Abstract
To render a diagnosis pediatricians rely upon reference standards for bone mineral density or bone mineral content, which are based on cross-sectional data from a relatively small sample of children. These standards are unable to adequately represent growth in a diverse pediatric population. Thus, the goal of this study was to develop sex and site specific standards for BMC using longitudinal data collected from four international sites in Canada and the United States. Data from four studies were combined; Saskatchewan Paediatric Bone Mineral Accrual Study (n=251), UBC Healthy Bones Study (n=382); Penn State Young Women’s Health Study (n=112) and Stanford’s Bone Mineral Accretion study (n=423). Males and females (8 to 25 years) were measured for whole body (WB), total proximal femur (PF), femoral neck (FN) and lumbar spine (LS) BMC (g). Data were analyzed using random effects models. Bland-Altman was used to investigate agreement in predicted and actual data. Age, height, weight and ethnicity independently predicted BMC accrual across sites (P <0.05). Compared to White males, Asian males had 31.8 (6.8) g less WB BMC accrual; Hispanic 75.4 (28.2) g less BMC accrual; Blacks 82.8 (26.3) g more BMC accrual with confounders of age, height and weight controlled. Similar findings were found for PF and FN. Female models for all sites were similar with age, height and weight all independent significant predictors of BMC accrual (P <0.05). We provide a tool to calculate a child’s BMC Z-score, accounting for age, size, sex and ethnicity. In conclusion, when interpreting BMC in paediatrics we recommend standards that are sex, age, size and ethnic specific.
Keywords: Children, DXA, growth, maturation, Z-score
Introduction
There are many diseases that deleteriously affect bone mass and bone mass accrual during the growing years [1]. Dual energy x-ray absorptiometry (DXA) is widely available, precise and safe and has become the preferred clinical instrument to assess bone mass in children [2]. However, DXA is a planar instrument and provides an areal bone mineral density (aBMD, g/cm2) that may be influenced by bone size, shape or body habitus [3]. Results may therefore misrepresent or be especially disadvantageous to healthy children who are late maturing or less than average height compared to same age peers [4]. To overcome the inherent size artifact in DXA images, researchers have since developed normative data for BMC and aBMD adjusted for body size and for estimated volumetric aBMD [3, 5–8].
To render a diagnosis, pediatricians may rely upon reference standards for young adult or pediatric aBMD or bone mineral content (BMC, g) [1, 3, 5–17]. Although these reference data address an important need in the clinical community they are based upon; i) cross-sectional data, ii) longitudinal data treated cross-sectionally [12] or, iii) a relatively small sample of children in a specific geographic region [9].
The limitations of using cross-sectional data to represent a longitudinal event, the growth of any tissue including bone, have been discussed at length over many years [18]. Indeed, longitudinal studies of children clearly demonstrated the wide variability in the pattern, magnitude and timing of bone mineral accrual in the human skeleton [19]. Comparing individuals to reference data may also be limited by the sex and/or ethnicity of the sample and the type of DXA instrument used to acquire the reference data [20]. Thus, to better represent sex differences [21] and the highly individualized process of maturation it may be more appropriate to develop normative growth curves from a diverse sample of children using repeated measurements of BMC across growth [10].
Therefore, our aim was to develop sex and site specific standards for BMC using longitudinal data collected from four international sites in Canada and the United States. These standards are unique in that they, i) derive from longitudinal data, ii) were acquired from a diverse sample of children aged 8 – 25 years, iii) were developed using multilevel modeling and, iv) account for ethnicity, age and size.
Materials and Methods
Research design
We undertook a collaborative multi-center project and integrated longitudinal bone mineral accrual data from four centers in Canada and the United States to create international standards for bone mineral content (BMC) in males and females aged 8–25 years. Although all studies were conducted with healthy participants and used Hologic (Hologic, Inc., Waltham, MA, USA) bone densitometers, there were differences in recruitment methods, data collection time frame and sample sizes. We introduce and outline specific methods for each site below.
1. University of British Columbia (UBC)
The UBC Healthy Bones Study (1999–2006) 7 year longitudinal study is described in detail elsewhere [22]. In brief, it used a mixed longitudinal design [23], and recruited 382 girls and boys across four age cohorts (9, 10, 11 and 12 years). Children were all pre-pubertal at baseline and recruited from two multi-ethnic communities in Vancouver. Forty-four percent of children were Caucasian, 38% were Asian and 18% were from other or mixed ethnic groups. A health history questionnaire was used to determine each child’s ethnicity based on parents’ or grandparents’ place of birth. Children were classified as ‘Asian’ if both parents or all 4 grandparents were born in Hong Kong or China, India, Philippines, Vietnam, Korea or Taiwan or ‘Caucasian’ if both parents or all 4 grandparents were born in North America or Europe. The relative composition of the ethnic clusters was consistent across the 7 years of data collection. Children visited the laboratory at UBC every 6 months for the first 3 years of the study and annually for the next 5 years. The study experienced 39% attrition rate from 1999–2006. The greatest attrition occurred between years 2000 and 2001 where teachers (rather than children) chose to discontinue their participation. There were no differences in any baseline measure between children who continued to participate and those who dropped out of the study. All eligible children were healthy, had no history of chronic disease or chronic medication use and had no medical conditions, allergies or medications known to influence bone metabolism or calcium balance. Children with recent fractures and who were casted at the time of measurement were excluded from the study. Previous history of fracture was not an exclusion criteria. The UBC Clinical and Behavioural Sciences Research Ethics Board approved the study. All parents/guardians and children completed and signed informed consents prior to study participation.
2. University of Saskatchewan (U of S)
The U of S Paediatric Bone Mineral Accrual Study (1991–1998) has been described in detail elsewhere [19]. In brief, this 7- year study utilised a mixed longitudinal design [24], and recruited 251 males and females from local elementary schools across eight age cohorts. Children were prepubertal, pubertal, and post-pubertal, aged between 8 – 15 years at baseline. The cohorts were all healthy Caucasian children from middle-income neighbourhoods. Ethnicity was determined based on parents’ place of birth. During the 7 years of data collection the relative composition of the clusters remained the same. As there were overlaps in ages between the clusters it was possible to estimate a consecutive 13-year developmental pattern over the shorter period of 7 years. The study experienced 39% attrition rate from 1991–1997. There were no differences in any baseline measure between children who continued to participate and those who dropped out of the study. Eligible children had no history of chronic disease or chronic medication use; and no medical conditions, allergies or medications known to influence bone metabolism or calcium balance. Children with recent fractures and who were casted at the time of measurement were excluded from the study. Previous history of fracture was not an exclusion criteria. The study received approval from the University of Saskatchewan Biomedical Research Ethics Board (Bio # 88-102). Between 1991 and 1993 written informed consent was obtained from parents of participating children for the whole study.
