Abstract
Objective
To determine the frequency of aortic arch calcification and it’s relationship with procedural times, angiographic recanalization, and discharge outcomes in acute ischemic stroke patients undergoing endovascular treatment.
Methods
The thoracic component of computed tomographic (CT) angiogram were reviewed by an independent reviewer to determine presence of any calcification; and the severity of calcification was graded as follows: mild, single small calcifications; moderate, multiple small calcifications; or severe, one or more large calcifications.
Results
Aortic arch calcification was present in 120 (62.4%) of 188 patients and severity was graded as mild (n=24), moderate (n=44), and severe (n=52). Compared with patients without calcification, the mean intracranial access time (minutes ± SD) was similar among patients with aortic arch calcification (70 ± 31 versus 64 ± 31, p=0.9). The mean time intracranial access time increased with increasing severity of aortic arch calcification (61±27, 67±29, and 74±34, p=0.3). Patients with aortic arch calcification had similar rates of complete or partial recanalization [85 (71%) versus 50 (76%)], p=0.6) but lower rates of favorable outcomes [modified Rankin scale 0–2] at discharge 27 (22%) versus 26 (39%), p=0.02).
Conclusions
A high proportion of acute ischemic stroke patients have aortic arch calcification which is associated with lower rates of favorable outcome following endovascular treatment.
Abbreviations:
- SD
standard deviation
- ICH
intracerebral hemorrhage
- NIHSS
National Institutes of Health Stroke Scale
- TIA
transient ischemic attack
- ICH
intracerebral hemorrhage
- mRS
modified Rankin scale
Keywords: Aortic calcification, Procedure time, Endovascular treatment, Acute ischemic strobe, MRV
Introduction
Recently, the recognition of relatively high rates futile recanalization in patients undergoing endovascular treatment for acute ischemic stroke has resulted in a greater emphasis on patient selection.1,2 Procedure time in patients with acute ischemic stroke appears to be a critical determinant of outcomes following endovascular treatment.3 Therefore, variables that can predict prolonged endovascular procedures may be valuable in risk stratification. Traversing the arch of the aorta to access the supra-aortic arteries is an essential component of endovascular treatment of acute ischemic stroke. Aortic atheromatous disease is expected to increase the procedural time particularly in the recent years owing to the increasing use of mechanical thrombectomy devices and flow arrest, both of which require large and relatively inflexible guide catheters. Aortic atheromatous disease is present in approximately 60% of patients with acute ischemic stroke.4,5 Either computed tomographic (CT) angiography of the chest or transesophageal echocardiography is used to identify aortic atheromatous disease, both of which are not performed emergently in ischemic stroke patients.6 Another novel approach may be to examine the aortic arch images that are acquired as part of CT angiogram performed emergently in a high proportion of acute ischemic stroke patients to identify the site of occlusion prior to endovascular treatment. Aortic arch calcification and its severity may be used as a surrogate marker of aortic atheromatous disease and thus used as a predictor for the complexity of the procedure.
We performed this study to determine the frequency of aortic arch calcification and its relationship with procedure times, angiographic recanalization, and discharge outcomes in a consecutive series of acute ischemic stroke patients undergoing endovascular treatment.
Methods
A retrospective study of consecutive acute ischemic stroke patients who underwent endovascular treatment performed between January 1, 2007 to May 1, 2012 at the University of Minnesota and Hennepin County Medical Centers was performed. The two institutions maintained a prospective endovascular procedure database which recorded information regarding the procedural components, devices used, and intraprocedural medication with dosages. The protocol for collecting data was reviewed and approved by the Institutional Review Board at each institution as part of a standardized database.
Data collected
The presence of cardiovascular risk factors (active smoking history, hypertension, atrial fibrillation, coronary artery disease, hyperlipidemia, diabetes mellitus, prior transient ischemic attack (TIA) or ischemic stroke, time interval between symptom onset and endovascular intervention, and use of IV rt-PA) were ascertained as previously described.3 We recorded admission, 24-h post-treatment, and discharge National Institutes of Health Stroke Scale (NIHSS) scores. We also recorded any occurrence of post-endovascular intracerebral hemorrhage (ICH) and in-hospital mortality. Symptomatic ICH was defined as noncontrast CT scan documented ICH resulting in neurological deterioration (greater than or equal to 4 point worsening on an NIHSS score compared with previous clinical assessment). Outcome at time of discharge was assessed using modified Rankin scale (mRS) ascertained using detailed descriptions provided by the vascular neurology team, and occupational, speech, and physical therapists. Favorable functional outcome was defined by mRs of 0–2 at discharge.
