deficiency
All GPxs investigated so far inhibited apoptosis. Thus, it was not surprising to find an increase in apoptotic cells also in the colon of GPx2 KO animals. Inhibition of apoptosis is in line with the postulated role of GPx2 in the maintenance of the self-renewal of the mucosal epithelium. The observation made here in GPx2 KO animals verifies this hypothesis. Also the gradually decline of GPx2 from the crypt base towards the luminal surface is in accordance with the continuous occurrence of physiological apoptosis at the luminal side. Anti-apoptotic effects of GPx2 have so far only been demonstrated in GPx2-overexpressing MCF7 cells [7] and in the ileum of 10-weeks-old GPx1/GPx2 DKO mice [14]. In the latter model also signs of increased mitotic cells have been observed. However, the number of ileal crypts was lowered indicating a severe intestinal damage. The higher mitosis index could not compensate for the crypt loss due to apoptosis. While we did not find increased mitotic cells in our 20-weeks-old Se-adequate or -supplemented GPx2 KO mice, the number was significantly increased in the middle of the crypts under selenium-restriction (Fig. 6B). The increase in mitosis in GPx2-KO mice does not support a specific function of GPx2 in the maintenance of cell proliferation. Like the support of tumor cell growth concluded from the development of larger tumors from wild-type cells compared to GPx2-knocked down cells [9], also the ‘proliferative’ action of GPx2 in the crypt grounds can be attributed to the capacity of GPx2 to inhibit apoptosis. Apparently the enhanced GPx1 expression is partially able to compensate for the loss of GPx2 when sufficient selenium is available, and might at least delay severe destruction of the intestinal mucosa. The major anti-apoptotic function of selenoproteins in colon crypts, however, can be attributed to GPx2. In the selenium-poor state, not only GPx1 but also other selenoproteins are decreased [17] and might not yet work at an optimum in the selenium-adequate state. In selenium-supplemented animals, only tiny effects on apoptosis (Fig. 6A) and GPx activity (Fig. 1) could be observed; all selenoproteins should work optimally. In this state, the only difference between wild-type and GPx2 KO mice is lack of GPx2 and the increase in apoptosis remains.
In summary, our data show that even a single knockout of GPx2 can impair intestinal mucosa integrity. GPx2, thus, is required to prevent undue apoptosis in the proliferative zone of colonic crypts which can contribute to the maintenance of the mucosal homeostasis. The up-regulation of GPx1 in GPx2-deficient mucosal epithelium especially in areas where otherwise GPx2 is located can be considered as attempt to counteract increased apoptosis although not completely successful in Se-adequacy or even -supplementation. The data also provide an explanation why both enzymes, GPx1 and GPx2, have to be deleted before inflammation and cancer can develop spontaneously.
Acknowledgments
The work was funded by the German Research Council (DFG), BR 778/8-1, and by R01 CA114569 (FFC). The authors thank Stefanie Deubel and the team of the animal facilities especially Elke Thom, Elisabeth Meyer and Swetlana König for excellent technical assistance.
Footnotes
To induce carcinogenesis, AOM in saline was injected i.p. to the experimental groups 5 weeks after adjusting the selenium status. One week later, AOM was followed by DSS application for 7 days as 1% DSS solution in the drinking water. In addition, groups of animals were fed sulforaphane in 0.1 M potassium phosphate buffer, pH 7.4, for 4 weeks, starting 1 week before AOM.
The authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esworthy RS, Swiderek KM, Ho YS, Chu FF. Selenium-dependent glutathione peroxidase-GI is a major glutathione peroxidase activity in the mucosal epithelium of rodent intestine. Biochim Biophys Acta. 1998;1381:213–226. doi: 10.1016/s0304-4165(98)00032-4. [DOI] [PubMed] [Google Scholar]
- 2.Florian S, Wingler K, Schmehl K, Jacobasch G, Kreuzer OJ, Meyerhof W, Brigelius-Flohé R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic Res. 2001;35:655–663. doi: 10.1080/10715760100301181. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu H, Okayasu I, Mitomi H, Imai H, Nakagawa Y, Obata F. Immunohistochemical detection of human gastrointestinal glutathione peroxidase in normal tissues and cultured cells with novel mouse monoclonal antibodies. J Histochem Cytochem. 2001;49:759–766. doi: 10.1177/002215540104900609. [DOI] [PubMed] [Google Scholar]
- 4.Chu FF, Esworthy RS, Doroshow JH. Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic Biol Med. 2004;36:1481–1495. doi: 10.1016/j.freeradbiomed.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 6.Kipp A, Banning A, Brigelius-Flohé R. Activation of the glutathione peroxidase 2 (GPx2) promoter by beta-catenin. Biol Chem. 2007;388:1027–1033. doi: 10.1515/BC.2007.137. [DOI] [PubMed] [Google Scholar]
- 7.Yan W, Chen X. GPX2, a direct target of p63, inhibits oxidative stress-induced apoptosis in a p53-dependent manner. J Biol Chem. 2006;281:7856–7862. doi: 10.1074/jbc.M512655200. [DOI] [PubMed] [Google Scholar]
- 8.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 9.Banning A, Kipp A, Schmitmeier S, Lowinger M, Florian S, Krehl S, Thalmann S, Thierbach R, Steinberg P, Brigelius-Flohe R. Glutathione peroxidase 2 inhibits cyclooxygenase-2-mediated migration and invasion of HT-29 adenocarcinoma cells but supports their growth as tumors in nude mice. Cancer Res. 2008;68:9746–9753. doi: 10.1158/0008-5472.CAN-08-1321. [DOI] [PubMed] [Google Scholar]
- 10.Brigelius-Flohé R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Al-Taie OH, Uceyler N, Eubner U, Jakob F, Mork H, Scheurlen M, Brigelius-Flohé R, Schottker K, Abel J, Thalheimer A, Katzenberger T, Illert B, Melcher R, Köhrle J. Expression profiling and genetic alterations of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis. Nutr Cancer. 2004;48:6–14. doi: 10.1207/s15327914nc4801_2. [DOI] [PubMed] [Google Scholar]
- 12.Naiki-Ito A, Asamoto M, Hokaiwado N, Takahashi S, Yamashita H, Tsuda H, Ogawa K, Shirai T. Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res. 2007;67:11353–11358. doi: 10.1158/0008-5472.CAN-07-2226. [DOI] [PubMed] [Google Scholar]
- 13.Walshe J, Serewko-Auret MM, Teakle N, Cameron S, Minto K, Smith L, Burcham PC, Russell T, Strutton G, Griffin A, Chu FF, Esworthy S, Reeve V, Saunders NA. Inactivation of glutathione peroxidase activity contributes to UV-induced squamous cell carcinoma formation. Cancer Res. 2007;67:4751–4758. doi: 10.1158/0008-5472.CAN-06-4192. [DOI] [PubMed] [Google Scholar]
- 14.Chu FF, Esworthy RS, Chu PG, Longmate JA, Huycke MM, Wilczynski S, Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 15.Esworthy RS, Yang L, Frankel PH, Chu FF. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr. 2005;135:740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 16.Esworthy RS, Mann JR, Sam M, Chu FF. Low glutathione peroxidase activity in Gpx1 knockout mice protects jejunum crypts from gamma-irradiation damage. Am J Physiol Gastrointest Liver Physiol. 2000;279:G426–G436. doi: 10.1152/ajpgi.2000.279.2.G426. [DOI] [PubMed] [Google Scholar]
- 17.Kipp A, Banning A, van Schothorst EM, Meplan C, Schomburg L, Evelo C, Coort S, Gaj S, Keijer J, Hesketh J, Brigelius-Flohe R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res. 2009;53:1561–1572. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- 18.Ritskes-Hoitinga M. Nutrition of Laboratory Mice. In: Hedrich H, editor. The laboratory mouse. San Diego: Elsevier Acad. Press; 2004. pp. 463–479. [Google Scholar]
