Abstract
The nonsteroidal antiinflammatory drug sulindac displays chemopreventive activity in patients with familial adenomatous polyposis (FAP). Sulindac metabolites induce apoptosis in colon tumor cells, in part, by a polyamine-dependent mechanism that can be suppressed with exogenous putrescine. To determine the relevance of this mechanism in animals, we treated ApcMin/+ mice, a model of human FAP, with sulindac alone or in combination with dietary putrescine. Sulindac increased steady-state RNA levels and enzymatic activity of the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase and intestinal levels of monoacetylspermidine, spermidine, and spermine in the small intestine of mice. Sulindac also decreased the activity of the biosynthetic enzyme ornithine decarboxylase but not adenosylmethionine decarboxylase (AMD). Dietary putrescine increased intestinal putrescine contents, whereas the combination of dietary putrescine and sulindac yielded the highest levels of intestinal putrescine and correlated with a statistically significant reduction in AMD enzyme activity. Dietary putrescine did not statistically significantly increase tumorigenesis, although it significantly increased the grade of adenoma dysplasia (P < 0.05). The effectiveness of sulindac to suppress intestinal carcinogenesis was partially abrogated by dietary putrescine. These data suggest that sulindac exerts at least some of its anticarcinogenic effects in mice via a polyamine-dependent mechanism. Because high concentrations of putrescine can be found in certain dietary components, it may be advantageous to restrict dietary putrescine consumption in patients undergoing treatment with sulindac.
Introduction
Familial adenomatous polyposis (FAP) is an inherited cancer syndrome, where afflicted individuals develop colon cancer with a high frequency (1). FAP is caused by a mutation or deletion of the adenomatous polyposis coli (APC) tumor suppressor gene (2). Although FAP syndrome accounts for less than 1% of colorectal cancers in the United States, somatic mutations of the APC tumor suppressor gene are found inmore than 90% of sporadic colorectal cancers (3). In both inherited and sporadic colonic tumors, the loss of function of the APC gene product appears to be an early step in tumorigenesis.
The nonsteroidal antiinflammatory drug (NSAID) sulindac has been shown in randomized clinical trials to induce polyp shrinkage in FAP patients (4–6). A number of studies suggest that the antitumor properties of NSAIDs result from their ability to induce apoptosis and to inhibit prostaglandin synthesis (7–10). In recent years, it has become evident that the chemopreventive action of NSAIDs also includes cyclooxygenase (COX)-independent mechanisms. Studies using colorectal cell lines show that piroxicam, aspirin, and indomethacin influence polyamine metabolism (11–14).
Polyamines (putrescine, spermidine, and spermine), which are important for cell proliferation and differentiation, are downstream mediators of genetic risk factors in human intestinal cancers (15). Cellular polyamine pools are derived from intercellular biosynthesis and uptake of amines from other host cell microorganisms in the intestinal tract and/or dietary contributions (16,17).
Recently, we have reported that sulindac decreases intracellular polyamine levels and induces apoptosis in colon cancer cell lines via the transcriptional activation of the key polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase (SSAT) (18). Sulindac is a prodrug metabolized by the liver and intestinal flora to a sulfide, which is the active antiinflammatory metabolite, primarily responsible for blockage of prostaglandin synthesis, and to a sulfone, which has no COX-inhibitory activity (19,20). The apoptosis induced by sulindac sulfone can be rescued by exogenous polyamines in colon cancer cell lines (18,21).
ApcMin/+ or Min (multiple intestinal neoplasia) mice are phenotypically similar to humans with FAP, in that they develop macroscopically visible pan-intestinal and desmoid intestinal tumors and, infrequently, colonic tumors (22,23). Reduction of COX enzyme activity by NSAIDs suppresses intestinal carcinogenesis in ApcMin/+ mice (24–26) as well as other models of intestinal cancer (7,27–30). Expression of polyamine biosynthetic enzyme, ornithine decarboxylase (ODC), is elevated in the intestinal tissues of ApcMin/+ mice and in apparently normal colonicmucosal biopsies from FAP patients (31,32). Inhibition of ODC activity, using the enzyme- activated irreversible inhibitor α-difluoromethylornithine, suppresses intestinal carcinogenesis in this model (32). Exogenous polyamines from the diet and intestinal bacteria, through intestinal transport, can reverse the growth-inhibiting effect of the ODC inhibitor on transplanted tumors and promote the growth of azoxymethane-induced aberrant crypt foci in rat colon (16,17,33–35).
Our goal in this study was to evaluate the polyamine-dependentmechanisms of antitumor activity of sulindac in vivo using the ApcMin/+ mouse model of FAP.
Materials and Methods
Rodent Model
C57BL/6J-ApcMin/+ mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in the University of Arizona’s Animal Care Facility in accordance with The University of Arizona Institutional Animal Care and Use Committee guidelines. These mice have a dominant mutation at codon 850 of themurine Apc gene that converts a leucine into a stop codon and causes the truncation of anAPC protein. Affected ApcMin/+ animals were obtained by crossing ApcMin/+ males with B6 females. The presence of the mutant APC allele was detected in the DNA extracted from tail tissue using an allele-specific polymerase chain reaction (PCR) assay (23). Animals were housed in groups of one to four in microisolator cages under fluorescent lighting on a 12-h cycle. Mice were fed the defined synthetic diet AIN93G (Harlan Teklad, Indianapolis, IN). Irradiated distilled water was available ad libitum for the duration of the experiment.