3. Stanford University Medical Center (Stanford)
The Stanford study has been described in detail elsewhere [1]. In brief, this 4 year study utilised a mixed longitudinal design and recruited 423 males and females (aged 9 – 25 years) from the community. Recruitment occurred between May 1992 and February 1996 and data collection was completed in February 1997. Subjects were encouraged to return annually and were included if they had at least four visits or until they had reached age 25 years. Children were from low to upper-middle income families. The cohort at entry were 24% non-Hispanic whites, 24% Hispanics, 24% East Asians (Chinese, Japanese), and 27% non-Hispanic blacks; aged 8.8 – 25.9 years [1]. Ethnicity was determined by self-identity of the parents (both had to have the same self-identity as the child), with mixed race families excluded. Participants were excluded if they completed fewer than four visits, refused to participate, relocated, or reached age 25 during the study period. Participants who were recruited late in the study did not undertake 4 visits prior to the end of the funding period and they were excluded from analysis. Therefore, 73% of the samples were not included in this analysis. Eligible children had no history of chronic disease or chronic medication use: and no medical conditions or medications known to influence bone metabolism. Children with recent fractures and who were casted at the time of measurement were excluded from the study. Previous history of fracture was not an exclusion criteria. The study protocol was approved by the Stanford University Administrative Panel on Human Subjects in Medical Research. Written consent was obtained from all participants and from the parents of participants who were younger than age 18 years.
4. Pennsylvania State University Young Women’s Health Study
The Penn State University (PSU) Young Women’s Health Study (YWHS) has been described in detail elsewhere [25]. Briefly, the YWHS was a 10 year prospective study that enrolled 112 healthy, premenarcheal females who were aged 11.9 (0.5) years, on average, at baseline (1990). Participants were Caucasian adolescent females who attended public school in central Pennsylvania. Participants were excluded if they were not within 80 – 120% of the ideal weight for height (based on the most recent US National Health and Nutrition Examination Survey), and not of Northern European descent (determined if all grandparents were descendants of Northern Europeans). Participants were assessed every 6 months across years 1–4 and annually across years 6–10. The study experienced 31% attrition rate across the 10 year study. There were no differences in any baseline measure between girls who continued to participate and those who dropped out of the study [26]. Eligible children had no medical history known to effect bone development and did not have any known disorders of dietary behaviour. Children with recent fractures and who were casted at the time of measurement were excluded from the study. Previous history of fracture was not an exclusion criteria. The study was approved by the PSU College of Medicine Institutional Review Board. Participants and their parents provided informed consent.
5. All Datasets
In the present analysis we included all participants who were assessed on at least two measurement occasions with complete data on those occasions. Thus, for our analysis we included 814 males who were measured on 1944 occasions and 1061 females who were measured on 3229 occasions.
Descriptive data
Chronological age
Age in years was determined as a decimal age by subtracting date of birth from the measurement date. In the models age (Agec) was centred around the mid age of the subjects, 13 years.
Anthropometry
At all sites, standard anthropometric techniques were used to measure height (to the nearest 0.1cm), and body mass (to the nearest 0.1kg). Participants were assessed at study entry and reassessed using identical techniques at subsequent follow-up visits. We created the height by weight index (Weight/height*100) to represent the interaction between height (Agec*height) and weight (Agec*weight).
Bone Mineral Content measurement
The UBC study assessed BMC (g) at the whole body (WB), lumbar spine (L1–L4; LS), proximal femur (PF) and femoral neck (FN) using DXA in array mode (Hologic QDR 4500W, Hologic, Inc., Waltham, MA, USA). Trained and qualified technologists conducted and analyzed all scans using standard procedures (Software version 12.1). Short-term precision for WB, LS, PF and FN BMC was assessed in-vivo, by measuring healthy young adults. Coefficients of variation (CV) for BMC at the WB and LS were <0.7% and at the PF and FN precision ranged from 1.4 to 3.5%. A spine anthropomorphic phantom was scanned daily to maintain quality assurance of the QDR 4500W.
The U of S study assessed BMC (g) of the WB, LS (L1–L4) and FN using DXA in array mode (Hologic QDR 2000, Hologic, Inc., Waltham, MA, U.S.A). Trained and qualified technologists conducted and analyzed all scans using standard procedures. Whole body scans were analyzed using software version 5.67A, with LS and FN analysed using software version 4.66A. Short-term precision for WB, LS, PF and FN BMC was assessed in vivo, by measuring healthy young adults. The CV for BMC at the WB and LS were <0.61%, and at the FN precision was 0.91%. [27]. Lumbar spine BMC was not measured in the first year hence there less data for this variable at each chronological and maturity age band. A spine anthropomorphic phantom was scanned daily to maintain quality assurance of the QDR 2000W.
The Stanford study assessed BMC (g) for the WB, LS (L2–L4) and PF using DXA using the pencil beam mode (Hologic QDR 1000W, Hologic Inc., Waltham, MA, USA). One DXA technologist acquired 95% of the DXA scans and analyzed all scans using software version 6.10. A standardized protocol was used to analyze PF so as to reduce measurement inconsistencies that may arise from changes in FN geometry during growth [1]. Short-term precision for WB, LS and FN BMC was assessed in vivo by measuring healthy adolescents and young adults. The CV for BMC was 0.6% at all skeletal sites. A spine anthropomorphic phantom was scanned daily to maintain quality assurance of the QDR 1000W.
The Penn State study assessed BMC (g) at the WB, LS (L1–L4) and PF using DXA (Hologic QDR-2000W, Hologic Inc., Waltham, MA, USA). Whole body scans were acquired in pencil beam mode in the presence of the manufacturer’s three-step acrylic and aluminum tissue phantom. Bi-lateral PF and LS scans were acquired in array mode. The same two clinical research coordinators acquired and analysed all the scans. Whole body and LS scans were acquired every 6 months from baseline through age 16 years. A proximal femur protocol was added in the last 6 years of the study. The observed CV assessed in vitro, was less than 0.7% for the day to day quality control scans. A spine anthropomorphic phantom was scanned daily to maintain quality assurance of the QDR 2000W.
