Abstract
Analysis of genes and proteins involved in lipid biosynthesis in mammary epithelial cells (MECs) is complicated by the presence of adipose tissue in the mammary gland, which may be predominant in whole tissue lysates depending upon developmental stage. We have developed a method based on protocols used to establish primary mammary epithelial cell cultures that allows for analysis of MECs depleted of adipose. Adipose depletion yields enriched MECs that are suitable for gene expression profiling and protein analysis from a single mouse. Additionally, the phosphorylation of proteins is maintained, allowing investigation of signal transduction events. Application of this method to the analysis of MECs from genetically modified mice will aid in the identification of factors controlling tissue-specific events in the mammary gland. In contrast to other methods such as laser capture microdissection, the MEC enrichment method described here is performed using standard lab supplies, equipment, and techniques.
Keywords: Mammary epithelial cell, Enrichment, Pregnancy, Lactation, Lipid metabolism, Epithelial specific, mRNA, Protein, Profiling
Introduction
The primary function of the mammary gland is to provide sustenance required for optimal growth of offspring who represent the next generation of that mammal. In the mouse, nearly 80% of calories needed for growth and brain development of the neonate is derived from lipids present in the milk primarily as triglycerides (TAGs). The mammary gland is a heterogeneous tissue that consists of multiple cell types. The epithelium and adipose are of principal importance to lipid metabolism and change dramatically in proportion to one another during pregnancy and lactation relative to the nulliparous gland. Also complicating the analysis of lipid metabolism in mammary epithelial cells is that both cell types employ the same suite of enzymes for TAG biosynthesis [1]. We have observed that the expression of these genes at the RNA and protein levels in MECs is often masked when examining whole tissue lysates (WTLs). In order to study lipid metabolism in the epithelium, a method was needed that depletes the MECs of ‘contaminating’ adipocytes. This adipose depletion method is derived from the initial steps of the MEC preparation used to make primary cultures developed by Owens [2], Pallan and Struli [3], and also outlined by Darcy et al. [4], capable of producing organoids or MECs to culture in vitro. For adipose depletion, generally MECs are separated from adipocytes directly ex-vivo, but rather than placing MECs in organoid culture, they are utilized for direct molecular analysis of mRNA and protein.
Materials
Two buffers are used in this method: MEC digestion buffer and Mammary Gland (MG) lysis buffer. Digestion buffer is used to deplete the minced mammary gland of adipose, while MG lysis buffer is used for protein analysis (see “Methods”). Additionally, Trizol (Invitrogen) is used for the extraction of total RNA to conduct gene expression profiling according to the manufacturer’s protocol. Trizol LS may also be used, but it should be noted that sodium acetate must be added to a final concentration of 0.3M, when the chloroform phase is transferred to a new tube but prior to isopropanol addition. It is also worth noting that hyaluronidase may be substituted for trypsin (http://www.bcm.edu/rosenlab/protocols/primaryMEC.pdf), because long incubations with trypsin will ultimately result in single MECs. Hyaluronidase should be used if intact multicellular organoids are desired. Standard disposables such as 15 mL and 50 mL conical tubes, 1.75 mL Eppendorf tubes, scalpels, and barrier pipette tips are used in this procedure.
Methods
Isolation of Mammary Epithelial Cells
Because these preparations are not used for culture, minimal sterile technique is required.
Anesthetize mice using carbon dioxide prior to euthanasia by cervical dislocation. (Follow your institutional guidelines).
Excise the 4th and 5th mammary glands using standard techniques and remove lymph nodes.
Snap-freeze two portions, approximately 30 mg each, for use in preparing WTLs.
Mince the remaining tissue to a medium-fine consistency (approx 2 min) using scalpels.
Add minced samples to 5 mL of pre-cooled MEC digestion buffer (Table 1) in a 50 mL conical tube.
Incubate samples for 25–30 min in a 37°C shaker at 150–180 rpm.
Transfer contents to 15 mL conical tube, bring up the digested cell suspension to 14 mL with ice cold PBS, and centrifuge at 275–300×G for 6 min.