Study Design
Experimental protocols were approved by the Institutional Animal Care and Use Committee. ApcMin/+ mice were treated with the nonselective COX-2 inhibitor sulindac at a dose of 167 ppm administered in the custom-prepared AING93G diet (Harlan Teklad). The dietary preparations of sulindac have been successfully used in murine cancer models in many prior chemoprevention studies and are based on the recommended human doses (36–38). Putrescine (Sigma-Aldrich, St. Louis, MO) was administered continuously as a 1% solution in the drinking water. The treatments started at 5 wk of age. For the SSAT expression study, mice were treated for 1, 4, or 10 wk. For biochemical studies, animals were treated for 1 wk. For the tumorigenesis study, animals received treatments until 110–115 days old. Animals were housed in groups of one to four in microisolator cages under fluorescent lighting on a 12-h cycle. Throughout the experiment, animals had access to drinking water and food ad libitum. Food and water were replaced weekly or as needed.
Tissue Collection
Mice were consistently sacrificed (at approximately 10:00 am) byCO2 inhalation after being without food for 1 h. The intestinal tract was removed, opened, and flushed with ice-cold saline. The proximal and distal parts of the small intestine were used for measuring polyamine contents. For enzyme activity assays, the distal portion of the small intestine was used. The middle part of the distal portion of the small intestine was used for RNA isolation. Only the apparently normal intestinal tissue was used for these analyses.
RNA Isolation and Analysis
Intestinal tissue samples were homogenized in TRIzol® reagent (Gibco, BLR) using a Polytron homogenizer and were processed for the isolation of total tissue RNA (39). Twentymicrograms of total RNA from each sample was separated on a 1%agarose/formaldehyde gel and transferred to a nylon membrane (Hybone-N, Amersham, Arlington Heights, IL). Membranes were subsequently hybridized with 32P-labeled cDNA encoding for the human SSAT gene (0.67-kb EcoRI-EcoRI fragment) by random priming using a kit from Boehringer Mannheim (Indianapolis, IN). 32P-labeled cDNA for the glyceraldehyde-3′-phosphate dehydrogenase (GAPDH) gene (0.75-kb PstI-XbaI fragment) was used to normalize for the variation in loading. Northern blot autoradiographs were quantitated by densitometric analysis (Imagequant Densitometer, Molecular Dynamics, Sunnyvale, CA). Amounts of SSAT RNA were expressed as relative arbitrary units of integrated density bands normalized to GAPDH bands.
Real-Time PCR Analysis of SSAT RNA
Total RNA was isolated from control and sulindac-treated animals using the Qiagen RNeasyKit according to themanufacturer’s protocol. Intestinal tissue samples were homogenized in RLT buffer using a Polytron homogenizer. Reverse transcription was completed using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA). One microgram of total RNA was transcribed into cDNA in a 100-μl reaction using random hexamers under thermal conditions recommended by the protocol. Real-time PCR amplification was performed with the ABI PRISM 7700 SDS instrument (Applied Biosystems) under the universal thermal cycling conditions recommended by the Assay- on-Demand products protocol. Each 50-μl reaction in-cluded 25 μl TaqMan Universal PCRmastermix, 10 μl of the resulting cDNA from the reverse transcription step, and 15 μl diluted SSAT primer and probe mixes ordered from Assay- on-Demand products (Applied Biosystems). No-template controls were included in each plate to monitor the potential PCR contamination. SSAT gene expression was analyzed in animals treated with sulindac for 1 or 4 wk. Each reaction was run in duplicate. To determine the relative expression level of the SSAT gene, the comparative CT method was used. The CT values of the SSAT gene in control and treated animals were normalized by the endogenous reference (ΔCT = CT(target) − CT(18S)). Normalized CT values from treated animals were compared with ones from control animals (ΔΔCT = ΔCT(treatment) − ΔCT(control)). The relative expression of the SSAT gene was calculated via the equation 2− ΔΔCT. Data were presented as a ratio of the relative expression of SSAT in sulindac-treated animals to the relative expression SSAT in control animals.
SSAT Enzyme Activity
Measurement of SSAT enzyme activity was performed according to the protocol previously described (40). Briefly, distal portions of the small intestine (100–120 mg of wet weight) were opened longitudinally, and their luminal contents were removed by washing the tissues in phosphate-buffered saline (140 mM NACl, 2 mM KCl, 8.1 mM Na2HPO4, and 0.9 mM K2HPO4). Tissues were homogenized in lysis buffer [50 mMTris-HCl, 2.5 mMDTT, and 0.1 mMEDTA (pH 7.5)] and frozen at −80°C until the SSAT enzyme analysis was performed. After thawing, samples were spun for 10 min at 13,200 rpm at 4°C, and supernatants were used for SSAT activity assay. The radiochemical assay of SSAT activity was performed by estimation of labeled acetylspermidine synthesized from [14C]-acetylcoenzyme A and unlabeled spermidine. One unit of enzyme activity is defined as the amount that catalyzes the formation of 1 pmol acetylspermidine per minute per milligram of protein at 30°C.