Precision and cross calibration of DXA instruments
We used the European Spine Phantom (ESP) to compare DXA instruments and to establish precision across sites. A trained DXA technologist acquired ten repeat measurements, without repositioning the ESP at each geographical site. All DXA instruments demonstrated excellent precision within-site with coefficients of variation between 0.32% (PSU) and 0.59% (Stanford). Based on QC data from each site, there was excellent stability over the study periods. Although all data were collected on Hologic instruments there was a small (6%) systematic difference in bone outcomes between the Stanford and Hologic instruments at other sites. To correct for differences between instruments in vivo, the U of S and Stanford determined precision across different Hologic instruments at their sites. The U of S compared results obtained from 9 males and 15 females assessed on Hologic 2000 and 4500 instruments. Stanford compared results obtained from 20 males and 20 females assessed on Hologic 1000 and 4500 instruments. From these data we developed conversion factors so that all outcomes used for analysis represented Hologic 4500 equivalent values (Table 1). Each site undertook standard data cleaning and screened for outliers. In addition, one researcher at UBC (re)examined all scans to identify any discrepancies in scan analyses between sites. Questionable scans were reanalyzed using standard procedures.
Table 1.
Conversion factors for Hologic 1000 and 2000 to 4500 machines
| Variables | 4500 = slope ± 2000 | 4500 = slope ± 1000 |
|---|---|---|
| Whole Body BMC (g) | = 1.019 ± 2000 | = 1.053 ± 1000 |
| Proximal femur BMC (g) | = 1.063 ± 2000 | = 0.989 ± 1000 |
| Femoral Neck BMC (g) | = 1.037 ± 2000 | = 0.978 ± 1000 |
| Lumber Spine BMC (g) | = 1.035 ± 2000 | = 0.979 ± 1000 |
Key: BMC – Bone mineral content; Slope – Slope of the regression of either 1000 or 2000 on 4500.
Statistical analysis
We used SPSS software version 15.0 (SPSS Inc.) to generate descriptive data for the whole sample. We report values as means (SD) unless otherwise noted. For the longitudinal analyses, we constructed hierarchical (multi-level) random effects models using a multilevel modeling approach (MlwiN version 1.0, Multi-level Models Project; Institute of Education, University of London, UK [28, 29]. We previously used multilevel (hierarchical) regression to develop bone accrual models [30, 31] and described this approach in detail [32]. In brief, we obtained repeated measures of BMC and other independent variables from within individuals (level 1 of the hierarchy) and between individuals (level 2 of the hierarchy). Specifically, we developed 8 random effects multi-level models to describe these longitudinal events at the total proximal femur, femoral neck, lumbar spine and whole body for males and females. The general additive, sex and site-specific random effects multi-level regression model we used to describe developmental changes in BMC across chronological ages is described below;-
where: y is the BMC parameter on measurement occasion i in the j-th individual; αj is the constant for the jth individual; βjxij is the slope of the BMC parameter with centred chronological age (years) for j-th individual; and k1 to kn are the coefficients of various explanatory variables (e.g. height, weight and ethnicity) at assessment occasion i in the j-th individual. These are the fixed parameters in the model. Both μj and εij are random quantities, whose means are equal to zero; they form the random parameters in the model. They are assumed to be uncorrelated and follow a normal distribution and thus their variances can be estimated; μj is the level-2 (between subjects variance) and εij the level-1 residual (within individual variance) for the i-th assessment of lean mass in the j-th individual. We centered age around the mean age of the sample (Agec), where Agec=decimal age − 13.00 years. We built models in a stepwise procedure, i.e. predictor variables (κ-fixed effects) were added one at a time and the log-likelihood ratio statistic was used to judge improvement of the model [32]. Predictor variables (κ) were accepted as significant if the estimated mean coefficient was greater than twice the standard error of the estimate (SEE) i.e. P < 0.05. If the retention criteria were not met we discarded the predictor variable (shown as non-significant (NS) in Tables 3 and 4). To allow for the non-linearity of growth, we introduced chronological age centred power functions into the linear models (agec2, agec3). To allow for maturity-associated changes in proportionality we added interaction terms: agec*height interaction, agec*weight interaction and weight divided by height. Ethnicity was added as a dummy variable where Caucasians were the base category and compared with Asians’, Blacks and Hispanics, respectively.
Table 3.
Sex-specific multilevel regression models for bone mineral content of the (a) Whole Body, (b) Total Proximal Femur, (c) Femoral Neck and (d) Lumbar Spine in males
| Variables | (a) Whole Body | (b) Total Proximal Femur | (c) Femoral Neck | (d) Lumbar Spine | ||||
|---|---|---|---|---|---|---|---|---|
| Fixed Effect | Estimates | Estimates | Estimates | Estimates | ||||
| Constant | −1271.18(223.45) | −38.41(5.69) | −4.19(0.30) | −108.41(3.92) | ||||
| Agec | −554.33(38.65) | −10.56(0.94) | −0.98(0.10) | −33.73(1.36) | ||||
| Agec2 | −9.39(1.02) | −0.23(0.02) | −0.02(0.003) | −0.54(0.04) | ||||
| Agec3 | 0.44(0.12) | 0.003(0.003) | 0.00001(0.0004) | 0.04(0.005) | ||||
| Height | 14.28(1.46) | 0.37(0.04) | 0.05(0.002) | 0.90(0.03) | ||||
| Weight | 31.06(4.39) | 0.58(0.11) | 0.01(0.002) | 0.04(0.02) | ||||
| Agec*Height | 3.69(0.24) | 0.07(0.006) | 0.006(0.0006) | 0.21(0.008) | ||||
| Agec*Weight | NS | NS | 0.001(0.0005) | NS | ||||
| Weight by Height | −31.83(6.82) | −0.72(0.17) | NS | NS | ||||
| White vs Asian | −31.36(9.97) | −0.70(0.22) | −0.22(0.03) | NS | ||||
| White vs Hispanic | −75.49(28.21) | 1.57(0.65) | 0.29(0.09) | NS | ||||
| White vs Black | 82.81(26.26) | 4.20(0.58) | 0.63(0.08) | NS | ||||
| Random Effects | Level 1 | Level 1 | Level 1 | Level 1 | ||||
| Constant | 2640.6(132.36) | 2.90(0.14) | 0.03(0.001) | 2.84(0.17) | ||||
| Level 2 | Level 2 | Level 2 | Level 2 | |||||
| Constant | Agec | Constant | Agec | Constant | Agec | Constant | Agec | |
| Constant | 23594.47(1542.09) | 3938.13(370.75) | 14.320(0.95) | 2.656(0.23) | 0.206(0.01) | 0.03(0.002) | 39.29(2.97) | 9016(0.86) |
| Agec | 3938.13(370.75) | 1264.66(124.50) | 2.656(0.23) | 0.560(0.07) | 0.028(0.002) | 0.007(0.010) | 9.16(0.86) | 2.979(0.29) |
Fixed effect values are Estimated Mean Coefficients (SEE - Standard Error Estimate); Random effects values Estimated Mean Variance (SEE). Level 1 – within individuals; Level 2 – between individuals. Agec – Age centred (Age – 13 years); Agec2 – Age centred squared; Agec3 - Age centred cubed; Height (cm); Weight (kg); Agec*Height – Agec multiplied by height; Agec*Weight – Agec multiplied by weight; Weight by height − Weight/height*100; White vs Asian - White 0, Asian = 1; White vs Hispanic - White 0, Hispanic = 1; White vs Black - White 0, Black = 1; P< 0.05 (mean > 2*SEE). NS = not significant and removed from the model.
