Abstract
Digital breast tomosynthesis (DBT) has strong promise to improve sensitivity for detecting breast cancer. DBT reconstruction estimates the breast tissue attenuation using projection views (PVs) acquired in a limited angular range. Because of the limited field of view (FOV) of the detector, the PVs may not completely cover the breast in the x-ray source motion direction at large projection angles. The voxels in the imaged volume cannot be updated when they are outside the FOV, thus causing a discontinuity in intensity across the FOV boundaries in the reconstructed slices, which we refer to as the truncated projection artifact (TPA). Most existing TPA reduction methods were developed for the filtered backprojection method in the context of computed tomography. In this study, we developed a new diffusion-based method to reduce TPAs during DBT reconstruction using the simultaneous algebraic reconstruction technique (SART). Our TPA reduction method compensates for the discontinuity in background intensity outside the FOV of the current PV after each PV updating in SART. The difference in voxel values across the FOV boundary is smoothly diffused to the region beyond the FOV of the current PV. Diffusion-based background intensity estimation is performed iteratively to avoid structured artifacts. The method is applicable to TPA in both the forward and backward directions of the PVs and for any number of iterations during reconstruction. The effectiveness of the new method was evaluated by comparing the visual quality of the reconstructed slices and the measured discontinuities across the TPA with and without artifact correction at various iterations. The results demonstrated that the diffusion-based intensity compensation method reduced the TPA while preserving the detailed tissue structures. The visibility of breast lesions obscured by the TPA was improved after artifact reduction.
1. Introduction
Digital breast tomosynthesis (DBT) is an emerging imaging modality that provides quasi-three-dimensional (3D) structural information of the breast. It has been demonstrated that DBT can reduce tissue overlap and improve the differentiation of normal tissue and suspicious masses. DBT has strong promise to improve sensitivity for detecting subtle mass lesions, especially in dense breasts, compared to mammography (Niklason et al 1997, Kopans 2001, Rafferty et al 2002, Wu et al 2003, Kopans 2005, Helvie et al 2006, 2007, Poplack et al 2007, Andersson et al 2008, Gennaro et al 2008, Helvie et al 2008, Smith et al 2008, Gur et al 2009, Kopans 2009, Helvie et al 2010, Chan et al 2010, Wallis et al 2012).
DBT is an application of the cone-beam limited-angle computed tomography (CT) technology. A stack of tomosynthesized slices is reconstructed from a sequence of low-dose x-ray projections of the breast acquired at a small number of angles over a limited angular range. A number of reconstruction methods have been implemented for DBT. These methods can be classified into two categories: iterative and non-iterative methods. Non-iterative reconstruction methods include simple backprojection (BP) algorithms (Kolitsi et al 1992) and filtered backprojection (FBP) algorithms (Wu et al 2004a, Chen and Ning 2002). The simple BP method provides smooth reconstructed images with low contrast structures. In FBP, image features are enhanced by special filters but the design of these filters depends strongly on the imaging and sampling geometries. Iterative methods successively estimate the tissue attenuation data with increasing number of iterations. These methods can be easily adapted to any imaging geometry. Previous studies have shown that simultaneous algebraic reconstruction technique (SART) (Zhang et al 2006, Sompel and Brady 2008, Han et al 2009, Lu et al 2010) and iterative statistical reconstruction algorithms such as the maximum likelihood-convex (ML-convex) method (Wu et al 2004a, 2004b, Chan et al 2005, Zhang et al 2006) can enhance the contrast and edges of structures and lesions in the breast. The degree of enhancement of the iterative methods depends on the reconstruction parameters and the number of iterations chosen.
There exist several different artifacts arising from DBT system geometry (Lu et al 2012). The field of view (FOV) of the projection views (PVs) in DBT is determined by the area of the detector and the projection angle. For each PV, the collimated x-ray beam only exposes the breast volume within the detector FOV. For a given PV updating, the voxel values beyond its FOV boundary cannot be updated, causing a discontinuity in voxel values across the FOV boundary. If uncorrected, the reconstructed DBT slices will contain step artifacts, which we refer to as truncated projection artifacts (TPAs). The TPA affects the quality of reconstructed DBT images and may distract radiologists during image interpretation.
Reduction of the TPA has been studied for different tomography systems and various reconstruction techniques. Theoretical studies of truncation artifacts in parallel beam imaging can be traced back to the work by Bracewell et al over three decades ago (Bracewell and Wernecke 1975). Their work was further extended to fan-beam and cone-beam imaging via FBP reconstruction (Müller and Arce 1996). The existing methods for truncation artifact reduction can be grouped into two categories: correction in the projection space or in the imaged volume. Correction in the projection space includes extension of truncated projections (Ogawa et al 1984, Kadrmas et al 1995, Loncaric et al 1995, Chang et al 1995, Ohnesorge et al 2000, Hsieh et al 2004, Sourbelle et al 2005, Lewitt 1979) and weighted normalization of projections (Li et al 2007). To extend the truncated projections, extrapolation methods were employed via polynomial functions (Lewitt 1979, Kadrmas et al 1995), symmetric mirroring (Ohnesorge et al 2000), cylindrical water object fitting (Hsieh et al 2004) and square root function (Sourbelle et al 2005). Alternatively, the missed data of one truncated projection were estimated from other PVs (Ogawa et al 1984), or from its opposite projection in a dedicated asymmetric fan-beam CT system (Loncaric et al 1995, Chang et al 1995). However, extension of truncated projections may introduce approximation error and blurred breast tissue, especially for large truncated area on a PV as often occurring in DBT scan. In the weighted normalization approach proposed by Li et al, the number of PVs that contributed information to each imaged voxel was counted and used to normalize the backprojected projections (Li et al 2007). However, weighted normalization of projections cannot be applied to linear reconstruction methods using individual PV updating, such as the SART method, or nonlinear reconstruction methods, such as the ML method. On the other hand, compensation in the imaged volume is feasible for iterative reconstruction (Kunze et al 2007, Zhang et al 2009). Kunze et al used a statistical model and designed a constraint term of the object logarithmic-likelihood function to regularize the reconstructed images (Kunze et al 2007). Their method reduced the truncation artifacts in CT reconstruction of thoracic images. However, their algorithm used the probability density function of human tissue attenuation values as a priori information for the object to be reconstructed. Zhang et al used a local intensity equalization strategy to compensate for voxel values outside the FOV after each PV updating in SART (Zhang et al 2009). However, the local intensity equalization strategy did not retain the structural information from previous SART iteration so that it would not be useful for TPA reduction in iterative DBT reconstruction with multiple iterations. The purpose of this study is to develop a general diffusion-based TPA reduction method for SART that can be used for any number of iterations.