ODC Enzyme Activity
Tissue samples were collected and homogenized as described in SSAT Enzyme Activity. ODC activity was determined bymeasuring the liberation of 14CO2 from l-[14C]ornithine (Du Pont-New England Nuclear), as described by Sertich et al. (41). The volume of 150 μl of ODC buffer (0.5 Msodium phosphate buffer containing 0.1mMEDTA, 1mM dithiothreitol, 0.05 mg/ml pyridoxal-5-phosphate, and 0.1 mM phenylmethylsulfonyl fluoride) was added to 100 μl of tissue lysate. The samples were mixed on ice with 0.5 μCi labeled ornithine. The tubes were capped and incubated at 37°C for 1 h. The reaction was terminated by adding 500 μl of 1 M citric acid on ice. Tubes were left overnight, and the released 14CO2 was captured on Whatman no. 3 (2.5-cm filters) treated with 75 μl of NCS-II Tissue Solubilizer (Amersham Biosciences) suspended from the cup. The filters were then counted in 10 ml of UniverSol (ICN, Costa Mesa, CA) on a liquid scintillation counter, and ODC activity was expressed as picomoles 14CO2 per milligram of protein per minute.
AMD Enzyme Activity
Tissue samples were collected and homogenized as described in SSAT Enzyme Activity. Adenosylmethionine decarboxylase (AMD) activity was determined by measuring the liberation of 14CO2 from S-adenosyl-l-[carboxy-14C] methionine (Amersham Biosciences), as described by Shantz and Pegg (42). The volume of 150 μl of AMDbuffer (0.5Mphosphate buffer containing 25 mMdithiothreitol, 15 mM putrescine, 4 mM S-adenosylmethionine, and 0.2 μCi S-adenosyl-l-[carboxy-14C] methionine) was added to 100 μl of tissue lysates. Tubes were incubated at 37°C for 2 h. The reactionwas terminated by adding 300 μl of 5Msulfuric acid on ice. Tubes were incubated for an additional 30 min at 37°C, and the released 14CO2 was captured onWhatman no. 3 (2.5-cm filters) treated with 75 μl of NCS-II Tissue Solubilizer (Amersham Biosciences) suspended from the cup. The filters were then counted in 10 ml of UniverSol (ICN) on a liquid scintillation counter, andAMDactivitywas expressed as picomoles 14CO2 per milligram of protein per minute.
Polyamine Analysis
After collection, tissue samples were stored frozen at −80°C. Samples were processed and assayed for polyamine (putrescine, spermidine, and spermine) content by reverse- phase high-performance liquid chromatography (HPLC) with 1,7-diaminoheptane as an internal standard (39). Protein content in each sample was determined by the BCA protein assay kit (Pierce, Rockford, IL). Data are expressed as nanomoles of polyamine permilligram of protein.
Tumor Scoring
Mice were sacrificed at the age of 110–115 days by CO2 inhalation, and whole small intestine and colon segments were removed, flushed with buffered saline, opened longitudinally, and spread out, mucosal surface up. Tissues were fixed in 10% neutral buffered formalin for 24 h and then placed in 70% ethanol. Tumor number per mouse was assessed using an LSGA epiiluminator dissecting microscope (Olympus Optical, Tokyo) at ×20 magnification. Tumors larger than 0.5 mm were counted, and their maximal diameter was measured. All assessments were done by the same observer, who was blinded to the mice genotypes and treatments. Afterward, a Swiss gut roll of small intestine and all grossly evident colon tumors were fixed in 10% buffered formalin, processed, and paraffin embedded, and 5-μm sections were stained with hematoxylin and eosin and evaluated by a veterinary pathologist. The grade of dysplasia for each adenoma examined histologically was determined on the ba-sis of criteria outlined in a recent review on the pathology of mouse models of intestinal cancer (43).
Statistics
All of the statistical analyses were carried out with Statistical Analysis System software (version 9.1 for Windows, SAS Institute, Cary, NC), and P values of <0.05 were considered significant. A nonparametric test (Wilcoxon rank test) was performed for the analysis of SSAT gene expression measured using Northern blotting due to the small sample sizes. For multiple comparison, the Tukey Student range test was used. For the difference in the number of tumors between control and treated mice, Poisson regression analysis was used. For the tumor-grade analysis, Dunnett’s test and Bonferroni multiple comparison test were used. The analysis of variance (ANOVA) test was used for the statistical analysis of enzyme activities.
Results
Sulindac Induces SSAT Expression in Intestinal Tissues of Min Mice
Mice carrying the wild-type (Apc+/+) or mutant Apc (ApcMin/+) allele were given sulindac mixed with the AIN93G diet at a concentration of 167 ppm starting at 5 wk of age. Mice weremaintained on the sulindac-containing diet for 10 wk and sacrificed at the age of 110–115 days. Total RNA was isolated from distal portions of the small intestine, which were free of visible tumors, and analyzed for SSAT gene expression by Northern blot analysis. Figure 1A shows that steady-state SSAT RNA levels were elevated in the small intestine of both the Apc wild-type (P = 0.02) and ApcMin/+ animals (P = 0.004) after administration of sulindac.
Figure 1.