Table 4.
Sex specific multilevel regression models for bone mineral content at the (a) Whole Body, (b) Total Proximal Femur, (c) Femoral Neck and (d) Lumbar Spine in females
| Variables | (a) Whole Body | (b) Total Proximal Femur | (c) Femoral Neck | (d) Lumbar Spine | ||||
|---|---|---|---|---|---|---|---|---|
| Fixed Effect | Estimates | Estimates | Estimates | Estimates | ||||
| Constant | ||||||||
| Agec | −230.68(22.59) | −2.45(0.52) | −0.45(0.07) | −11.55(1.03) | ||||
| Agec2 | −1.48(0.58) | −0.04(0.01) | −0.005(0.002) | −0.07(0.03) | ||||
| Agec3 | −0.78(0.08) | −0.002(0.002) | 0.0008(0.0003) | −0.02(0.004) | ||||
| Height | 5.17(0.13) | 0.12(0.003) | 0.02(0.0004) | 0.20(0.006) | ||||
| Weight | 55.38(1.50) | 1.03(0.04) | 0.08(0.005) | 2.15(0.07) | ||||
| Agec*Height | 2.09(0.15) | 0.03(0.004) | 0.004(0.0004) | 0.10(0.01) | ||||
| Agec*Weight | −0.73(0.10) | −0.02(0.002) | −0.002(0.0003) | −0.03(0.005) | ||||
| Weight by Height | −62.48(2.47) | −1.40(0.064) | −0.09(0.008) | −3.08(0.11) | ||||
| White vs Asian | −20.74(9.97) | −1.22(0.20) | −0.2(0.03) | NS | ||||
| White vs Hispanic | NS | 1.84(0.55) | 0.34(0.07) | NS | ||||
| White vs Black | 182.96(21.79) | 2.31(0.44) | 0.39(0.06) | NS | ||||
| Random Effects | Level 1 | Level 1 | Level 1 | Level 1 | ||||
| Constant | 2618.82(90.95) | 1.41(0.06) | 0.025(0.001) | 3.55(0.15) | ||||
| Level 2 | Level 2 | Level 2 | Level 2 | |||||
| Constant | Agec | Constant | Agec | Constant | Agec | Constant | Agec | |
| Constant | 23531.90(1200.26) | 2527.44(210.47) | 0.28(0.03) | 1.40(0.11) | 0.16(0.01) | 0.02(0.002) | 38.73(2.20) | 5.64(0.45) |
| Agec | 2527.44(210.47) | 622.26(53.83) | 1.40(0.11) | 10.20(0.57) | 0.02(0.002) | 0.004(0.005) | 5.64(0.45) | 1.28(0.12) |
Fixed effect values are Estimated Mean Coefficients (SEE - Standard Error Estimate); Random effects values Estimated Mean Variance (SEE). Level 1 – within individuals; Level 2 – between individuals. Agec – Age centred (Age – 13 years); Agec2 – Age centred squared; Agec3 - Age centred cubed; Height (cm); Weight (kg); Agec*Height – Agec multiplied by height; Agec*Weight – Agec multiplied by weight; Weight by height − Weight/height*100; White vs Asian - White 0, Asian = 1; White vs Hispanic - White 0, Hispanic = 1; White vs Black - White 0, Black = 1; P< 0.05 (mean > 2*SEE). NS = not significant and removed from the model.
To determine the accuracy of our BMC prediction equations we applied our prediction models for BMC to two separate populations; a) a random selection of half of the original sample and, b) an independent sample of same age children recruited into the UBC Healthy Bones Study in 2009 (40 males, 10.4 (±0.5) years; and 78 females, 10.4 (±0.6) years; 50% Caucasian and 50% Asian). The independent sample of children were all prepubertal and were assessed using the same testing protocol as the original UBC sample. We compared predicted to actual BMC in the original (Figures 1 and 2) and the independent (Figure 3), samples using the procedure described by Bland and Altman [33]. Finally, we developed models using the original data (Tables 3 and 4). We calculated Z-scores for the sample as [Z =observed BMC − predicted BMC)/BMC standard deviation].