The organization of this paper is as follows. Section 2 reviews the breast tomosynthesis system and the SART for DBT reconstruction used in this study, and describes the TPA and the proposed reduction method. Section 3 discusses the experimental design and results. The improvement in TPA was measured and visually assessed for DBT of different views. Finally, discussion and conclusions are presented in sections 4 and 5, respectively.
2. Methods
2.1. Breast tomosynthesis system
A GE prototype GEN2 DBT system in the breast imaging research laboratory at the University of Michigan was used to acquire DBT scans in this study. The imaging geometry of this DBT system is illustrated in figure 1. The distance from the x-ray focal spot to the fulcrum of rotation is 64 cm and the x-ray source rotation plane is parallel to the chest wall and perpendicular to the detector plane. The system has a CsI phosphor/a:Si active matrix flat panel digital detector with a matrix size of 1920 × 2304 pixels and a pixel pitch of 0.1 mm × 0.1 mm. The digital detector is stationary during image acquisition. The system uses a step-and-shoot design and acquires PV images from a total of 21 angles in 3° increments over a ± 30° range in less than 8 s. The DBT system uses an Rh-target/Rh-filter x-ray source for all breast thicknesses.
Figure 1.
Geometry of the GE prototype GEN2 DBT system used in this study.
DBT imaging of human subjects was performed with IRB approval and informed consent. Patients who were recommended for biopsy of a suspicious lesion in the breast were eligible. DBT in both the cranio-caudal (CC) view and mediolateral oblique (MLO) view were obtained for the breast with the lesion.
In our reconstruction, the voxel dimensions of the imaged volume in both the X- and Y-directions were chosen to be 0.1 mm, the same as the pixel pitch of the detector. The slice spacing in the Z-direction was chosen to be 1 mm. A ray-tracing method similar to the Siddon algorithm was employed for calculating the contribution of each voxel to the forward projection (Siddon 1985). Logarithmic transformation was applied to the raw pixel intensities of the PV image before reconstruction. The projection model assumed a monoenergetic x-ray source and ignored the effects of scattering and beam hardening, similar to the approach by Wu et al (2004a, 2003).
2.2. DBT reconstruction
We applied SART to DBT reconstruction in this study (Kak and Slaney 1988). The linear attenuation coefficient of each voxel is updated simultaneously using all rays in one projection. The number of imaged volume updates in one SART iteration is equal to the number of projections, N. In the kth SART iteration, the updating of the reconstructed image volume by the nth projection can be described as follows:
| (1) |
where An = (Aij,n) is the projection matrix for the nth PV (1 ≤ n ≤ N), fi,n is the ith pixel of the nth projection, is the linear attenuation coefficient of the jth voxel after updating by the nth projection in the kth iteration, I is the number of pixels in the detector, and J is the total number of voxels in the reconstructed volume. The output voxel values of the imaged volume are scaled to 4096 gray levels. The details of our implementation of the SART method were described in the literature (Zhang et al 2006, Lu et al 2010).
2.3. Truncated projection artifacts
The (truncated projection artifacts) TPAs arise from the transition between the exposed and unexposed parts of the imaged volume which varies as the x-ray source position changes. The strength of the artifacts depends on the reconstruction method and the parameters used. A brief review of the TPA in SART reconstruction is given below.
For each projection, the FOV boundary is determined by the boundary of the detector and the x-ray source position in cone-beam geometry, with the x-ray beam defined by the collimator. At large angles, the FOV may not cover the entire breast volume (see figure 2(a)). Therefore, the two sides of the breast may be covered by fewer PVs than the full set of N projections, and the number of PVs seen by a given voxel varies with its location. During updating of voxel values with a given PV, only the voxels within the FOV will be updated, whereas the voxels outside maintain their previous values. This will cause a discontinuity of voxel values across the FOV boundary in the reconstructed volume. The truncation artifacts from the set of PVs finally appear as parallel staircase strips on each tomosynthesized slice. The spacing of the staircase strips decreases as the slice gets closer to the detector. Figure 2 illustrates an example for which SART updating used PVs sequentially from the left to the right. In the following discussion, we refer to the right side and left side as the forward direction and backward direction, respectively.
Figure 2.
(a) The change in FOV coverage from different x-ray source locations resulting in unexposed regions in the imaged volume for some PVs and truncated projections of the breast at large projection angles. At the FOV boundary of an individual PV (e.g., right side of (n−1)th and nth PV), the lack of information for image updating in SART outside the FOV will cause a discontinuity in the voxel values. The discontinuities from the set of PVs will appear as staircase strips parallel to the detector (or collimator) boundary on each tomosynthesized slice. (b) One slice of DBT with TPAs appearing as staircase strips at the top (forward direction of PV updating) and bottom (backward direction of PV updating) parts of the image marked by arrows.