Classification of aortic arch calcification
CT angiograms of the head and neck with contrast were obtained. Each study consisted of intravenous injection of 80 cc bolus of nonionic iodinated contrast medium. Concurrently, axial images were obtained with 1.5 mm slice thickness, 1.0 mm collimation, and 0.5 mm overlap, from below the arch of the aorta and up through the head to a level above the Circle of Willis. Three-dimensional (3-D) reconstructions and multiplanar image reformations were performed by the neuroradiologist using the Vitrea workstation. The images were retrieved and analyzed using the isite and Imagecast image viewing softwares. The thoracic component of CT angiogram images was reviewed by an independent reviewer (HR) to determine presence of any calcification and severity of calcification. All of the thoracic images were reviewed to select the image that provided the best image of the aortic arch. The severity of calcification was graded as follows: mild, single small calcification; moderate, multiple small calcifications; severe, one or more large calcifications (see Figure 1). The difference between small and large calcification was based on whether the calcification was larger than the radius of the aortic arch. A total of 98 consecutive CT angiograms were reviewed by another investigator. The interobserver reliability between the two reviewers was high (kappa=0.7).
Figure 1.
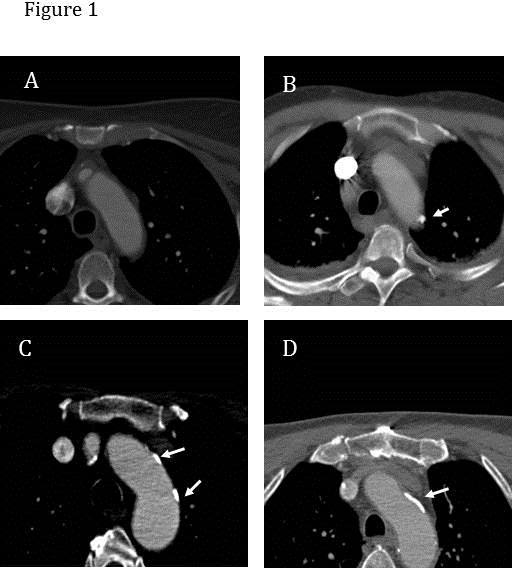
The severity of calcification was graded as follows: (A) no calcification; (B) mild, single small calcification; (C) moderate, multiple small calcifications; and (D) severe, one or more large calcifications. The difference between small and large calcification was based on whether the calcification was larger than the radius of the aortic arch. The white arrows indicate areas of calcification in the aortic arch.
Intracranial access and procedure times
A typical procedure was initiated by femoral catheterization using a 6-French (Fr) introducer sheath in the common femoral artery. An angiogram was performed to confirm the position of the introducer sheath in anticipation of placement of closure device post-procedure. A 6-Fr multipurpose device guide catheter was introduced into the internal carotid artery or vertebral artery. Once the arterial occlusion was identified, a microcatheter ranging in diameter from 1.4 to 2.3 Fr was advanced over a 0.014 microwire into the vessel of interest, in close proximity with the thrombus. Subsequently, a combination of pharmacological agents and/or mechanical thrombus disruption and/or retrieval was used in varying paradigms.
Intracranial access time was defined as the time interval between femoral artery catheterization and microcatheter placement. The time was determined using angiographic images archived in the electronic medical systems. Each image demonstrates the time in hours and minutes at the time of acquisition. Procedural time was defined by the time interval between femoral artery catheterization and partial or complete recanalization or procedure termination. The times were identified by two authors (AEH and JTM) individually reviewing the angiographic images and recording the individual times of microcatheter placement and recanalization or completion of the procedure. Angiographic occlusion and recanalization were classified by the treating physician by using either the thrombolysis in myocardial infarction (TIMI) grading scale7 or the Qureshi grading scale.8 Complete recanalization was defined by post-treatment TIMI grade9 of 3 which is equivalent to thrombolysis in cerebral infarction (TICI) reperfusion grade10 of 3 or by Qureshi grade of 0. Partial recanalization was defined by an improvement of 1 grade or more on either the TIMI or Qureshi grading scale. This methodology has been used in previous publications.1,11
Statistical analysis
All data was descriptively presented using mean ± standard deviation for continuous data and frequencies for categorical data. The frequency of baseline demographic and clinical characteristics, admission NIHSS score, intracranial access time, procedure time, and rates of symptomatic ICH and favorable outcome at discharge were compared among patients with or without aortic calcification. Another analysis was performed to compare the aforementioned variables among patients without aortic calcification to those with aortic calcification stratified by severity of calcification. We performed a multivariate analysis evaluating the independent effect of aortic calcification on favorable outcome at discharge after adjusting for age and admission NIHSS score. We subsequently added intracranial access time in the model to determine the influence of intracranial access time on the association between aortic calcification and favorable outcome. A p value < .05 was considered significant. All analyses were performed by using SAS statistical software (SAS, Cary, NC).