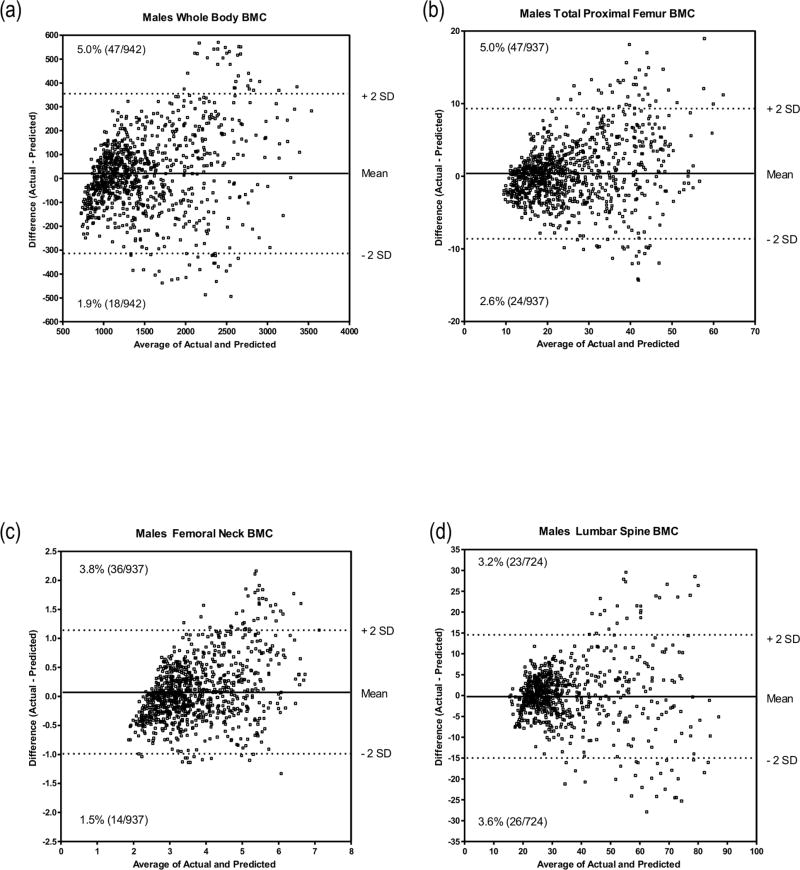
Figure 1.
Bland and Altman procedure showing the agreement between predicted BMC and observed BMC for all four skeletal sites in males from the original sample; (a) Whole body; (b) Total proximal femur; (c) Femoral neck; (d) Lumbar spine.
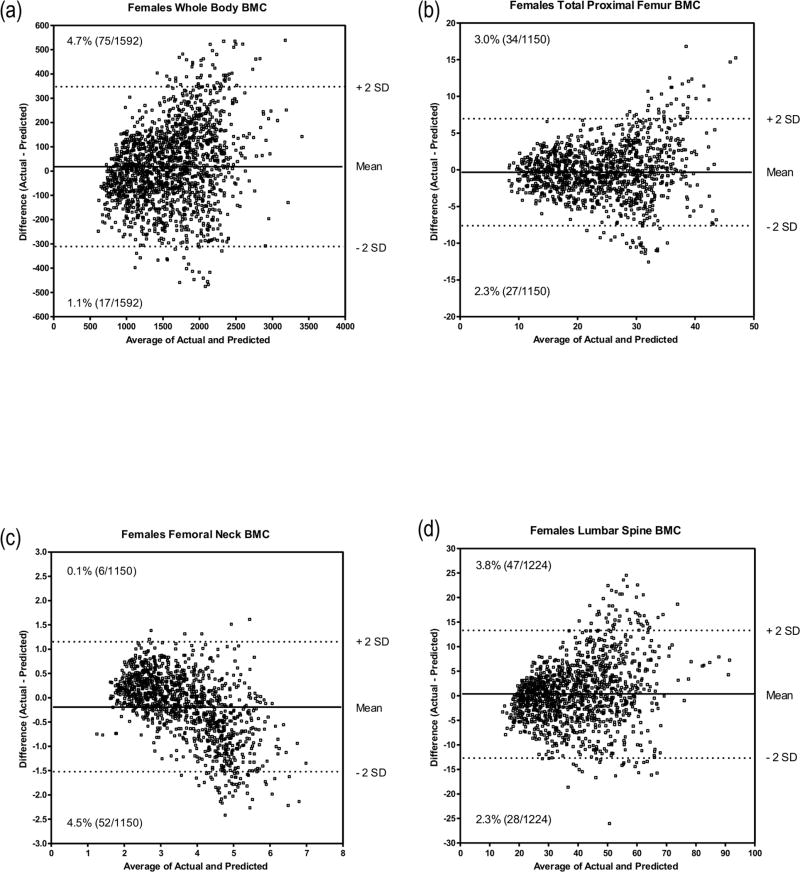
Figure 2.
Bland and Altman procedure showing the agreement between predicted BMC and observed BMC for all four skeletal sites in females from the original sample; (a) Whole body; (b) Total proximal femur; (c) Femoral neck; (d) Lumbar spine.
Figure 3.
Bland and Altman procedure showing the agreement between predicted BMC and observed BMC for all four skeletal sites in males (a–d) and females (e–h) from the new sample.
Results
We provide participant descriptives in Table 2.
Table 2.
Participant descriptives, Means (SD)
| Males (n = 1944) | Females (n = 3229) | |
|---|---|---|
| Age (years) | 13.3 (2.6) | 12.58 (2.6) |
| Height (cm) | 155.4 (11.5) | 155.1 (15.5) |
| Weight (kg) | 48.5 (13.0) | 48.2 (16.0) |
| Whole body BMC (g) | 1686.9 (511.2) | 1689.9 (675.1) |
| Total proximal femur BMC (g) | 25.9 (8.6) | 29.2 (12.4) |
| Femoral neck BMC (g) | 3.7 (1.0) | 4.1 (1.2) |
| Lumbar spine BMC (g) | 42.7 (14.7) | 41.4 (19.2) |
| Ethnicity (W/A/H/B) (%) | 25/8/2/2 | 50/8/2/3 |
Key: BMC – Bone mineral content; n = number of observation. W – White; A – Asian; H – Hispanic; B – Black.
Figures 1 and 2 illustrate the outcomes of the Bland-Altman procedure where we compared predicted with actual values in males and females, from the original cohort, at the four measured bone sites. We plotted the mean difference between predicted and actual BMC against the average of the two BMC values. For males’ WB BMC the mean difference between actual and predicted values was 21.2 g with a standard deviation of 178.6 g. The mean and standard deviations for PF, FN and LS were 0.26 (4.65) g, 0.07 (0.56) g and −0.39 (7.28) g respectively. For females, the mean difference between actual and predicted WB BMC was 15.6 g with a standard deviation of 173.4 g. The mean and standard deviations for PF, FN and LS were −0.41 (3.43) g, −0.19 (0.57) g and 0.34 (6.59) g, respectively.
Figure 3 illustrates the outcomes of the Bland-Altman procedure where we compared predicted with actual values for males and females using data from an independent sample of children at the four measured bone sites. We plotted the mean difference between predicted and actual BMC against the average of the two BMC values. For males’ WB BMC the mean difference between actual and predicted values was 69.2 g with a standard deviation of 111.1 g. The mean and standard deviations for PF, FN and LS were 0.66 (3.15) g, −0.17 (0.43) g and 0.91 (2.86) g respectively. For females, the mean difference between actual and predicted WB BMC was 60.1 g with a standard deviation of 126.1 g. The mean and standard deviations for PF, FN and LS were 1.21 (2.49) g, −0.15 (0.35) g and 1.31 (4.06) g, respectively.