Resuspend the cell pellet with ice cold PBS, bring to 14 mL, and centrifuge again (repeat twice).
Decant PBS and transfer approximately half of the resulting 200–400 μL packed mammary epithelial cells into 1 mL of Trizol for RNA analysis. Transfer the second half into 500 μL of MG lysis buffer (Table 1) for immunoblot analysis of proteins.
Table 1.
List of mammary epithelial cell digestion and mammary gland lysis buffer reagents used in the adipose depletion method.
| MEC digestion buffer | Final |
|---|---|
| DMEM/F12 medium (Sigma D6421) | 20 mL |
| Trypsin (Gibco 840-7250) | 30 mg |
| Collagenase A (Boehringer 1088793) | 60 mg |
| Sodium fluoride (Aldrich 450022) | 50 mM |
| Sodium orthovanadate (Aldrich 450243) | 1 mM |
| MG lysis buffer | Final |
| Tris pH 7.4 | 50 mM |
| Sodium chloride | 150 mM |
| EDTA | 2 mM |
| Triton X-100 | 1% |
| Deoxycholate | 1% |
| SDS | 0.1% |
| Sodium fluoride (Aldrich 450022) | 50 mM |
| Sodium orthovanadate (Aldrich 450243) | 1 mM |
| Phenylmethanesulfonyl fluoride (Sigma P7626) | 0.57 mM |
| EDTA-free Protease Inhibitor cocktail (Roche) | 20 μL/mL |
Note: it is important not to saturate the Trizol or the MG lysis buffer with too much tissue. Samples might need an additional half volume of Trizol or MG lysis buffer following homogenization.
Protein Isolation and Immunoblot Analysis
MG lysis buffer (Table 1) is used to extract proteins from both the MEC-enriched fraction and the WTL. Samples are homogenized using a Brinkmann Polytron (www.brinkmann.com) for 20–45 s on medium setting and lysates are cleared at 13,000×G for 25 min at 4°C. Protein concentrations are determined using the 660 nm Protein Assay (ThermoFisher #22660) according to the manufacturer’s protocols. Proteins are resolved using glycerol-based acrylamide SDS-PAGE 8% gels, and then transferred to PVDF membranes (Millipore). Immunoblotting is carried out using standard techniques. Antibodies directed towards phospho/total ACLY were obtained from Cell Signaling Technology (www.cellsignal.com; #4331 and #4332); anti-perilipin from Fitzgerald (www.fitzgerald-fii.com; #70R-PR004); anti-cytokeratin18 and actin from Santa Cruz (www.scbt.com; #sc-28264 and #sc-1616).
RNA Isolation
Each sample is homogenized using a Brinkmann Polytron for 20–45 s on medium setting and lysates are cleared at 13,000×G for 15 min at 4°C. RNA is isolated using Trizol, according to the manufacturer’s protocol. Washed RNA pellets are resuspended in 40–100 μL (volume depending on pellet size) of nuclease-free water and contaminants removed using the Qiagen RNeasy mini Plus protocol (www.qiagen.com; # 74134). Total RNA is quantified using the Nanodrop 1000 spectrophotometer (www.nanodrop.com). Total RNA integrity is examined using the Agilent Bioanalyzer Nanoscale Microfluidics Chip Assay (www.chem.agilent.com, 2100 Bioanalyzer). In our experience, all samples from MEC-enriched portions have been of sufficient integrity for use in quantitative real-time PCR (qPCR) analysis [5].