Results
A total of 232 patients underwent endovascular treatment for acute ischemic stroke during the study duration. CT angiogram was performed in 188 patients prior to treatment. The CT angiogram images were adequate for analysis in all 188 patients (mean age ± SD of 65 ± 16 years). Aortic arch calcification was present in 120 (62.4%) of 188 patients and severity was graded as mild (n=24), moderate (n=44), or severe (n=52). The mean age was lower among patients with no aortic calcification (51 ± 14 versus 73 ± 11 years, p=0.06). Patients with aortic arch calcification were more likely to have underlying hypertension, atrial fibrillation, coronary artery disease, hyperlipidemia, and diabetes mellitus. The proportion of underlying cardiovascular risk factors appeared to increase with increased severity of aortic arch calcification.
The proportion of patients with NIHSS score 20–42 was higher among patients with aortic arch calcification (36% versus 15%, p=0.002). Compared with patients without calcification, the intracranial access time (minutes ± SD) was similar among patients with aortic arch calcification (70 ± 31 versus 64 ± 31, p=0.9). The mean time interval increased with increasing severity of aortic arch calcification (61 ± 27, 67 ± 29, and 74 ± 34, p=0.3). There was no difference in the procedural time between patients with and those without aortic arch calcification. Patients with aortic arch calcification had similar rates of complete or partial recanalization [85 (71%) versus 50 (76%)], p=0.6) but lower rates of favorable outcomes [modified Rankin scale 0–2] at discharge 27 (22%) versus 26 (39%), p=0.02).
In the multivariate analysis, presence of aortic calcification was not significantly associated with favorable outcome at discharge (odds ratio [OR] 0.7, 95% confidence interval [CI] 0.2–3.7). Adding the procedural time into the model further reduced the strength of association (OR 1.1 from OR of 0.7).
Discussion
The effect of aortic calcification on intracranial access and procedural time was not significantly different from those without aortic calcification. There are several potential explanations. First, aortic calcification may be not be a sensitive marker of severity of aortic atheromatous disease and tortuosity, However, studies have found a high correlation between aortic arch calcification and presence of atheromatous disease.12 Second, the new generation of guide catheters and sheaths are more flexible and can be navigated with greater ease than those of previous generations and therefore unlikely to be hindered by aortic atheromatous disease. Physician bias in adjusting treatment strategies based on anticipation of complexity of traversing the arch of aorta may also have obscured the relationship. Third, the influence of aortic atheromatous disease may be different for different supra-aortic arteries. Catheterizing the left subclavian artery may not be influenced by aortic atheromatous disease to the same extent as right subclavian artery catheterization.
The inverse relationship between aortic arch calcification and favorable outcome at discharge may be explained by several factors. Aortic calcification and atheromatous disease are more frequent in older patients.13,14 Several studies have demonstrated that older patients have a higher rate of death and disability following endovascular treatment for acute ischemic stroke.15,16 We also found that patients with aortic arch calcification had higher proportion of patients with NIHSS score 20–42. The lower rate of favorable outcomes among patients with aortic arch calcification may also be due to the high NIHSS score at baseline. The results of the multivariate analysis confirmed the lack of independent effect of aortic arch calcification on clinical outcome. Therefore, aortic calcification appears to be a bystander in the relationship between age, NIHSS score strata, and clinical outcomes following endovascular treatments.
Aortic arch calcification may be associated with atheromatous disease in other distributions and diffuse atherosclerosis in cerebral arterial beds, and thus the lower rates of favorable outcomes is not directly related to aortic arch disease.17,18 In contrast to cardioembolic occlusions, atherothrombotic occlusions may be more resistant to recanalization by endovascular treatment.19 In our analysis, we did not find any difference in the rates of recanalization among patients with or without aortic arch calcification. Aortic calcification may also be associated with microvascular intracranial disease secondary to common underlying risk factors such as hypertension14 and diabetes mellitus.20,21 These patients may be more prone to the nonreflow phenomenon after successful recanalization and thus experience greater ischemic injury at the tissue level.
Our study demonstrated the feasibility of identifying aortic arch calcification using data that is acquired as part of CT angiograms in acute ischemic stroke patients. Using the current design, aortic arch calcification did not predict the complexity of the endovascular procedures. Interestingly, the rates of favorable outcomes were lower in patients with aortic arch calcification. However, this relationship appears to be related to older age, greater severity of neurological deficits, and other associated factors seen in patients with aortic arch calcification.