We provide site-specific prediction models using all original data at each skeletal site (Tables 3 and 4). For male’s WB, a 1 year age centred (agec) increment predicted −554.3 (38.6) g of BMC accrual (i.e. at 12 years agec=−1 and predicted +554.3 (38.6) g, 13 years agec=0 and predicted 0 g, and at 14 years agec=+1 and predicted −554.3 (38.6) g etc), each cm of height predicted 14.2 (1.4) g of BMC accrual and each kg of weight predicted 31.1 (4.4) g of BMC accrual. We noted significant interactions between agec and height, and weight and height (P >0.05). On average, Hispanics had 75.5 (28.2) g less BMC accrual than Caucasians, Asians had 31.8 (6.8) g less BMC accrual than Caucasians and Caucasians had 82.8 (26.3) g less BMC accrual than Blacks, once agec, height and weight were controlled. A similar trend emerged with accrual differences noted between Blacks, Caucasians, Asians and Hispanics in descending order of value at the PF and FN. There were no differences between ethnicities at the LS. In all models based on random effects at level 1 (within individuals), BMC increased over time and at level 2 (between individuals) individuals had significantly different intercepts and slopes (P >0.05) of BMC accrual; the higher the intercept, the greater the slope (P >0.05). For females, models at the four sites (Table 4) were similar to those of the males. That is, agec, height and weight were all significant predictors of BMC accrual (P <0.05), as were the interaction terms (age height interaction, age weight interaction and weight divided by height). We also noted similar relationship between ethnicities for females as we did for boys (Table 3 and 4, P <0.05).
Discussion
We developed longitudinal BMC accrual models for WB, LS, PF and FN, derived from a large (n=5173 observations) national dataset of mixed ethnic samples of children and adolescents. These models account for sex, age, size and ethnicity and can be used to generate a Z-score to identify children with compromised bone health. The Bland and Altman analysis demonstrated that the models were excellent predictors of BMC as there was close agreement between the predicted and actual BMC. This close association was noted in both the international reference sample and in an independent, sample of healthy children. To our knowledge, our models are unique, and are especially important for clinicians whose patients may be small for age or as a result of disease and where growth (but not necessarily bone health) may be affected by a medical condition or a drug therapy.
We used multi-level statistical modeling to generate BMC curves so as to maximize the value of our longitudinal data. That is, our mixed-effects model allowed us to use data if they were complete, from all time points regardless of the number of study visits. Longitudinal data are the best means to capture the nuances of growth and to represent the substantial differences in the timing and magnitude of BMC accrual over time within and between the sexes. Although we recognize that physical activity, dietary intake and heredity may influence BMC accrual, these outcomes were not available across all studies. Thus, we were unable to include them in our models.
Our models reinforce what other excellent longitudinal studies have reported in smaller or single ethnicity cohorts [1, 9, 14]. However, our findings extend these models by increasing the numbers of children utilized to develop the models and by including age, sex, size and ethnicity in the model. As has been previously reported, BMC increases over time across all skeletal sites and in both sexes; although individuals had substantially different rates of BMC accrual [19]. For both males and females at the WB, PF and FN, the most significant independent predictors of BMC were age, ethnicity, weight and height; the most significant interaction terms were age/height and weight/height. These models highlight the already established relationships between age, size and BMC [17]. A novel component of our model was the ethnic mix that illustrated the difference in BMC accrual across Hispanic, Asian, Caucasian and Black children (except the lumbar spine) beginning at a young age. Our findings across ethnicities were consistent with previous cross-sectional and longitudinal studies [1, 7].
One aim of our study was to provide clinicians (or others) with a simple tool to calculate a child’s BMC Z-score. By accounting for age, size, sex and ethnicity the likelihood of misclassifying small (as a function of sex or ethnicity) or later maturing children as being “at risk” for bone health might be diminished. We also provide this calculation on a website (http://taurus.usask.ca/growthutility/) so that available clinical data can be entered and a standard score can be calculated for any child. The derived Z-score will allow the user to interpret DXA BMC data without the need for more statistical adjustments for bone area, body size or lean mass [3, 10, 17]. We provide a working example of the Z-score calculation at the end of the discussion.
Others have alerted clinicians to the many problems related to utilizing DXA to assess bone mass without correcting for its inherent size artifact [34]. This is especially important when children’s growth, but not their bone mass is affected by a clinical condition or a medication. This is also important when a child’s bone mass may be compromised but is masked by other factors, such as a larger body size. Depending on variables entered into the equation, the Z-score indicates BMC that is relative to children the same age, sex, height, weight and/or ethnicity. We provide prediction equations at 4 anatomical sites so that users have the opportunity to undertake the calculation most relevant for their patient’s individual circumstance. We do not suggest that our prediction models be used outside of standard medical practice that includes a complete medical history and collection of physical activity and dietary intake data.
We acknowledge that our study has a number of limitations. First, the prediction models we derived are specific to BMC data collected on Hologic QDR 4500 instruments. That is, only data acquired with Hologic instruments should be entered into the model. We provide cross-calibration factors (Table 1) so that data from other Hologic systems can be converted to Hologic 4500 values. Second, it would be ideal if all study BMC data were collected on the same generation instrument, calibrated across geographic measurement sites. Indeed, we accept that adjusting values across instruments is an imperfect science. To minimize error, we measured a standard calibration phantom across all study sites and conducted a precision study and standardized BMC values across study sites. Values for all but Stanford were within close range (Stanford values differed by 6% on average). Third, BMC assessed by DXA has inherent limitations. DXA provides a planar representation of a structure (bone) that is growing and developing in three dimensions. It would be useful in future to develop normative data collected from instruments such as peripheral quantitative computed tomography (pQCT) and high resolution peripheral quantitative computed tomography (HR-pQCT). This would provide a better understanding of the role of elements that underpin bone strength (total bone area and density and cortical and trabecular parameters). Fourth, we highlight the importance of dietary calcium intake and physical activity for bone accrual. The potentially significant differences in dietary and physical activity habits within and between countries should be considered when applying the prediction models across diverse groups. Fifth, we did not exclude children with a history of previous fracture. However, our target sites for measurement in this healthy cohort were the proximal femur and lumbar spine. As proximal femur fractures account for less than 1% of all fractures in children [35], and the overall incidence of spine fractures in children is 1.5% [36] we would expect these to be rare events in our cohort. Lastly, although we present BMC data from the largest cohort to date, our sample size is still relatively small when extrapolating to the larger population. This applies especially to the relatively small number of Hispanic, Asian and Black children and adolescents in any age group. However, our longitudinal, repeated measures design where one participant is measured in some cases over an extended period, substantially overcomes this limitation. On these two points there probably are significant differences between the different ethnicities living in North America. These habits may be even more different in children living in other countries. These aspects must be considered if this database is to be used also in other countries.
