Abstract
Lipid antigens are presented to T cells by the non-polymorphic MHC class I-related CD1 molecules. Microsomal triglyceride transfer protein (MTP) is an endoplasmic reticulum (ER)-resident chaperone that has been shown to lipidate the group 2 CD1 molecule CD1d and thus to regulate its function. We now report that MTP also regulates the function of group 1 CD1 molecules CD1a, CD1b, and CD1c. Pharmacological inhibition of MTP in monocyte-derived dendritic cells and lymphoblastoid B cell lines transfected with group 1 CD1 resulted in a substantial decrease in endogenous self lipid antigen presentation to several CD1-restricted T cell lines. Silencing MTP expression in CD1c-transfected HeLa cells similarly resulted in decreased self reactivity. Unexpectedly, inhibition of ER-resident MTP, which was confirmed by confocal microscopy, also markedly decreased presentation of exogenous, endosomally loaded, mycobacterial lipid antigens by CD1a and CD1c to T cells. Thus, these studies indicate that MTP, despite its ER localization, regulates endogenous as well as exogenous lipid antigen presentation, and suggest a broad role for MTP in the regulation of CD1 antigen presentation.
Keywords: CD1, Lipid antigen presentation, Microsomal triglyceride transfer protein
Introduction
The immune system has evolved a number of different strategies to recognize self (endogenous) and foreign (exogenous) antigens. Major histocompatibility class (MHC) class I molecules serve the function of presenting intracellularly derived peptides to CD8+ T cells, whereas MHC class II molecules present peptides sampled from the endocytic system to CD4+ T cells [1]. Endogenous and foreign lipid antigens are recognized by the immune system through presentation by CD1 molecules, which are an MHC class I-related and non-polymorphic class of molecules that like MHC class I molecules consist of a heavy chain non-covalently linked to β2 microglobulin (β2m) [2]. The human genome encodes five CD1 isoforms, which are grouped based upon sequence homology into either group 1 (CD1a, CD1b, and CD1c) or group 2 (CD1d), with CD1e being an intermediate between both groups [3]. Mice, in contrast, only contain CD1d orthologues [2]. CD1 molecules survey different endocytic compartments for lipid antigens, with CD1a trafficking through the early endocytic system, CD1b and murine CD1d exhibiting a localization to late endosomes and lysosomes, and CD1c and human CD1d having a broad distribution throughout the endocytic system [2, 4].
Group 1 CD1 molecules present microbial fatty acids, glycolipids, phospholipids, and lipopeptide antigens to T cells expressing diverse TCR-α and TCR-β chains [2, 5]. T cells that recognize group 1 CD1-antigen complexes are either double-negative, CD4+ or CD8+, making it clear that group 1 CD1-restricted T cells are a component of all major phenotypic groups of T cells previously thought to be MHC class I- and class II-restricted [2]. Most group 1 CD1-restricted T cell lines and clones derived thus far recognize lipid antigens purified from the lipid-rich cell walls of Mycobacterium species.
The newly synthesized CD1 heavy chains are translocated into the endoplasmic reticulum (ER), where N-linked glycans are attached and interactions occur with ER-resident chaperones (ERp57, calnexin, calreticulin), leading to association with β2m [4, 6]. In contrast to MHC class I, CD1d association with β2m occurs after chaperone-mediated folding of the CD1d heavy chain [2]. This is consistent with the differences in the requirement of CD1b and CD1d for an association with β2m before exiting the ER: CD1b heavy chains are confined to the ER in β2m-deficient cells in contrast to CD1d heavy chains that can exit the ER and reach the cell surface in the absence of β2m [7–9]. Newly synthesized CD1 molecules are rapidly delivered from the Golgi apparatus to the plasma membrane, most likely along the secretory pathway [4].
The exit of MHC class I molecules from the ER requires occupation of their peptide-binding grooves with proteasome-derived peptides that are translocated into the ER by the transporter associated with antigen processing (TAP) [1, 10]. Similarly, the peptide-binding groove of MHC class II molecules is occupied through its association with invariant chain, which also serves to target the multimeric complex to the endocytic system [1, 10].
Due to the hydrophobic nature of the CD1 antigen-binding groove, occupation of the CD1 antigen-binding groove by self lipids in the ER has been postulated [11, 12]. Specifically, self lipids (phosphatidylinositol and phosphatidylethanolamine) associate with CD1d during assembly in the ER and are thought to occupy the hydrophobic antigen-binding groove during traffic through the secretory and endocytic systems [12]. Consistent with this, CD1d-restricted T cells have been described that are specific for phosphatidylinositol [13]. Similarly, autoreactive human T cells clones have been identified that are restricted by CD1a, CD1b, and CD1c, suggesting that lipid loading also occurs within the ER for these molecules as autoreactivity is still evident with deletion at the cytoplasmic tail [14]. However, it is unclear whether such lipid loading constitutes an essential step in the assembly and folding of CD1 before exit from the ER. Likewise, it is unknown whether composition of the lipids that occupy the CD1 groove during assembly and secretion influences the exchange of self and foreign lipids in the endocytic system by the saposins [15–17] or the stability of CD1 molecules on the cell surface.