Table 1. The demographic and clinical characteristics of patients undergoing endovascular treatment according to presence and severity of aortic arch calcification.
| Patients with no aortic calcification | Patients with aortic calcification | P value | Patients with severe aortic calcification | Patients with moderate aortic calcification | Patients with mild aortic calcification | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 67 | 120 | 52 | 44 | 24 | |||||||
| Age years (mean ± SD ) | 51 ±14 | 73 ±11 | 0.06 | 76±10 | 73±12 | 69±13 | ||||||
| Women | 27 (40) | 61 (51) | 0.1 | 32 (62) | 20 (45) | 9 (37) | ||||||
| Hypertension | 33 (49) | 92 (77) | 0.0001 | 43 (83) | 34 (77) | 15 (62) | ||||||
| Atrial fibrillation | 6 (9) | 47 (39) | <.0001 | 24 (46) | 17 (39) | 6 (25) | ||||||
| Coronary artery disease | 5 (8) | 34 (28) | 0.0008 | 19 (37) | 11 (25) | 4 (17) | ||||||
| Hyperlipidemia | 14 (21) | 53 (44) | 0.001 | 25 (48) | 20 (46) | 8 (33) | ||||||
| Diabetes mellitus | 9 (13) | 34 (28) | 0.01 | 15 (29) | 17 (39) | 2 (8) | ||||||
| Previous TIA or stroke | 12 (18) | 21 (17) | 0.9 | 5 (10) | 12 (27) | 4 (17) | ||||||
| Admission NIHSS score strata | ||||||||||||
| NIHSS score 0–10 | 27 (40) | 26 (22) | 0.002 | 14 (27) | 7 (16) | 5 (21) | ||||||
| NIHSS score 11–19 | 30 (45) | 51 (42) | 20 (38) | 19 (43) | 12 (50) | |||||||
| NIHSS score 20–42 | 10 (15) | 43 (36) | 18 (35) | 18 (41) | 7 (29) | |||||||
| Treated artery | ||||||||||||
| Anterior cerebral artery | 0 (0) | 2 (2) | 0.1 | 1 (2) | 1 (2) | 0 (0) | ||||||
| Internal carotid artery | 8 (12) | 33 (27) | 16 (310 | 10 (23) | 7 (29) | |||||||
| Middle cerebral artery | 48 (72) | 72 (60) | 30 (58) | 29 (66) | 13 (54) | |||||||
| Posterior cerebral artery | 2 (3) | 3 (3) | 1 (2) | 1 (3) | 1 (4) | |||||||
| Basilar artery | 6 (9) | 6 (5) | 4 (8) | 1 (3) | 1 (4) | |||||||
| Treatment Times | ||||||||||||
| Time interval between symptom onset and procedure initiation (mean ± SD) | 297±234 | 342±273 | 0.2 | 296±231 | 356±266 | 409±353 | ||||||
| Intracranial access time (mean ± SD) | 64±31 | 70±31 | 0.9 | 74±34 | 67±29 | 61±27 | ||||||
| Procedural time (mean ± SD) | 124±53 | 127±47 | 0.3 | 134±48 | 120±49 | 126±42 | ||||||
| Type of treatment | ||||||||||||
| Intra-arterial thrombolytic | 55 (82) | 89 (74) | 0.2 | 42 (81) | 29 (66) | 18 (75) | ||||||
| Mechanical thrombectomy | 30 (45) | 64 (53) | 0.2 | 25 (48) | 23 (52) | 16 (67) | ||||||
| Stent placement | 7 (10) | 6 (5) | 0.2 | 3 (6) | 2 (5) | 1 (4) | ||||||
| Angiographic recanalization | ||||||||||||
| Partial or complete | 50 (76) | 85 (71) | 0.6 | 33 (64) | 34 (77) | 18 (78) | ||||||
| Intravenous thrombolytic administered | 22 (33) | 54 (45) | 0.1 | 24 (46) | 18 (41) | 12 (50) | ||||||
| ICH symptomatic | 6 (9) | 14 (12) | 0.6 | 7 (13) | 3 (7) | 4 (16) | ||||||
| Favorable outcome at discharge (mRS 0–2) | 26 (39) | 27 (22) | 0.02 | 6 (12) | 15 (34) | 6 (25) | ||||||
References
- Hussein HM, Georgiadis AL, Vazquez G, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;3166(3):454–458. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina CA. Futile recanalization in mechanical embolectomy trials: a call to improve selection of patients for revascularization. Stroke. 2010;41(5):842–843. doi: 10.1161/STROKEAHA.110.580266. [DOI] [PubMed] [Google Scholar]
- Hassan AE, Chaudhry SA, Miley JT, et al. Microcatheter to Recanalization (Procedure Time) Predicts Outcomes in Endovascular Treatment in Patients with Acute Ischemic Stroke: When Do We Stop? . AJNR Am J Neuroradiol. 2012 2012 Jul 19; doi: 10.3174/ajnr.A3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzikonstantinou A, Krissak R, Schaefer A, Schoenberg SO, Fink C, Hennerici MG. Coexisting large and small vessel disease in patients with ischemic stroke of undetermined cause. Eur Neurol. 2012;68(3):162–165. doi: 10.1159/000339945. [DOI] [PubMed] [Google Scholar]