(A) Effect of sulindac on steady-state levels of spermidine/spermine N1-acetyltransferase (SSAT) RNA in the small intestine of wild-type Apc (Apc+/+) and ApcMin/+ mice. Wild-type Apc (n = 9 per group) and ApcMin/+ mice (n = 6 per group) 110 days old were fed AIN93G diet (control) or AIN93G diet supplemented with 167 ppm of sulindac. Intestinal tissues were collected and processed for total RNA isolation and Northern blotting as described in Materials andMethods. SSAT expression in each animal was normalized using glyceraldehyde-3′-phosphate dehydrogenase (GAPDH) and presented as SSAT/GAPDH ratios of relative arbitrary units. P values were calculated between control and treatment groups using theWilcoxon rank nonparametric test (*P = 0.02 for Apc wild-type mice; **P < 0.004 for ApcMin/+ mice). (B) Real-time polymerase chain reaction (PCR) analysis of SSAT gene expression in ApcMin/+ control animals and animals treated with 167 ppm of sulindac for 1 (n = 4 per group) or 4 (n = 6 per treatment group) wk. Intestinal and colonic tissues were harvested, and total RNA was extracted according to a protocol supplied with the Qiagen RNeasy Kit. RT and real-time PCR were performed as described in Materials and Methods. Individual bars represent the relative expression of the SSAT gene in the intestinal tissue of sulindac-treated animals to the SSAT expression in untreated animals. P values were calculated with the analysis of variance test. *P ≤ 0.001.
Subsequently, ApcMin/+ mice were treated with the same dose of sulindac for shorter terms, 1 or 4 wk, and the SSAT expression was assessed in the small intestine by real-time PCR analysis. SSAT transcript levels were induced by sulindac in the small intestine of ApcMin/+ mice in a statistically significant manner as early as after 1 wk of treatment (Fig. 1B, P ≤ 0.001).
SSAT Enzyme Activity Is Induced in the Intestinal Tissue of ApcMin/+ Mice
We measured the SSAT enzyme activity in ApcMin/+ mice treated with 167 ppm of sulindac for 1 wk. Animals (n = 6) were put on study when 5 wk old. The SSAT enzyme activity was measured in the distal portion of the small intestine. The statistically significant increase in the SSAT enzyme activity (P < 0.003) was observed in the small intestine of treated ApcMin/+ animals compared with untreated littermates (Fig. 2). We also evaluated the effect of sulindac on enzymatic activity of two polyamine biosynthetic enzymes, ODC and AMD. TheODC enzyme activitywas decreased (P < 0.0001) in ApcMin/+mice treated with sulindac for 1 wk; the AMDenzyme activity was not changed in a statistically significant manner.
Figure 2.
Sulindac and putrescine combination suppresses polyamine biosynthesis enzyme activities. Ornithine decarboxylase (ODC), spermidine/spermine N1-acetyltransferase (SSAT), and adenosylmethionine decarboxylase (AMD) enzyme activities were measured in 6-wk-old control mice (n = 4 per group) and mice receiving 167 ppm sulindac, 1% putrescine, or both for 1 wk (n = 6 per group). The intestinal tissues were harvested and processed for enzyme activities as described in Materials and Methods. P values between control and treatment groups were calculated with the analysis of variance test (*P < 0.05; **P < 0.003; ***P < 0.0001).
Sulindac Induces Spermidine Acetylation and Putrescine Supplementation Reverses It
We determined the sulindac effect on the intestinal polyamine pool in ApcMin/+ mice with and without putrescine supplementation. Mice were given the AIN93G diet, which is deficient in polyamines, or the same diet supplemented with a 1% putrescine and/or 167 ppm sulindac for 1 wk. Adding either sulindac or 1% putrescine or both to mice did not affect their weight. All the mice weighed 19–20 g, that is, they absorbed similar amounts of food and water in the different treatments. Spermidine, spermine, and N1-acetylspermidine contents were increased with sulindac treatment (Table 1; P < 0.001 for spermidine and spermine; P = 0.002 for N1-acetylspermidine). ApcMin/+ mice treated with 1% putrescine showed a significant increase in the intestinal putrescine and spermidine levels with no changes in N1-acetylspermidine compared with untreated animals. Putrescine and spermidine levels were further increased in the small intestine of mice treated with both sulindac and putrescine compared with untreated mice (Table 1; P < 0.05 for putrescine; P < 0.001 for spermi-dine). When compared with sulindac-only–treated mice, mice that consumed the diet supplemented with both sulindac and putrescine had significantly increased putrescine levels and decreased spermine levels (Table 1; P < 0.001 for both). The level of N1-acetylspermidine was significantly lower in animals maintained on the combined sulindac and putrescine diet compared with the sulindac-only diet (Table 1; P = 0.008).
Table 1.
Polyamine Contents (mean ± SD) in the Small Intestine of ApcMin/+ Mice Treated With Sulindac and/or Putrescine for 1 Weeka
| Group | Polyamine Content (nmol/mg protein)
|
|||
|---|---|---|---|---|
| Putrescine | Spermidine | Spermine | N1 -Acetylspermidine | |
| Control | 0.27 ± 0.29 | 6.14 ± 1.31 | 4.76 ± 0.73 | 0.12 ± 0.1 |
| Sulindac | 0.96 ± 0.17 | 8.42 ± 1.15* | 6.32 ± 0.86* | 0.32 ± 0.21** |
| Putrescine | 1.61 ± 1.06*** | 8.47 ± 0.90* | 4.09 ± 0.31*** | 0.15 ± 0.12 |
| Sulindac + putrescine | 3.83 ± 1.84*** | 8.20 ± 1.01* | 4.80 ± 0.90 | 0.13 ± 0.14 |
P < 0.001 for putrescine and spermine; P = 0.008 for N1-acetylspermidine between sulindac and sulindac + putrescine groups.
P < 0.001 compared with control;
P = 0.002 compared with control;
P < 0.05 compared with control.