Quantitative Real Time PCR (qPCR) Analysis
Each cDNA reaction consists of 2.0 μg total RNA in 10 μL of nuclease free water plus 1.0 μL of random hexamers (50 μM) and 1.0 μL oligo dT (0.5 μg/μL) incubated at 70°C for 5 min. Reaction tubes are chilled on ice and spun down. A master mix contains 4 μL of 5× 1st strand buffer (Invitrogen), 2 μL of 0.1 M DTT, 1 μL of 10 mM dNTP blend (Roche), 1 μL RNase inhibitor (40 units/μL, Promega), and 1 μL MuLV reverse transcriptase (50 units/μL, Roche) per reaction. cDNA is diluted 1:10 and 5 μL are input into the qPCR reactions (representing 50 ng total RNA/reaction). All primer/probe sets have been ordered from Integrated DNA Technologies (www.IDTDNA.com). A reaction master mix is made including 12.5 μL of Absolute Fast qPCR Mix-Lox Rox (www.ThermoFisher.com), 2.5 μL of 10× primer/probe mix (5.0 μM forward, 5.0 μM reverse, and 2.5 μM probe), 5.0 μL of nuclease-free water, and 5.0 μL of 1:10 diluted cDNA per reaction. qPCR data are collected on the Applied Biosystems 7500 Fast thermocycler (www.appliedbiosystems.com). Copy numbers are calculated using the standard curve method (1.204 e7 stepping down 5-fold to 7.70 e2). In our hands, all standard curves have had near optimal slopes of −3.3, y-intercepts near 40, R correlations near 1, PCR efficiencies near 100%, and each target amplified within the standard curve.
Results
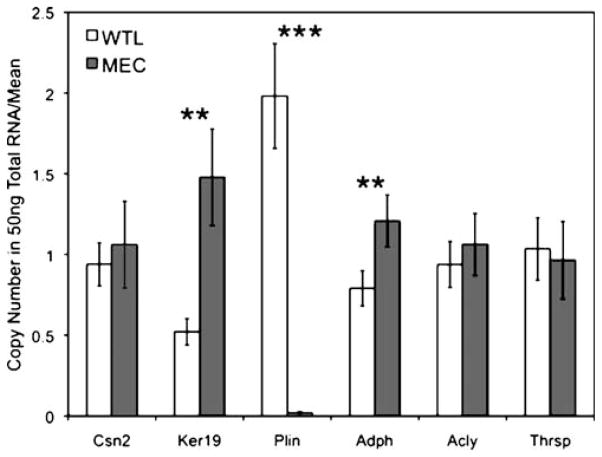
Minced whole mammary gland tissue digested in a collagenase and trypsin solution yields ductal and alveolar epithelium depleted of adipocytes to produce a MEC-enriched fraction from an otherwise heterogeneous tissue. qPCR based gene expression profiling was used to measure the copy number for several target genes (Fig. 1). WTL and MEC samples from lactation day 4 are from the same mouse, and the data are plotted as a ratio to the mean expression of each gene due to the dynamic range of absolute expression (error bars are SEM, n=4 mice). Figure 1 shows the level of two MEC specific genes, β-casein (Csn2) and cytokeratin 19 (Ker19). In this case, the levels of Csn2 mRNA did not change; perhaps because the mRNA for this major milk protein is highly expressed at lactation day 4 (108 copies/50 ng total RNA). The lack of any statistically significant reduction in Csn2 mRNA following adipose depletion suggests that this differentiation-specific gene, known to be regulated by ECM attachment [6], is not affected by dissociation from the ECM during the incubation time required in this protocol. Consistent with epithelial enrichment, the amount of the less abundant Ker19 and ADPH mRNAs (104 copies/50 ng total RNA) increased statistically (p=0.011 and 0.03, respectively) following adipose depletion. The level of the adipocyte-specific gene perilipin (PLIN) is noticeably diminished (<110 fold difference, p=0.00046) in the adipocyte-depleted fraction, indicating the significant loss of adipose contribution to gene expression profiles using this method. Interestingly, two genes known to be highly expressed in tissues that actively synthesize fatty acids, ACLY and THRSP (also known as Spot14) [7, 8], are unchanged in the MEC-enriched fraction.
Figure 1.
Quantitative PCR analysis of gene expression in WTLs and MEC-enriched samples. Copy numbers for β-casein (Csn2), cytokeratin 19 (Ker19), perilipin (Plin), adipophilin (Adph), ATP citrate lyase (Acly) and thyroid-hormone responsive protein (Thrsp) are plotted as the ratio to the mean copy number over all samples for that respective gene in order to display all targets on a comparable scale. Error bars are SEM, n=4 mice per group, ** is p≤0.03, ***p≤0.0005.