- The French Study of Aortic Plaques in Stroke Group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N Engl J Med. 1996;334(19):1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- Chatzikonstantinou A, Krissak R, Fluchter S, et al. CT angiography of the aorta is superior to transesophageal echocardiography for determining stroke subtypes in patients with cryptogenic ischemic stroke. Cerebrovasc Dis. 2012;33(4):322–328. doi: 10.1159/000335828. [DOI] [PubMed] [Google Scholar]
- TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings TIMI Study Group. N Engl J Med. 1985;312(14):932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke . Neurosurgery. 2002;50(6):1405–1414. doi: 10.1097/00006123-200206000-00049. discussion 1414–1405. [DOI] [PubMed] [Google Scholar]
- Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36(11):2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29(3):582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis AL, Memon MZ, Shah QA, et al. Intra-arterial tenecteplase for treatment of acute ischemic stroke: feasibility and comparative outcomes. J Neuroimaging. 2012;22(3):249–254. doi: 10.1111/j.1552-6569.2011.00628.x. [DOI] [PubMed] [Google Scholar]
- Litmanovich D, Bankier AA, Cantin L, Raptopoulos V, Boiselle PM. CT and MRI in diseases of the aorta . AJR Am J Roentgenol. 2009;193(4):928–940. doi: 10.2214/AJR.08.2166. [DOI] [PubMed] [Google Scholar]
- Gorich J, Zuna I, Merle M, et al. [Aortic calcification in CT. Correlation with risk factors and cardiovascular diseases] Radiologe. 1989;29(12):614–619. [PubMed] [Google Scholar]
- Tsakiris A, Doumas M, Nearchos N, Mavrokefalos A, Mpatakis N, Skoufas P. Aortic calcification is associated with age and sex but not left ventricular mass in essential hypertension. J Clin Hypertens (Greenwich) 2004;6(2):65– 70. doi: 10.1111/j.1524-6175.2004.03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra RV, Leslie-Mazwi TM, Oh DC, et al. Elderly patients are at higher risk for poor outcomes after intra-arterial therapy. Stroke. 2012;43(9):2356–2361. doi: 10.1161/STROKEAHA.112.650713. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Georgiadis AL, Vazquez G, Janjua NA. Intra-arterial recanalization techniques for patients 80 years or older with acute ischemic stroke: pooled analysis from 4 prospective studies. AJNR Am J Neuroradiol. 2009;30(6):1184– 1189. doi: 10.3174/ajnr.A1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Smale SE, Odink AE, Wieberdink RG, et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam Study. Atherosclerosis. 2010;212(2):656–660. doi: 10.1016/j.atherosclerosis.2010.06.037. [DOI] [PubMed] [Google Scholar]
- Odink AE, van der Lugt A, Hofman A, et al. Association between calcification in the coronary arteries, aortic arch and carotid arteries: the Rotterdam study. Atherosclerosis. 2007;193(2):408–413. doi: 10.1016/j.atherosclerosis.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Cho KH, Lee DH, Kwon SU, et al. Factors and outcomes associated with recanalization timing after thrombolysis. Cerebrovasc Dis. 2012;33(3):255–261. doi: 10.1159/000334666. [DOI] [PubMed] [Google Scholar]
- Taniwaki H, Ishimura E, Tabata T, et al. Aortic calcification in haemodialysis patients with diabetes mellitus. Nephrol Dial Transplant. 2005;20(11):2472–2478. doi: 10.1093/ndt/gfi039. [DOI] [PubMed] [Google Scholar]
- Farrag A, Bakhoum S, Salem MA, El-Faramawy A, Gergis E. The association between extracoronary calcification and coronary artery disease in patients with type 2 diabetes mellitus . [Nov 3;2011 ];Heart Vessels. 2011; doi: 10.1007/s00380-011-0205-6. [DOI] [PubMed] [Google Scholar]


