Abstract
Background
Reversible cerebral vasoconstriction syndrome was first described by Call, Fleming, and colleagues. Clinically this entity presents acutely, with severe waxing and waning headaches (“thunderclap”), and occasional fluctuating neurological signs.
Case presentation
We present four subsequent cases of patients with severe thunderclap headache and brain tomography with evidence of subarachnoid hemorrhage. The brain angiogram showed no aneurysm but intracranial vasculopathy consistent with multiple areas of stenosis and dilatation (angiographic beading) in different territories.
Conclusion
Neurologists should be aware of Call Fleming syndrome presenting with severe headache and associated convexity subarachnoid hemorrhage. After other diagnoses are excluded, patients can be reassured about favorable prognosis with symptomatic management.
Abbreviations
- RCVS
Reversible cerebral vasoconstriction syndrome
- CT
Computed tomography
- SAH
Subarachnoid hemorrhage
- MR
Magnetic resonance
- CTA
Computed tomography angiography
- MRA
Magnetic resonance angiography
Keywords: Call Fleming syndrome, nonaneurysmatic subarachnoid hemorrhage, cerebral vasoconstriction, thunderclap headache
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) was first described by Call, Fleming, and colleagues as a transient and fully reversible cerebral intracranial arteriopathy. Clinically this entity presents acutely, with severe waxing and waning headaches (“thunderclap”) and occasional fluctuating neurological signs. Angiographically there are reversible segments of vasoconstriction affecting several cerebral arteries in multiple territories.1,2 RCVS can occasionally present with associated subarachnoid hemorrhage (SAH). Here we present four middle-aged women referred for abrupt onset of severe headache and noncontrast head computed tomography (CT) showing acute subarachnoid hemorrhage.
Case 1
A 58-year-old right-handed female with prior medical history remarkable for dyslipidemia, chronic tensional headaches presented with an abrupt onset of severe thunderclap headache followed by projectile vomiting. Two days later she had a rebound of severe headache and confusion. Her physical examination and vital signs were normal. A noncontrast head CT showed a focal area of subarachnoid hemorrhage around the right parietal lobe convexity. Magnetic resonance angiogram (MRA) was normal. A cerebral angiogram was negative for intracranial aneurysm but showed areas of subtle focal narrowing and dilatation of the right pericallosal, callosomarginal, and parieto-occipital arteries. Her clinical and neurological course was marked by headache that required symptomatic treatment and patient was discharged without neurological deficits.
Case 2
A 62-year-old right-handed female, with a history of anxiety disorder, and a previous mild to moderate head trauma 8 years ago; presented an abrupt onset of severe thunderclap headache accompanied by projectile vomiting. Her neurological examination showed no abnormal neurological finding. A noncontrast head CT showed subarachnoid hemorrhage in the bilateral frontoparietal convexities, mainly around the parasagittal region. Computed tomography angiogram (CTA) was negative. Five days later she relapsed with another bout of acute thunderclap headache. A four-vessel cerebral angiogram showed intracranial vasculopathy consistent with multiple areas of stenosis and dilatation (angiographic beading) involving the cortical branches of bilateral anterior and middle cerebral artery and in a lesser degree the posterior cerebral arteries. Symptomatic headache treatment was instituted and the patient recovered quickly without residual neurologic deficits.
Case 3
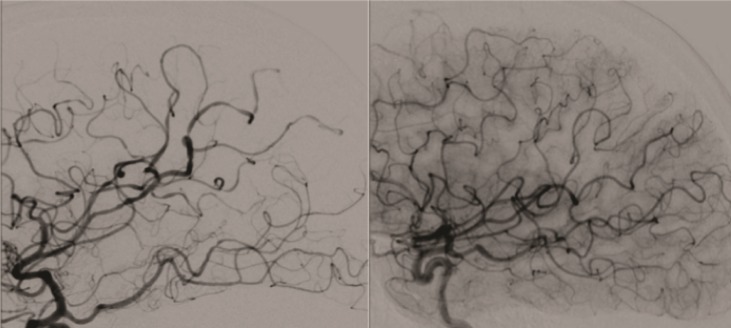
A 54-year-old right-handed female smoker, with history of attention deficit disorder (started sympathomimetic–pseudoephedrine-medication a months prior) and episodic attacks of migraine was admitted to the hospital after an abrupt onset of thunderclap headache followed by nausea, vomiting, and temporal disorientation. The neurological examination as well as fundoscopic examination was normal. A noncontrast head CT showed subarachnoid hemorrhage involving the convexity of the brain in the parasagittal region on the right side (Figure 1). CTA of the head showed no evidence of vascular abnormalities. MR venogram of the head showed no evidence of sinus thrombosis of cortical vein thrombosis. A four-vessel cerebral angiogram showed intracranial vasculopathy consistent with multiple areas of stenosis and dilatation involving the cortical branches of bilateral anterior and middle cerebral arteries, and in lesser degree the posterior cerebral arteries [ Figure 2(A)]. The headache was symptomatically treated and the patient was discharged without neurologic deficits and a followup angiogram 3 months later showed complete reversal of the angiographic abnormalities [ Figure 2(B)].