Microsomal triglyceride transfer protein (MTP), previously known for its role in the lipidation of apolipoprotein (Apo)B48 and ApoB100 and hence the generation of chylomicrons and very-low-density lipid particles [18–20], respectively, has recently been shown to regulate CD1d function in vivo and in vitro [21]. In addition to its expression in the intestinal epithelium and hepatocytes, MTP is expressed by professional antigen-presenting cells (APC), including dendritic cells (DC) [22, 23]. Inhibition of MTP in these cells reduced their ability to activate both self-reactive and α-galactosylceramide-reactive CD1d-restricted NKT cells. Furthermore, using an in vitro reductionist system, MTP was able to transfer phospholipids, but not triglycerides to CD1d [22, 23]. These data suggest a model whereby, during assembly in the ER, MTP lipidates CD1d in a step that is critical for CD1d to present both endogenous (ER-loaded) and exogenous (endosomal or surface-loaded) antigens to CD1d-restricted NKT cells. As a consequence of the regulation of CD1d by MTP, mice with a conditional deletion of MTP are protected from CD1d-mediated immunopathologies associated with hepatitis and colitis [21].
We hypothesized that MTP, in addition to regulating CD1d function, could have a broad role as the primary lipid transfer protein in the ER responsible for the loading of self lipids into CD1 molecules. Indeed, inhibition of MTP lipid transfer activity by a panel of small-molecule MTP inhibitors (MTPi) and RNA interference-mediated silencing of MTP expression resulted in diminished activation of CD1a-, CD1b-, and CD1c-restricted self-reactive T cell clones and strikingly decreased presentation of exogenous mycobacterial lipid antigens by CD1b and CD1c. These findings implicate a general role for MTP in the generation of CD1-restricted T cell responses to both self lipids and exogenous antigens.
Results
MTP affects group 1 CD1-restricted autoreactivity
A panel of group 1 CD1-restricted T cells that recognize endogenous lipid antigens and release IFN-γ upon stimulation with human monocyte-derived DC have been described [24, 25]. The precise intracellular site(s) where CD1a–c acquires endogenous lipids is not known. However, it is likely that these events occur co- or post-translationally within the ER, rendering MTP as a candidate accessory molecule involved in these processes. To test whether MTP regulates group 1 CD1 presentation of endogenous antigens, human monocyte-derived DC were differentiated from CD14+ monocyte precursors in the presence of BMS212122, a specific chemical inhibitor of MTP lipid transfer function [26], for 4 days. The resulting cells were tested for their ability to activate self-reactive CD1-restricted T cell clones with BMS212122 present during the assay. As shown in Fig. 1A, MTPi-treated DC induced significantly less IFN-γ secretion from both a CD1a-restricted T cell clone, Mt1.50, and a CD1c-restricted T cell clone, Ye2.3, compared to vehicle-treated DC. Importantly, there was no effect of MTP inhibition on the presentation of tetanus toxoid antigen in the context of HLA-DR to SPF3, an MHC class II-restricted T cell clone [27], indicating a specific role for MTP in CD1-restricted antigen presentation (Fig. 1B).
Figure 1.
Inhibition of MTP abrogates autoreactivity of DC. (A) CD14+ monocytes were differentiated to DC in the presence of IL-4 and GM-CSF; the indicated number of DC were co-cultured with 1×105 CD1a-restricted autoreactive Mt1.50 or CD1c-restricted Ye2.3 T cells. MTPi BMS212122 (open circles) or DMSO (vehicle; filled circles) was present throughout DC differentiation and co-culture with T cells. Supernatants were assayed for IFN-γ release by ELISA. (B) Using similar experimental setup as described in part A, the tetanus toxoid-reactive, MHC class II-restricted T cell line SPF3 (1×105) was co-cultured with tetanus-toxoid loaded DC (1×105) treated with either BMS212122 or DMSO. Activation of SPF3 was analyzed by IFN-γ release into the supernatant. (C) To exclude the possibility that the presence of BMS212122 might affect T cell activation, DC (1×105) were fixed with glutaraldehyde at day 4 of differentiation from monocytes, and co-cultured with autoreactive T cell clones (1×105) restricted by CD1a (Mt1.50), CD1b (Ec2.55), and CD1c (Ye2.3). Activation was assayed by IFN-γ release into the supernatant.