Our previous studies show that SART tends to enhance the high-frequency signals (Zhang et al 2006), which results in enhancement of the step edges of the TPA, at each update. The TPAs in the forward and backward directions, however, are enhanced by different number of PV updates. In the backward direction, the discontinuity across the FOV boundary generated by the update from the (n−1)th PV is not enhanced by the updates from subsequent PVs in the same iteration. The enhancement of this step edge occurs only by the updates from the first to (n−1)th PVs in the subsequent iterations. On the other hand, in the forward direction a discontinuity across the FOV boundary is enhanced by updates from all subsequent PVs in the same iteration, in addition to the updates from the nth to Nth PVs in the subsequent iterations. Therefore, as demonstrated in figure 2(b), the TPAs in the backward direction of PV updating (bottom part) are not as prominent as those in the forward direction (top part).
2.4. Diffusion-based TPA reduction method
Our approach to reducing the TPA is to compensate for the difference in the voxel values outside the FOV, which are not updated by the current PV, using the updated voxel values within the FOV of the current PV.
Let Ωn,m denote the intersection of the FOV of the nth PV image with the mth tomosynthesized slice, where the boundary of the intersection can be calculated from the imaging geometry and the depth of the mth slice. In the following, we describe a two-dimensional (2D) diffusion method to estimate the compensation needed for the voxel values outside Ωn,m using the voxel values in Ωn,m on each slice. The same method is applicable to the slices at any depth. For simplicity, the slice index m is not explicitly included in the following discussion. We consider the intersections of a given slice with the FOVs of three consecutive PVs as shown in figure 3. Let Xn denote the slice after the nth PV updating. The three consecutive intersections on Xn are defined as Ωn−1, Ωn and Ωn+1, respectively. For two sets A and B, let A\B denote the relative complement of B in A. Since the voxels in Ωn are updated by the nth PV while the voxels in the two areas Ωn+1\Ωn (forward direction) and Ωn−1\Ωn (backward direction) are not updated (shaded areas in figure 3), the discontinuity in voxel values appears as a step at the boundaries between Ωn and these two areas.
Figure 3.

The intersection of a DBT slice with the FOV of the (n−1)th PV (short dashed boundary), of the nth PV (solid boundary), or of the (n+1)th PV (dashed boundary). The forward direction is on the right. The shaded area on the right is Ωn+1\Ωn, and that on the left is Ωn−1\Ωn.
To estimate the missing updates to the voxel values within Ωn+1\Ωn and Ωn−1\Ωn after the nth PV updating, we designed an algorithm to diffuse the differences in voxel values from Ωn to Ωn+1\Ωn and Ωn−1\Ωn, as described in the following.
Algorithm 1. (Diffusion algorithm)
After the nth PV updating, Xn−1 is updated to Xn in the region Ωn. However, the un-updated regions Ωn+1\Ωn and Ωn−1\Ωn of Xn remain the same as Xn−1, thereby creating step artifacts between the regions. Consider the difference image Y = Xn − Xn−1, within the region Ωn, the difference image represents the change in voxel values due to updating with the nth PV, while in the regions Ωn+1\Ωn and Ωn−1\Ωn, the differences of voxel values are zero because there are no updated values. To reduce the step artifacts, we estimate the expected voxel values in regions Ωn+1\Ωn and Ωn−1\Ωn of the difference image to produce a smooth compensating difference image over the three regions, which can then be added to Xn−1 to obtain an updated image X̃n without TPA. However, to preserve the image details already reconstructed in the regions Ωn+1\Ωn and Ωn−1\Ωn of Xn−1 from the previous updating, the compensating difference image should only modify the low-frequency background. The following diffusion-based method is designed to estimate the low-frequency background in regions Ωn+1\Ωn and Ωn−1\Ωn of the difference image Y using the information from the neighboring region in Ωn.
Given an image Y and a region in Y as S, let the projection operator PS be defined as
| (2) |
where i is the index of a voxel in Y and SY is the domain of the image Y. The projection operator PS is illustrated in figure 4.
Figure 4.

Projection operator PS preserves the pixel values in the region S and set the pixel values in the relative complement of S in Y to be zero.
Let F denote a 2D low-pass filter, T denote the number of iterations chosen for generating the diffused image and I denote the identity operator. For the tth iteration of diffusion, we define
| (3) |
where ** denotes 2D convolution, t = 1, …, T, and Y0 = Y. S is the region Ωn+1\Ωn or Ωn−1\Ωn where the missing information is being estimated from its neighboring region in Y. We denote the diffused difference image YT as CS(Y). Note that YT = Y0 = Y in the region Ωn.
Algorithm 2. (TPA reduction algorithm)
The compensated image X̃n after updating with the nth PV and TPA reduction with the estimated background difference in regions Ωn+1\Ωn and Ωn−1\Ωn is given by X̃n = Xn−1 + YT = Xn−1 + CS(Y). Using algorithm 1, it can be seen that X̃n can be written as
| (4) |
Note that X̃n = Xn in region Ωn. In regions Ωn+1\Ωn and Ωn−1\Ωn, Xn−1 contains the detailed image features and the background intensity after updating up to the (n−1)th PV, and CS(Xn −Xn−1) is the diffused difference image, as described above, to compensate for the missing update of the low-frequency background expected from the nth PV updating. Alternatively, if we denote L(Xn−1) as the low-frequency background image of Xn−1 and L̂(Xn) as the expected low-frequency background image of Xn in region Ωn+1\Ωn or Ωn−1\Ωn, CS(Xn − Xn−1) is an approximation of L̂(Xn) − L(Xn−1). One may consider that Xn−1 + CS(Xn − Xn−1) ≈ Xn−1 − L (Xn−1) + L̂(Xn). Note that Xn−1 − L (Xn−1) contains the structured details of the step region, which is superimposed on the expected updated low-frequency background L̂(Xn) estimated by diffusion to obtain the overall step image with a background level as if update has been performed. The diffusion process in the forward or backward directions to reduce the TPA is basically the same except for the difference discussed next.