Figure 1. Admission noncontrast brain CT shows SAH involving the convexity of the brain in the parasagittal region of the right side (black arrow).
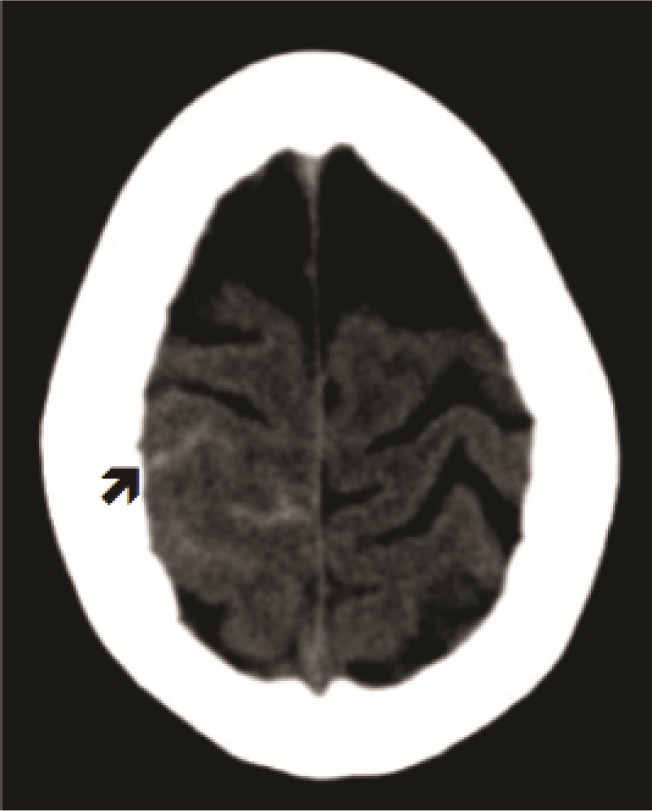
Figure 2. (A) Conventional angiogram shows multiple vessel narrowings in the anterior and middle cerebral arteries. (B) Followup angiogram 3 months later shows complete reversion of the angiographic abnormalities.
Case 4
A 58-year-old previously healthy female has an abrupt onset of severe headache and self-limited vomiting. Forty-eight hours later, she had a relapsing bout of severe thunderclap headache with confusion. A noncontrast head CT showed SAH around the parietal lobe convexity. MR angiogram was negative. Cerebral angiogram showed multiple areas stenosis and dilatation (angiographic beading) of the right pericallosal, callosal marginal, and bilateral parieto-occipital arteries. There were also lumen irregularities of bilateral distal cervical internal carotid arteries consistent with fibromuscular dysplasia. Patient recovered back to normal in the subsequent days without long-term neurological deficits.
Discussion
All patients had clinical and angiographic characteristics consistent with RCVS. RCVS comprise of a group of disorders characterized by prolonged but reversible vasoconstriction of the cerebral arteries, usually associated with acute-onset of severe (often referred as thunderclap headache), fluctuating headaches, with or without additional neurologic signs and symptoms.1,3 The incidence of seizures is low and there is a clear female predominance.3 Thunderclap headache is a common presentation of ruptured intracranial sacular aneurysm but also can happen in certain cases of nonaneurysmal intracranial vascular disease including cerebral sinus thrombosis, RCVS, and occasionally migraine attacks.2–5 Neurovascular imaging studies are warranted in all cases of abrupt onset of severe headache associated with SAH to investigate the presence of ruptured intracranial aneurysm. The majority of the cases of RCVS will cause an isolated severe attack of headache that occasionally will be accompanied by a small ischemic or hemorrhagic stroke including SAH.2,3 RCVS is an increasing recognizing cause of nonaneurysmal SAH. The classic presentation encompasses an abrupt onset of severe headache, nausea, vomiting, mild to moderate meningeal signs, a relative good neurologic status (Hunt and Hess grade I–II), and a minimal amount of subarachnoid hemorrhage (Fisher II). Interestingly, the location of the SAH is mainly in the convexity of the brain surface.6 Cerebral angiogram is the key to confirm the angiographic finding of RCVS and to definitely rule out the presence of intracranial aneurysms, including those sacular or mycotic. Unfortunately, the resolution of the current noninvasive neurovascular imaging studies (CTA and MRA) is limited to diagnose arterial disease beyond the proximal area around the circle of Willis. Central nervous system vasculitis can produce very similar angiographic findings. However, the clinical presentation and neurologic course help to differentiate between these two entities and to avoid unnecessary investigations like a leptomeningeal biopsy and/or potential harmful immunosuppressive treatments. The clinical course in case of SAH associated with RCVS is much more benign than that of the aneurysmal SAH.3,5 Complications such as vasospasm, communicating hydrocephalus, cerebral edema, or intracranial hypertension are not present (see Table I). The pathophysiology is unclear, but it has been associated with spontaneous alterations in vascular tone and also related to endogenous and exogenous factors such as sympathomimetic and serotonergic drugs, tumors, endocrine factors, brain trauma, or poorly controlled hypertension.2,4,5 If an offender agent is identified (sympathomimetic or serotonergic agent) it should be immediately discontinued.