To rule out an effect of BMS212122 on the responding T cells, monocyte-derived DC were fixed with glutaraldehyde after inhibitor treatment and co-cultured with T cells in the absence of inhibitor. As shown in Fig. 1C, inhibition of MTP with BMS212122 resulted in a similar decrease in presentation to self-reactive CD1a-, CD1b-, and CD1c-restricted T cell clones. Control experiments with anti-CD3 stimulation did not show any difference in IFN-γ secretion between MTPi-treated and untreated cells (data not shown).
To confirm and extend the observations obtained with BMS212122, we tested several other MTPi for their potential to regulate group 1 CD1 function. As shown in Fig. 2A and B, BMS195183 [28], 197636 [29], and 200150 [30] inhibited CD1b-and CD1c-restricted autoreactivity of T cell clones in a dose-dependent fashion to a level that was similar to that observed with BMS212122. Importantly, BMS197567 (Bristol-Myers-Squibb, Princeton, NJ; Fig. 2c), which is structurally similar to the other compounds tested, did not affect CD1 function at any concentration tested. This latter compound is less effective than the other compounds at inhibiting triglyceride transfer to ApoB with an IC50 of 20 μM, compared to the other inhibitors, which have IC50 for triglyceride transfer to ApoB in the range used in this assay (1.3–13 μM; Bristol-Myers Squibb, personal communication). This observation supports the likelihood that the observed phenotype is specific to blocking MTP function rather than due to a non-specific effect of this class of compounds on CD1 function. Taken together, these data support a role for MTP in endogenous lipid antigen presentation by group 1 CD1-restricted proteins.
Figure 2.
Dose dependency of various MTPi in regulating CD1b and CD1c autoreactivity. (A, B) DC (1×105) were differentiated from monocytes over 4 days in the presence of various MTPi as indicated, at final concentrations of 13 μM, 4.1 μM, 1.3 μM, or 0.41 μM. Cells were washed, fixed with glutaraldehyde, and co-cultured with 1×105 autoreactive CD1b-restricted Ec2.55 (A) and CD1c-restricted Ye2.3 (B) T cells, and supernatants assayed for IFN-γ release from T cells. (C) Structure of the novel MTPi BMS197567. (D) DC on day 4 of differentiation were analyzed for CD1, MHC class I, and MHC class II surface expression after incubation with vehicle (DMSO) or MTPi BMS212122.
Given that chemical inhibition of MTP alters the surface expression of CD1d on human and mouse DC [22], we assessed group 1 CD1 surface expression on inhibitor-treated monocyte-derived DC as a possible explanation of the observed functional defect. To this end, CD14+ monocytes were differentiated to DC in the presence of BMS212122 or with a vehicle control, and CD1 surface expression examined by FACS. As shown in Fig. 2D, the differentiation of monocyte-derived DC in the presence of BMS212122 significantly reduced, but did not abrogate, expression of CD1a, CD1b, and CD1c. In contrast, BMS212122 treatment had no effect on MHC class I and II expression, and did not alter expression of the DC activation marker CD83 (data not shown), indicating a specific effect of inhibitor treatment on CD1 expression.
To gain insights into the mechanistic basis of decreased surface expression of CD1a, CD1b, and CD1c in BMS212122-treated DC, we assessed mRNA expression of CD1 molecules. As depicted in Fig. 3A, expression of all three CD1 mRNA species was decreased in BMS212122-treated compared to DMSO-treated DC. The effect was marked for CD1a, but minor for CD1b and CD1c. Confocal microscopy revealed pronounced surface expression of CD1 molecules in DMSO-treated DC (Fig. 3A). As predicted from FACS analysis, CD1 surface staining was markedly reduced upon MTPi treatment, and exhibited a pronounced intracellular staining pattern partially co-localizing with the ER marker calnexin, an observation that was most pronounced for CD1b (Fig. 3A). Messenger RNA expression for β2m, HLA-A, and the MHC class I-like protein HFE was similar and slightly increased, respectively, in DC differentiated in the presence of BMS212122 compared to DMSO (Fig. 3B).
Figure 3.
MTP inhibition leads to intracellular redistribution of CD1 molecules in DC. (A) DC were differentiated from monocytes over 4 days in the presence of BMS212122 (final concentration 13 μM) or DMSO, fixed, and stained with anti-CD1a, anti-CD1b, and anti-CD1c as indicated (green), the ER marker anti-calnexin (red), and the nuclear marker TO-PRO3 (blue). Messenger RNA expression of CD1a, CD1b, and CD1c was determined by qPCR at the same time point and expressed as ratio to GAPDH expression. (B) Messenger RNA expression of β2m, HLA-A, and MHC class I-like HFE in DC treated with DMSO and BMS212122 as described in part A.