As shown in figure 2(b), the TPA in the backward direction is less obvious than that in the forward direction. However, it has a larger artifact zone to be compensated. In the forward direction on a given slice, only the strip artifact zone Ωn+1\Ωn needs to be compensated before the (n+1)th PV updating; while in the backward direction, all strip artifact zones Ωn−1\Ωn, Ωn−2\Ωn−1, …, Ω1\Ω2 have to be compensated at the same time to avoid new discontinuities created by, for example, compensating for Ωn−1\Ωn without compensating for Ωn−2\Ωn−1. This brings large computational cost. To improve the efficiency, we first obtained a good compensating difference image in the artifact zone Ωn−1\Ωn and then propagated the average intensity difference in zone Ωn−1\Ωn columnwise followed by low-pass filtering to estimate the intensity difference values in the other artifact zones Ωn−2\Ωn−1, …, Ω1\Ω2. As discussed above, the image features in the TPA zones are mainly updated by the subsequent iterations in SART, and the average intensity compensation approximately matching the un-updated background level to the updated background level is sufficient for artifact reduction purpose. This provides an efficient method to compensate for the intensity differences in the backward direction.
2.5. Study conditions
To evaluate the artifact reduction method, DBT images in both CC and MLO views were reconstructed by SART with and without TPA reduction. For the DBT volume reconstructed from each set of PV images, one to five SART iterations were performed. The parameters used for SART were based on our previous study (Lu et al 2010) with relaxation parameter λ of 0.5 for the first iteration and 0.3 for the subsequent iterations. The number of iterations in the diffusion process is adaptively determined by measuring the average voxel values in the diffused region S. When the change in the average voxel values of two consecutive diffused images is less than a given threshold, the iterative diffusion process is terminated. The choice of the low-pass filter kernel F in equation (3) for diffusing the difference image Y is not critical but small kernels will require more diffusion iterations. The role of the low-pass filter in the diffusion method is to remove the structured high-frequency information in the difference image Y. Any simple low-pass filter, such as a relatively large box filter or a wide Gaussian filter, in combination with proper diffusion will serve the purpose. We chose a box filter with a kernel size of 41 × 41 pixels because its constant kernel weights allow box filtering to be implemented using a simple sliding window algorithm which is substantially faster than a convolution algorithm used by general low-pass filters. Based on our experiments, the threshold for stopping the diffusion iteration was chosen to be 0.01.
3. Results
We applied the proposed algorithm in both the forward and backward directions of the PV updating to the DBT reconstruction. The improvement in TPA was measured and visually assessed for breasts of various densities.
3.1. Breast DBT images
An MLO-view DBT slice of a breast without and with TPA reduction after one, three and five SART iterations is shown in figure 5. The compressed thickness of the breast was measured by the DBT system as 7.6 cm. The slice shown was at 3.7 cm above the breast support plate. The x-ray source direction moved from the bottom to the top so that the top part was the forward direction and the bottom part was the backward direction in this reconstruction. Without artifact reduction, the TPAs decreased in the forward direction gradually (figures 5(a)–(c)), but were enhanced in the backward direction as the number of iterations increased. The artifact reduction method successfully removed the TPA in both directions (figures 5(d)–(f)). The structured tissue background was preserved as seen by visual comparison. Note that the voxel values of the pectoral muscle boundary at the top and the tissue structures at the bottom of the slice varied smoothly across the FOV boundaries after TPA removal.
Figure 5.
A DBT slice obtained by SART reconstruction for the MLO view of a breast. The upper row shows the tomosynthesized slice without TPA reduction after (a) one, (b) three and (c) five SART iterations. The lower row shows the same tomosynthesized slice with TPA reduction after (d) one, (e) three and (f) five SART iterations. The white lines indicated the locations where the gray-level profiles were analyzed in figure 6.
The gray-level profiles along the selected lines in figure 5 (white vertical lines across the steps with the voxel index relative to the line), averaged over a three-voxel-wide strip centered about the line, are compared in figure 6 for the reconstruction with and without TPA reduction in five iterations. To further demonstrate the effect of TPA reduction, the average step heights with and without artifact correction over the five iterations are shown in figures 6(e) and (f), respectively, for the forward and backward directions. The average step height of a given step was estimated as the difference in the average voxel values across the step and averaged along the entire step. Our TPA reduction method reduced the step heights in both the forward and backward directions of PV updating. In the forward direction (figure 6(e)), the step heights of the TPA were reduced by more than 95% compared to those without correction for the different iterations. In the backward direction (figure 6(f)), the TPA was enhanced by two to three times with increasing number of iterations without correction. Our method reduced the step heights by 90% to 95%. With TPA reduction, the steps were not visually apparent over the iterations in either direction (figures 5(d)–(f)).
Figure 6.
Left column line profiles along the white line at the top part in figure 5 (in the forward direction of PV updating) after (a) one iteration, (c) five iterations and (e) the trend of the average step height along the selected step (voxel index = 85 in (a)) with the increasing number of iterations with (TPAR) and without (NR) TPA reduction. Right column: line profiles along the white line at the bottom part in figure 5 (in the backward direction of PV updating) after (b) one iteration, (d) five iterations and (f) the trend of the average step height along the selected step (voxel index = 112 in (b)) with increasing number of iterations with and without TPA reduction.