Table 1. Differential diagnosis in SAH related to aneurysmal rupture or RCVS.
| Aneurysmal SAH | SAH associated with RCVS | |
|---|---|---|
| Onset | Hyperacute | Hyperacute |
| Mean age | 55 years | 45 years |
| Female/male ratio | 1.5/1 | 3–4/1 |
| Hunt and Hess Grade | I–V | Usually I |
| Location of SAH | Basilar cisterns | Convexity of brain surface |
| Vasospasm | Common | Uncommon |
| Non communicating hydrocephalus | Likely | Unlikely |
| Neurovascular diagnostic imaging | CTA-MRA or cerebral angiogram | Cerebral angiogram |
In a prospective series of 67 patients with RCVS, the complications were subarachnoid hemorrhage (22%), intracerebral hemorrhage (6%), seizures (6%), and reversible posterior leukoencephalopathy syndrome (9%).6,7 Although there is no exact data, it is estimated that one out of four cases of RCVS has some degree of SAH around the cortical surface.7 Symptomatic treatment of the headache is usually enough until the SAH is reabsorbed. Some studies have reported benefit with calcium-channel blockers, brief courses of glucocorticoids or magnesium sulfate.3 In our series, all patient were referred under the diagnosis of aneurysmal subarachnoid hemorrhage and the cerebral angiogram was able to elucidate the typical angiographic findings of RVCS and rule out ruptured intracranial aneurysms. With the exemption of one case of concomitant extracranial cervical fibromuscular dysplasia there was no other intra or extracranial associated arteriopathy. The clinical course was benign in our entire patient case series and recovery within days to weeks was the rule.
Conclusion
RCVS-associated SAH is one of the spectrums of presentation of this entity, and diagnostic workup should be made to rule out this condition Neurologists should be aware of RCVS presenting with severe headache and associated convexity subarachnoid hemorrhage. After an offending agent is ruled out as the cause, patients can be reassured about a favorable prognosis with symptomatic management.
Acknowledgment
The project described was supported by Grant Number D43TW008333 from the Fogarty International Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
References
- Call G, Fleming M, Sealfon S, Levine H, Kistler P, Fisher C. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19:1159–70. doi: 10.1161/01.str.19.9.1159. [DOI] [PubMed] [Google Scholar]
- Moustafa R, Allen C, Baron J. Call-Fleming syndrome associated with subarachnoid haemorrhage: three new cases. J Neurol Neurosurg Psychiatry. 2008;79:602–5. doi: 10.1136/jnnp.2007.134635. [DOI] [PubMed] [Google Scholar]
- Calabrese L, Dodick D, Schwedt T, Singhal A. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- Singhal A, Caviness V, Begleiter A, Mark E, Rordorf G, Koroshetz W. Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology. 2002;58:130–3. doi: 10.1212/wnl.58.1.130. [DOI] [PubMed] [Google Scholar]
- Noskin O, Jafarimojarrad E, Libman R, Nelson J. Diffuse cerebral vasoconstriction (Call-Fleming syndrome) and stroke associated with antidepressants. Neurology. 2006;67:159–60. doi: 10.1212/01.wnl.0000223648.76430.27. [DOI] [PubMed] [Google Scholar]
- Ducros A, Boukobza M, Porcher R, Saroy M, Valade D, Bousser M.2007The clinical and radiological sepectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients Brain1303091–101. [DOI] [PubMed] [Google Scholar]
- Kazuyuki N, Fukae J, Fujishima K, Mori K, Urabe T, Nobutaka H, et al. Reversible cerebral vasoconstriction syndrome presenting as subarachnoid hemorrhage, reversible posterior leukoencephalopaty and cerebral infarction. Intern Med. 2011;50:1227–33. doi: 10.2169/internalmedicine.50.4812. [DOI] [PubMed] [Google Scholar]