The finding that CD1a, CD1b, and CD1c surface levels are reduced, but still present at high levels on DC differentiated in the presence of MTPi makes it unlikely that surface levels alone account for the results of the functional experiments. To clarify the effect of CD1 surface levels following MTP inhibition, we tested the effect of BMS212122 on CD1 surface expression in the B lymphoblastoid cell line C1R transfected with CD1a, CD1b, or CD1c [31] (Fig. 4A). After 3 days of culture in the presence of BMS212122, CD1a, CD1b, and CD1c were expressed on the cell surface at levels comparable to the vehicle control cells. Strikingly, functional experiments using inhibitor-treated C1R-CD1a and C1R-CD1c transfectants resulted in decreased activation of Mt1.50 and Ye2.3 T cells to a comparable extent as seen with monocyte-derived DC (Fig. 4B). BMS212122 had no effect on co-cultures of mock-transfected C1R cells and Ye2.3 and Mt1.50 T cells (data not shown).
Figure 4.
MTP regulates autoreactivity of C1R and HeLa transfectants without affecting CD1 surface expression. (A) B lymphoblastoid C1R cells stably transfected with CD1a, CD1b, or CD1c were cultured with BMS212122 or vehicle (DMSO) for 72 h and surface expression of CD1 molecules analyzed by flow cytometery. (B) C1R cells were treated as above, and subsequently co-cultured at indicated numbers with 1×105 autoreactive CD1a-restricted Mt1.50 and CD1c-restricted Ye2.3 T cells in the continued presence of MTPi (open circles) or DMSO (filled circles). Activation of T cells was measured by IFN-γ release into the supernatant. (C) HeLa cells stably transfected with CD1c were electroporated with MTP siRNA (MTP 8259) or mock siRNA, and MTP mRNA expression analyzed 60 h later by quantitative PCR. Data are presented normalized to β-actin mRNA expression. (D) MTP-silenced or mock-silenced HeLa CD1c transfectants (1×104 per well) were co-cultured with 5×104 autoreactive CD1c-restricted Ye2.3 cells for 6 h, and IFN-γ release as a measure of T cell activation measured in the supernatant. (E) Surface CD1c expression on MTP- and mock-silenced HeLa CD1c transfectants was analyzed by flow cytometry.
To independently confirm the role of MTP in the presentation of endogenous self lipids by group 1 CD1 molecules, we silenced MTP in HeLa cells stably transfected with CD1c and tested the ability of MTP-silenced cells to activate Ye2.3 T cells. Although HeLa cells were previously considered a cell line that did not express MTP, we could detect MTP mRNA transcripts and transfection of MTP small interfering RNA (siRNA) into HeLa-CD1c cells reduced MTP transcript levels by 98% (Fig. 4C). Silencing of MTP in these cells resulted in decreased activation of Ye2.3 T cells compared to control-treated cells, without a concomitant reduction in CD1c surface levels (Fig. 4D and E). Collectively, these studies demonstrate a role for MTP in the generation of CD1-endogenous antigen complexes and suggest that while MTP inhibition alters CD1 surface expression in primary cells, such changes do not fully explain the observed functional defects.
MTP regulates exogenous antigen presentation by group 1 CD1 molecules
Previous work demonstrated that MTP regulates the presentation of both self and exogenously added foreign lipid antigens to NKT cells by CD1d [21, 22, 32]. T cells restricted by group 1 CD1 molecules recognize both self antigens and foreign antigens, including a number of lipids and lipopeptides from Mycobacterium spp. Unlike endogenous lipid antigens, which may be loaded into CD1 during assembly in the ER, CD1a, CD1b, and CD1c are believed to acquire foreign antigens either at the cell surface or in the endocytic system [4]. We therefore tested whether MTP regulates the presentation of Mycobacterium antigens by group 1 CD1 molecules.