- 19.Nell S, Bahtz R, Bossecker A, Kipp A, Landes N, Bumke-Vogt C, Halligan E, Lunec J, Brigelius-Flohé R. PCR-verified microarray analysis and functional in vitro studies indicate a role of alpha-tocopherol in vesicular transport. Free Radic Res Commun. 2007;41:930–942. doi: 10.1080/10715760701416988. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Hrdina J, Banning A, Kipp A, Loh G, Blaut M, Brigelius-Flohe R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J Nutr Biochem. 2009;20:638–648. doi: 10.1016/j.jnutbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Maiorino M, Gregolin C, Ursini F. Phospholipid hydroperoxide glutathione peroxidase. Methods Enzymol. 1990;186:448–457. doi: 10.1016/0076-6879(90)86139-m. [DOI] [PubMed] [Google Scholar]
- 23.Bauer-Marinovic M, Florian S, Müller-Schmehl K, Glatt H, Jacobasch G. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis. 2006;27:1849–1859. doi: 10.1093/carcin/bgl025. [DOI] [PubMed] [Google Scholar]
- 24.Böcher M, Boldicke T, Kiess M, Bilitewski U. Synthesis of mono- and bifunctional peptide-dextran conjugates for the immobilization of peptide antigens on ELISA plates: properties and application. J Immunol Methods. 1997;208:191–202. doi: 10.1016/s0022-1759(97)00149-x. [DOI] [PubMed] [Google Scholar]
- 25.Potten CS. What is an apoptotic index measuring? A commentary. Br J Cancer. 1996;74:1743–1748. doi: 10.1038/bjc.1996.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drew JE, Farquharson AJ, Arthur JR, Morrice PC, Duthie GG. Novel sites of cytosolic glutathione peroxidase expression in colon. FEBS Lett. 2005;579:6135–6139. doi: 10.1016/j.febslet.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 27.Chu FF, Esworthy RS. The expression of an intestinal form of glutathione peroxidase (GSHPx-GI) in rat intestinal epithelium. Arch Biochem Biophys. 1995;323:288–294. doi: 10.1006/abbi.1995.9962. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Montes E, Pollard SE, Vauzour D, Jofre-Montseny L, Rota C, Rimbach G, Weinberg PD, Spencer JP. Activation of glutathione peroxidase via Nrf1 mediates genistein's protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346:851–859. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]
- 29.Throm SL, Klemsz MJ. PU.1 regulates glutathione peroxidase expression in neutrophils. J Leukoc Biol. 2003;74:111–117. doi: 10.1189/jlb.0203061. [DOI] [PubMed] [Google Scholar]
- 30.Tan M, Li S, Swaroop M, Guan K, Oberley LW, Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274:12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 31.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensaad K, Vousden KH. Savior and slayer: the two faces of p53. Nat Med. 2005;11:1278–1279. doi: 10.1038/nm1205-1278. [DOI] [PubMed] [Google Scholar]
- 33.Chu FF, Esworthy RS, Lee L, Wilczynski S. Retinoic acid induces Gpx2 gene expression in MCF-7 human breast cancer cells. J Nutr. 1999;129:1846–1854. doi: 10.1093/jn/129.10.1846. [DOI] [PubMed] [Google Scholar]
- 34.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang X, DeWeese TL, Nelson WG, Abate-Shen C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005;65:6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 36.Christensen MJ, Burgener KW. Dietary selenium stabilizes glutathione peroxidase mRNA in rat liver. J Nutr. 1992;122:1620–1626. doi: 10.1093/jn/122.8.1620. [DOI] [PubMed] [Google Scholar]
- 37.Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, Hesketh JE. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J. 1995;311:425–430. doi: 10.1042/bj3110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingler K, Bocher M, Flohé L, Kollmus H, Brigelius-Flohé R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem. 1999;259:149–157. doi: 10.1046/j.1432-1327.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 39.Müller C, Wingler K, Brigelius-Flohé R. 3′UTRs of glutathione peroxidases differentially affect selenium-dependent mRNA stabilities and selenocysteine incorporation efficiency. Biol Chem. 2003;384:11–18. doi: 10.1515/BC.2003.002. [DOI] [PubMed] [Google Scholar]
- 40.Squires JE, Stoytchev I, Forry EP, Berry MJ. SBP2 binding affinity is a major determinant in differential selenoprotein mRNA translation and sensitivity to nonsense-mediated decay. Mol Cell Biol. 2007;27:7848–7855. doi: 10.1128/MCB.00793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