In addition to being applicable for gene expression profiling, the adipocyte depletion method also provides protein samples for immunoblot analysis. In Fig. 2a, evaluation of MEC enriched samples at both late pregnancy and early lactation (P17 and Lac2) confirmed that adipose-specific perilipin is markedly reduced in the MEC samples but is abundant in WTLs. The levels of both adipophilin and cytokeratin 18 (Ker18) are preserved in the MEC fraction, as anticipated when the epithelium is depleted of adipose (actin was used as a loading control). Figure 2b shows a comparison of WTLs versus MECs across a developmental time series from late pregnancy (P17) through early and into mid lactation (Lac2 and Lac9, respectively). In each case, both the WTL and the MEC fractions were isolated from a single mouse to allow direct comparison, and were analyzed for protein levels of THRSP, with Ker18 shown as a loading control. During this developmental series, the amount of THRSP protein appears to be unchanged in the WTLs; however, in the MEC fraction the amount of THRSP is strongly induced by Lac9. Importantly, these changes in the pattern of MEC-specific expression would not have been observed using only WTLs.
Figure 2.
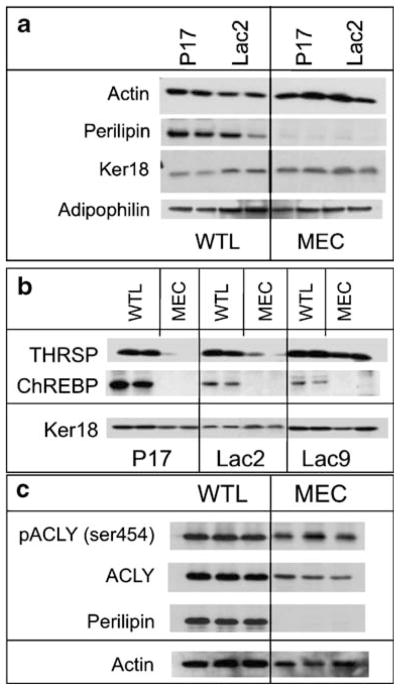
Immunoblot analysis of proteins in WTL and enriched MEC fractions during late pregnancy and lactation. a Immunoblot analysis of perilipin, cytokeratin 18, and adipophilin in duplicate WTL and adipose-depleted MEC preparations at pregnancy day 17 (P17) and lactation day 2 (Lac2). Note the reduction of adipocyte-specific perilipin following adipose depletion in the MEC fraction. Ker18 and ADPH remain in both fractions and actin was used as a loading control. b Immunoblot analysis of THRSP and ChREBP in WTL and MEC fractions from duplicate mice at pregnancy day 17 (P17) and lactation days 2 and 9 (Lac2 and Lac9, respectively). Ker18 was used as a loading control. c Immunoblot analysis in triplicate of phospho-ACLY (ser454), total ACLY, and perilipin in WTL and MEC preparations at lactation day 4. Actin was used as a loading control.
In contrast to the levels of THRSP, the carbohydrate responsive element binding protein (ChREBP), known to regulate the de novo fatty acid synthesis pathway in the liver [9], does not appear to be expressed in the MECs and its expression in WTLs decreases from the levels seen in late pregnancy. These observations indicate the adipose-specific expression of ChREBP, and suggest that activity of the transcription factor in MECs is dispensable during late pregnancy and lactation. Conversely, THRSP protein exists in both epithelium and adipocytes, and the robust change in its MEC expression suggests that it is important during mid-lactation as evidenced by the protein abundance at Lac9 (Fig. 2b). This observation is also consistent with the lactation defect documented by Zhu et al. in THRSP null mice [10].
Another feature of the adipose depletion method is that post-translational protein phosphorylation is preserved by the addition of phosphatase inhibitors to both the MEC digestion and MG lysis buffers. Figure 2c shows the levels of phosphorylated ACLY (ser454) and total ACLY at Lac4 in WTL and MEC samples. Again, perilipin denotes the marked depletion of adipocytes from the MEC fraction, and actin was used as a loading control. In each case, this method reliably depleted the adipose compartment from the whole mammary gland, which allowed dissection of lipid metabolism components unique to the mammary epithelium.