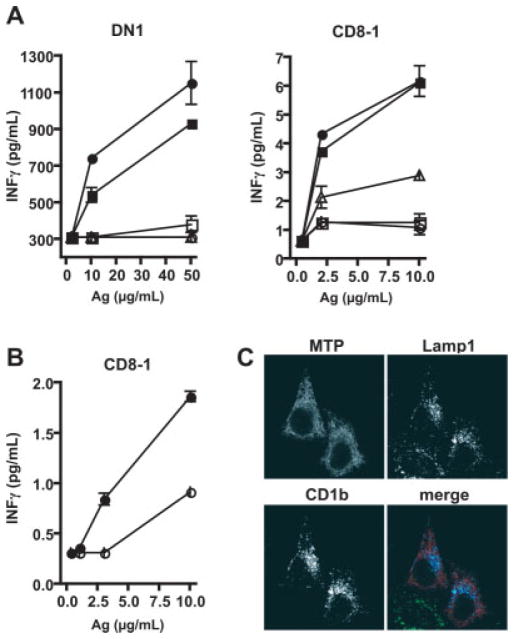
Human monocyte-derived DC were generated in the presence or absence of MTPi and pulsed for 4 h with lipid antigens, prior to fixation and incubation with either DN1 or CD8-1 T cells. DN1 is a double-negative CD1b-restricted TCR-αβ+ T cell clone that recognizes mycolic acid derived from mycobacterial cell walls [31, 33, 34] and CD8-1 is a CD8+ TCR-αβ+ T cell clone that recognizes mycoketides derived from mycobacteria in a CD1c-restricted fashion [35, 36]. As shown in Fig. 5A, three different MTPi, BMS197636, BMS200150, and BMS212122, completely blocked presentation of mycolic acid by CD1b to DN1 T cells compared to vehicle-treated DC or DC treated with BMS197567. Similarly, the same three MTPi reduced the presentation of mannosyl-β-1-phosphomycoketide to CD8-1 T cells, with a more striking effect observed with BMS200150 and BMS212122 (Fig. 5A). To substantiate findings with monocyte-derived DC, C1R-CD1c transfectants were cultured for 3 days with BMS200150, pulsed with antigen overnight in the presence of BMS200150, fixed with glutaraldehyde, and then co-cultured with CD8-1 T cells. BMS200150 decreased presentation by 50% at varying antigen doses up to the highest concentration tested of 10 μg/mL (Fig. 5B).
Figure 5.
MTP regulates exogenous antigen presentation by DC and C1R transfectants. (A) Day-4 DC (1×105) differentiated from monocytes in the presence of vehicle (DMSO; filled circles) or various MTPi (BMS197567, filled squares; BMS197636, open triangles; BMS200150, open circles; BMS212122, open squares), were loaded with antigen (mycobacterial extracts) for 4 h at the indicated concentrations, washed, fixed with glutaraldehyde, and co-cultured with 1×105 mycolic acid-reactive CD1b-restricted DN1 T cells [41] and mannosyl-β-1-phos-soprenoid-reactive CD1c-restricted CD8-1 T cells [42]. T cell activation was measured by IFN-γ release into the supernatant. (B) CD1c-transfected C1R cells (3×105) were treated with BMS200150 (open circles) or vehicle (DMSO; filled circles) for 72 h, loaded with antigen overnight at the indicated concentrations, washed, fixed with glutar-aldehyde, co-cultured with the CD1c-restricted CD8-1 T cell line (1×105), and IFN-γ measured in the supernatant. (C) MTP localizes to the ER in HeLa transfectants. N-terminally HA-tagged MTP (red) was transiently expressed in HeLa cells stably transfected with CD1b (blue) and analyzed for co-localization of CD1b and a lysosomal marker, Lamp1 (green). A reticular staining pattern indicative of ER localization is observed for MTP. MTP-HA does not co-localize with CD1b or Lamp1.
Taken together, the functional data utilizing pharmacological inhibition of MTP and siRNA knockdown indicate that MTP regulates both endogenous and physiologic exogenous CD1-restricted antigen presentation. Since CD1 intersects lipid antigens in various intracellular compartments, the intracellular localization of MTP and CD1b was examined. HA-tagged MTP was expressed in HeLa cells stably transfected with CD1b, and the localization of MTP and CD1b visualized by confocal microscopy. Consistent with previous descriptions of MTP localization in epithelial cells, MTP-HA staining exhibited a reticular pattern indicative of ER localization and failed to co-localize with CD1b and lysosomal-associated membrane protein 1 (Lamp1) in lysosomal vesicles (Fig. 5C).
Discussion
Here we report that MTP has a profound role in the regulation of group 1 CD1 proteins as we and others have previously shown for group 2 CD1 proteins of both mouse and human [21–23, 32]. Using a panel of pharmacological MTPi as well as by silencing MTP expression, we demonstrate that ER-resident MTP in APC and group 1 CD1-transfected cell lines is required for activation of group 1 CD1-restricted T cells harboring TCR that recognize endogenous self lipid antigens. Furthermore, we provide evidence that MTP also affects presentation of exogenous lipid antigens derived from the microbial world, highlighting a distant function of MTP on the presentation of non-ER-derived antigenic lipids presented by CD1b and CD1c molecules. Coupled with previous reports on the regulation of CD1d function [21–23, 32], the data presented here suggest that MTP has a general function in regulating lipid antigen presentation by both group 1 and group 2 CD1 molecules.