Sulindac and Putrescine Combination Suppresses Polyamine Biosynthesis Enzyme Activities in ApcMin/+ Mice
We assessed the activities of ODC, AMD, and SSAT in ApcMin/+mice treated for 1 wk with putrescine alone or with the combination of sulindac and putrescine starting at 5 wk of age (Fig. 2). Mice supplemented with putrescine had lower ODC enzyme activity compared with untreated mice (P < 0.0001). Both ODC and AMD enzyme activities were significantly suppressed in the small intestine of mice on the combined sulindac and putrescine diet compared with untreated littermates (P < 0.0001). SSAT enzyme activity was elevated in the small intestine of mice treated with the combination of sulindac and putrescine in a similar manner as in the sulindac-treated animals (P < 0.05 compared with untreated mice).
Effect of Sulindac on Tumorigenesis in Min Mice Is Polyamine Dependent
We assessed the effect of sulindac on intestinal tumorigenesis in ApcMin/+ animals fed the polyamine-deficient AIN93G diet. Sulindac significantly suppressed small intestinal tumorigenesis in these mice, as shown in Fig. 3. Untreated mice developed 32.5 ± 4.74 (mean ± SE) intestinal tumors per mouse, whereas the sulindac-containing diet reduced this number to 2.5 ± 0.9 tumors per mouse (P < 0.0001). In contrast to the small intestine, sulindac had no effect on colonic tumorigenesis in this model (data not shown). No sulindac-related toxicity was observed in treated mice as measured by body weight changes.
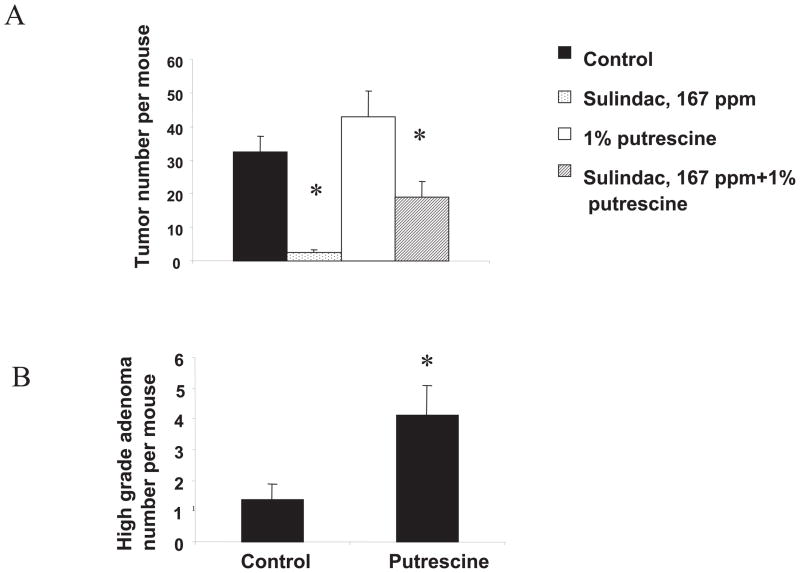
Figure 3.
Suppression of intestinal tumorigenesis by sulindac can be reversed by exogenous putrescine. (A) Tumor counts in small intestine of ApcMin/+ control mice (n = 11 mice per group) or mice treated with either sulindac (167 ppm in AIN93G diet, n = 10 mice per group), putrescine (1% in the drinking water, n = 8 mice per group), or a combination of sulindac and putrescine (n = 10 mice per group). Tumor number was determined as described in Materials and Methods. Poisson regression analysis was used to calculate P values between control and sulindac- or sulindac plus putrescine–treated groups as well as between sulindac- and sulindac plus putrescine–treated groups (*P < 0.0001 for all comparisons). (B) The effect of putrescine on high-grade adenoma number in the small intestine of ApcMin/+ mice. Mice were fed AIN93G diet with or without supplementation of 1% putrescine in the drinking water. Tumor grade was evaluated as described in Materials and Methods. The Bonferroni multiple comparison test was used to calculate the P value between control and putrescine-treated groups. *P < 0.05.
To determine the effect of dietary polyamine modifications on the antitumor effect of sulindac, we established two groups of ApcMin/+ mice that were put on the AIN93G diet either with a 1% putrescine solution in the drinking water or else on the AIN93G diet supplemented with sulindac. Feeding with these diets commenced at 5 wk of age and was terminated at 110–115 days. As summarized in Fig. 3, putrescine-fed ApcMin/+ mice exhibited an increase in tumor number per animal compared with control mice, but this increase was not statistically significant. We also analyzed the effect of sulindac and/or putrescine supplementation on adenoma grade in the small intestine of ApcMin/+ animals. Sulindac treatment decreased the number of low-grade adenomas in a statistically significant manner (P < 0.05). Putrescine-fed animals had a threefold increase in the number of high-grade adenomas compared with control animals (P < 0.05) (Fig. 3B). Mice, which were maintained on both sulindac and putrescine diet, had a decreased number of both low- and high-grade adenomas, but this decrease was not statistically significant. The histopathological analysis of intestinal and colon tumors in Min mice treated with putrescine revealed the presence of neutrophilic infiltrates in lamina propria of three of the total four colon tumors developed in this treatment group (n = 6 mice per group). The average intestinal tumor number per mouse in the group treated with the combination of sulindac and putrescine (19 ± 4.79 tumors per animal) was substantially higher compared with the sulindac-only–treated group (2.5 ± 0.9; P < 0.0001; or 7.6-fold increase). No sign of inflammation was observed in the colon of combined sulindac and putrescine–treated mice by histopathological analysis.