Discussion
Lipid biosynthesis in the mammary gland occurs in both the alveolar epithelium and the adipocytes, and both compartments synthesize TAGs from small molecule precursors and preformed fatty acids, although not necessarily at the same developmental stage. During lactation the majority of the gland appears to be epithelial in nature; however, adipocytes remain present and may be less metabolically active. Potential uses for this method include examination of samples from mid- and late-pregnant mice versus samples from lactating mice for developmental changes that occur at secretory activation that are unique to the epithelium. The true utility of this method is the investigation of MEC samples from genetically modified mice in comparison to wildtype controls. In that context, genes that control developmental changes unique to the epithelium could be identified. Recently, we used this technique successfully to analyze MEC-specific events that occur in conditional knockout mice with severely impaired lipid biosynthesis [11].
While we have used this method to analyze MEC-specific events in mice bearing a conditional MEC knockout, this approach has great utility to examine changes in the MEC compartment of transgenic or whole animal knockouts. For example, tissue specific analysis of Src family kinases or Akt isoform deletions would be informative since these genes are expressed in both the epithelial and adipose compartments. Likewise, the transcription factors C/EBPβ and C/EBPδ are both involved in mammary epithelial function during pregnancy [12] and involution [13], respectively, and each is induced during the early stages of pre-adipocyte differentiation [14, 15]. The use of whole tissues to analyze developmental changes that occur in specific compartments throughout the cycle of mammary gland development is likely to provide new insights that could not have been elucidated without this approach using standard gene expression profiling or immunoblotting techniques. For example, models incorporating mammary epithelial expression of ChREBP can be discarded when one understands that the transcription factor does not appear to be expressed in mammary epithelium. Clearly, this method of MEC enrichment by adipose depletion is critical for any experiments designed to study processes involving lipid and fatty acid metabolism in one tissue compartment versus the other.
As an alternative (or supplement) to the method we have described, one could use laser capture microdissection (LCM) to isolate the different cell types. Investigators at our institution have successfully used LCM; however, this approach is more complicated, it requires specialized equipment plus costly downstream reagents, and it does not generate a high yield of total RNA or protein. In contrast, the method outlined here to isolate adipocyte-depleted MECs can be done using standard lab equipment and reagents, and both total RNA and protein can be extracted from whole tissue and adipose depleted samples excised from a single animal. Thus, the adipose depletion method allows direct comparison of MECs with whole tissue from the same animal that can be used for rapid and inexpensive screening of MEC specific genes and proteins. Finally, adipose depletion nicely complements additional, more time consuming and/or costly assays, including immunofluorescence, in situ hybridization, and LCM.
Limitations/Improvements
Some limitations to the adipose depletion method do exist. One disadvantage is that an enriched population of adipocytes cannot be prepared. During the digestion and wash portion of the procedure, the adipocytes float, as does the milk fat and other buoyant debris, making analysis of this fraction problematic. In addition, other cell types may inadvertently spin down with the MECs, such as red blood cells and myoepithelium; and these cells may obscure any desired results. Furthermore, this method may be relevant only to studies of lipid metabolism or studies that examine the MECs relative to adipocyte components. We have not yet evaluated this method for components outside of lipid metabolism, and the possibility exists that genes induced during early stages of involution could be activated by this protocol. The analysis of nulliparous and early pregnancy samples is impractical because there is not enough alveolar epithelium present for high yields from a single mouse. In contrast to these limitations, since the MECs are alive following disaggregation of the whole gland, these samples could be treated with pharmacological agents, isotope tracers, etc. to analyze downstream effects. Potentially one could acutely stimulate the isolated organoids with growth factors or steroid hormones to observe any induction of immediate early response genes. However, long-term incubation of organoids could induce apoptotic responses similar to involution. None of these possibilities have yet been evaluated since we have immediately lysed the isolated MECs for direct biochemical analysis of lipid metabolism components. Finally, we are pursuing studies to couple this method following up-the-teat adenoviral injections [16] into late pregnant glands to investigate effects of exogenous transgenes and RNAi’s during lactation in lieu of making transgenic mice.