As mentioned above, MTP has a significant role in the presentation of both foreign and self lipids presented by CD1. Recently, Sagiv et al. [32] confirmed a critical role of MTP in CD1d antigen presentation, and proposed that MTP deficiency diminishes the presentation of lysosome-dependent exogenous lipids by reducing CD1d recycling from the lysosome to the plasma membrane. This was supported by an observed increase in intracellular CD1d, relative to cell surface CD1d expression, and functionally, using a panel of TCR invariant and non-invariant CD1d-restricted T cell clones [32]. Specifically, CD1d presentation of lysosomally processed lipid antigens was found to be MTP-dependent, whereas the presentation of antigens that do not require lysosomal trafficking by CD1d was unaffected [32].
In the current report we observed that MTP affects both endogenous and exogenous antigen presentation by group 1 CD1 proteins, suggesting that MTP affects acquisition of antigens by CD1a, CD1b, and CD1c broadly within the secretory and endocytic system. Whether this is due to an effect of MTP that originates within the ER and which is carried onto distal functions of CD1 in other intracellular organelles, or is derived primarily from MTP function outside of the ER, remains to be characterized. However, given that MTP is an ER-resident protein with an established role in the lipidation of ApoB in the ER [19, 20], as confirmed here, it is likely that MTP acts on CD1 molecules during their biosynthesis and assembly prior to entering the secretory pathway.
Several mechanisms may account for the observed effects of MTP on the presentation of exogenous lipid antigens acquired in endosomal compartments. First, pharmacological inhibition of MTP in primary monocyte-derived DC diminishes CD1a, CD1b, and CD1c surface expression relative to vehicle-treated controls. However, these cells maintain moderate to high levels of CD1 surface expression that would be expected to be competent for stimulation of T cells. Alternatively, the absence of MTP may alter the nature of the endogenous lipids loaded into CD1, resulting in either decreased stability of the CD1-lipid complex or an altered CD1 conformation. These changes might have distal effects at the level of CD1-mediated antigen presentation on the plasma membrane, at saposin- or GM2-mediated lipid exchange in the endocytic pathway, or might also contribute to a recycling defect of CD1 molecules. These findings support a more general role for MTP in antigen presentation for all CD1 isoforms including the presentation of self and microbial lipid antigens that may be loaded in both the ER and non-ER compartments.
Materials and methods
Reagents and antibodies
The MTPi BMS212122 [22], BMS197567, BMS195183 (compound 20 in [28]), BMS197636 [37], and BMS200150 [30, 37] were kindly provided by Bristol-Myers-Squibb, dissolved in DMSO, and used at a final concentration of 13 μM [22] if not otherwise indicated. DMSO or the structural analogue 9-fluorenyl carboxylic acid (Acros Chemicals) [22] was used as a control. The following antibodies were used: anti-CD1a (10H3.9, mouse IgG2a) [38], anti-CD1b (BCD1b3.2, mouse IgG1) [33], anti-CD1c (F10/21A, mouse IgG1) [34], anti-CD83 (BD Biosciences), FITC-conjugated anti-HLA-A,B,C (BD Biosciences), anti-HLA-DR,DP,DQ and anti-Lamp1 (BD Biosciences), FITC-anti-mouse IgG+IgM (Biosource International), anti-HA (12ca5; Roche Applied Science, Indianapolis, IN), anti-calnexin (rabbit IgG; Cell Signaling), AlexaFluor488-conjugated goat anti-mouse IgG (Molecular Probes), and AlexaFluor568-conjugated goat anti-rabbit IgG (Molecular Probes). Primer sequences were obtained from PrimerBank (http://pga.mgh.harvard.edu/primerbank/) [39].
Cell culture
Cells were maintained in RPMI 1640 supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (R-10). Human monocytes were harvested from the peripheral blood of healthy laboratory volunteers by positive selection on CD14-magnetic beads (Miltenyi Biotec) and cultured in R-10 medium at 106 cells/mL, supplemented with 200 U/mL recombinant human IL-4 and 300 U/mL recombinant human GM-CSF (PeproTech). After 4 days, cells were dislodged by gentle pipetting, analyzed by flow cytometry, and assayed for antigen presentation. As indicated, MTPi were added at the start of monocyte culture and were present until harvesting of DC. The MHC class I-deficient B lymphoblastoid cell line C1R transfected with CD1a, CD1b, CD1c, or mock-transfected were cultured in R-10 (supplemented with 1 mg/mL G-418) with MTPi present as indicated during 72 h.
HeLa cells transfected with CD1c or mock-transfected [40] were maintained in Dulbecco’s modified essential medium supplemented with 10% FBS, penicillin/streptomycin, 2 mM L-glutamine, HEPES buffer, and non-essential amino acids (D-10), supplemented with 1 mg/mL G418. The T cell clones Mt1.50 [24] (CD1a-restricted, autoreactive), Ec2.55 [24] (CD1b-restricted, autoreactive), Ye2.3 [24] (CD1c-restricted, autoreactive), DN1 [31, 33, 34] (CD1b-restricted, mycolic acid-specific), CD8-1 [35, 36] (CD1c-restricted, mycopeptide-specific), and SPF3 [27] (MHC class II-restricted, tetanus toxoid-specific) were expanded and maintained as previously described [24].