In conclusion, we have developed a useful tool to assess relative bone health (presented as BMC by DXA) in paediatric populations. Despite the noted limitations of our study, we feel there is an urgent need for normative bone data adjusted for key variables to overcome the potential misclassification of both healthy and unhealthy children and adolescents.
Worked examples (http://taurus.usask.ca/growthutility)
Example 1
An 11.9 years old Caucasian female (born June 5th 1997 and measured on May 5th 2009) has received long-term steroid medication. Her BMC was assessed using DXA (Hologic QDR 4500). Her femoral neck BMC was 2.80 g; she was 140 cm tall (5th percentile) and weighed 35 kg (25th percentile). Order of calculation; 1) calculate age centred; 2) predict BMC value; 3) determine SD for FN BMC; 4) calculate age and size specific Z-score.
-
1)
Agec = 11.9 years – 13.00 years = −1.1 years.
From Table 4:
-
2)
Predicted FN BMC = (agec*−0.449)+(agec2*0.005)+(agec3*0.00075)+(height*0.0157)+ (weight*0.083)+(agec*height*0.0039)+(agec*weight*−0.0019)+ ((weight/height*100)*−0.094)= 2.71 g.
-
3)
FN BMC SD at 11.9 years= (0.161 + (2*(0.018*−1.1)) + (0.0037*(−1.12)0.5 = 0.36 g.
-
4)
To calculate the Z-score = (actual FN BMC − predicted FN BMC)/FN BMC SD.
Z-score = (2.80 − 2.71)/0.36 = 0.26.
Interpretation: Despite long-term steroid use this girl has a femoral neck BMC that is within the normal range for girls of the same age, size and ethnicity.
Example 2
An 11.9 years old Caucasian female (born June 5th 1997 and measured on May 5th 2009) has received long-term medication and treatment for Crohns disease. Her BMC was assessed using DXA (Hologic QDR 4500). Her femoral neck BMC was 1.89 g; she was 145 cm tall (25th percentile) and weighed 35 kg (25th percentile). Order of calculation; 1) calculate age centred; 2) predict BMC value; 3) determine SD for FN BMC; 4) calculate age and size specific Z-score.
-
1)
Agec = 11.9 years – 13.00 years = −1.1 years.
From Table 4:
-
2)
Predicted FN BMC = (agec*−0.449)+(agec2*0.005)+(agec3*0.00075)+(height*0.0157)+ (weight*0.083)+(agec*height*0.0039)+(agec*weight*−0.0019)+ ((weight/height*100)*−0.094)= 2.85 g.
-
3)
FN BMC SD at 11.9 years= (0.161 + (2*(0.018*−1.1)) + (0.0037*(−1.12)0.5 = 0.36 g.
-
4)
To calculate the Z-score = (actual FN BMC − predicted FN BMC)/FN BMC SD.
Z-score = (1.89 − 2.85)/0.36 = −2.71
Interpretation: The long-term medication and treatment for Crohns disease in this girl has a resulted in a femoral neck BMC that is over 2SD below the normal range for girls of the same age, size and ethnicity. Such a Z-score would warrant further investigation for low BMC.
Acknowledgments
Funding disclosures:
The UBC Healthy Bones Study (1999–2006) was supported by the Canadian Institute for Health Research project grant (20RNO793). Additional support was provided by the Michael Smith Foundation for Health Research grant no (20R41770). PBMAS (1991–1998) was supported by the Canadian National Health and Research Development Program (NHRDP), grant no. 6608-1261. PBMAS (2002–2005) was supported by the Canadian Institute of Health Research, grant no. MOP 57671. Additional support was provided by the Saskatchewan Health Research Foundation. Stanford bone study was funded by the NIH, grant DK 45226. The Penn State YWHS was supported by NIH grants from the NICHD-R01 HD25973 (T. Lloyd), M01-RR-10732 (Penn State University GCRC), and NIAMS-K23 AR49040-01A1 (M. Petit). The BUGSY study was supported by a NIH RO1, grant no AR45655-08.