Discussion
Epidemiological studies have demonstrated that NSAIDs’ use in the general population is associated with a reduced risk of colon cancer death (4,44). The understanding of themechanisms through which NSAIDs exert their protective action is important for further progress in the area of prevention of colon cancer. Established mechanisms by which NSAIDs alter intestinal and colonic tumorigenesis include the inhibition of COXs and the synthesis of prostaglandins (24,27,45). However, NSAIDs also work by COX-independent mechanisms to induce apoptosis (9,18), and one of these latter mechanisms involves the activation of the polyamine pathway (15,46).
Endogenous polyamine synthesis is controlled by activities of three key enzymes in polyamine metabolism. These include the biosynthetic enzymes ODC and AMD, which catalyze the production of putrescine, and decarboxylated S-adenosylmethionine (dcSAM), respectively. dcSAM serves as the donor of aminopropyl groups in spermidine and spermine synthesis. The catabolic enzyme SSAT acetylates spermidine and spermine and targets these molecules for export in some cases (47).
We reported recently that the sulfone metabolite of sulindac induces the transcription of SSAT via activation of peroxisome proliferator-activated receptor gamma (18). In Caco-2 colon cancer cells, induction of the SSAT gene transcription resulted in a decrease in intracellular polyamine levels. Sulindac sulfone also induced apoptosis in this cell line, partially via a polyamine-dependent mechanism because exogenous putrescine could partially rescue cells from apoptosis.
In this study, we evaluated the effects of the nonselective COX-2 inhibitor sulindac on polyamine metabolism in vivo using the ApcMin/+mouse model of FAP. Northern blot analysis showed induction of steady-state levels of SSAT RNA in the intestinal tissue of ApcMin/+mice treated with sulindac for 10 wk. The sulindac effect on SSAT was independent of the mutation in the APC gene. The 1-wk treatment with sulindac was sufficient to induce SSAT RNA levels, SSAT enzyme activity, and spermidine acetylation in the small intestine of ApcMin/+ mice. In sulindac-treated animals, spermidine and spermine levels were increased in a statistically significant manner. In our cell culture models, we provided evidence that these steps lead to polyamine export and decreased levels of the longer-chain amines spermidine and spermine. Because we found that sulindac suppresses the activity of polyamine biosynthetic enzymes, the observed increase in spermidine and spermine is likely not due to drug-induced biosynthesis. An alternative explanation is that intestinal spermidine and spermine pools increase due to increased uptake, possibly associated with sulindac-induced polyamine export into other tissues.
Our animals were fed the restricted AIN93G diet, which contains a very low amount of polyamines, as assessed by HPLC analysis (1.5 pmol/mg diet of putrescine, 1.2 pmol/mg diet of spermidine, and 0.85 pmol/mg diet of spermine). The ApcMin/+ mice express a functional intestinal diamine oxidase, which is capable of metabolizing intestinal luminal putrescine (data not shown). Addition of 1%putrescine to the drinking water of mice increased intestinal putrescine and spermidine contents (P < 0.05 for putrescine and P < 0.001 for spermidine compared with control mice). Dietary putrescine did not statistically significantly increase tumorigenesis, although it significantly increased the number of high-grade adenomas (P < 0.05). However, the consumption of 1% putrescine by the sulindac-treated mice resulted in the significant increase in the intestinal tumor number (P < 0.0001 compared with sulindac-only–treated mice) and suppression of N1-acetylspermidine levels. Sulindac suppressed polyamine biosynthetic ODC enzyme activity and induced the catabolic SSAT activity while reducing intestinal tumor formation. When ApcMin/+ mice were provided dietary putrescine in combination with sulindac, the antitumor effects of sulindac were at least partially reversed. This combination caused disregulation of polyamine metabolic flux and resulted in more than 10-fold elevation in the intestinal putrescine concentrations in treated ApcMin/+ mice compared with controls. Suppression of AMD by the combined action of putrescine and sulindac likely contributed to the high tissue levels of putrescine via reduction of dcSAM pools.
Gastrointestinal polyamines can be synthesized by intestinal epithelial cells and bacteria or can come from the diet. Analysis of luminal polyamines in the intestine of germ-free and conventional rats showed that the polyamine content in the gut of conventional rats is highly influenced by the intestinal bacteria (48). Noack et al. (48) estimated that the total amount of polyamines that might be synthesized by the intestinal flora in human colon would contain 155 μmol spermidine and 670 μmol putrescine. Polyamines synthesized by intestinal bacteria may contribute significantly to the intestinal polyamine pool if the dietary source of polyamines is low and polyamine synthesis is pharmacologically inhibited (16,48). Dietary polyamines can easily be absorbed by the gut and could play an important role in maintaining the optimal levels of polyamines in various organs (49,50). The importance of dietary polyamines in tumorigenesis was originally suggested by animal studies when the antitumor effect of polyamine biosynthetic inhibitors was enhanced with the use of a polyamine-deficient diet (51).