Antigen presentation assay
Monocyte-derived DC or C1R transfectants as APC were cultured in the presence of indicated MTPi or control compounds as described above. Co-culture of APC and T cell clones was performed at the indicated cell density in 96-well round bottom plates with MTPi present, unless APC were fixed in glutaraldehyde [16] as indicated. As required, APC were primed with antigen for 16 h, and washed, before start of the co-cultures. Supernatants were harvested after 18–20 h, and assayed using IFN-γ-specific antibody pairs as recommended by the manufacturers (Pierce, Rockford, IL, and BD Pharmingen).
Flow cytometry
Cells were stained on ice for 30 min with 50 μg/mL of primary antibodies as indicated and 50 μg/mL FITC-anti-mouse IgG+IgM as secondary antibody. Immediately after staining, cells were collected on a FACSort (Becton Dickinson) flow cytometer, and CellQuest and FloJo software used for data analysis.
Silencing
HeLa CD1c transfectants were passaged 24 h before electroporation of siRNA. Cells (1×106) were resuspended in 100 μL of proprietary “solution R” (Amaxa, Gaithersburg, MD) and Mtp-specific siRNA oligonucleotides (GGAAAAAGCCCAUCUAAAAtt and UUUUAGAUGGGCUUUUUCCtt; Ambion) or non-specific mock siRNA added to a final concentration of 1 μM, and electroporated with program I-013 according to recommendations by the manufacturer (Amaxa). Cells were then cultured for 60 h in 1.5 mL D-10 in 6-well plates, then trypsinized, seeded at 1×104 cells per well in 96-well plates, allowed to adhere for 2 h, and then 5×104 Ye2.3 cells added for 6 h of co-culture. Culture supernatants were collected and assayed for IFN-γ by sandwich ELISA. A portion of the 60-h silenced cells were used for RNA extraction, and assessment of Mtp transcript knockdown measured by quantitative PCR using SYBR green PCR reagents (BioRad) on an iCycler (BioRad).
Plasmid construction and confocal microscopy
A full-length cDNA of human MTP in pSV7d was kindly provided by Dr. Nicolas Davidson (Washington University, St. Louis, MO). The full-length insert containing the endogenous signal peptide sequence was PCR-amplified with an HA-tag sequence (5′-TACCCATACGACGTCCCAGACTACGCT-3′) engineered in-frame at the 3′-OH end and cloned in pcDNA3.1(+) vector (Invitrogen), and confirmed by sequencing and western blotting of protein expressed in COS-7 cells. Primer sequences are available upon request. Stable CD1b-HeLa cell transfectants were transiently transfected with the MTP-HA construct using electroporation conditions as recommended by the manufacturer (Amaxa). Cells were fixed and analyzed 16 h later, using anti-CD1b, anti-Lamp1, and 12ca5 anti-HA-tag antibodies. For confocal analysis of DMSO-and BMS212122-treated monocyte-derived DC, cells were fixed and analyzed using anti-calnexin, anti-CD1a, anti-CD1b, and anti-CD1c. Nuclei were stained with TO-PRO3 (Molecular Probes).
Acknowledgments
We thank Drs. Thomas W. Harrity and Richard E. Gregg (Bristol-Myers Squibb) for discussions and reagents. This work was supported by NIH grants DK51362, DK44319, DK53056, the Harvard Digestive Diseases Center (to R.S.B.), and R01CA47724 and R37AI28973 (to M.B.B). D.H. and A.K. were supported by the Damon Runyon Cancer Research Foundation (DRG-1814-04) and the Crohn’s and Colitis Foundation of America, respectively.