Footnotes
All authors do not perceive or have any real potential conflicts of interests. The study sponsors had no input into the study design, collection, analysis and interpretation of the data, the writing of the report, or the decision to submit the paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. Journal of Clinical Endocrinology and Metabolism. 1999;84:4702–12. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 2.Specker B, Schoenau E. Quantitative bone analysis in children: current methods and recommendations. The Journal of Pediatrics. 2005:726–731. doi: 10.1016/j.jpeds.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–45. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 4.Leonard MBPK, Zemel BS, Stallings VA, Feldman HI. Discrepancies in pediatric bone mineral density reference data: potential for misdiagnosis of osteopenia. Journal of Pediatrics. 1999:135. doi: 10.1016/s0022-3476(99)70020-x. [DOI] [PubMed] [Google Scholar]
- 5.Kroger H, Kotaniemi A, Vainio P, Alhava E. Bone densitometry of the spine and femur in children by dual-energy x-ray absorptiometry. Bone and Mineral. 1992;17:75–85. doi: 10.1016/0169-6009(92)90712-m. [DOI] [PubMed] [Google Scholar]
- 6.Molgaard C, Thomsen BL, Prentice A, Cole TJ, Michaelsen KF. Whole body bone mineral content in healthy children and adolescents. Archives of Disease in Childhood. 1997;76:9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis K, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, Abram SA. Z score prediction model for assessment of bone mineral content in pediatric diseases. Journal of Bone and Mineral Research. 2001;16:1658–1664. doi: 10.1359/jbmr.2001.16.9.1658. [DOI] [PubMed] [Google Scholar]
- 8.Schoenau E, Neu CM, Rauch F, Manz F. Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone. 2002;31:110–113. doi: 10.1016/s8756-3282(02)00802-5. [DOI] [PubMed] [Google Scholar]
- 9.Faulkner R, Bailey DA, Drinkwater DT, McKay HA, Arnold C, Wilkinson AA. Bone densitometry in Canadian children 8–17 years of age. Calcified Tissue International. 1996;59:344–351. doi: 10.1007/s002239900138. [DOI] [PubMed] [Google Scholar]
- 10.Molgaard C, Thomsen BL, Michaelsen KF. Influence of weight, age and puberty on bone size and bone mineral content in healthy children and adolescents. Acta Paediatr. 1998;87:494–9. doi: 10.1080/08035259850158173. [DOI] [PubMed] [Google Scholar]
- 11.Binkley TL, Specker BL, Wittig TA. Centile curves for bone densitometry measurements in healthy males and females ages 5–22 yr. J Clin Densitom. 2002;5:343–53. doi: 10.1385/jcd:5:4:343. [DOI] [PubMed] [Google Scholar]
- 12.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. The Bone Mineral Density in Childhood Study: Bone Mineral Content and Density According to Age, Sex, and Race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 13.Lu P, Cowell CT, Lloyd-Jones SA, Briody JN, Howan-Giles R. Volumetric bone mineral density in normal subjects aged 5–27 years. Journal of Clinical Endocrinology and Metabolism. 1996;81:1586–1590. doi: 10.1210/jcem.81.4.8636372. [DOI] [PubMed] [Google Scholar]
- 14.Maynard LM, Guo SS, Chumlea WC, Roche AF, Wisemandle WA, Zeller CM, Towne B, Siervogel RM. Total-body and regional bone mineral content and areal bone mineral density in children aged 8–18 y: the Fels Longitudinal Study. Am J Clin Nutr. 1998;68:1111–7. doi: 10.1093/ajcn/68.5.1111. [DOI] [PubMed] [Google Scholar]
- 15.Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: Normative values for the 2–20 year old population. Bone. 1995;16:S393–399. doi: 10.1016/8756-3282(95)00082-o. [DOI] [PubMed] [Google Scholar]
- 16.Boot AM, Engels MA, Boerma GJ, Krenning EP, De Muinck Keizer-Schrama SM. Changes in bone mineral density, body composition, and lipid metabolism during growth hormone (GH) treatment in children with GH deficiency. J Clin Endocrinol Metab. 1997;82:2423–8. doi: 10.1210/jcem.82.8.4149. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree NJ, Kibirige MS, Fordham JN, Banks LM, Muntoni F, Chinn D, Boivin CM, Shaw NJ. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–72. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DA, Baxter-Jones AD, Mirwald RL, Faulkner RA. Bone growth and exercise studies: The complications of maturation. Journal of Musculoskeletal and Neurological Interactions. 2003;3:335–7. discussion 356. [PubMed] [Google Scholar]
- 19.Bailey DA, McKay HA, Mirwald RL, Crocker PR, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan Bone Mineral Accrual Study. Journal of Bone Mineral Research. 1999;14:1672–9. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 20.Bachrach L. Osteoporosis and measurement of bone mass in children and adolescents. Endocrinology and Metabolism Clinics of North America. 2005;34:521–535. doi: 10.1016/j.ecl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Bailey D, Faulkner RA, McKay HA. Growth, physical activity and bone mineral acquisition. Exerc Sport Sci Rev. 1996;24:233–266. [PubMed] [Google Scholar]
- 22.MacKelvie KJ, McKay HA, Khan KM, Crocker PR. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. Journal of Pediatrics. 2001;139:501–8. doi: 10.1067/mpd.2001.118190. [DOI] [PubMed] [Google Scholar]
- 23.Baxter-Jones A, Mirwald RM. Multilevel modeling. In: Hauspie RC, Cameron N, Molinari L, editors. Methods in Human Growth Research. Cambridge University Press; 2004. pp. 306–329. [Google Scholar]
- 24.Kemper H, Twisk JWR, Van Mechelen W, Post GB, Roos JC, Lips P. A fifteen-year longitudinal study in young adults on the relation of physical activity and fitness with the development of the bone mass: the Amsterdam growth and health longitudinal study. Bone. 2000;27:847–853. doi: 10.1016/s8756-3282(00)00397-5. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd T, Rollings N, Andon MB, Demers LM, Eggle DF, Kieselhorst K, Kulin H, Landis JR, MarteL JK, Orr G, Smith P. Determinants of bone density in young women. I. Relationships among pubertal development, totat body bone mass, and total body bone density in premenarchal females. Journal of Clinical Endocrinology and Metabolism. 1992;75:383–387. doi: 10.1210/jcem.75.2.1639940. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–82. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Wallace B. College of Physical Education. Saskatoon: University of Saskatchewan; 1995. Precision of bone mineral and soft tissue measurements using an Hologic QDR 2000 in array mode. [Google Scholar]
- 28.Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8–19-year-old boys and girls. Ann Hum Biol. 2003;30:160–175. doi: 10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein H. Multilevel statistics models. London: Arnold; 1995. [Google Scholar]
- 30.Baxter-Jones AD, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8–19-year-old boys and girls. Ann Hum Biol. 2003;30:160–75. doi: 10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- 31.Forwood MR, Baxter-Jones AD, Beck TJ, Mirwald RL, Howard A, Bailey DA. Physical activity and strength of the femoral neck during the adolescent growth spurt: A longitudinal analysis. Bone. 2006;38:576–583. doi: 10.1016/j.bone.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Forwood MR, Bailey DA, Beck TJ, Mirwald RL, Baxter-Jones AD, Uusi-Rasi K. Sexual dimorphism of the femoral neck during the adolescent growth spurt: a structural analysis. Bone. 2004;35:973–981. doi: 10.1016/j.bone.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- 34.Rauch F. Bone growth in length and width: the yin and yang of bone stability. J Musculoskelet Neuronal Interact. 2005;5:194–201. [PubMed] [Google Scholar]
- 35.Canale S. Fractures of the hip in children and adolescents. Orthopedic Clinics of North America. 1990;21:341–352. [PubMed] [Google Scholar]
- 36.Patel JC, Tepas JJ, Mollitt DL, Pieper P. Pediatric cervical spine injuries: defining the disease. Journal of Pediatric Surgery. 2001;36:373–376. doi: 10.1053/jpsu.2001.20720. [DOI] [PubMed] [Google Scholar]