Figure 7 shows an example of a CC view DBT slice without and with the TPA reduction after one, three and five SART iterations. The thickness of the compressed breast was measured by the DBT system as 5.7 cm. The selected slice was at 3.8 cm above the breast support plate. The x-ray source direction in this reconstruction also moved from bottom to the top. Without TPA reduction, the staircase artifacts were enhanced gradually in the backward direction as the number of iterations increased. The TPA reduction method reduced the visibility of the steps in both the forward and backward directions of PV updating. The breast tissue structures are preserved with smooth transition across the steps (see the arrow in figure 7(a)). This is consistent with what we observed in figure 5.
Figure 7.
A DBT slice obtained by SART reconstruction for the CC view of a breast. The upper row shows the tomosynthesized slice without TPA reduction after (a) one, (b) three and (c) five SART iterations. The lower row shows the same tomosynthesized slice with TPA reduction after (d) one, (e) three and (f) five SART iterations. The white lines indicated the locations where the gray-level profiles were analyzed in figure 8.
The average gray-level profiles along the selected lines in figure 7 are compared in figure 8 for the reconstruction with and without artifact reduction in five iterations. The average step heights of a typical step with and without artifact correction over the five iterations are shown in figures 8(e) and (f), respectively, for the forward and backward directions. This example demonstrates that the TPA does not always monotonically change with increasing number of iterations. In the forward direction, the TPA decreased in the second and third iterations but increased again as the iterations continued. However, regardless of the trend of the TPA, using our TPA reduction method in the forward direction of PV updating (left column of figure 8), the average step heights were reduced by 80–90% compared to those without TPA reduction. In the backward direction (right column of figure 8), the TPA was enhanced by two to three times with increasing number of iterations without correction. Our method reduced the step heights by 70–80%. These examples demonstrate that our diffusion-based method could reduce the TPA to a level that was not visually apparent over the iterations in both directions (figures 7(d)–(f)).
Figure 8.
Left column line profiles along the white vertical line at the top part in figure 7 (in the forward direction of PV updating) after (a) one iteration, (c) five iterations and (e) the trend of the average step height along the selected step (voxel index = 56 in (a)) with increasing number of iterations with (TPAR) and without (NR) TPA reduction. Right column: line profiles along the white vertical line at the bottom part in figure 7 (in the backward direction of PV updating) after (b) one iteration, (d) five iterations and (f) the trend of the average step height along the selected step (voxel index = 32 in (b)) with and without TPA reduction.
The average performance of the TPA reduction method was evaluated by analysis of ten DBT views from ten different subjects. All ten DBT views are MLO views since MLO view contains larger TPA regions, especially in the pectoral muscle area, compared to CC view of the same subject. For the TPA in a given direction (forward or backward) and a given DBT view, the average step height of two representative steps (the closet step to the center of FOV and one step from the middle of the artifact region) was estimated as the step height of that TPA region. The mean and standard deviation of the step height in a given direction were then estimated from the ten views. The dependence of the average step heights in each direction with and without correction on the number of iterations has been plotted in figures 9(a) and (b), respectively. The average trends demonstrate that, in the forward direction, the TPA decreased at the initial iterations but the enhancement dominated as the iteration continued. In the backward direction, the TPA was enhanced with increasing number of iterations. The TPA was more prominent and had larger variations in the forward than in the backward direction. With correction, the average step heights in both the forward and backward directions were reduced to similar levels with small variations. The efficiency of the TPA correction algorithm was also estimated from the ten DBT views. The computational time of TPA correction was, on average, 0.69 ± 0.14% of the computational time of the SART reconstruction when the reconstruction was performed on a workstation with six-core CPU of 3.47 GHz and 24 GB of system memory.
Figure 9.

The trend of the average step height with increasing number of iterations with (TPAR) and without (NR) TPA reduction estimated from ten DBT views of ten different subjects in the (a) forward direction and (b) backward direction. The error bars indicate one standard deviation of the measurements. The standard deviations after correction were smaller than two voxel values.
The effectiveness of our artifact reduction method on improving the image quality of breast lesions, in case the lesion is located at the TPA region, is demonstrated in figure 10 for two subjects, who were a part of the ten-subject set used above. The TPAs obscured the microcalcification cluster and the spiculated masses in figures 10(a) and (c). The correction method successfully removed the TPAs and restored the quality of the microcalcifications and masses.
Figure 10.
Top row a region of interest containing a cluster of microcalcifications in a spiculated mass (a) without and (b) with TPA reduction. Bottom row: a region of interest containing a spiculated mass (c) without and (d) with TPA reduction. Both examples are shown after five iterations.