Our data support the conclusion that the polyamine pathway influences the antitumor activity of the nonselective COX-2 inhibitor sulindac. Our results also emphasize the potential impact of a diet on the efficacy of anticancer treatment regimes. Estimation of the dietary contribution of polyamines may be important for evaluating the effects of NSAIDs such as sulindac on the treatment of FAP patients. Polyamines are components of a variety of foods (17,49), and they may negate the anticancer effects of these drugs. For example, putrescine is found in high concentrations in grapefruit and orange juices (0.2–0.3 mmol/cup) (Dr. Cheryl Rock, personal communication, and manuscript submitted). Mice supplemented with 1% putrescine in the drinking water consume between 1 and 2 mmol/day of this amine. Even without considering the difference in metabolic rates between mice and humans, our results suggest that certain sources of dietary putrescine could reach levels capable of modifying sulindac responses in humans. Our data add to the growing recognition of the potential importance of dietary polyamines for their effects on cancer progression (52,53).
Acknowledgments
This research was supported by NIH Grant CA 72008 and Arizona Disease Control Research Commission (ADCRC) contract #8004.
Contributor Information
Natalia A. Ignatenko, Department of Cell Biology and Anatomy, Arizona Cancer Center, The University of Arizona, Tucson
David G. Besselsen, University Animal Care, The University of Arizona, Tucson, AZ
Upal K. Basu Roy, Department of Biochemistry and Molecular Biophysics, The University of Arizona, Tucson, AZ
David E. Stringer, Cancer Biology Division, Arizona Cancer Center, The University of Arizona, Tucson, AZ
Karen A. Blohm-Mangone, Cancer Biology Division, Arizona Cancer Center, The University of Arizona, Tucson, AZ
Jose L. Padilla-Torres, Cancer Biology Division, Arizona Cancer Center, The University of Arizona, Tucson, AZ
Jose M. Guillen-R, Cancer Biology Division, Arizona Cancer Center, The University of Arizona, Tucson, AZ
Eugene W. Gerner, Department of Cell Biology and Anatomy, Arizona Cancer Center, The University of Arizona, Tucson. Department of Biochemistry and Molecular Biophysics, The University of Arizona, Tucson, AZ
References
- 1.Jass JR. Familial colorectal cancer: pathology and molecular characteristics. Lancet Oncol. 2000;1:220–226. doi: 10.1016/s1470-2045(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 2.Fodde R. The APC gene in colorectal cancer. Eur J Cancer. 2002;38:867–871. doi: 10.1016/s0959-8049(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 3.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Offerhaus JA, Tersmette AC, Hylind LM, Krush AJ, et al. Sulindac induced regression of colorectal adenomas in familial adenomatous polyposis: evaluation of predictive factors. Gut. 1996;38:578–581. doi: 10.1136/gut.38.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, et al. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 6.Nugent KP, Farmer KC, Spigelman AD, Williams CB, Phillips RK. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- 7.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon. [PubMed] [Google Scholar]
- 8.Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26:2–10. doi: 10.1016/s0049-0172(97)80046-7. [DOI] [PubMed] [Google Scholar]
- 9.Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–2459. [PubMed] [Google Scholar]
- 10.Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–3116. [PubMed] [Google Scholar]
- 11.Carbone PP, Douglas JA, Larson PO, Verma AK, Blair IA, et al. Phase I chemoprevention study of piroxicam and alpha-difluoromethylornithine. Cancer Epidemiol Biomarkers Prev. 1998;7:907–912. [PubMed] [Google Scholar]
- 12.Turchanowa L, Dauletbaev N, Milovic V, Stein J. Nonsteroidal anti-inflammatory drugs stimulate spermidine/spermine acetyltransferase and deplete polyamine content in colon cancer cells. Eur J Clin Invest. 2001;31:887–893. doi: 10.1046/j.1365-2362.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinez ME, O’Brien TG, Fultz KE, Babbar N, Yerushalmi H, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci USA. 2003;100:7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394:317–324. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babbar N, Gerner EW. Polyamines asmodifiers of genetic risk factors in human intestinal cancers. Biochem Soc Trans. 2003;31:388–392. doi: 10.1042/bst0310388. [DOI] [PubMed] [Google Scholar]
- 16.Sarhan S, Knodgen B, Seiler N. The gastrointestinal tract as polyamine source for tumor growth. Anticancer Res. 1989;9:215–223. [PubMed] [Google Scholar]
- 17.Bardocz S, Duguid TJ, Brown DS, Grant G, Pusztai A, et al. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- 18.Babbar N, Ignatenko NA, Casero RA, Jr, Gerner EW. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J Biol Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 19.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 20.Duggan DE, Hooke KF, Risley EA, Shen TY, Arman CG. Identification of the biologically active form of sulindac. J Pharmacol Exp Ther. 1977;201:8–13. [PubMed] [Google Scholar]