Abbreviations
- ApoB
apolipoprotein B
- Lamp1
lysosomal-associated membrane protein 1
- MTP
microsomal triglyceride transfer protein
- MTPi
microsomal triglyceride transfer protein inhibitor
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 2.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 3.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 4.Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. CD1 assembly and the formation of CD1-antigen complexes. Curr Opin Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O’Connor PB, Zajonc DM, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 6.Kang SJ, Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. 2002;277:44838–44844. doi: 10.1074/jbc.M207831200. [DOI] [PubMed] [Google Scholar]
- 7.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, Colgan SP, Blumberg RS. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–262. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem. 1999;274:9289–9295. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 9.Sugita M, Porcelli SA, Brenner MB. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J Immunol. 1997;159:2358–2365. [PubMed] [Google Scholar]
- 10.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 11.Park JJ, Kang SJ, De Silva AD, Stanic AK, Casorati G, Hachey DL, Cresswell P, Joyce S. Lipid-protein interactions: Biosynthetic assembly of CD1 with lipids in the endoplasmic reticulum is evolutionarily conserved. Proc Natl Acad Sci USA. 2004;101:1022–1026. doi: 10.1073/pnas.0307847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S. Lipid protein interactions: The assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol. 2002;168:723–733. doi: 10.4049/jimmunol.168.2.723. [DOI] [PubMed] [Google Scholar]
- 13.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 14.Jackman RM, Stenger S, Lee A, Moody DB, Rogers RA, Niazi KR, Sugita M, et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity. 1998;8:341–351. doi: 10.1016/s1074-7613(00)80539-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D, Cantu C, III, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SJ, Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. 2004;5:175–181. doi: 10.1038/ni1034. [DOI] [PubMed] [Google Scholar]
- 17.Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, Porcelli SA, et al. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. 2004;5:169–174. doi: 10.1038/ni1035. [DOI] [PubMed] [Google Scholar]
- 18.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 19.Gordon DA, Wetterau JR, Gregg RE. Microsomal triglyceride transfer protein: A protein complex required for the assembly of lipoprotein particles. Trends Cell Biol. 1995;5:317–321. doi: 10.1016/s0962-8924(00)89054-6. [DOI] [PubMed] [Google Scholar]
- 20.Hussain MM, Fatma S, Pan X, Iqbal J. Intestinal lipoprotein assembly. Curr Opin Lipidol. 2005;16:281–285. doi: 10.1097/01.mol.0000169347.53568.5a. [DOI] [PubMed] [Google Scholar]
- 21.Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 22.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 25.Vincent MS, Xiong X, Grant EP, Peng W, Brenner MB. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol. 2005;175:6344–6351. doi: 10.4049/jimmunol.175.10.6344. [DOI] [PubMed] [Google Scholar]
- 26.Robl JA, Sulsky R, Sun CQ, Simpkins LM, Wang T, Dickson JK, Jr, Chen Y, et al. A novel series of highly potent benzimidazole-based microsomal triglyceride transfer protein inhibitors. J Med Chem. 2001;44:851–856. doi: 10.1021/jm000494a. [DOI] [PubMed] [Google Scholar]
- 27.Roncarolo MG, Yssel H, Touraine JL, Bacchetta R, Gebuhrer L, de Vries JE, Spits H. Antigen recognition by MHC-incompatible cells of a human mismatched chimera. J Exp Med. 1988;168:2139–2152. doi: 10.1084/jem.168.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnin DR, Biller SA, Wetterau J, Robl JA, Dickson JK, Jr, Taunk P, Harrity TW, et al. Microsomal triglyceride transfer protein inhibitors: Discovery and synthesis of alkyl phosphonates as potent MTP inhibitors and cholesterol lowering agents. Bioorg Med Chem Lett. 2003;13:1337–1340. doi: 10.1016/s0960-894x(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 29.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779–1785. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK, Jr, Chen Y, Ricci B, et al. An inhibitor of the microsomal triglyceride transfer protein inhibits ApoB secretion from HepG2 cells. Proc Natl Acad Sci USA. 1996;93:11991–11995. doi: 10.1073/pnas.93.21.11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 32.Sagiv Y, Bai L, Wei DG, Agami R, Savage PB, Teyton L, Bendelac A. A distal effect of microsomal triglyceride transfer protein deficiency on the lysosomal recycling of CD1d. J Exp Med. 2007;204:921–928. doi: 10.1084/jem.20061568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behar SM, Porcelli SA, Beckman EM, Brenner MB. A pathway of costimulation that prevents anergy in CD28− T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, Porcelli SA, Brenner MB. Molecular recognition of lipid antigens by T cell receptors. J Exp Med. 1999;189:195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, et al. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 36.Matsunaga I, Bhatt A, Young DC, Cheng TY, Eyles SJ, Besra GS, Briken V, et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J Exp Med. 2004;200:1559–1569. doi: 10.1084/jem.20041429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetterau JR, Gregg RE, Harrity TW, Arbeeny C, Cap M, Connolly F, Chu CH, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 1998;282:751–754. doi: 10.1126/science.282.5389.751. [DOI] [PubMed] [Google Scholar]
- 38.Olive D, Dubreuil P, Mawas C. Two distinct TL-like molecular subsets defined by monoclonal antibodies on the surface of human thymocytes with different expression on leukemia lines. Immunogenetics. 1984;20:253–264. doi: 10.1007/BF00364207. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugita M, Jackman RM, van Donselaar E, Behar SM, Rogers RA, Peters PJ, Brenner MB, Porcelli SA. Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science. 1996;273:349–352. doi: 10.1126/science.273.5273.349. [DOI] [PubMed] [Google Scholar]
- 41.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 42.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]