3.2. Phantom DBT images
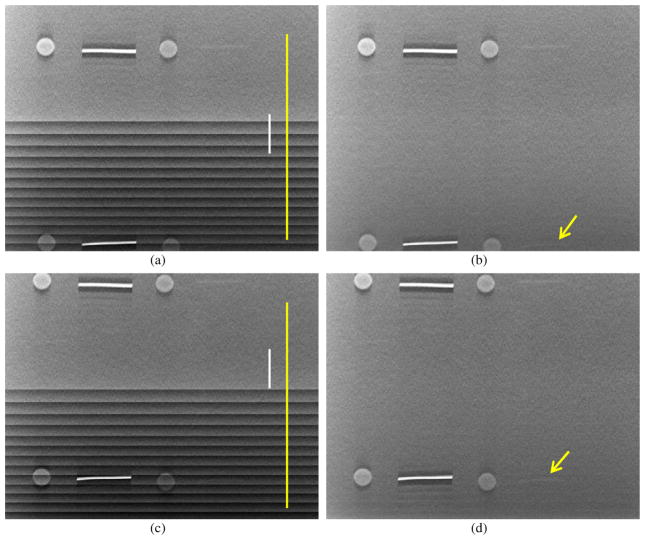
We custom built a phantom containing test objects on a thin feature layer sandwiched between one 1 cm thick homogeneous Lucite plate on top and another five 1 cm thick homogeneous Lucite plates below the layer. To evaluate the effectiveness of the TPA correction algorithm for artifact regions at different location, we shifted the phantom and acquired DBT scans of the phantom at two positions of the FOV. The phantom was shifted parallel to the chest wall edge of the stationary detector such that the test objects in the lower part of the phantom were not covered in 12 PVs at one position (figure 11(a)) and not covered in 8 PVs at another position (figure 11(c)). Figure 11 compared the reconstructed slice of the phantoms at the focal plane of the test objects without and with TPA correction at the end of the second iteration. The steps were reduced with the TPA reduction algorithm and the visual image quality of all features was improved, especially the subtle fibril (see arrows in figures 11(b) and (d)) which was completely obscured by the TPA without correction. Figure 12 compared the line profiles along the vertical lines in figures 11(a) and (c) without and with correction. Figures 12(a) and (b) show that with TPA correction, the slice images of phantoms placed at different positions obtained similar background voxel value profiles, except for the small difference around voxel indices of 750–800, close to the lower boundary of the images (figures 11(a) and (c)). Figures 12(c) and (d) compared the profiles at the same physical locations of the phantom in the two different scans before and after TPA correction. One line was located at the TPA region of the phantom slice and another line outside the TPA region of the corresponding phantom slice. The same physical location of the phantom appeared at different locations of the FOV because the phantom was shifted between the two scans. With TPA correction, the voxel value profile within the TPA region became similar to that outside the TPA region.
Figure 11.
Top row a region of interest of reconstructed phantom images at the location where the objects at the lower part were not covered by 12 of the 21 PVs (a) without and (b) with artifact correction. Bottom row: a region of interest of reconstructed phantom images at the location where the objects at the lower part were not covered in 8 of the 21 PVs (c) without and (d) with artifact correction. A very subtle fibril (arrow) can be seen in the corrected images but is invisible in the presence of the step artifacts.
Figure 12.
Line profiles along the vertical lines in figure 11, (a) without and (b) with artifact correction along the yellow lines, (c) without and (d) with artifact correction along the vertical white lines.
4. Discussion
In this study, we developed a new diffusion-based artifact correction method to reduce the TPAs in DBT reconstruction by SART in the image domain. The discontinuity across the boundary of the FOV between the PVs is smoothened in the corrected images. This study demonstrates that the TPA correction method can reduce the staircase artifacts in both the forward and backward directions while preserving structured background tissues without introducing additional artifacts.
The proposed method used the information in the updated area to compensate for the low-frequency background intensity difference in the un-updated zone. The aim of the truncation artifact reduction problem is close to that of the image inpainting problem which was well studied in image science (Masnou and Morel 1998, Bertalmio et al 2000, Chan et al 2006). However, in the image inpainting problem, the data are missed in the incomplete zone, while in the current artifact reduction problem the image feature information exists after the first SART iteration and the existing image features need to be preserved for the rest of the SART iterations. The truncation artifact reduction method designed by Zhang et al for DBT reconstruction is much similar to the image inpainting method (Zhang et al 2009) in that the existing detailed image features are not preserved. Therefore, the method by Zhang et al works well only in the forward direction of the FOV boundaries of the processed PVs in the first iteration. Our new correction method preserves the detailed features in the images already reconstructed from the previous iterations up to the latest PV updating. In addition, the artifact reduction problem is a dynamic procedure incorporated with the reconstruction process while image inpainting is relatively static. The image features in the artifact zone are accumulated by iterative reconstruction from PVs that cover the zone within their FOVs rather than by extrapolating the information from neighboring areas as in image inpainting problems.
The TPA may distort the appearance of breast lesions and reduce the visibility of subtle features if the lesions are imaged in the TPA regions. We have demonstrated that our TPA reduction method can reduce the distortion and improve the visibility of subtle details in both the phantom and breast DBT images. The correction of the TPA would likely reduce distraction to radiologists during image interpretation. However, whether TPA reduction will have an impact on lesion detection and diagnosis in clinical practice has yet to be assessed by observer study, which is out of the scope of the current study.
In this study, we incorporated the TPA reduction method into the SART framework and evaluated its effectiveness over a number of iterations. As can be seen, the diffusion-based intensity compensation method is independent of the SART reconstruction process. It is therefore expected that the same approach can be applied to other iterative reconstruction techniques to correct for truncation artifacts and achieve similar effects. The usefulness of the TPA reduction method with other iterative reconstruction techniques will have to be validated in future studies.
5. Conclusion
We developed a new TPA reduction method for DBT reconstruction with SART. The proposed method used iterative diffusion to smoothly compensate for the discontinuity across the FOV boundary of the PV images used for image volume updating. Image features accumulated in the reconstructed images obtained from the previous SART iterations up to the latest PV updating were preserved, and the expected updated voxel values in the step region as if the PV image was not truncated were estimated by diffusing the difference between the current and the last updated images in the updated zone to the step region. The estimated correction was then used to compensate for the background voxel discontinuity before the next updating. The results demonstrate that the proposed method can reduce the TPA successfully at both the front and back FOV boundaries in the x-ray source motion direction while preserving the detailed structured features. The visibility of breast structures obscured by the TPA was improved. Reduction of the TPAs can improve the image quality of DBT, reduce the distraction during radiologists’ interpretation and potentially may improve detection and assessment of subtle breast lesions that are in the areas with the TPAs.