- 21.Hughes A, Smith NI, Wallace HM. Polyamines reverse non-steroidal anti-inflammatory drug-induced toxicity in human colorectal cancer cells. Biochem J. 2003;374:481–488. doi: 10.1042/BJ20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich WF, Lander ES, Smith JS, Moser AR, Gould KA, et al. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 24.Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 25.Evans JF. Rofecoxib (Vioxx), a specific cyclooxygenase-2 inhibitor, is chemopreventive in a mouse model of colon cancer. Am J Clin Oncol. 2003;26:S62–S65. doi: 10.1097/01.COC.0000074159.05087.50. [DOI] [PubMed] [Google Scholar]
- 26.Beazer-Barclay Y, Levy DB, Moser AR, Dove WF, Hamilton SR, et al. Sulindac suppresses tumorigenesis in the Min mouse. Carcinogenesis. 1996;17:1757–1760. doi: 10.1093/carcin/17.8.1757. [DOI] [PubMed] [Google Scholar]
- 27.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams CS, Watson AJ, Sheng H, Helou R, Shao J, et al. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 29.Charalambous D, Farmer C, O’Brien PE. Sulindac and indomethacin inhibit formation of aberrant crypt foci in the colons of dimethyl hydrazine treated rats. J Gastroenterol Hepatol. 1996;11:88–92. doi: 10.1111/j.1440-1746.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 30.Skinner SA, Penney AG, O’Brien PE. Sulindac inhibits the rate of growth and appearance of colon tumors in the rat. Arch Surg. 1991;126:1094–1096. doi: 10.1001/archsurg.1991.01410330048007. [DOI] [PubMed] [Google Scholar]
- 31.Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, et al. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57:199–201. [PubMed] [Google Scholar]
- 32.Erdman SH, Ignatenko NA, Powell MB, Blohm-Mangone KA, Holubec H, et al. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in theMin mouse. Carcinogenesis. 1999;20:1709–1713. doi: 10.1093/carcin/20.9.1709. [DOI] [PubMed] [Google Scholar]
- 33.Seiler N, Sarhan S, Grauffel C, Jones R, Knodgen B, et al. Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 1990;50:5077–5083. [PubMed] [Google Scholar]
- 34.Quemener V, Moulinoux JP, Havouis R, Seiler N. Polyamine deprivation enhances antitumoral efficacy of chemotherapy. Anticancer Res. 1992;12:1447–1453. [PubMed] [Google Scholar]
- 35.Paulsen JE, Reistad R, Eliassen KA, Sjaastad OV, Alexander J. Dietary polyamines promote the growth of azoxymethane-induced aberrant crypt foci in rat colon. Carcinogenesis. 1997;18:1871–1875. doi: 10.1093/carcin/18.10.1871. [DOI] [PubMed] [Google Scholar]
- 36.Rao CV, Rivenson A, Simi B, Zang E, Kelloff G, et al. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995;55:1464–1472. [PubMed] [Google Scholar]
- 37.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 38.Saukkonen K, Tomasetto C, Narko K, Rio MC, Ristimaki A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003;63:3032–3036. [PubMed] [Google Scholar]
- 39.Seiler N, Knodgen B. High-performance liquid chromatography procedure for the simultaneous determination of the natural polyamines and their monoacetylderivatives. J Chromatogr. 1980;221:227–238. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- 40.Ignatenko NA, Gerner EW. Growth arrest- and polyamine-dependent expression of spermidine/spermine N1-acetyltransferase in human tumor cells. Cell Growth Differ. 1996;7:481–486. [PubMed] [Google Scholar]
- 41.Sertich GJ, Glass JR, Fuller DJ, Gerner EW. Altered polyamine metabolism in Chinese hamster cells growing in a defined medium. J Cell Physiol. 1986;127:114–120. doi: 10.1002/jcp.1041270115. [DOI] [PubMed] [Google Scholar]
- 42.Shantz LM, Pegg AE. Assay of mammalian S-adenosylmethionine decarboxylase activity. Polyamine Protocols. 1998;79:45–49. doi: 10.1385/0-89603-448-8:45. [DOI] [PubMed] [Google Scholar]
- 43.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 44.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl JMed. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 45.Oshima M, Murai N, Kargman S, Arguello M, Luk P, et al. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733–1740. [PubMed] [Google Scholar]
- 46.Wallace HM, Caslake R. Polyamines and colon cancer. Eur J Gastroenterol Hepatol. 2001;13:1033–1039. doi: 10.1097/00042737-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 48.Noack JKB, Blaut M. Contribution of the intestinal microflora to polyamine formation in the gut. In: Bardocz SKJ, Grillo M, White A, editors. COST 91—Biogenically Active Amines in Food. Vol. 3. Luxembourg: Office of Official Publications of the European Communities; 1999. pp. 55–59. [Google Scholar]
- 49.Nishimura K, Shiina R, Kashiwagi K, Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J Biochem. 2006;139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- 50.Wery I, Kaouass M, Deloyer P, Buts JP, Barbason H, et al. Exogenous spermine induces maturation of the liver in suckling rats. Hepatology. 1996;24:1206–1210. doi: 10.1002/hep.510240537. [DOI] [PubMed] [Google Scholar]
- 51.Nakaike S, Kashiwagi K, Terao K, Iio K, Igarashi K. Combined use of alpha-difluoromethylornithine and an inhibitor of S-adenosylmethionine decarboxylase in mice bearing P388 leukemia or Lewis lung carcinoma. Jpn J Cancer Res. 1988;79:501–508. doi: 10.1111/j.1349-7006.1988.tb01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cipolla B, Guilli F, Moulinoux JP. Polyamine-reduced diet inmetastatic hormone-refractory prostate cancer (HRPC) patients. Biochem Soc Trans. 2003;31:384–387. doi: 10.1042/bst0310384. [DOI] [PubMed] [Google Scholar]
- 53.Landete JM, Ferrer S, Polo L, Pardo I. Biogenic amines in wines from three Spanish regions. J Agric Food Chem. 2005;53:1119–1124. doi: 10.1021/jf049340k. [DOI] [PubMed] [Google Scholar]