Acknowledgments
The authors thank the members of the Mammary Gland Biology Program Project Grant at the University of Colorado Denver for discussions during the development of this technique. SMA is supported by a grant from the Public Health Service PO1-HD38129. MCR is supported by a Department of Defense Predoctoral Fellowship BC 810596.
Abbreviations
- ADPH
Adipophilin
- ACLY
ATP Citrate Lyase
- Csn2
β-casein
- ChREBP
Carbohydrate Responsive Element Binding Protein
- C/EBPβ and C/EBPδ
CCAAT/enhancer-binding protein beta and delta
- Ker18
Ker19, Cytokeratin 18 and 19
- LCM
Laser Capture Micro-dissection
- MECs
Mammary Epithelial Cells
- PLIN
Perilipin
- qPCR
quantitative Real Time PCR
- THRSP, Spot14
Thyroid Hormone Responsive Protein Spot 14
- TAGs
triglycerides
- WTLs
Whole mammary Tissue Lysates
Contributor Information
Michael C. Rudolph, Department of Pathology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045, USA. Program in Molecular Biology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045, USA
Elizabeth A. Wellberg, Department of Pathology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045, USA
Steven M. Anderson, Email: Steve.Anderson@ucdenver.edu, Department of Pathology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045, USA. Program in Molecular Biology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO 80045, USA. School of Medicine, Department of Pathology, University of Colorado Denver, MS 8104, 12801 East 17th Avenue, P.O. Box 6500, Aurora, CO 80045, USA
References
- 1.Rudolph MC, Neville MC, Anderson SM. Lipid synthesis in lactation: diet and the fatty acid switch. J Mammary Gland Biol Neoplasia. 2007;12(4):269–81. doi: 10.1007/s10911-007-9061-5. [DOI] [PubMed] [Google Scholar]
- 2.Owens IS, Vonderhaar BK, Topper YJ. Concerning the necessary coupling of development to proliferation of mouse mammary epithelial cells. J Biol Chem. 1973;248(2):472–7. [PubMed] [Google Scholar]
- 3.Harris A. Handbooks in practical animal cell biology. Cambridge: Cambridge University Press; 1996. Epithelial cell culture; p. xii.p. 182. [Google Scholar]
- 4.Ip MM, Asch BB. Methods in mammary gland biology and breast cancer research. New York: Kluwer Academic/Plenum; 2000. p. xvi.p. 329. [4] leaves of col. plates. [Google Scholar]
- 5.Schroeder A, et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pujuguet P, et al. Trichostatin A inhibits beta-casein expression in mammary epithelial cells. J Cell Biochem. 2001;83(4):660–70. doi: 10.1002/jcb.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berwick DC, et al. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277(37):33895–900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 8.Brown SB, Maloney M, Kinlaw WB. “Spot 14” protein functions at the pretranslational level in the regulation of hepatic metabolism by thyroid hormone and glucose. J Biol Chem. 1997;272 (4):2163–6. [PubMed] [Google Scholar]
- 9.Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63 (12):2075–80. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, et al. The Spot 14 protein is required for de novo lipid synthesis in the lactating mammary gland. Endocrinology. 2005;146(8):3343–50. doi: 10.1210/en.2005-0204. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph M, et al. Mammary epithelial specific deletion of escort protein SCAP reveals SREBF-dependent and independent fatty acid synthesis control during lactation. 2009 Submitted. [Google Scholar]
- 12.Raught B, Liao WS, Rosen JM. Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence beta-casein gene expression. Mol Endocrinol. 1995;9(9):1223–32. doi: 10.1210/mend.9.9.7491114. [DOI] [PubMed] [Google Scholar]
- 13.Thangaraju M, et al. C/EBPdelta is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development. 2005;132(21):4675–85. doi: 10.1242/dev.02050. [DOI] [PubMed] [Google Scholar]
- 14.Yeh WC, et al. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9(2):168–81. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T, et al. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16 (24):7432–43. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell TD, et al. Transduction of the mammary epithelium with adenovirus vectors in vivo. J Virol. 2003;77(10):5801–9. doi: 10.1128/JVI.77.10.5801-5809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]