Acknowledgments
This work is supported by USPHS grants RO1 CA151443 and RO1 CA91713. The DBT system was developed by the GE Global Research Group, with input and some revisions from the University of Michigan investigators, through the Biomedical Research Partnership (USPHS grant CA91713, PI: Paul Carson, PhD) collaboration. The content of this paper does not necessarily reflect the position of the funding agencies and no official endorsement of any equipment and product of any companies mentioned should be inferred.
References
- Andersson I, Ikeda DM, Zackrisson S, Ruschin M, Svahn T, Timberg P, Tingberg A. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol. 2008;18:2817–25. doi: 10.1007/s00330-008-1076-9. [DOI] [PubMed] [Google Scholar]
- Bertalmio M, Sapiro G, Caselles V, Ballester C. Image inpainting. Computer Graphics (SIGGRAPH 2000) 2000:417–24. [Google Scholar]
- Bracewell RN, Wernecke SJ. Image reconstruction over a finite field of view. J Opt Soc Am. 1975;65:1342–6. [Google Scholar]
- Chan H-P, Wei J, Wu T, Sahiner B, Rafferty EA, Hadjiiski LM, Helvie MA, Roubidoux MA, Moore RH, Kopans DB. Computer-aided detection on digital breast tomosynthesis (DBT) mammograms: dependence on image quality of reconstruction. RSNA Program Book 2005. 2005:269. [Google Scholar]
- Chan H-P, Wu YT, Sahiner B, Wei J, Helvie MA, Zhang Y, Moore RH, Kopans DB, Hadjiiski L, Way T. Characterization of masses in digital breast tomosynthesis: comparison of machine learning in projection views and reconstructed slices. Med Phys. 2010;37:3576–86. doi: 10.1118/1.3432570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TF, Shen J, Zhou H-M. Total variation wavelet inpainting. J Math Imaging Vis. 2006;25:107–25. [Google Scholar]
- Chang W, Loncaric S, Huang G, Sanpitak P. Asymmetric fan transmission CT on SPECT systems. Phys Med Biol. 1995;40:913–28. doi: 10.1088/0031-9155/40/5/013. [DOI] [PubMed] [Google Scholar]
- Chen B, Ning R. Cone-beam volume CT breast imaging: feasibility study. Med Phys. 2002;29:755–70. doi: 10.1118/1.1461843. [DOI] [PubMed] [Google Scholar]
- Gennaro G, Baldan E, Bezzon E, La Grassa M, Pescarini L, di Maggio C. Clinical performance of digital breast tomosynthesis versus full-field digital mammography: preliminary results. Proc 9th Int Workshop on Digital Mammography (Lecture Notes in Computer Science. 2008;5116:477–82. [Google Scholar]
- Gur D, Abrams GS, Chough DM, Ganott MA, Hakim CM, Perrin RL, Rathfon GY, Sumkin JH, Zuley ML, Bandos AI. Digital breast tomosynthesis: observer performance study. Am J Roentgenol. 2009;193:586–91. doi: 10.2214/AJR.08.2031. [DOI] [PubMed] [Google Scholar]
- Han T, Zhong Y, Chen L, Lai C, Liu X, Shen Y, Ge S, Yi Y, Shaw C. SU-FF-I-41: accuracy and computing time of a ray-driven projector/back-projector for simulation and reconstruction in tomosynthesis and cone beam CT imaging. Med Phys. 2009;36:2443–4. [Google Scholar]
- Helvie MA, Chan H-P, Hadjiiski L, Goodsitt MM, Roubidoux MA, Carson PL. Digital breast tomosynthesis mammography: effect of breast density on breast mass visibility and characterization. RSNA Program Book 2010. 2010:436. [Google Scholar]
- Helvie MA, Roubidoux MA, Hadjiiski LM, Zhang Y, Carson PL, Chan H-P. Tomosynthesis mammography vs conventional mammography: comparison of breast masses detection and characterization. RSNA Program Book 2007. 2007:381. [Google Scholar]
- Helvie MA, Roubidoux MA, Hadjiiski LM, Zhang Y, Carson PL, Chan H-P. Research digital tomosynthesis mammography: detection of t1 invasive breast carcinomas not diagnosed by conventional breast imaging or physical exam. RSNA Program Book 2008. 2008:468. [Google Scholar]
- Helvie MA, Roubidoux MA, Zhang Y, Carson PL, Chan H-P. Tomosynthesis mammography vs conventional mammography: lesion detection and reader preference. Initial experience RSNA Program Book 2006. 2006:335. [Google Scholar]
- Hsieh J, Chao E, Thibault J, Grekowicz B, Horst A, McOlash S, Myers TJ. A novel reconstruction algorithm to extend the CT scan field-of-view. Med Phys. 2004;31:2385–91. doi: 10.1118/1.1776673. [DOI] [PubMed] [Google Scholar]
- Kadrmas DJ, Jaszczak RJ, McCormick JW, Coleman RE, Lim CB. Truncation artifact reduction in transmission CT for improved SPECT attenuation compensation. Phys Med Biol. 1995;40:1085–104. doi: 10.1088/0031-9155/40/6/009. [DOI] [PubMed] [Google Scholar]
- Kak A, Slaney M. Principle of Computerized Tomographic Imaging. New York: IEEE Press; 1988. [Google Scholar]
- Kolitsi Z, Panayiotakis G, Anastassopoulus V, Scodras A, Pallikarakis N. A multiple projection method for digital tomosynthesis. Med Phys. 1992;19:1045–50. doi: 10.1118/1.596822. [DOI] [PubMed] [Google Scholar]
- Kopans DB. Novel approaches and newer imaging modalities. Course 815: Problem-solving Breast Imaging. RSNA Program Book 2001. 2001:97. [Google Scholar]
- Kopans DB. Digital tomosynthesis and other applications. Categorical Course in Diagnostic Radiology: Breast Imaging-Digital Mammography. RSNA Program Book 2005. 2005:130. [Google Scholar]
- Kopans DB. Digital breast tomosynthesis: experience with imaging over 3500 subjects. Categorical Course in Diagnostic Radiology Physics: Advances in Digital Tomosynthesis—From Physics to Clinical Applications. RSNA Program Book 2009. 2009:288. [Google Scholar]
- Kunze H, Härer W, Stierstorfer K. Iterative extended field of view reconstruction. Proc SPIE. 2007;6510:65105X. [Google Scholar]
- Lewitt RM. Processing of incomplete measurement data in computed tomography. Med Phys. 1979;6:412–7. doi: 10.1118/1.594519. [DOI] [PubMed] [Google Scholar]
- Li B, Avinash G, Claus B, Metz S. 3-D view weighted cone-beam filtered backprojection reconstruction for digital tomosynthesis. Proc SPIE. 2007;6510:65104X. [Google Scholar]
- Loncaric S, Chang W, Huang G. A processing technique for the truncated projections of asymmetric-fan-beam transmission imaging. IEEE Trans Nucl Sci. 1995;42:2292–7. [Google Scholar]
- Lu Y, Chan H-P, Wei J, Hadjiiski LM. Selective-diffusion regularization for enhancement of microcalcifications in digital breast tomosynthesis reconstruction. Med Phys. 2010;37:6003–14. doi: 10.1118/1.3505851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chan H-P, Wei J, Hadjiiski LM. Improving image quality of digital breast tomosynthesis by artifact reduction. Proc 11th Workshop on Digital Mammography (Lecture Notes in Computer Science. 2012;7361:745–52. [Google Scholar]
- Masnou S, Morel J-M. Level-lines based disocclusion. Proc 5th IEEE Int Conf on Image Processing. 1998:259–63. [Google Scholar]
- Müller M, Arce GR. Truncation artifacts in tomographic reconstruction from projections. Appl Opt. 1996;35:3902–14. doi: 10.1364/AO.35.003902. [DOI] [PubMed] [Google Scholar]
- Niklason LT, et al. Digital tomosynthesis in breast imaging. Radiology. 1997;205:399–406. doi: 10.1148/radiology.205.2.9356620. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nakajima M, Yuta SI. A reconstruction algorithms from truncated projections. IEEE Trans Med Imaging. 1984;3:34–40. doi: 10.1109/TMI.1984.4307648. [DOI] [PubMed] [Google Scholar]
- Ohnesorge B, Flohr T, Schwarz K, Heiken JP, Bae KT. Efficient correction for CT image artifacts caused by objects extending outside the scan field of view. Med Phys. 2000;27:39–46. doi: 10.1118/1.598855. [DOI] [PubMed] [Google Scholar]
- Poplack SP, Tosteson TD, Kogel CA, Nagy HM. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. Am J Roentgenol. 2007;189:616–23. doi: 10.2214/AJR.07.2231. [DOI] [PubMed] [Google Scholar]
- Rafferty EA, Georgian-Smith D, Kopans DB, Hall DA, Moore R, Wu T. Comparison of full-field digital tomosynthesis with two view conventional film screen mammography in the prediction of lesion malignancy. Radiology. 2002;225:268. [Google Scholar]
- Siddon RL. Fast calculation of the exact radiological path for a three-dimensional CT array. Med Phys. 1985;12:252–5. doi: 10.1118/1.595715. [DOI] [PubMed] [Google Scholar]
- Smith AP, Rafferty EA, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. Proc 9th Int Workshop on Digital Mammography (Lecture Notes in Computer Science. 2008;5116:61–6. [Google Scholar]
- Sompel DVd, Brady M. Systematic performance analysis of SART as applied to digital breast tomosynthesis. Proc 9th Int Workshop on Digital Mammography (Lecture Notes in Computer Science. 2008;5116:561–9. [Google Scholar]
- Sourbelle K, Kachelriess M, Kalender WA. Reconstruction from truncated projections in CT using adaptive detruncation. Eur Radiol. 2005;15:1008–14. doi: 10.1007/s00330-004-2621-9. [DOI] [PubMed] [Google Scholar]
- Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution x-ray imaging observer study. Radiology. 2012;262:788–96. doi: 10.1148/radiol.11103514. [DOI] [PubMed] [Google Scholar]
- Wu T, Moore RH, Rafferty EA, Kopans DB. A comparison of reconstruction algorithms for breast tomosynthesis. Med Phys. 2004a;31:2636–47. doi: 10.1118/1.1786692. [DOI] [PubMed] [Google Scholar]
- Wu T, Zhang J, Moore R, Rafferty E, Kopans D. Digital tomosynthesis mammography using a parallel maximum-likelihood reconstruction method. Proc SPIE. 2004b;5368:1–11. [Google Scholar]
- Wu T, et al. Tomographic mammography using a limited number of low-dose cone-beam projection images. Med Phys. 2003;30:365–80. doi: 10.1118/1.1543934. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chan H-P, Sahiner B, Wei J, Goodsitt MM, Hadjiiski LM, Ge J, Zhou C. A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis. Med Phys. 2006;33:3781–95. doi: 10.1118/1.223754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chan H-P, Sahiner B, Wei J, Zhou C, Hadjiiski LM. Artifact reduction methods for truncated projections in iterative breast tomosynthesis reconstruction. J Comput Assist Tomogr. 2009;33:426–35. doi: 10.1097/RCT.0b013e3181838000. [DOI] [PMC free article] [PubMed] [Google Scholar]